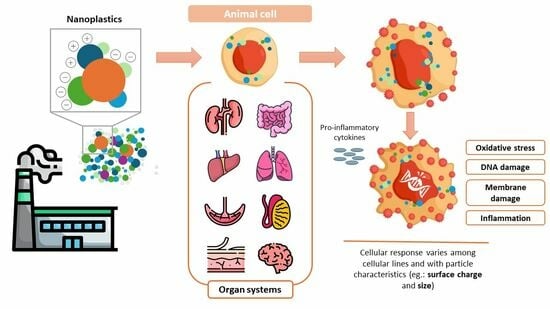
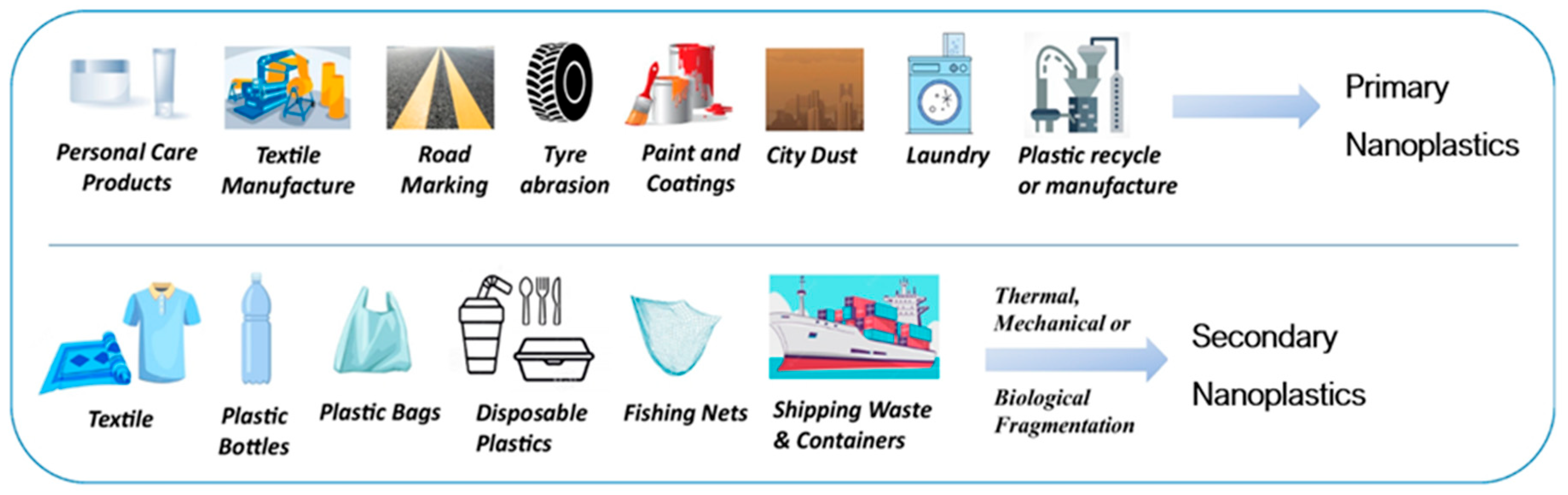
Assessing the Impact of Nanoplastics in Biological Systems: Systematic Review of In Vitro Animal Studies
Abstract
1. Introduction
2. Materials and Methods
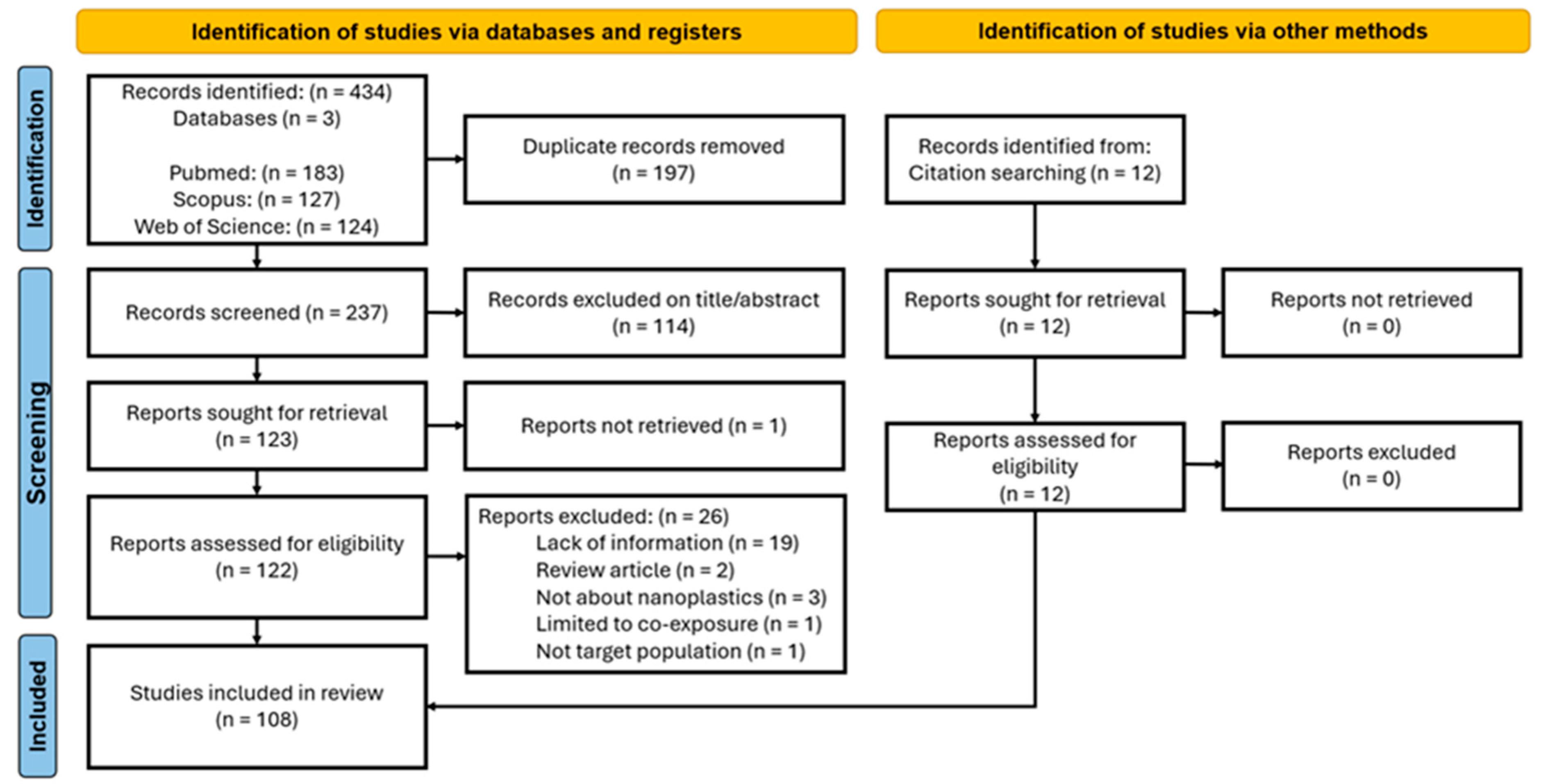
2.1. Searches and Eligibility Criteria
2.2. Data Extraction and Quality Assessment
2.3. Data Visualization
3. Results
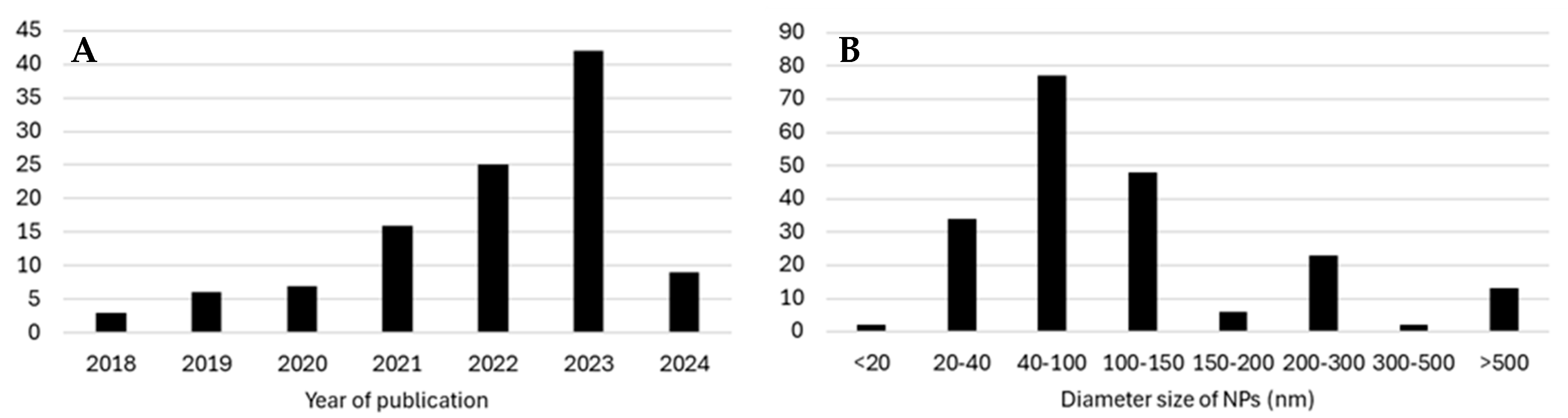
3.1. Overall Results
3.2. Results by NP Polymer Type and Size
3.3. Results by Biological System
3.4. Reporting Quality of the Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AOP | adverse outcome pathway |
| A-PS | amine-modified polystyrene |
| C-PS | carboxyl-modified polystyrene |
| ECHA | European Chemicals Agency |
| MMP | mitochondrial membrane potential |
| NP | nanoplastics |
| PET | polyethylene terephthalate |
| PE | polyethylene |
| PC | polycarbonate |
| PLA | polylactic acid |
| PMMA | polymethyl methacrylate |
| PP | polypropylene |
| PS | polystyrene |
| PTFE | polytetrafluoroethylene |
| ROS | reactive oxygen species |
| Sa-PS | sulfonic acid-modified polystyrene |
| S-PS | sulfate-modified polystyrene |
| uPS | unmodified polystyrene |
| wPET | weathered polyethylene terephthalate |
| wPS | weathered polystyrene |
References
- Sangkham, S.; Faikhaw, O.; Munkong, N.; Sakunkoo, P.; Arunlertaree, C.; Chavali, M.; Mousazadeh, M.; Tiwari, A. A Review on Microplastics and Nanoplastics in the Environment: Their Occurrence, Exposure Routes, Toxic Studies, and Potential Effects on Human Health. Mar. Pollut. Bull. 2022, 181. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Moore, C.J.; vom Saal, F.S.; Swan, S.H. Plastics, the Environment and Human Health: Current Consensus and Future Trends. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L.; Neal, M.A. Applications and Societal Benefits of Plastics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- COMMISSION REGULATION (EU) …/… of XXX Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Synthetic Polyme. Available online: https://ec.europa.eu/transparency/comitology-register/screen/documents/083921/1/consult?lang=en (accessed on 5 May 2024).
- Pelegrini, K.; Carneiro, T.; Pereira, B.; Garcia, T.; De Souza, L.; Regina, N.; Basso, D.S.; Ligia, G.; De Galland, B.; Angelica, R.; et al. Science of the Total Environment Micro- and Nanoplastic Toxicity: A Review on Size, Type, Source, and Test-Organism Implications. Sci. Total Environ. 2023, 878. [Google Scholar] [CrossRef]
- Logvina, Y.; Matas, I.M.; Ribeiro, H.; Pinto da Silva, L.; Rodrigues, P.; Leitão, J.; da Silva, J.E. Micro- and Nanoplastics in the Atmosphere: Methodology for Microplastics Size-Fractionation Sampling. Microplastics 2024, 3, 82–97. [Google Scholar] [CrossRef]
- Gigault, J.; ter Halle, A.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current Opinion: What Is a Nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Yong, C.Q.; Valiyaveettil, S.; Tang, B.L. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef]
- Panel on Contaminants in the Food Chain, E. Presence of Microplastics and Nanoplastics in Food, with Particular Focus on Seafood. EFSA J. 2016, 14, e04501. [Google Scholar] [CrossRef]
- Rios Mendoza, L.M.; Karapanagioti, H.; Álvarez, N.R. Micro(Nanoplastics) in the Marine Environment: Current Knowledge and Gaps. Curr. Opin. Environ. Sci. Health 2018, 1, 47–51. [Google Scholar] [CrossRef]
- Ali, N.; Katsouli, J.; Marczylo, E.L.; Gant, T.W.; Wright, S.; Bernardino de la Serna, J. The Potential Impacts of Micro-and-Nano Plastics on Various Organ Systems in Humans. EBioMedicine 2024, 99. [Google Scholar] [CrossRef]
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence That the Great Pacific Garbage Patch Is Rapidly Accumulating Plastic. Sci. Rep. 2018, 8, 4666. [Google Scholar] [CrossRef] [PubMed]
- Richard, C. Thompson Microplastics in the Marine Environment: Sources, Consequences and Solutions. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 185–200. ISBN 978-3-319-16509-7. [Google Scholar]
- Bermúdez, J.R.; Swarzenski, P.W. A Microplastic Size Classification Scheme Aligned with Universal Plankton Survey Methods. MethodsX 2021, 8, 101516. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.S. Micro- and Nano-Plastics and Human Health. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 343–366. ISBN 978-3-319-16510-3. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2024, 3, e1700782. [Google Scholar] [CrossRef]
- Yee, M.S.-L.; Hii, L.-W.; Looi, C.K.; Lim, W.-M.; Wong, S.-F.; Kok, Y.-Y.; Tan, B.-K.; Wong, C.-Y.; Leong, C.-O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Sharma, S.; Bhardwaj, A.; Thakur, M.; Saini, A. Understanding Microplastic Pollution of Marine Ecosystem: A Review. Environ. Sci. Pollut. Res. 2023, 31, 41402–41445. [Google Scholar] [CrossRef]
- Moeck, C.; Davies, G.; Krause, S.; Schneidewind, U. Microplastics and Nanoplastics in Agriculture—A Potential Source of Soil and Groundwater Contamination? Grundwasser 2023, 28, 23–35. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, L.; Li, J. A Critical Review on the Sources and Instruments of Marine Microplastics and Prospects on the Relevant Management in China. Waste Manag. Res. 2018, 36, 898–911. [Google Scholar] [CrossRef]
- Tamburri, M.N.; Soon, Z.Y.; Scianni, C.; Øpstad, C.L.; Oxtoby, N.S.; Doran, S.; Drake, L.A. Understanding the Potential Release of Microplastics from Coatings Used on Commercial Ships. Front. Mar. Sci. 2022, 9, 1074654. [Google Scholar] [CrossRef]
- Mayer, P.M.; Moran, K.D.; Miller, E.L.; Brander, S.M.; Harper, S.; Garcia-Jaramillo, M.; Carrasco-Navarro, V.; Ho, K.T.; Burgess, R.M.; Thornton Hampton, L.M.; et al. Where the Rubber Meets the Road: Emerging Environmental Impacts of Tire Wear Particles and Their Chemical Cocktails. Sci. Total Environ. 2024, 927, 171153. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wu, T.; Wang, X.; Song, Z.; Zong, C.; Wei, N.; Li, D. Consistent Transport of Terrestrial Microplastics to the Ocean through Atmosphere. Environ. Sci. Technol. 2019, 53, 10612–10619. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Santos, T.; Duarte, A.C. A Critical Overview of the Analytical Approaches to the Occurrence, the Fate and the Behavior of Microplastics in the Environment. TrAC Trends Anal. Chem. 2015, 65, 47–53. [Google Scholar] [CrossRef]
- Cunningham, B.E.; Sharpe, E.E.; Brander, S.M.; Landis, W.G.; Harper, S.L. Critical Gaps in Nanoplastics Research and Their Connection to Risk Assessment. Front. Toxicol. 2023, 5, 1154538. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Fang, T.; Xu, P.; Zhu, L.; Li, D. Source and Potential Risk Assessment of Suspended Atmospheric Microplastics in Shanghai. Sci. Total Environ. 2019, 675, 462–471. [Google Scholar] [CrossRef]
- Bhat, M.A.; Gedik, K.; Gaga, E.O. Atmospheric Micro (Nano) Plastics: Future Growing Concerns for Human Health. Air Qual. Atmos. Health 2023, 16, 233–262. [Google Scholar] [CrossRef]
- Sun, X.; Song, R.; Liu, J.; Yan, S.; Li, Y.; Jin, X.; Liang, Y.; Wu, Y.; Mei, L.; Pan, R.; et al. Characterization of Airborne Microplastics at Different Workplaces of the Poly(Ethylene:Propylene:Diene) (EPDM) Rubber Industry. Environ. Sci. Pollut. Res. Int. 2023, 30, 78839–78848. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic Contamination in an Urban Area: A Case Study in Greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar] [CrossRef]
- Bhat, M.A.; Eraslan, F.N.; Gedik, K.; Gaga, E.O. Impact of Textile Product Emissions: Toxicological Considerations in Assessing Indoor Air Quality and Human Health. In Ecological and Health Effects of Building Materials; Malik, J.A., Marathe, S., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 505–541. ISBN 978-3-030-76073-1. [Google Scholar]
- Bergmann, M.; Mützel, S.; Primpke, S.; Tekman, M.B.; Trachsel, J.; Gerdts, G. White and Wonderful? Microplastics Prevail in Snow from the Alps to the Arctic. Sci. Adv. 2024, 5, eaax1157. [Google Scholar] [CrossRef]
- Peng, M.; Vercauteren, M.; Grootaert, C.; Catarino, A.I.; Everaert, G.; Rajkovic, A.; Janssen, C.; Asselman, J. Bioenergetic Effects of Pristine and Ultraviolet-Weathered Polydisperse Polyethylene Terephthalate and Polystyrene Nanoplastics on Human Intestinal Caco-2 Cells. Sci. Total Environ. 2024, 908, 168267. [Google Scholar] [CrossRef]
- Hua, T.; Kiran, S.; Li, Y.; Sang, Q.-X.A. Microplastics Exposure Affects Neural Development of Human Pluripotent Stem Cell-Derived Cortical Spheroids. J. Hazard. Mater. 2022, 435, 128884. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Liu, Z.; Yu, B.; Zhang, Y.; Yang, H.; Han, Y.; Wang, B.; Liu, Z.; Zhang, H. Emergence of Nanoplastics in the Aquatic Environment and Possible Impacts on Aquatic Organisms. Sci. Total Environ. 2024, 906, 167404. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Wang, L.; Gao, J.; Jiang, Y.; Adeel, M.; Hou, D. Nanoplastic–Plant Interaction and Implications for Soil Health. Soil Use Manag. 2023, 39, 13–42. [Google Scholar] [CrossRef]
- Sarma, H.; Basumatary, T.; Yousaf, B.; Narayan, M. Nanoplastics and Lithium Accumulation in Soil–Plant Systems: Assessing Uptake, Toxicological Effects, and Potential Synergistic Interactions. Curr. Res. Biotechnol. 2024, 7, 100170. [Google Scholar] [CrossRef]
- Bhat, R.A.H.; Sidiq, M.J.; Altinok, I. Impact of Microplastics and Nanoplastics on Fish Health and Reproduction. Aquaculture 2024, 590, 741037. [Google Scholar] [CrossRef]
- Fang, M.; Liao, Z.; Ji, X.; Zhu, X.; Wang, Z.; Lu, C.; Shi, C.; Chen, Z.; Ge, L.; Zhang, M.; et al. Microplastic Ingestion from Atmospheric Deposition during Dining/Drinking Activities. J. Hazard. Mater. 2022, 432, 128674. [Google Scholar] [CrossRef]
- He, T.; Qu, Y.; Yang, X.; Liu, L.; Xiong, F.; Wang, D.; Liu, M.; Sun, R. Research Progress on the Cellular Toxicity Caused by Microplastics and Nanoplastics. J. Appl. Toxicol. 2023, 43, 1576–1593. [Google Scholar] [CrossRef]
- D, M.; Tulasi, C.D.S.L.N.; Chepuri, K. Cellular and Animal Toxicities of Micro- and Nanoplastics. In Micro and Nanoplastics in Soil: Threats to Plant-Based Food; Maddela, N.R., Reddy, K.V., Ranjit, P., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 261–292. ISBN 978-3-031-21195-9. [Google Scholar]
- Li, Y.; Tao, L.; Wang, Q.; Wang, F.; Li, G.; Song, M. Potential Health Impact of Microplastics: A Review of Environmental Distribution, Human Exposure, and Toxic Effects. Environ. Health 2023, 1, 249–257. [Google Scholar] [CrossRef]
- Yang, Z.; DeLoid, G.M.; Zarbl, H.; Baw, J.; Demokritou, P. Micro- and Nanoplastics (MNPs) and Their Potential Toxicological Outcomes: State of Science, Knowledge Gaps and Research Needs. NanoImpact 2023, 32, 100481. [Google Scholar] [CrossRef]
- Pikuda, O.; Xu, E.G.; Berk, D.; Tufenkji, N. Toxicity Assessments of Micro- and Nanoplastics Can Be Confounded by Preservatives in Commercial Formulations. Environ. Sci. Technol. Lett. 2019, 6, 21–25. [Google Scholar] [CrossRef]
- Domenech, J.; de Britto, M.; Velázquez, A.; Pastor, S.; Hernández, A.; Marcos, R.; Cortés, C. Long-Term Effects of Polystyrene Nanoplastics in Human Intestinal Caco-2 Cells. Biomolecules 2021, 11, 1442. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Barguilla, I.; Domenech, J.; Marcos, R.; Hernández, A. Biological Effects, Including Oxidative Stress and Genotoxic Damage, of Polystyrene Nanoparticles in Different Human Hematopoietic Cell Lines. J. Hazard. Mater. 2020, 398, 122900. [Google Scholar] [CrossRef] [PubMed]
- Poma, A.; Vecchiotti, G.; Colafarina, S.; Zarivi, O.; Aloisi, M.; Arrizza, L.; Chichiriccò, G.; Di Carlo, P. In Vitro Genotoxicity of Polystyrene Nanoparticles on the Human Fibroblast Hs27 Cell Line. Nanomaterials 2019, 9, 1299. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, P.M.; Saranya, V.; Vijayakumar, S.; Mythili Meera, M.; Ruprekha, S.; Kunal, R.; Pranay, A.; Thomas, J.; Mukherjee, A.; Chandrasekaran, N. Assessment on Interactive Prospectives of Nanoplastics with Plasma Proteins and the Toxicological Impacts of Virgin, Coronated and Environmentally Released-Nanoplastics. Sci. Rep. 2019, 9, 8860. [Google Scholar] [CrossRef]
- Damaj, S.; Trad, F.; Goevert, D.; Wilkesmann, J. Bridging the Gaps between Microplastics and Human Health. Microplastics 2024, 3, 46–66. [Google Scholar] [CrossRef]
- Masseroni, A.; Rizzi, C.; Urani, C.; Villa, S. Nanoplastics: Status and Knowledge Gaps in the Finalization of Environmental Risk Assessments. Toxics 2022, 10, 270. [Google Scholar] [CrossRef]
- Dang, F.; Wang, Q.; Huang, Y.; Wang, Y.; Xing, B. Key Knowledge Gaps for One Health Approach to Mitigate Nanoplastic Risks. Eco-Environ. Health 2022, 1, 11–22. [Google Scholar] [CrossRef]
- Wang, Z.; Litterio, M.C.; Müller, M.; Vauzour, D.; Oteiza, P.I. (-)-Epicatechin and NADPH Oxidase Inhibitors Prevent Bile Acid-Induced Caco-2 Monolayer Permeabilization through ERK1/2 Modulation. Redox Biol. 2020, 28, 101360. [Google Scholar] [CrossRef]
- Meunier, V.; Bourrié, M.; Berger, Y.; Fabre, G. The Human Intestinal Epithelial Cell Line Caco-2; Pharmacological and Pharmacokinetic Applications. Cell Biol. Toxicol. 1995, 11, 187–194. [Google Scholar] [CrossRef]
- Lockwood, C.; Aromataris, E.; Porritt, K.; Pilla, B.; Jordan, Z. (Eds.) Editors JBI Manual for Evidence Synthesis; JBI: Miami, FL, USA, 2024; ISBN 9780648848820. [Google Scholar]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Chierrito, D.; Villas-Boas, C.B.; Tonin, F.S.; Fernandez-Llimos, F.; Sanches, A.C.C.; de Mello, J.C.P. Using Cell Cultures for the Investigation of Treatments for Attention Deficit Hyperactivity Disorder: A Systematic Review. Curr. Neuropharmacol. 2019, 17, 916–925. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualising risk-of-bias assessments. Res. Synth. Methods 2020, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhu, Y.; Zhang, J.; Xu, Y.; Wu, J.; Zeng, W.; Lin, Y.; Liu, X. The Potential Toxicity of Polystyrene Nanoplastics to Human Trophoblasts in Vitro. Environ. Pollut. 2022, 311, 119924. [Google Scholar] [CrossRef]
- Djapovic, M.; Apostolovic, D.; Postic, V.; Lujic, T.; Jovanovic, V.; Stanic-Vucinic, D.; van Hage, M.; Maslak, V.; Cirkovic Velickovic, T. Characterization of Nanoprecipitated PET Nanoplastics by 1H NMR and Impact of Residual Ionic Surfactant on Viability of Human Primary Mononuclear Cells and Hemolysis of Erythrocytes. Polymers 2023, 15, 4703. [Google Scholar] [CrossRef]
- Tan, Y.; Zhu, X.; Wu, D.; Song, E.; Song, Y. Compromised Autophagic Effect of Polystyrene Nanoplastics Mediated by Protein Corona Was Recovered after Lysosomal Degradation of Corona. Environ. Sci. Technol. 2020, 54, 11485–11493. [Google Scholar] [CrossRef]
- Ding, R.; Chen, Y.; Shi, X.; Li, Y.; Yu, Y.; Sun, Z.; Duan, J. Size-Dependent Toxicity of Polystyrene Microplastics on the Gastrointestinal Tract: Oxidative Stress Related-DNA Damage and Potential Carcinogenicity. Sci. Total Environ. 2024, 912, 169514. [Google Scholar] [CrossRef]
- Lim, S.L.; Ng, C.T.; Zou, L.; Lu, Y.; Chen, J.; Bay, B.H.; Shen, H.-M.; Ong, C.N. Targeted Metabolomics Reveals Differential Biological Effects of Nanoplastics and NanoZnO in Human Lung Cells. Nanotoxicology 2019, 13, 1117–1132. [Google Scholar] [CrossRef]
- Vela, L.; Villacorta, A.; Venus, T.; Estrela-Lopis, I.; Pastor, S.; García-Rodriguez, A.; Rubio, L.; Marcos, R.; Hernández, A. The Potential Effects of in Vitro Digestion on the Physicochemical and Biological Characteristics of Polystyrene Nanoplastics. Environ. Pollut. 2023, 329, 121656. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Z.; Liu, Y.; Mei, A.; Wang, X.; Shi, Q. Cellular Absorption of Polystyrene Nanoplastics with Different Surface Functionalization and the Toxicity to RAW264.7 Macrophage Cells. Ecotoxicol. Environ. Saf. 2023, 252, 114574. [Google Scholar] [CrossRef]
- Xu, X.; Feng, Y.; Han, C.; Yao, Z.; Liu, Y.; Luo, C.; Sheng, J. Autophagic Response of Intestinal Epithelial Cells Exposed to Polystyrene Nanoplastics. Environ. Toxicol. 2023, 38, 205–215. [Google Scholar] [CrossRef]
- Chen, W.; Chu, Q.; Ye, X.; Sun, Y.; Liu, Y.; Jia, R.; Li, Y.; Tu, P.; Tang, Q.; Yu, T.; et al. Canidin-3-Glucoside Prevents Nano-Plastics Induced Toxicity via Activating Autophagy and Promoting Discharge. Environ. Pollut. 2021, 274, 116524. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wen, Y.; Zhang, F.; Fu, Z.; Yuan, Y.; Kuang, H.; Kuang, X.; Huang, J.; Zheng, L.; Zhang, D. Exposure to Nanoplastics Induces Mitochondrial Impairment and Cytomembrane Destruction in Leydig Cells. Ecotoxicol. Environ. Saf. 2023, 255, 114796. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Cheng, Y.; Chen, Z.; Liu, T.; Yin, L.; Pu, Y.; Liang, G. In Vitro Evaluation of Nanoplastics Using Human Lung Epithelial Cells, Microarray Analysis and Co-Culture Model. Ecotoxicol. Environ. Saf. 2021, 226, 112837. [Google Scholar] [CrossRef] [PubMed]
- DeLoid, G.M.; Cao, X.; Bitounis, D.; Singh, D.; Llopis, P.M.; Buckley, B.; Demokritou, P. Toxicity, Uptake, and Nuclear Translocation of Ingested Micro-Nanoplastics in an in Vitro Model of the Small Intestinal Epithelium. Food Chem. Toxicol. 2021, 158, 112609. [Google Scholar] [CrossRef]
- Martin-Folgar, R.; González-Caballero, M.C.; Torres-Ruiz, M.; Cañas-Portilla, A.I.; de Alba González, M.; Liste, I.; Morales, M. Molecular Effects of Polystyrene Nanoplastics on Human Neural Stem Cells. PLoS ONE 2024, 19, e0295816. [Google Scholar] [CrossRef]
- Fu, Y.; Fan, M.; Xu, L.; Wang, H.; Hu, Q.; Jin, Y. Amino-Functionalized Polystyrene Nano-Plastics Induce Mitochondria Damage in Human Umbilical Vein Endothelial Cells. Toxics 2022, 10, 215. [Google Scholar] [CrossRef]
- Basini, G.; Bussolati, S.; Andriani, L.; Grolli, S.; Ramoni, R.; Bertini, S.; Iemmi, T.; Menozzi, A.; Berni, P.; Grasselli, F. Nanoplastics Impair in Vitro Swine Granulosa Cell Functions. Domest. Anim. Endocrinol. 2021, 76, 106611. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Duan, Z.; Wang, L. Pulmonary Toxicology Assessment of Polyethylene Terephthalate Nanoplastic Particles in Vitro. Environ. Int. 2022, 162, 107177. [Google Scholar] [CrossRef]
- Brandts, I.; Solà, R.; Garcia-Ordoñez, M.; Gella, A.; Quintana, A.; Martin, B.; Esteve-Codina, A.; Teles, M.; Roher, N. Polystyrene Nanoplastics Target Lysosomes Interfering with Lipid Metabolism through the PPAR System and Affecting Macrophage Functionalization. Environ. Sci. Nano 2023, 10, 2245–2258. [Google Scholar] [CrossRef]
- He, S.; Cai, J.; Jia, T.; Mao, Z.; Zhou, L.; Zhang, X.; Jiang, S.; Huang, P. New Sight of Renal Toxicity Caused by UV-Aged Polystyrene Nanoplastics: Induced Ferroptosis via Adsorption of Transferrin. Small 2024, 20, 2309369. [Google Scholar] [CrossRef]
- Yang, Q.; Dai, H.; Cheng, Y.; Wang, B.; Xu, J.; Zhang, Y.; Chen, Y.; Xu, F.; Ma, Q.; Lin, F.; et al. Oral Feeding of Nanoplastics Affects Brain Function of Mice by Inducing Macrophage IL-1 Signal in the Intestine. Cell Rep. 2023, 42. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, C.; Liu, D.; Wang, Y.; Qi, H.; Liu, X.; Zhang, Y.; Chen, H.; Zeng, Y.; Li, J. Polystyrene Nanoplastics-Induced Lung Apoptosis and Ferroptosis via ROS-Dependent Endoplasmic Reticulum Stress. Sci. Total Environ. 2024, 912, 169260. [Google Scholar] [CrossRef]
- Ballesteros, S.; Domenech, J.; Barguilla, I.; Cortés, C.; Marcos, R.; Hernández, A. Genotoxic and Immunomodulatory Effects in Human White Blood Cells after Ex Vivo Exposure to Polystyrene Nanoplastics. Environ. Sci. Nano 2020, 7, 3431–3446. [Google Scholar] [CrossRef]
- Ban, M.; Shimoda, R.; Chen, J. Investigation of Nanoplastic Cytotoxicity Using SH-SY5Y Human Neuroblastoma Cells and Polystyrene Nanoparticles. Toxicol. In Vitro 2021, 76, 105225. [Google Scholar] [CrossRef]
- Guo, X.; Cheng, C.; Chen, L.; Cao, C.; Li, D.; Fan, R.; Wei, X. Metabolomic Characteristics in Human CD34+ Hematopoietic Stem/Progenitor Cells Exposed to Polystyrene Nanoplastics. Food Chem. Toxicol. 2023, 177, 113817. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, R.; Li, B.; Du, Y.; Li, J.; Tong, X.; Wu, Y.; Ji, X.; Zhang, Y. Tissue Distribution of Polystyrene Nanoplastics in Mice and Their Entry, Transport, and Cytotoxicity to GES-1 Cells. Environ. Pollut. 2021, 280, 116974. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Shang, L.; Yin, J.; Qian, Z.; Chen, C.; Yang, Y. Size-Dependent Neurotoxicity of Micro- and Nanoplastics in Flowing Condition Based on an in Vitro Microfluidic Study. Chemosphere 2022, 303, 135280. [Google Scholar] [CrossRef]
- Zheng, T.; Yuan, D.; Liu, C. Molecular Toxicity of Nanoplastics Involving in Oxidative Stress and Desoxyribonucleic Acid Damage. J. Mol. Recognit. 2019, 32, e2804. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, B.; Li, Z.; Zhong, Y.; Wang, B.; Zhang, B.; Du, J.; Ye, R.; Xian, H.; Min, W.; et al. Polystyrene Nanoplastic Exposure Induces Excessive Mitophagy by Activating AMPK/ULK1 Pathway in Differentiated SH-SY5Y Cells and Dopaminergic Neurons in Vivo. Part Fibre Toxicol. 2023, 20, 44. [Google Scholar] [CrossRef]
- Soto-Bielicka, P.; Tejeda, I.; Peropadre, A.; Hazen, M.J.; Fernández Freire, P. Detrimental Effects of Individual versus Combined Exposure to Tetrabromobisphenol A and Polystyrene Nanoplastics in Fish Cell Lines. Environ. Toxicol. Pharmacol. 2023, 98, 104072. [Google Scholar] [CrossRef]
- He, Y.; Li, Z.; Xu, T.; Luo, D.; Chi, Q.; Zhang, Y.; Li, S. Polystyrene Nanoplastics Deteriorate LPS-Modulated Duodenal Permeability and Inflammation in Mice via ROS Drived-NF-ΚB/NLRP3 Pathway. Chemosphere 2022, 307, 135662. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Chen, K.-F.; Lin, K.-Y.A.; Tsang, Y.F.; Hsu, Y.-F.; Lin, C.-H. Evaluation of the Pulmonary Toxicity of PSNPs Using a Transwell-Based Normal Human Bronchial Epithelial Cell Culture System. Sci. Total Environ. 2023, 895, 165213. [Google Scholar] [CrossRef] [PubMed]
- Magrì, D.; Veronesi, M.; Sánchez-Moreno, P.; Tolardo, V.; Bandiera, T.; Pompa, P.P.; Athanassiou, A.; Fragouli, D. PET Nanoplastics Interactions with Water Contaminants and Their Impact on Human Cells. Environ. Pollut. 2021, 271, 116262. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Liao, R.; Shi, Y.; Li, J.; Cao, J.; Liao, B.; Wu, J.; Li, G. Polystyrene Nanoplastics Induce Apoptosis of Human Kidney Proximal Tubular Epithelial Cells via Oxidative Stress and MAPK Signaling Pathways. Environ. Sci. Pollut. Res. 2023, 30, 110579–110589. [Google Scholar] [CrossRef]
- Banaei, G.; García-Rodríguez, A.; Tavakolpournegari, A.; Martín-Pérez, J.; Villacorta, A.; Marcos, R.; Hernández, A. The Release of Polylactic Acid Nanoplastics (PLA-NPLs) from Commercial Teabags. Obtention, Characterization, and Hazard Effects of True-to-Life PLA-NPLs. J. Hazard. Mater. 2023, 458, 131899. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Q.; Liu, X.; Wang, L.; He, Y.; Tang, J. Perfluorooctane Sulfonate (PFOS) Enhanced Polystyrene Particles Uptake by Human Colon Adenocarcinoma Caco-2 Cells. Sci. Total Environ. 2022, 848, 157640. [Google Scholar] [CrossRef]
- González-Fernández, C.; Tallec, K.; Le Goïc, N.; Lambert, C.; Soudant, P.; Huvet, A.; Suquet, M.; Berchel, M.; Paul-Pont, I. Cellular Responses of Pacific Oyster (Crassostrea Gigas) Gametes Exposed in Vitro to Polystyrene Nanoparticles. Chemosphere 2018, 208, 764–772. [Google Scholar] [CrossRef]
- Xu, D.; Ma, Y.; Han, X.; Chen, Y. Systematic Toxicity Evaluation of Polystyrene Nanoplastics on Mice and Molecular Mechanism Investigation about Their Internalization into Caco-2 Cells. J. Hazard. Mater. 2021, 417, 126092. [Google Scholar] [CrossRef]
- González-Fernández, C.; Díaz Baños, F.G.; Esteban, M.Á.; Cuesta, A. Functionalized Nanoplastics (NPs) Increase the Toxicity of Metals in Fish Cell Lines. Int. J. Mol. Sci. 2021, 22, 7141. [Google Scholar] [CrossRef]
- Cui, M.; He, Q.; Wang, Z.; Yu, Y.; Gao, H.; Liu, Z.; Peng, H.; Wang, H.; Zhang, X.; Li, D.; et al. Mucin2 Regulated by Ho1/P38/IL-10 Axis Plays a Protective Role in Polystyrene Nanoplastics-Mediated Intestinal Toxicity. Environ. Pollut. 2023, 330, 121808. [Google Scholar] [CrossRef]
- Hu, R.; Yao, C.; Li, Y.; Qu, J.; Yu, S.; Han, Y.; Chen, G.; Tang, J.; Wei, H. Polystyrene Nanoplastics Promote CHIP-Mediated Degradation of Tight Junction Proteins by Activating IRE1α/XBP1s Pathway in Mouse Sertoli Cells. Ecotoxicol. Environ. Saf. 2022, 248, 114332. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ma, Y.; Ye, S.; Su, Y.; Hu, D.; Xiao, F. Endogenous Hydrogen Sulfide Counteracts Polystyrene Nanoplastics-Induced Mitochondrial Apoptosis and Excessive Autophagy via Regulating Nrf2 and PGC-1α Signaling Pathway in Mouse Spermatocyte-Derived GC-2spd(Ts) Cells. Food Chem. Toxicol. 2022, 164, 113071. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, J.; Chen, J.; Miao, X.; Li, G.; He, Q.; Xu, H.; Li, H.; Wei, Y. Cytotoxic Effects of Polystyrene Nanoplastics with Different Surface Functionalization on Human HepG2 Cells. Sci. Total Environ. 2020, 723, 138180. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Shan, H.; Xiong, S.; Zhao, Y.; van Gestel, C.A.M.; Qiu, H.; Wang, Y. Polystyrene Nanoparticle Exposure Accelerates Ovarian Cancer Development in Mice by Altering the Tumor Microenvironment. Sci. Total Environ. 2024, 906, 167592. [Google Scholar] [CrossRef]
- Sun, R.; Liu, M.; Xiong, F.; Xu, K.; Huang, J.; Liu, J.; Wang, D.; Pu, Y. Polystyrene Micro- and Nanoplastics Induce Gastric Toxicity through ROS Mediated Oxidative Stress and P62/Keap1/Nrf2 Pathway. Sci. Total Environ. 2024, 912, 169228. [Google Scholar] [CrossRef]
- Cortés, C.; Domenech, J.; Salazar, M.; Pastor, S.; Marcos, R.; Hernández, A. Nanoplastics as a Potential Environmental Health Factor: Effects of Polystyrene Nanoparticles on Human Intestinal Epithelial Caco-2 Cells. Environ. Sci. Nano 2020, 7, 272–285. [Google Scholar] [CrossRef]
- Florance, I.; Chandrasekaran, N.; Gopinath, P.M.; Mukherjee, A. Exposure to Polystyrene Nanoplastics Impairs Lipid Metabolism in Human and Murine Macrophages in Vitro. Ecotoxicol. Environ. Saf. 2022, 238, 113612. [Google Scholar] [CrossRef]
- Brandts, I.; Garcia-Ordoñez, M.; Tort, L.; Teles, M.; Roher, N. Polystyrene Nanoplastics Accumulate in ZFL Cell Lysosomes and in Zebrafish Larvae after Acute Exposure, Inducing a Synergistic Immune Response In Vitro without Affecting Larval Survival in Vivo. Environ. Sci. Nano 2020, 7, 2410–2422. [Google Scholar] [CrossRef]
- Stock, V.; Böhmert, L.; Coban, G.; Tyra, G.; Vollbrecht, M.-L.; Voss, L.; Paul, M.B.; Braeuning, A.; Sieg, H. Microplastics and Nanoplastics: Size, Surface and Dispersant – What Causes the Effect? Toxicol. In Vitro 2022, 80, 105314. [Google Scholar] [CrossRef]
- Xiao, M.; Li, X.; Zhang, X.; Duan, X.; Lin, H.; Liu, S.; Sui, G. Assessment of Cancer-Related Signaling Pathways in Responses to Polystyrene Nanoplastics via a Kidney-Testis Microfluidic Platform (KTP). Sci. Total Environ. 2023, 857, 159306. [Google Scholar] [CrossRef]
- Halimu, G.; Zhang, Q.; Liu, L.; Zhang, Z.; Wang, X.; Gu, W.; Zhang, B.; Dai, Y.; Zhang, H.; Zhang, C.; et al. Toxic Effects of Nanoplastics with Different Sizes and Surface Charges on Epithelial-to-Mesenchymal Transition in A549 Cells and the Potential Toxicological Mechanism. J. Hazard. Mater. 2022, 430, 128485. [Google Scholar] [CrossRef] [PubMed]
- Tavakolpournegari, A.; Annangi, B.; Villacorta, A.; Banaei, G.; Martin, J.; Pastor, S.; Marcos, R.; Hernández, A. Hazard Assessment of Different-Sized Polystyrene Nanoplastics in Hematopoietic Human Cell Lines. Chemosphere 2023, 325, 138360. [Google Scholar] [CrossRef] [PubMed]
- Englert, F.H.; Mueller, F.A.; Dugershaw-Kurzer, B.; Kissling, V.M.; Boentges, S.; Gupta, G.S.; Fontana, G.A.; Diedrich, S.; Suter-Dick, L.; Sturla, S.J.; et al. Environmentally Relevant UV-Light Weathering of Polystyrene Micro- and Nanoplastics Promotes Hepatotoxicity in a Human Cell Line. Environ. Sci. Nano 2023, 10, 1644–1659. [Google Scholar] [CrossRef]
- Almeida, M.; Martins, M.A.; Soares, A.M.V.; Cuesta, A.; Oliveira, M. Polystyrene Nanoplastics Alter the Cytotoxicity of Human Pharmaceuticals on Marine Fish Cell Lines. Environ. Toxicol. Pharmacol. 2019, 69, 57–65. [Google Scholar] [CrossRef]
- Domenech, J.; Hernández, A.; Rubio, L.; Marcos, R.; Cortés, C. Interactions of Polystyrene Nanoplastics with in Vitro Models of the Human Intestinal Barrier. Arch. Toxicol. 2020, 94, 2997–3012. [Google Scholar] [CrossRef]
- Li, Y.; Xu, M.; Zhang, Z.; Halimu, G.; Li, Y.; Li, Y.; Gu, W.; Zhang, B.; Wang, X. In Vitro Study on the Toxicity of Nanoplastics with Different Charges to Murine Splenic Lymphocytes. J. Hazard. Mater. 2022, 424, 127508. [Google Scholar] [CrossRef]
- Sui, A.; Yao, C.; Chen, Y.; Li, Y.; Yu, S.; Qu, J.; Wei, H.; Tang, J.; Chen, G. Polystyrene Nanoplastics Inhibit StAR Expression by Activating HIF-1α via ERK1/2 MAPK and AKT Pathways in TM3 Leydig Cells and Testicular Tissues of Mice. Food Chem. Toxicol. 2023, 173, 113634. [Google Scholar] [CrossRef]
- Jeong, B.; Baek, J.Y.; Koo, J.; Park, S.; Ryu, Y.-K.; Kim, K.-S.; Zhang, S.; Chung, C.; Dogan, R.; Choi, H.-S.; et al. Maternal Exposure to Polystyrene Nanoplastics Causes Brain Abnormalities in Progeny. J. Hazard. Mater. 2022, 426, 127815. [Google Scholar] [CrossRef]
- Paul, M.B.; Böhmert, L.; Hsiao, I.-L.; Braeuning, A.; Sieg, H. Complex Intestinal and Hepatic in Vitro Barrier Models Reveal Information on Uptake and Impact of Micro-, Submicro- and Nanoplastics. Environ. Int. 2023, 179, 108172. [Google Scholar] [CrossRef]
- Ma, T.; Liu, X.; Xiong, T.; Li, H.; Zhou, Y.; Liang, J. Polystyrene Nanoplastics Aggravated Dibutyl Phthalate-Induced Blood-Testis Barrier Dysfunction via Suppressing Autophagy in Male Mice. Ecotoxicol. Environ. Saf. 2023, 264, 115403. [Google Scholar] [CrossRef]
- Nikolic, S.; Gazdic-Jankovic, M.; Rosic, G.; Miletic-Kovacevic, M.; Jovicic, N.; Nestorovic, N.; Stojkovic, P.; Filipovic, N.; Milosevic-Djordjevic, O.; Selakovic, D.; et al. Orally Administered Fluorescent Nanosized Polystyrene Particles Affect Cell Viability, Hormonal and Inflammatory Profile, and Behavior in Treated Mice. Environ. Pollut. 2022, 305, 119206. [Google Scholar] [CrossRef] [PubMed]
- Florance, I.; Ramasubbu, S.; Mukherjee, A.; Chandrasekaran, N. Polystyrene Nanoplastics Dysregulate Lipid Metabolism in Murine Macrophages in Vitro. Toxicology 2021, 458, 152850. [Google Scholar] [CrossRef] [PubMed]
- Tolardo, V.; Magrì, D.; Fumagalli, F.; Cassano, D.; Athanassiou, A.; Fragouli, D.; Gioria, S. In Vitro High-Throughput Toxicological Assessment of Nanoplastics. Nanomaterials 2022, 12, 1947. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Sun, X.; Wang, Y.; Su, J.; Li, G.; Wang, X.; Yang, Y.; Zhang, Y.; Li, B.; Zhang, G.; et al. Biological Interactions of Polystyrene Nanoplastics: Their Cytotoxic and Immunotoxic Effects on the Hepatic and Enteric Systems. Ecotoxicol. Environ. Saf. 2023, 264, 115447. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Zhao, T.; Sun, M.; Xu, M.; Che, S.; Pan, Z.; Wu, C.; Shen, L. Polystyrenenanoplastics Lead to Ferroptosis in the Lungs. J. Adv. Res. 2024, 56, 31–41. [Google Scholar] [CrossRef]
- Guanglin, L.; Shuqin, W. Polystyrene Nanoplastics Exposure Causes Inflammation and Death of Esophageal Cell. Ecotoxicol. Environ. Saf. 2024, 269, 115819. [Google Scholar] [CrossRef]
- Yang, S.; Lee, S.; Lee, Y.; Cho, J.-H.; Kim, S.H.; Ha, E.-S.; Jung, Y.-S.; Chung, H.Y.; Kim, M.-S.; Kim, H.S.; et al. Cationic Nanoplastic Causes Mitochondrial Dysfunction in Neural Progenitor Cells and Impairs Hippocampal Neurogenesis. Free Radic. Biol. Med. 2023, 208, 194–210. [Google Scholar] [CrossRef]
- Giannandrea, D.; Parolini, M.; Citro, V.; De Felice, B.; Pezzotta, A.; Abazari, N.; Platonova, N.; Sugni, M.; Chiu, M.; Villa, A.; et al. Nanoplastic Impact on Bone Microenvironment: A Snapshot from Murine Bone Cells. J. Hazard. Mater. 2024, 462, 132717. [Google Scholar] [CrossRef]
- Shan, S.; Zhang, Y.; Zhao, H.; Zeng, T.; Zhao, X. Polystyrene Nanoplastics Penetrate across the Blood-Brain Barrier and Induce Activation of Microglia in the Brain of Mice. Chemosphere 2022, 298, 134261. [Google Scholar] [CrossRef]
- Banerjee, A.; Billey, L.O.; Shelver, W.L. Uptake and Toxicity of Polystyrene Micro/Nanoplastics in Gastric Cells: Effects of Particle Size and Surface Functionalization. PLoS ONE 2022, 16, e0260803. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, Y.; Bai, H.; Shimizu, K.; Li, R.; Zhang, C. Investigation of Pulmonary Toxicity Evaluation on Mice Exposed to Polystyrene Nanoplastics: The Potential Protective Role of the Antioxidant N-Acetylcysteine. Sci. Total Environ. 2023, 855, 158851. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ren, X.-M.; He, H.; Li, F.; Liu, K.; Zhao, F.; Hu, H.; Zhang, P.; Huang, B.; Pan, X. Cytotoxicity and Pro-Inflammatory Effect of Polystyrene Nano-Plastic and Micro-Plastic on RAW264.7 Cells. Toxicology 2023, 484, 153391. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Kelpsiene, E.; Gupta, G.; Dobryden, I.; Cedervall, T.; Fadeel, B. Label-Free Detection of Polystyrene Nanoparticles in Daphnia Magna Using Raman Confocal Mapping. Nanoscale Adv. 2023, 5, 3453–3462. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhou, C.; Xu, W.; Huang, Y.; Wang, W.; Ma, Z.; Huang, J.; Li, J.; Hu, L.; Xue, Y.; et al. The Ovarian-Related Effects of Polystyrene Nanoplastics on Human Ovarian Granulosa Cells and Female Mice. Ecotoxicol. Environ. Saf. 2023, 257, 114941. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Li, J.; Song, Z.; Zhang, C.; Guan, B. Toxicity of Polystyrene Nanoplastics to Human Embryonic Kidney Cells and Human Normal Liver Cells: Effect of Particle Size and Pb2+ Enrichment. Chemosphere 2023, 328, 138545. [Google Scholar] [CrossRef]
- Ruan, Y.; Zhong, Z.; Liu, X.; Li, Z.; Li, J.; Sun, L.; Sen, H. Correlation between Cellular Uptake and Cytotoxicity of Polystyrene Micro/Nanoplastics in HeLa Cells: A Size-Dependent Matter. PLoS ONE 2023, 18, e0289473. [Google Scholar] [CrossRef]
- Peng, L.; Xu, T.; Fan, X.; Chi, Q.; Tang, X.; Li, Z.; Bai, Z.; Li, S. Polystyrene Nanoplastics Exacerbate Lipopolysaccharide-Induced Myocardial Fibrosis and Autophagy in Mice via ROS/TGF-Β1/Smad. Toxicology 2022, 480, 153338. [Google Scholar] [CrossRef]
- Xuan, L.; Luo, J.; Qu, C.; Guo, P.; Yi, W.; Yang, J.; Yan, Y.; Guan, H.; Zhou, P.; Huang, R. Predictive Metabolomic Signatures for Safety Assessment of Three Plastic Nanoparticles Using Intestinal Organoids. Sci. Total Environ. 2024, 913, 169606. [Google Scholar] [CrossRef]
- Domenech, J.; Cortés, C.; Vela, L.; Marcos, R.; Hernández, A. Polystyrene Nanoplastics as Carriers of Metals. Interactions of Polystyrene Nanoparticles with Silver Nanoparticles and Silver Nitrate, and Their Effects on Human Intestinal Caco-2 Cells. Biomolecules 2021, 11, 859. [Google Scholar] [CrossRef]
- Shi, Q.; Tang, J.; Wang, L.; Liu, R.; Giesy, J.P. Combined Cytotoxicity of Polystyrene Nanoplastics and Phthalate Esters on Human Lung Epithelial A549 Cells and Its Mechanism. Ecotoxicol. Environ. Saf. 2021, 213, 112041. [Google Scholar] [CrossRef]
- Yan, L.; Yu, Z.; Lin, P.; Qiu, S.; He, L.; Wu, Z.; Ma, L.; Gu, Y.; He, L.; Dai, Z.; et al. Polystyrene Nanoplastics Promote the Apoptosis in Caco-2 Cells Induced by Okadaic Acid More than Microplastics. Ecotoxicol. Environ. Saf. 2023, 249, 114375. [Google Scholar] [CrossRef] [PubMed]
- Roursgaard, M.; Hezareh Rothmann, M.; Schulte, J.; Karadimou, I.; Marinelli, E.; Møller, P. Genotoxicity of Particles From Grinded Plastic Items in Caco-2 and HepG2 Cells. Front. Public Health 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Palacios, M.; Almeida, M.; Martins, M.A.; Oliveira, M.; Esteban, M.Á.; Cuesta, A. Establishment of a Brain Cell Line (FuB-1) from Mummichog (Fundulus heteroclitus) and Its Application to Fish Virology, Immunity and Nanoplastics Toxicology. Sci. Total Environ. 2020, 708, 134821. [Google Scholar] [CrossRef]
- Huang, J.; Dong, G.; Liang, M.; Wu, X.; Xian, M.; An, Y.; Zhan, J.; Xu, L.; Xu, J.; Sun, W.; et al. Toxicity of Micro(Nano)Plastics with Different Size and Surface Charge on Human Nasal Epithelial Cells and Rats via Intranasal Exposure. Chemosphere 2022, 307, 136093. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Z.; Gao, X.; Zhao, J.; Zhang, H. Polystyrene Nanoparticles Induced Mammalian Intestine Damage Caused by Blockage of BNIP3/NIX-Mediated Mitophagy and Gut Microbiota Alteration. Sci. Total Environ. 2024, 907, 168064. [Google Scholar] [CrossRef]
- Dusza, H.M.; Katrukha, E.A.; Nijmeijer, S.M.; Akhmanova, A.; Vethaak, A.D.; Walker, D.I.; Legler, J. Uptake, Transport, and Toxicity of Pristine and Weathered Micro- and Nanoplastics in Human Placenta Cells. Environ. Health Perspect. 2024, 130, 97006. [Google Scholar] [CrossRef]
- Babonaitė, M.; Čepulis, M.; Kazlauskaitė, J.; Lazutka, J.R. Evaluation of In Vitro Genotoxicity of Polystyrene Nanoparticles in Human Peripheral Blood Mononuclear Cells. Toxics 2023, 11, 627. [Google Scholar] [CrossRef]
- Basini, G.; Bussolati, S.; Andriani, L.; Grolli, S.; Bertini, S.; Iemmi, T.; Menozzi, A.; Quintavalla, F.; Ramoni, R.; Serventi, P.; et al. The Effects of Nanoplastics on Adipose Stromal Cells from Swine Tissues. Domest. Anim. Endocrinol. 2022, 81, 106747. [Google Scholar] [CrossRef]
- Alzaben, M.; Burve, R.; Loeschner, K.; Møller, P.; Roursgaard, M. Nanoplastics from Ground Polyethylene Terephthalate Food Containers: Genotoxicity in Human Lung Epithelial A549 Cells. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2023, 892, 503705. [Google Scholar] [CrossRef]
- Contino, M.; Ferruggia, G.; Indelicato, S.; Pecoraro, R.; Scalisi, E.M.; Bracchitta, G.; Dragotto, J.; Salvaggio, A.; Brundo, M.V. In Vitro Nano-Polystyrene Toxicity: Metabolic Dysfunctions and Cytoprotective Responses of Human Spermatozoa. Biology 2023, 12, 624. [Google Scholar] [CrossRef]
- Yang, M.; Wang, W.-X. Differential Cascading Cellular and Subcellular Toxicity Induced by Two Sizes of Nanoplastics. Sci. Total Environ. 2022, 829, 154593. [Google Scholar] [CrossRef] [PubMed]
- Ilić, K.; Krce, L.; Rodriguez-Ramos, J.; Rico, F.; Kalčec, N.; Aviani, I.; Turčić, P.; Pavičić, I.; Vinković Vrček, I. Cytotoxicity of Nanomixture: Combined Action of Silver and Plastic Nanoparticles on Immortalized Human Lymphocytes. J. Trace Elem. Med. Biol. 2022, 73, 127004. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Hu, J.; Zhang, M.; Meng, Z.; Shi, G.; Wang, Z.; Li, W. Maltol Attenuates Polystyrene Nanoplastic-Induced Enterotoxicity by Promoting AMPK/MTOR/TFEB-Mediated Autophagy and Modulating Gut Microbiota. Environ. Pollut. 2023, 322, 121202. [Google Scholar] [CrossRef] [PubMed]
- Hesler, M.; Aengenheister, L.; Ellinger, B.; Drexel, R.; Straskraba, S.; Jost, C.; Wagner, S.; Meier, F.; von Briesen, H.; Büchel, C.; et al. Multi-Endpoint Toxicological Assessment of Polystyrene Nano- and Microparticles in Different Biological Models in Vitro. Toxicol. In Vitro 2019, 61, 104610. [Google Scholar] [CrossRef]
- Li, Y.; Guo, M.; Niu, S.; Shang, M.; Chang, X.; Sun, Z.; Zhang, R.; Shen, X.; Xue, Y. ROS and DRP1 Interactions Accelerate the Mitochondrial Injury Induced by Polystyrene Nanoplastics in Human Liver HepG2 Cells. Chem. Biol. Interact. 2023, 379, 110502. [Google Scholar] [CrossRef]
- Hou, Z.; Meng, R.; Chen, G.; Lai, T.; Qing, R.; Hao, S.; Deng, J.; Wang, B. Distinct Accumulation of Nanoplastics in Human Intestinal Organoids. Sci. Total Environ. 2022, 838, 155811. [Google Scholar] [CrossRef]
- Xu, M.; Halimu, G.; Zhang, Q.; Song, Y.; Fu, X.; Li, Y.; Li, Y.; Zhang, H. Internalization and Toxicity: A Preliminary Study of Effects of Nanoplastic Particles on Human Lung Epithelial Cell. Sci. Total Environ. 2019, 694, 133794. [Google Scholar] [CrossRef]
- Li, C.; Huang, X.; Min, W.; Zhong, H.; Yan, X.; Gao, Y.; Wang, J.; Zhou, H.; Yan, B. Inflammatory Responses Induced by Synergistic Actions between Nanoplastics and Typical Heavy Metal Ions in Human Cells. Environ. Sci. Nano 2023, 10, 1599–1613. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y.; Du, Y.; Zhang, W.; Liu, Z.; Bai, J.; Cui, G.; Du, Z. Involvement of the JNK/HO 1/FTH1 Signaling Pathway in Nanoplastic induced Inflammation and Ferroptosis of BV2 Microglia Cells. Int. J. Mol. Med. 2023, 52, 61. [Google Scholar] [CrossRef]
- Tolardo, V.; Romaldini, A.; Fumagalli, F.; Armirotti, A.; Veronesi, M.; Magrì, D.; Sabella, S.; Athanassiou, A.; Fragouli, D. Polycarbonate Nanoplastics and the in Vitro Assessment of Their Toxicological Impact on Liver Functionality. Environ. Sci. Nano 2023, 10, 1413–1427. [Google Scholar] [CrossRef]
- Busch, M.; Bredeck, G.; Kämpfer, A.A.M.; Schins, R.P.F. Investigations of Acute Effects of Polystyrene and Polyvinyl Chloride Micro- and Nanoplastics in an Advanced in Vitro Triple Culture Model of the Healthy and Inflamed Intestine. Environ. Res. 2021, 193, 110536. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, X.; Huang, R.; Tang, C.; Hu, C.; Ning, P.; Wang, F. Cytotoxicity and Genotoxicity of Polystyrene Micro- and Nanoplastics with Different Size and Surface Modification in A549 Cells. Int. J. Nanomed. 2022, 17, 4509–4523. [Google Scholar] [CrossRef] [PubMed]
- Murano, C.; Bergami, E.; Liberatori, G.; Palumbo, A.; Corsi, I. Interplay Between Nanoplastics and the Immune System of the Mediterranean Sea Urchin Paracentrotus Lividus. Front. Mar. Sci. 2021, 8. [Google Scholar] [CrossRef]
- Bai, H.; Wu, Y.; Li, H.; Zhu, Y.; Che, R.; Wang, F.; Zhang, C. Cerebral Neurotoxicity of Amino-Modified Polystyrene Nanoplastics in Mice and the Protective Effects of Functional Food Camellia Pollen. Sci. Total Environ. 2024, 912, 169511. [Google Scholar] [CrossRef]
- Li, S.; Liu, L.; Luo, G.; Yuan, Y.; Hu, D.; Xiao, F. The Crosstalk between M1 Macrophage Polarization and Energy Metabolism Disorder Contributes to Polystyrene Nanoplastics-Triggered Testicular Inflammation. Food Chem. Toxicol. 2023, 180, 114002. [Google Scholar] [CrossRef]
- Magrì, D.; Sánchez-Moreno, P.; Caputo, G.; Gatto, F.; Veronesi, M.; Bardi, G.; Catelani, T.; Guarnieri, D.; Athanassiou, A.; Pompa, P.P.; et al. Laser Ablation as a Versatile Tool To Mimic Polyethylene Terephthalate Nanoplastic Pollutants: Characterization and Toxicology Assessment. ACS Nano 2018, 12, 7690–7700. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef]
- Courtioux, P.; Métivier, F.; Reberioux, A. Scientific Competition between Countries: Did China Get What It Paid For? 2019. Available online: https://shs.hal.science/halshs-02307534v1/document (accessed on 5 May 2024).
- Morrison, J. China Becomes World’s Third-Largest Producer of Research Articles. Nature 2014. [Google Scholar] [CrossRef]
- Baker, S. China Seeks Global Impact and Recognition. Nature Index 2024. Available online: https://pubmed.ncbi.nlm.nih.gov/38840020/ (accessed on 5 May 2024).
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic Waste Inputs from Land into the Ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Merdy, P.; Delpy, F.; Bonneau, A.; Villain, S.; Iordachescu, L.; Vollertsen, J.; Lucas, Y. Nanoplastic Production Procedure for Scientific Purposes: PP, PVC, PE-LD, PE-HD, and PS. Heliyon 2023, 9, e18387. [Google Scholar] [CrossRef] [PubMed]
- Erni-Cassola, G.; Zadjelovic, V.; Gibson, M.I.; Christie-Oleza, J.A. Distribution of Plastic Polymer Types in the Marine Environment; A Meta-Analysis. J. Hazard. Mater. 2019, 369, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Bråte, I.L.; Halsband, C.; Allan, I.; Thomas, K. Report Made for the Norwegian Environment Agency: Microplastics in Marine Environments. Occurrence, Distribution and Effects. 2014. Available online: https://www.researchgate.net/publication/273089847_Report_made_for_the_Norwegian_Environment_Agency_Microplastics_in_marine_environments_Occurrence_distribution_and_effects (accessed on 5 May 2024).
- Plastics—The Fast Facts. 2023. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/ (accessed on 5 May 2024).
- Heddagaard, F.E.; Møller, P. Hazard Assessment of Small-Size Plastic Particles: Is the Conceptual Framework of Particle Toxicology Useful? Food Chem. Toxicol. 2020, 136, 111106. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.; Kim, I.-S.; Yu, J.; Chung, H.E.; Choy, J.-H.; Choi, S.-J. Effect of Different Forms of Anionic Nanoclays on Cytotoxicity. J. Nanosci. Nanotechnol. 2011, 11, 1803–1806. [Google Scholar] [CrossRef]
- Schaeublin, N.M.; Braydich-Stolle, L.K.; Schrand, A.M.; Miller, J.M.; Hutchison, J.; Schlager, J.J.; Hussain, S.M. Surface Charge of Gold Nanoparticles Mediates Mechanism of Toxicity. Nanoscale 2011, 3, 410–420. [Google Scholar] [CrossRef]
- Baek, M.; Kim, M.K.; Cho, H.J.; Lee, J.A.; Yu, J.; Chung, H.E.; Choi, S.J. Factors Influencing the Cytotoxicity of Zinc Oxide Nanoparticles: Particle Size and Surface Charge. J. Phys. Conf. Ser. 2011, 304, 12044. [Google Scholar] [CrossRef]
- Martin, L.M.A.; Gan, N.; Wang, E.; Merrill, M.; Xu, W. Materials, Surfaces, and Interfacial Phenomena in Nanoplastics Toxicology Research. Environ. Pollut. 2022, 292, 118442. [Google Scholar] [CrossRef]
- Eisenberg, S.; Haimov, E.; Walpole, G.F.W.; Plumb, J.; Kozlov, M.M.; Grinstein, S. Mapping the Electrostatic Profiles of Cellular Membranes. Mol. Biol. Cell 2021, 32, 301–310. [Google Scholar] [CrossRef]
- Roshanzadeh, A.; Park, S.; Ganjbakhsh, S.E.; Park, J.; Lee, D.-H.; Lee, S.; Kim, E.-S. Surface Charge-Dependent Cytotoxicity of Plastic Nanoparticles in Alveolar Cells under Cyclic Stretches. Nano Lett. 2020, 20, 7168–7176. [Google Scholar] [CrossRef]
- Reznickova, A.; Novotna, Z.; Kolska, Z.; Svorcik, V. Immobilization of Silver Nanoparticles on Polyethylene Terephthalate. Nanoscale Res. Lett. 2014, 9, 305. [Google Scholar] [CrossRef]
- Ferrante, M.C.; Monnolo, A.; Del Piano, F.; Mattace Raso, G.; Meli, R. The Pressing Issue of Micro- and Nanoplastic Contamination: Profiling the Reproductive Alterations Mediated by Oxidative Stress. Antioxidants 2022, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Palić, D. Micro- and Nano-Plastics Activation of Oxidative and Inflammatory Adverse Outcome Pathways. Redox Biol. 2020, 37, 101620. [Google Scholar] [CrossRef] [PubMed]
- Waller, W.T.; Allen, H.J. Acute and Chronic Toxicity. In Encyclopedia of Ecology; Houghton, R.A., Jørgensen, S.E., Fath, B.D., Eds.; Academic Press: Oxford, UK, 2008; pp. 32–43. ISBN 978-0-08-045405-4. [Google Scholar]
- Zhang, X.; Wen, K.; Ding, D.; Liu, J.; Lei, Z.; Chen, X.; Ye, G.; Zhang, J.; Shen, H.; Yan, C.; et al. Size-Dependent Adverse Effects of Microplastics on Intestinal Microbiota and Metabolic Homeostasis in the Marine Medaka (Oryzias melastigma). Environ. Int. 2021, 151, 106452. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; He, G.; Mai, K. Modulation of Lipid Metabolism, Immune Parameters, and Hepatic Transferrin Expression in Juvenile Turbot (Scophthalmus maximus L.) by Increasing Dietary Linseed Oil Levels. Aquaculture 2016, 464, 489–496. [Google Scholar] [CrossRef]
- Yin, L.; Liu, H.; Cui, H.; Chen, B.; Li, L.; Wu, F. Impacts of Polystyrene Microplastics on the Behavior and Metabolism in a Marine Demersal Teleost, Black Rockfish (Sebastes schlegelii). J. Hazard. Mater. 2019, 380, 120861. [Google Scholar] [CrossRef]
- Warrillow, S.; Fisher, C.; Bellomo, R. Correction and Control of Hyperammonemia in Acute Liver Failure: The Impact of Continuous Renal Replacement Timing, Intensity, and Duration. Crit. Care Med. 2020, 48, 218–224. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, J.; Wei, X.; Chang, L.; Liu, S. Proinflammatory Properties and Lipid Disturbance of Polystyrene Microplastics in the Livers of Mice with Acute Colitis. Sci. Total Environ. 2021, 750, 143085. [Google Scholar] [CrossRef]
- Brandts, I.; Teles, M.; Tvarijonaviciute, A.; Pereira, M.L.; Martins, M.A.; Tort, L.; Oliveira, M. Effects of Polymethylmethacrylate Nanoplastics on Dicentrarchus Labrax. Genomics 2018, 110, 435–441. [Google Scholar] [CrossRef]
- Luo, T.; Wang, C.; Pan, Z.; Jin, C.; Fu, Z.; Jin, Y. Maternal Polystyrene Microplastic Exposure during Gestation and Lactation Altered Metabolic Homeostasis in the Dams and Their F1 and F2 Offspring. Environ. Sci. Technol. 2019, 53, 10978–10992. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Galloway, T.S. The Impact of Polystyrene Microplastics on Feeding, Function and Fecundity in the Marine Copepod Calanus Helgolandicus. Environ. Sci. Technol. 2015, 49, 1130–1137. [Google Scholar] [CrossRef]
- Wright, S.L.; Rowe, D.; Thompson, R.C.; Galloway, T.S. Microplastic Ingestion Decreases Energy Reserves in Marine Worms. Curr. Biol. 2013, 23, R1031–R1033. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Ma, Y.; Li, T.; Sun, M.; Sun, Z.; Duan, J. The Detrimental Effects of Micro-and Nano-Plastics on Digestive System: An Overview of Oxidative Stress-Related Adverse Outcome Pathway. Sci. Total Environ. 2023, 878, 163144. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Paul, M.B.; Böhmert, L.; Thünemann, A.F.; Loeschner, K.; Givelet, L.; Fahrenson, C.; Braeuning, A.; Sieg, H. Influence of Artificial Digestion on Characteristics and Intestinal Cellular Effects of Micro-, Submicro- and Nanoplastics. Food Chem. Toxicol. 2024, 184, 114423. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, S.; Liu, Q.; Wei, J.; Jin, Y.; Wang, X.; Zhang, L. Polystyrene Microplastics Cause Cardiac Fibrosis by Activating Wnt/β-Catenin Signaling Pathway and Promoting Cardiomyocyte Apoptosis in Rats. Environ. Pollut. 2020, 265, 115025. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, J.; Wang, W.; Gonzalez-Gil, G.; Vrouwenvelder, J.S.; Li, Z. Effects of Nano- and Microplastics on Kidney: Physicochemical Properties, Bioaccumulation, Oxidative Stress and Immunoreaction. Chemosphere 2022, 288, 132631. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, R.; Pu, Q.; Jiang, S.; Yu, F.; Yang, Z.; Han, T. Investigation of Nephrotoxicity on Mice Exposed to Polystyrene Nanoplastics and the Potential Amelioration Effects of DHA-Enriched Phosphatidylserine. Sci. Total Environ. 2023, 892, 164808. [Google Scholar] [CrossRef]
- Cheng, W.; Li, X.; Zhou, Y.; Yu, H.; Xie, Y.; Guo, H.; Wang, H.; Li, Y.; Feng, Y.; Wang, Y. Polystyrene Microplastics Induce Hepatotoxicity and Disrupt Lipid Metabolism in the Liver Organoids. Sci. Total Environ. 2022, 806, 150328. [Google Scholar] [CrossRef]
- Ge, Y.; Yang, S.; Zhang, T.; Wan, X.; Zhu, Y.; Yang, F.; Yin, L.; Pu, Y.; Liang, G. The Hepatotoxicity Assessment of Micro/Nanoplastics: A Preliminary Study to Apply the Adverse Outcome Pathways. Sci. Total Environ. 2023, 902, 165659. [Google Scholar] [CrossRef]
- Matthews, S.; Mai, L.; Jeong, C.-B.; Lee, J.-S.; Zeng, E.Y.; Xu, E.G. Key Mechanisms of Micro- and Nanoplastic (MNP) Toxicity across Taxonomic Groups. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 247, 109056. [Google Scholar] [CrossRef]
- Koner, S.; Florance, I.; Mukherjee, A.; Chandrasekaran, N. Cellular Response of THP-1 Macrophages to Polystyrene Microplastics Exposure. Toxicology 2023, 483, 153385. [Google Scholar] [CrossRef] [PubMed]
- Adler, M.Y.; Issoual, I.; Rückert, M.; Deloch, L.; Meier, C.; Tschernig, T.; Alexiou, C.; Pfister, F.; Ramsperger, A.F.R.M.; Laforsch, C.; et al. Effect of Micro- and Nanoplastic Particles on Human Macrophages. J. Hazard. Mater. 2024, 471, 134253. [Google Scholar] [CrossRef] [PubMed]
- Wolff, C.M.; Singer, D.; Schmidt, A.; Bekeschus, S. Immune and Inflammatory Responses of Human Macrophages, Dendritic Cells, and T-Cells in Presence of Micro- and Nanoplastic of Different Types and Sizes. J. Hazard. Mater. 2023, 459, 132194. [Google Scholar] [CrossRef] [PubMed]
- Falahati, M.; Hasan, A.; Zeinabad, H.A.; Serpooshan, V.; von der Thüsen, J.H.; ten Hagen, T.L.M. Engineering of Pulmonary Surfactant Corona on Inhaled Nanoparticles to Operate in the Lung System. Nano Today 2023, 52, 101998. [Google Scholar] [CrossRef]
- Cao, J.; Yang, Q.; Jiang, J.; Dalu, T.; Kadushkin, A.; Singh, J.; Fakhrullin, R.; Wang, F.; Cai, X.; Li, R. Coronas of Micro/Nano Plastics: A Key Determinant in Their Risk Assessments. Part Fibre Toxicol. 2022, 19, 55. [Google Scholar] [CrossRef]
- Zha, S.; Liu, H.; Li, H.; Li, H.; Wong, K.-L.; All, A.H. Functionalized Nanomaterials Capable of Crossing the Blood–Brain Barrier. ACS Nano 2024, 18, 1820–1845. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, S.; Liu, J.; Liu, Z. The Effects of Micro- and Nanoplastics on the Central Nervous System: A New Threat to Humanity? Toxicology 2024, 504, 153799. [Google Scholar] [CrossRef]
- Revel, M.; Châtel, A.; Mouneyrac, C. Micro(Nano)Plastics: A Threat to Human Health? Curr. Opin. Environ. Sci. Health 2018, 1, 17–23. [Google Scholar] [CrossRef]
- Domenech, J.; Marcos, R. Pathways of Human Exposure to Microplastics, and Estimation of the Total Burden. Curr. Opin. Food Sci. 2021, 39, 144–151. [Google Scholar] [CrossRef]
- Try, C.; Moulari, B.; Béduneau, A.; Fantini, O.; Pin, D.; Pellequer, Y.; Lamprecht, A. Size Dependent Skin Penetration of Nanoparticles in Murine and Porcine Dermatitis Models. Eur. J. Pharm. Biopharm. 2016, 100, 101–108. [Google Scholar] [CrossRef]
- Netzlaff, F.; Schaefer, U.F.; Lehr, C.-M.; Meiers, P.; Stahl, J.; Kietzmann, M.; Niedorf, F. Comparison of Bovine Udder Skin with Human and Porcine Skin in Percutaneous Permeation Experiments. Altern. Lab. Anim. 2006, 34, 499–513. [Google Scholar] [PubMed]
- Grote, K.; Brüstle, F.; Vlacil, A.-K. Cellular and Systemic Effects of Micro- and Nanoplastics in Mammals-What We Know So Far. Materials 2023, 16, 3123. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Celli, A.; Zhu, H.; Elmahdy, A.; Cao, Y.; Hui, X.; Maibach, H. Confocal Laser Scanning Microscopy to Estimate Nanoparticles’ Human Skin Penetration in Vitro. Int. J. Nanomed. 2017, 12, 8035–8041. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yin, R. The Reproductive and Transgenerational Toxicity of Microplastics and Nanoplastics: A Threat to Mammalian Fertility in Both Sexes. J. Appl. Toxicol. 2024, 44, 66–85. [Google Scholar] [CrossRef]
- Ali, W.; Buriro, R.S.; Gandahi, J.A.; Chen, Y.; ul Aabdin, Z.; Bhutto, S.; Sun, J.; Zhu, J.; Liu, Z.; Zou, H. A Critical Review on Male-Female Reproductive and Developmental Toxicity Induced by Micro-Plastics and Nano-Plastics through Different Signaling Pathways. Chem. Biol. Interact. 2024, 394, 110976. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, Y.; Chen, J.; Liu, X.; Nie, H.; Li, K.; Liu, H.; Lai, W.; Shi, Y.; Xi, Z.; et al. Effects of Nanoplastic Exposure during Pregnancy and Lactation on Neurodevelopment of Rat Offspring. J. Hazard. Mater. 2024, 474, 134800. [Google Scholar] [CrossRef]
- Harvey, N.E.; Mercer, G.V.; Stapleton, D.; Steeves, K.L.; Hanrahan, J.; Cui, M.; Aghaei, Z.; Spring, S.; Helm, P.A.; Simpson, A.J.; et al. Maternal Exposure to Polystyrene Nanoplastics Impacts Developmental Milestones and Brain Structure in Mouse Offspring. Environ. Sci. Adv. 2023, 2, 622–628. [Google Scholar] [CrossRef]
- Nie, J.-H.; Shen, Y.; Roshdy, M.; Cheng, X.; Wang, G.; Yang, X. Polystyrene Nanoplastics Exposure Caused Defective Neural Tube Morphogenesis through Caveolae-Mediated Endocytosis and Faulty Apoptosis. Nanotoxicology 2021, 15, 885–904. [Google Scholar] [CrossRef]
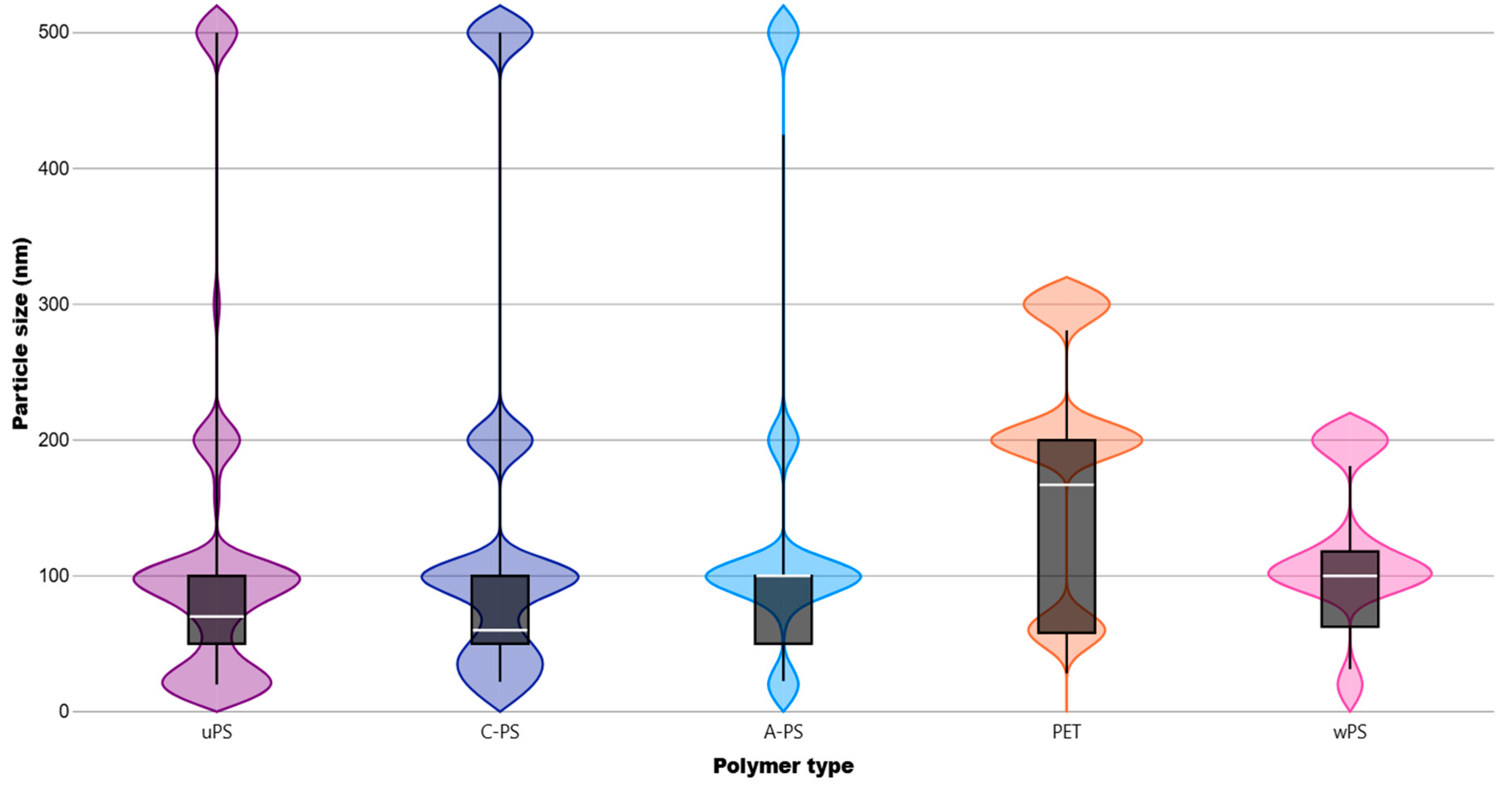
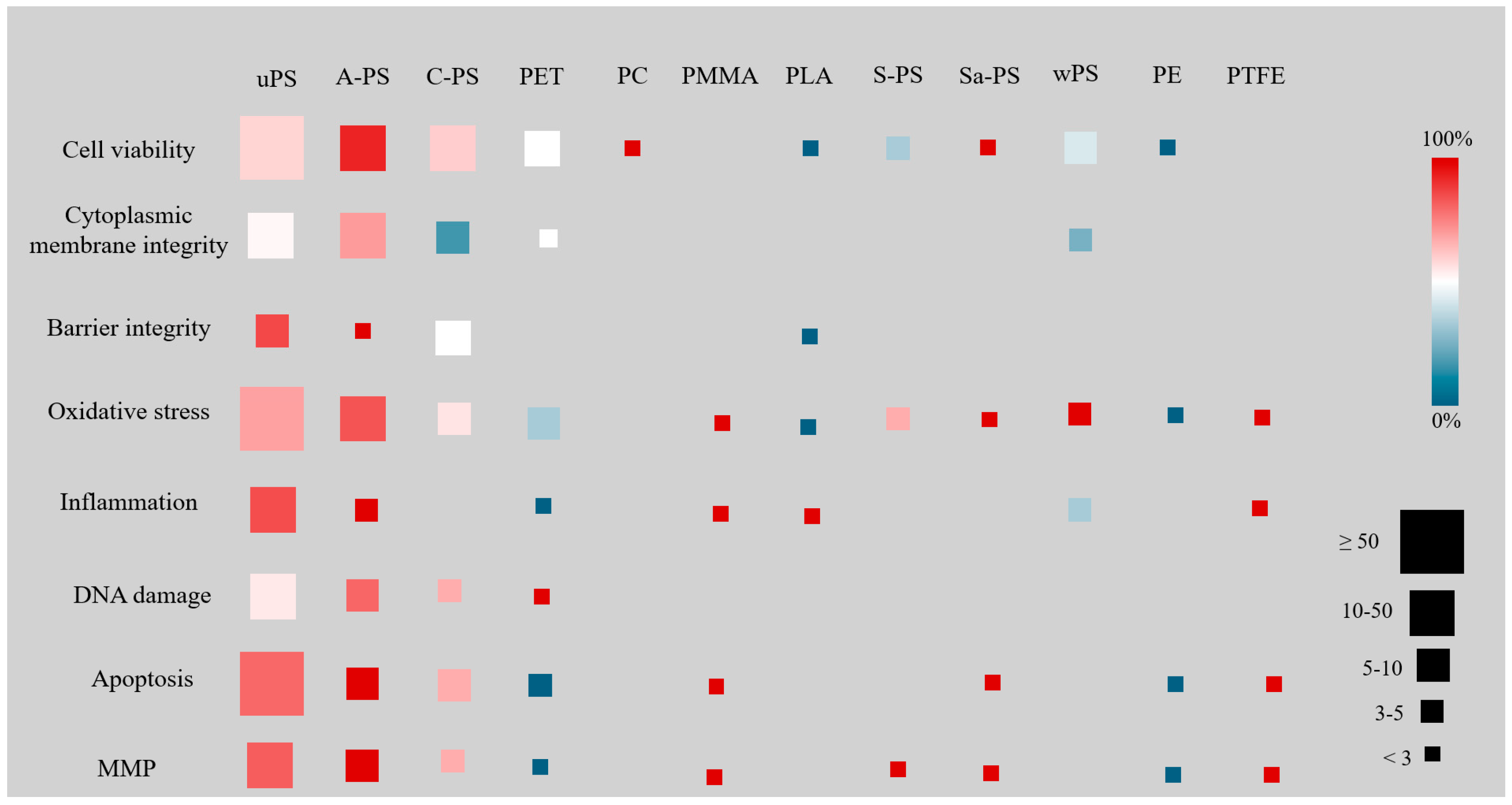
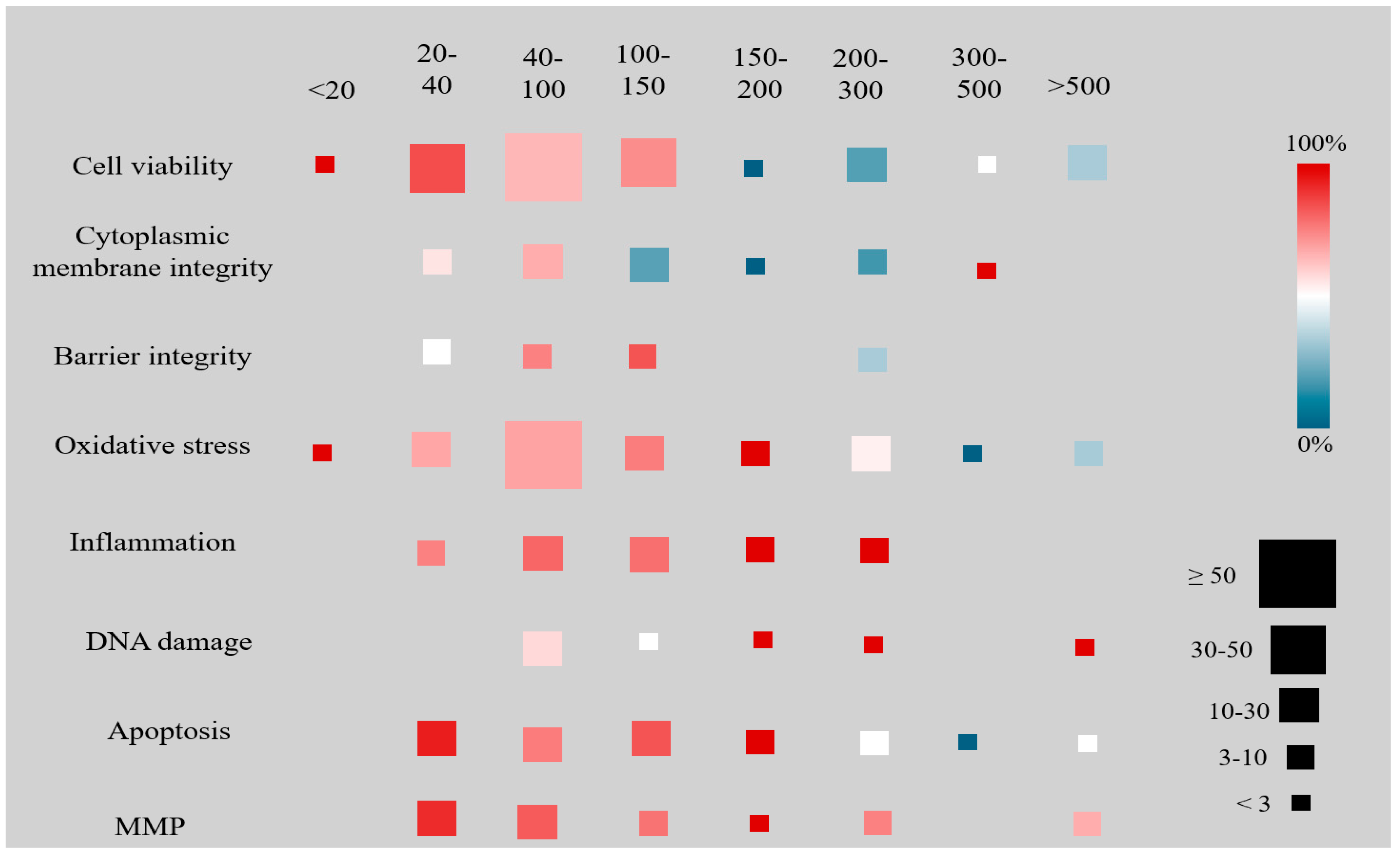
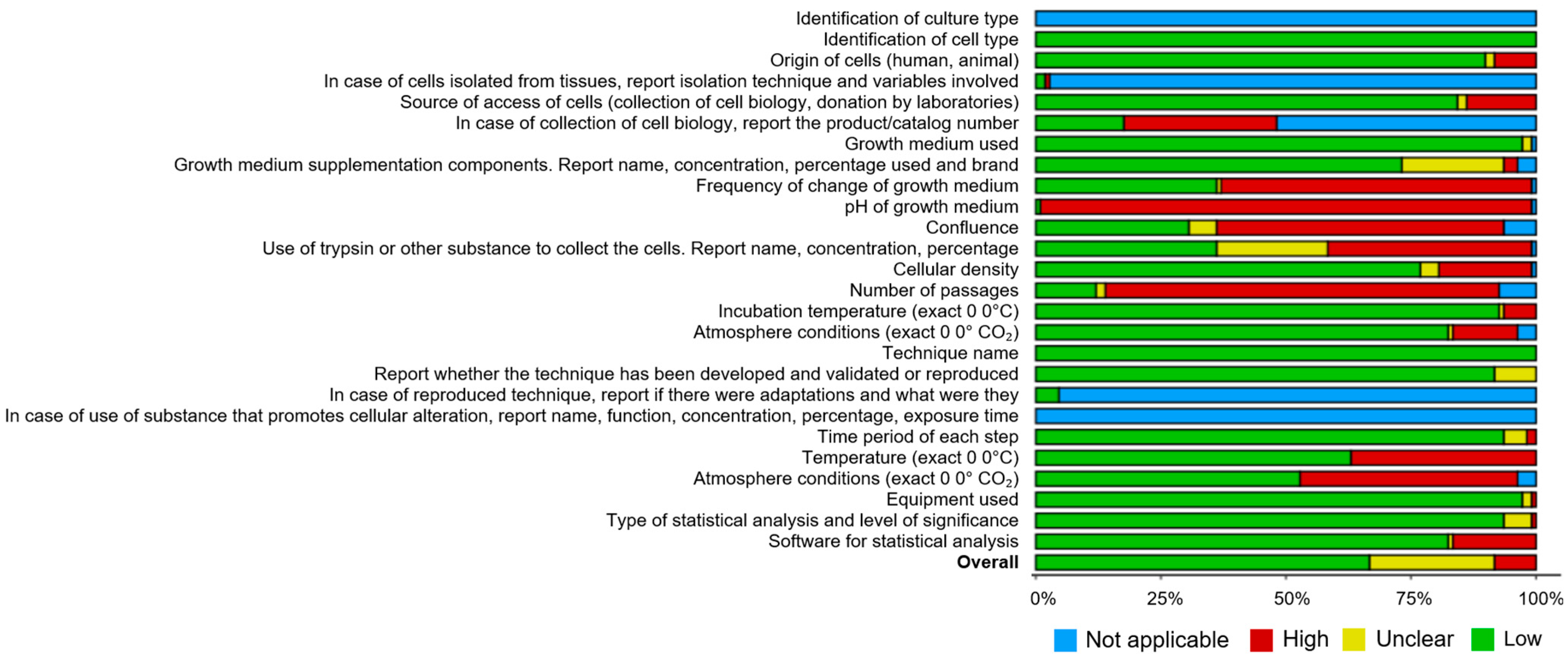
| System | N. Articles | N. Cellular Lines | NP Types | Global Negative Effects of the Most Reported NPs (% of Studies) # | ||
|---|---|---|---|---|---|---|
| C-PS | uPS | A-PS | ||||
| Hepatic | 12 | 7 | uPS, C-PS, A-PS, wPS, PET, wPET, PC, PMMA, PLA, PP, S-PS | 57 | 42 | 100 * |
| Urinary | 4 | 2 | uPS, wPS | - | 85 | - |
| Respiratory | 15 | 7 | uPS, C-PS, A-PS, PET | 64 | 100 | 100 |
| Digestive | 33 | 13 | uPS, C-PS, A-PS, wPS, PET, wPET, PC, PMMA, PLA, PP, PTFE | 41 | 62 | 75 |
| Immune | 22 | 11 | uPS, C-PS, A-PS, S-PS, Sa-PS, PE, PET | 78 | 52 | 85 |
| Reproductive | 13 | 11 | uPS, C-PS, A-PS | 100 * | 80 | 62.5 |
| Gestational tissues | 4 | 3 | uPS, wPS, A-PS, C-PS | 33 | 40 * | 100 * |
| Nervous | 12 | 10 | uPS, C-PS, A-PS, PE | 0 | 80 | 100 * |
| Connecting tissues | 6 | 7 | uPS, A-PS | - | 87 * | 100 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viana, M.; Tonin, F.S.; Ladeira, C. Assessing the Impact of Nanoplastics in Biological Systems: Systematic Review of In Vitro Animal Studies. J. Xenobiot. 2025, 15, 75. https://doi.org/10.3390/jox15030075
Viana M, Tonin FS, Ladeira C. Assessing the Impact of Nanoplastics in Biological Systems: Systematic Review of In Vitro Animal Studies. Journal of Xenobiotics. 2025; 15(3):75. https://doi.org/10.3390/jox15030075
Chicago/Turabian StyleViana, Maria, Fernanda S. Tonin, and Carina Ladeira. 2025. "Assessing the Impact of Nanoplastics in Biological Systems: Systematic Review of In Vitro Animal Studies" Journal of Xenobiotics 15, no. 3: 75. https://doi.org/10.3390/jox15030075
APA StyleViana, M., Tonin, F. S., & Ladeira, C. (2025). Assessing the Impact of Nanoplastics in Biological Systems: Systematic Review of In Vitro Animal Studies. Journal of Xenobiotics, 15(3), 75. https://doi.org/10.3390/jox15030075