Ames Assay Transferred from the Microtiter Plate to the Planar Assay Format
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Cultivation of the Two Bacterial Strains Using Cryostocks
2.3. Plate Pretreatment
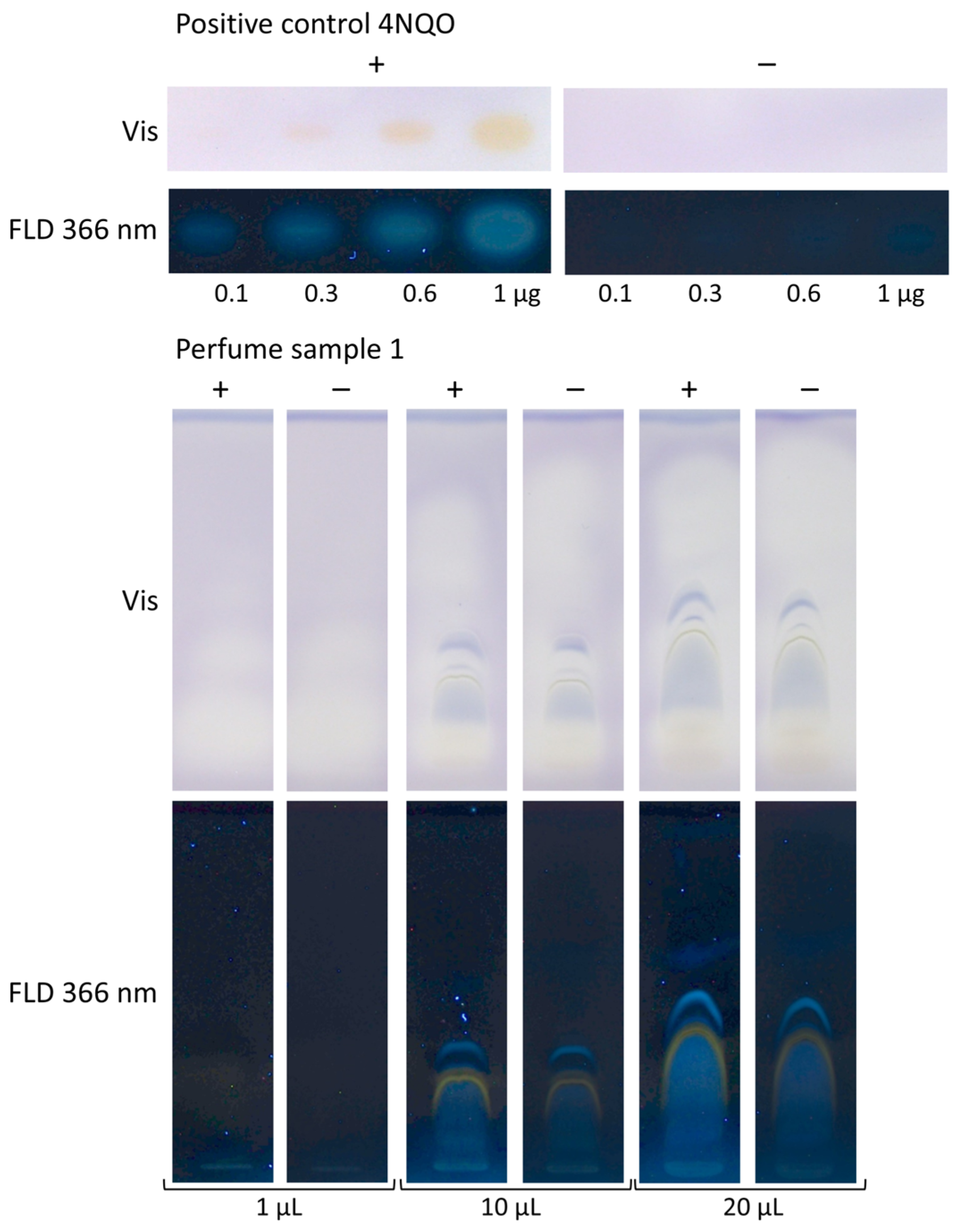
2.4. Preparation and Testing of the Positive Control Standards as Band Pattern
2.5. Preparation and Separation of the Four Different Sample Categories
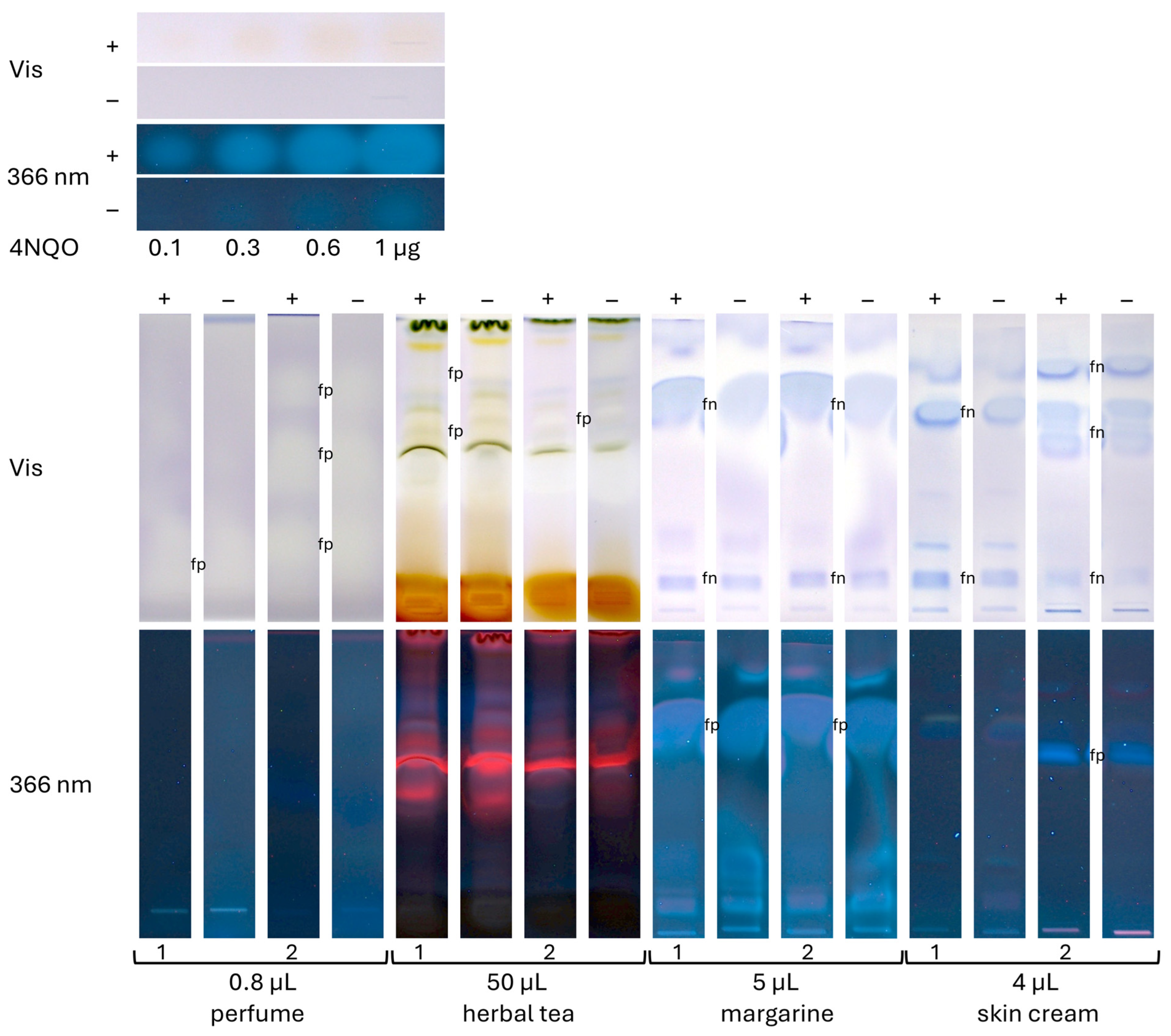
- The perfumes were transferred to vials without any sample preparation, applied (0.8, 1, 10, and 20 µL/band each), and developed with 7 mL of cyclohexane–ethyl acetate 19:1 (V/V) [33].
- The margarines and skin creams (100 mg each) were dissolved in 1.5 mL of isopropanol–pentane 1:1 (V/V), ultrasonificated (Sonorex Digiplus, Bandelin, Berlin, Germany) for 10 min, and centrifuged (17,000× g, 10 min). Each upper phase was transferred to a vial, where 5 µL was applied. Development was performed using 5 mL of pentane–diethyl ether 8:3 (V/V) after chamber saturation with 20 mL of the solvent system [31].
- The tea samples (500 mg each) were extracted with 5 mL of methanol, ultrasonicated (75 °C, 30 min), and centrifuged (17,000× g, 15 min). Each supernatant was filtered (sterile) into vials, where 50 µL was applied and developed using 7 mL of dichloromethane–methanol–ammonia 85:15:1 (V/V/V).
2.6. Planar Ames Bioassay Detection
3. Results and Discussion
3.1. Development of the Planar Ames Assay
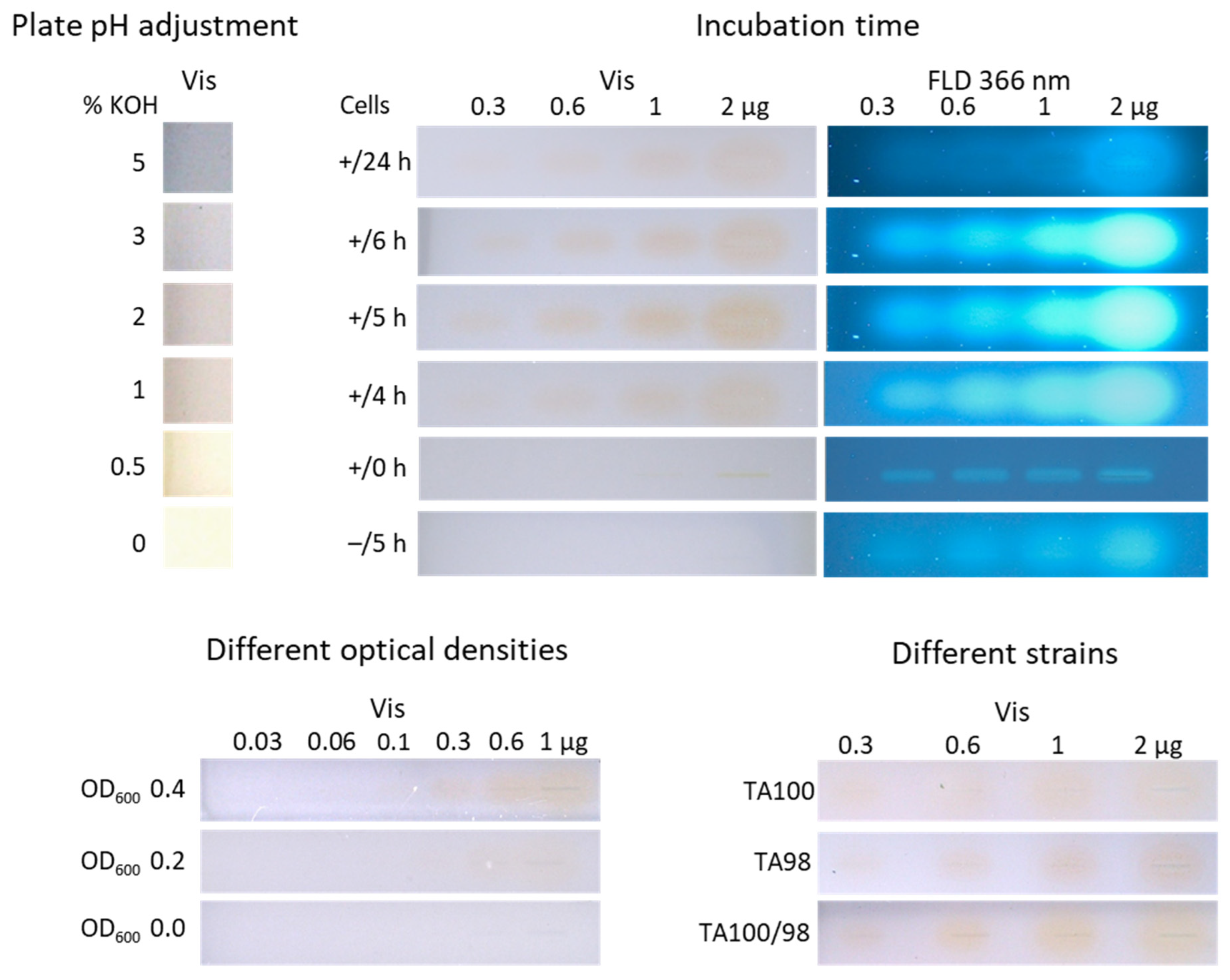
3.1.1. Adjustment of the Plate pH
3.1.2. Study of the Incubation Time
3.1.3. Negative Control as Proof of the Selectivity or Specificity of the Response
3.1.4. Adjustment of the Optical Density of the Cell Culture
3.1.5. Detection Limit of Individual Versus Combined Strains Towards 4NQO
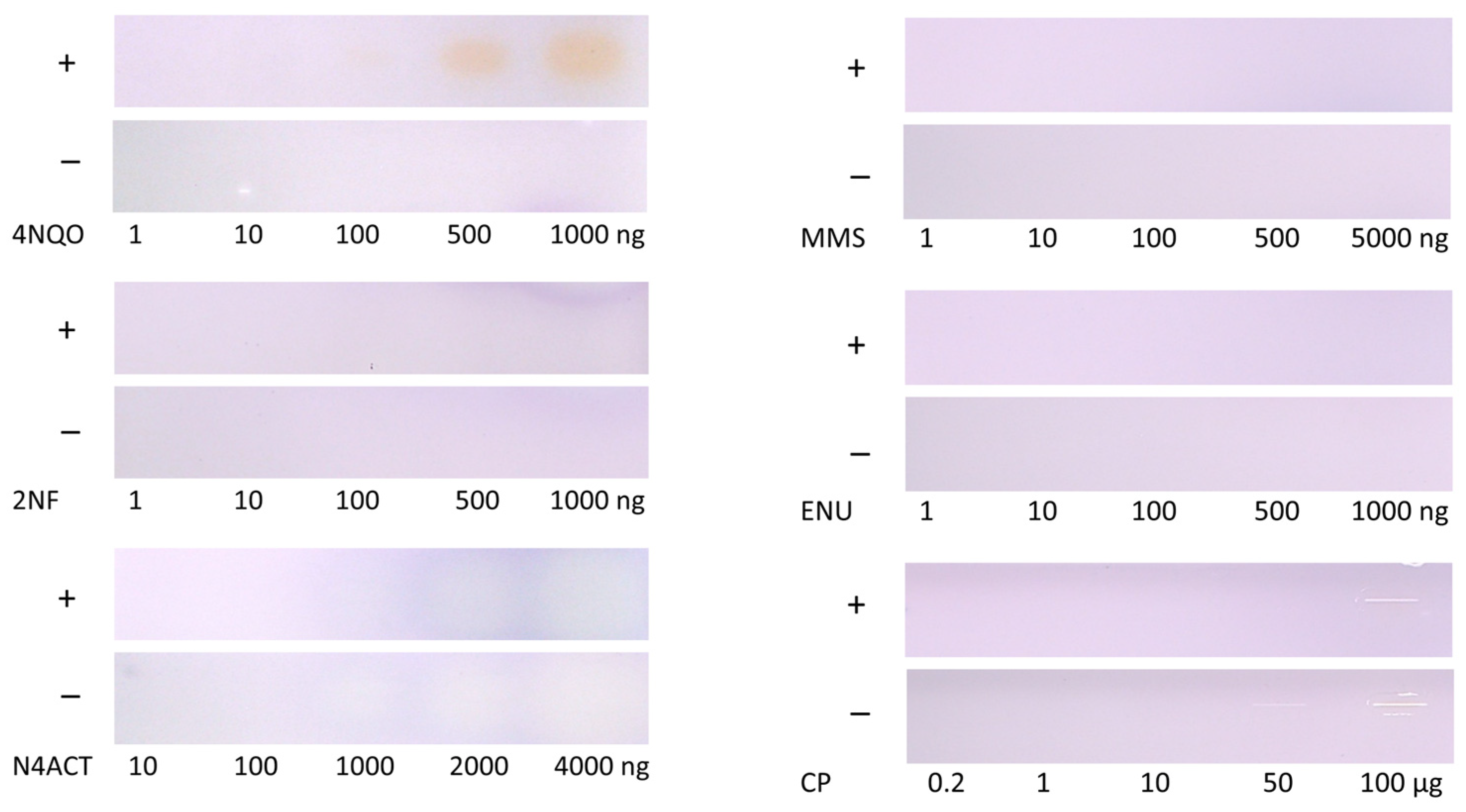
3.2. Screening of Further Mutagenic Reference Substances
3.3. Application to Four Different Sample Categories Pointing to False Positives/Negatives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Noncommunicable Diseases. 2023. Available online: www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 28 April 2025).
- Cancer Today. Absolute Numbers, Incidence, Both Sexes, in 2022. Available online: https://gco.iarc.who.int/today/en/dataviz/pie?mode=population&group_populations=0 (accessed on 26 April 2025).
- Bosetti, C.; Bertuccio, P.; Malvezzi, M.; Levi, F.; Chatenoud, L.; Negri, E.; La Vecchia, C. Cancer mortality in Europe, 2005–2009, and an overview of trends since 1980. Ann. Oncol. 2013, 24, 2657–2671. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Agents Classified by the IARC Monographs, Volumes 1–135. 2023. Available online: https://monographs.iarc.who.int/agents-classified-by-the-iarc/ (accessed on 26 April 2025).
- Ames, B.N.; Joyce, M.; Edith, Y. Methods for Detecting Carcinogens and Mutagens with the Salmonella/Mammalian-Microsome Mutagenicity Test. Mutat. Res. 1975, 31, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Durston, W.E.; Yamasaki, E.; Lee, F.D. Carcinogens are Mutagens: A Simple Test System Combining Liver Homogenates for Activation and Bacteria for Detection. Proc. Nat. Acad. Sci. USA 1973, 70, 2281–2285. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Charles, Y. The Detection of Chemical Mutagens with Enteric Bacteria. In Chemical Mutagens; Springer: Boston, MA, USA, 1971. [Google Scholar]
- Houk, V.S.; Claxton, L.D. Screening complex hazardous wastes for mutagenic activity using a modified version of the TLC/Salmonella assay. Mutat. Res. 1986, 169, 81–92. [Google Scholar] [CrossRef]
- Bjørseth, A.; Eidså, G.; Gether, J.; Landmark, L.; Møller, M. Detection of mutagens in complex samples by the Salmonella assay applied directly on thin-layer chromatography plates. Science 1982, 215, 87–89. [Google Scholar] [CrossRef]
- OECD. Test No. 471: Bacterial Reverse Mutation Test; OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2020. [Google Scholar] [CrossRef]
- Mortelmans, K.; Zeiger, E. The Ames Salmonella/microsome mutagenicity assay. Mutat. Res. 2000, 455, 29–60. [Google Scholar] [CrossRef]
- Williams, R.V.; DeMarini, D.M.; Stankowski, L.F.; Escobar, P.A.; Zeiger, E.; Howe, J.; Elespuru, R.; Cross, K.P. Are all bacterial strains required by OECD mutagenicity test guideline TG471 needed? Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 848, 503081. [Google Scholar] [CrossRef]
- Cross, K.P.; DeMarini, D.M. Analysis of chemical structures and mutations detected by Salmonella TA98 and TA100. Mutat. Res. 2023, 827, 111838. [Google Scholar] [CrossRef]
- Maron, D.M.; Ames, B.N. Revised methods for Salmonella mutagenicity test. Mutat. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Flückiger-Isler, S.; Baumeister, M.; Braun, K.; Gervais, V.; Hasler-Nguyen, N.; Reimann, R.; van Gompel, J.; Wunderlich, H.-G.; Engelhardt, G. Assessment of the performance of the Ames II assay: A collaborative study with 19 coded compounds. Mutat. Res. 2004, 558, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Flückiger-Isler, S.; Kamber, M. Direct comparison of the Ames microplate format (MPF) test in liquid medium with the standard Ames pre-incubation assay on agar plates by use of equivocal to weakly positive test compounds. Mutat. Res. 2012, 747, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Flückiger-Isler, S.; Kamber, M. The Ames MPF 98/100 Assay: Novel Mutagenicity Testing in Liquid Microplate Format Using S. typhimurium TA98 and TA100, (Poster Presentation); EEMS: Praque, Czech Republic, 2006. [Google Scholar]
- Flückiger-Isler, S.; Kamber, M. The Ames MPF™ Penta I Assay: Mutagenicity Testing in Liquid Microplate Format Using OECD Guideline 471 Compliant Strains, (Poster Presentation); ICEM: Florence, Italy, 2009. [Google Scholar]
- Spiliotopoulos, D.; Koelbert, C.; Audebert, M.; Barisch, I.; Bellet, D.; Constans, M.; Czich, A.; Finot, F.; Gervais, V.; Khoury, L.; et al. Assessment of the performance of the Ames MPF™ assay: A multicenter collaborative study with six coded chemicals. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2024, 893, 503718. [Google Scholar] [CrossRef]
- Yao, W.; Byrne, R.H. Spectrophotometric determination of freshwater pH using bromocresol purple and phenol red. Environ. Sci. Technol. 2001, 35, 1197–1201. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, Y.; Joshi, K.; Hickey, C.; Wahler, J.; Wall, B.; Etter, S.; Smith, B.; Griem, P.; Tate, M.; Jones, F.; et al. The BlueScreen HC assay to predict the genotoxic potential of fragrance materials. Mutagenesis 2022, 37, 13–23. [Google Scholar] [CrossRef]
- Morlock, G.E. High-performance thin-layer chromatography combined with effect-directed assays and high-resolution mass spectrometry as an emerging hyphenated technology: A tutorial review. Anal. Chim. Acta 2021, 1180, 38644. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G.E. Planar chromatographic super-hyphenations for rapid dereplication. Phytochem. Rev. 2022, 24, 1–12. [Google Scholar] [CrossRef]
- Varalda, G.M.; Zellmer, S.; Tietz, T. Risk assessment of food contact materials. EFSA J. 2024, 22, e221107. [Google Scholar] [CrossRef]
- Mayrhofer, E.; Prielinger, L.; Sharp, V.; Rainer, B.; Kirchnawy, C.; Rung, C.; Gruner, A.; Juric, M.; Springer, A. Safety Assessment of Recycled Plastics from Post-Consumer Waste with a Combination of a Miniaturized Ames Test and Chromatographic Analysis. Recycling 2023, 8, 87. [Google Scholar] [CrossRef]
- Rainer, B.; Mayrhofer, E.; Redl, M.; Dolak, I.; Mislivececk, D.; Czerny, T.; Kirchnawy, C.; Marin-Kuan, M.; Schilter, B.; Tacker, M. Mutagenicity assessment of food contact material migrates with the Ames MPF assay. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 1419–1432. [Google Scholar] [CrossRef]
- Dung, C.-H.; Wu, S.-C.; Yen, G.-C. Genotoxicity and oxidative stress of the mutagenic compounds formed in fumes of heated soybean oil, sunflower oil and lard. Toxicol. In Vitro 2006, 20, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G.E.; Meyer, D. Designed genotoxicity profiling detects genotoxic compounds in staple food such as healthy oils. Food Chem. 2023, 408, 135253. [Google Scholar] [CrossRef]
- Di Sotto, A.; Maffei, F.; Hrelia, P.; Di Giacomo, S.; Pagano, E.; Borrelli, F.; Mazzanti, G. Genotoxicity assessment of some cosmetic and food additives. Regul. Toxicol. Pharmacol. 2014, 68, 16–22. [Google Scholar] [CrossRef]
- Morlock, G.E.; Zoller, L. Fast unmasking toxicity of safe personal care products. J. Chromatogr. A 2025, 1752, 465886. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Al-Rajudi, T.; Al-Qudaihi, G.; Manogaran, P. Evaluating the potential genotoxicity of phthalates esters (PAEs) in perfumes using in vitro assays. Environ. Sci. Pollut. Res. Int. 2017, 24, 23903–23914. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G.E.; Heil, J. Fast unmasking hazards of safe perfumes. J. Chromatogr. A 2025, 465959. [Google Scholar] [CrossRef]
- Rainer, B.; Pinter, E.; Prielinger, L.; Coppola, C.; Marin-Kuan, M.; Schilter, B.; Apprich, S.; Tacker, M. Direct Comparison of the Lowest Effect Concentrations of Mutagenic Reference Substances in Two Ames Test Formats. Toxics 2021, 9, 152. [Google Scholar] [CrossRef] [PubMed]
- Matches, J.R.; Liston, J. Effect of pH on low temperature growth of Salmonella. Milk Food Technol. 1972, 35, 1. [Google Scholar]
- Schreiner, T.; Ronzheimer, A.; Friz, M.; Morlock, G.E. Multiplex planar bioassay with reduced diffusion on normal phase, identifying androgens, verified antiandrogens and synergists in botanicals via 12D hyphenation. Food Chem. 2022, 395, 133610. [Google Scholar] [CrossRef]

| Bioassay | Advantages of Complex Mixture Analysis | Disadvantages of Complex Mixture Analysis |
|---|---|---|
| Ames MPF (mutagenicity) | None | Not usable for complex mixtures Long incubation time (48 h) Limited to single substances Non-selective for acidic components Prone to matrix effects Dilution can lead to false negatives |
| Planar Ames (mutagenicity) | Separation from matrix components Selective detection of mutagens | About 10-fold lower sensitivity for 4NQO Partially co-eluting acidic components |
| Planar SOS-Umu-C (genotoxicity) | Separation from matrix components High sensitivity and selectivity by fluorescence detection Fast analysis (3 h) | Requires devices for fluorescence detection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidtmann, K.; Lemme, J.; Morlock, G.E. Ames Assay Transferred from the Microtiter Plate to the Planar Assay Format. J. Xenobiot. 2025, 15, 67. https://doi.org/10.3390/jox15030067
Schmidtmann K, Lemme J, Morlock GE. Ames Assay Transferred from the Microtiter Plate to the Planar Assay Format. Journal of Xenobiotics. 2025; 15(3):67. https://doi.org/10.3390/jox15030067
Chicago/Turabian StyleSchmidtmann, Katharina, Johanna Lemme, and Gertrud E. Morlock. 2025. "Ames Assay Transferred from the Microtiter Plate to the Planar Assay Format" Journal of Xenobiotics 15, no. 3: 67. https://doi.org/10.3390/jox15030067
APA StyleSchmidtmann, K., Lemme, J., & Morlock, G. E. (2025). Ames Assay Transferred from the Microtiter Plate to the Planar Assay Format. Journal of Xenobiotics, 15(3), 67. https://doi.org/10.3390/jox15030067






