COMT Genetic Variants and BDNF Level Associations with Cannabinoid Plasma Exposure: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Enrolled Patients
- Bedrocan® (THC level standardized at 19% and with a CBD level < 1%);
- Bediol® (THC and CBD levels standardized at concentrations of 6.5% and 8%, respectively);
- FM2® (THC and CBD levels standardized at concentrations of 5–8% and 7.5–12%, respectively);
- Pedanios® 22/1 (THC and CBD levels standardized at concentrations of 22% and <1%, respectively).
2.2. Pharmacokinetic Analyses
2.3. Genetic Polymorphism Analyses
2.4. ELISA Tests
2.5. SiMoA® Tests
2.6. Statistical Analyses
3. Results
3.1. Characteristics of Enrolled Patients
3.2. Biomarker Concentrations
3.3. Genetics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- WHO. Available online: https://www.who.int/teams/mental-health-and-substance-use/alcohol-drugs-and-addictive-behaviours/drugs-psychoactive/cannabis (accessed on 9 November 2023).
- Pisanti, S.; Bifulco, M. Medical Cannabis: A plurimillennial history of an evergreen. J. Cell Physiol. 2018, 234, 8342–8351. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, H.; Kobayashi, M.; Momohara, A.; Eguchi, S.; Okamoto, T.; Yanagisawa, S.; Okubo, S.; Kiyonage, J. Early Holocene coastal environment change in ferred from deposits at Okinoshima archeological site, Boso Peninsula, central Japan. Quat. Int. 2011, 230, 87–94. [Google Scholar] [CrossRef]
- G.U. Decreto Ministeriale 9 November 2015. Funzioni di Organisimo Statale per la Cannabis Previsto Dagli Articoli 23 e 28 Della Convenzione Unica Sugli Stupefacenti del 1961, Come Modificata Nel 1972. Available online: https://www.epicentro.iss.it/farmaci/pdf/Decreto%20uso%20medico%20Cannabis%20(GU%2030.11.2015)%20 (accessed on 3 March 2025).
- Aizpurua-Olaizola, O.; Elezgarai, I.; Rico-Barrio, I.; Zarandona, I.; Etxebarria, N.; Usobiaga, A. Targeting the endocannabinoid system: Future therapeutic strategies. Drug Discov. Today 2016, 22, 105–110. [Google Scholar] [CrossRef]
- Battista, N.; Di Tommaso, M.; Bari, M.; Maccarrone, M. The endocannabinoid system: An overview. Front. Behav. Neurosci. 2012, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W. The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases. Int. J. Mol. Sci. 2021, 22, 9472. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Batkai, S.; Kunos, G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef]
- Eid, B.G. Cannabinoids for Treating Cardiovascular Disorders: Putting Together a Complex Puzzle. J. Microsc. Ultrastruct. 2018, 6, 171–176. [Google Scholar] [CrossRef]
- Mendizabal, V.E.; Adler-Graschinsky, E. Cannabinoids as therapeutic agents in cardiovascular disease: A tale of passions and illusions. Br. J. Pharmacol. 2007, 151, 427–440. [Google Scholar] [CrossRef]
- Borowska, M.; Czarnywojtek, A.; Sawicka-Gutaj, N.; Wolinski, K.; Plazinska, M.T.; Mikolajczak, P.; Ruchala, M. The effects of cannabinoids on the endocrine system. Endokrynol. Pol. 2018, 69, 705–719. [Google Scholar] [CrossRef]
- Parsons, L.H.; Hurd, Y.L. Endocannabinoid signalling in reward and addiction. Nat. Rev. Neurosci. 2015, 16, 579–594. [Google Scholar] [CrossRef]
- Whiteside, G.T.; Lee, G.P.; Valenzano, K.J. The role of the cannabinoid CB2 receptor in pain transmission and therapeutic potential of small molecule CB2 receptor agonists. Curr. Med. Chem. 2007, 14, 917–936. [Google Scholar] [CrossRef] [PubMed]
- Romero, T.R.; Resende, L.C.; Guzzo, L.S.; Duarte, I.D. CB1 and CB2 cannabinoid receptor agonists induce peripheral antinociception by activation of the endogenous noradrenergic system. Anesth. Analg. 2013, 116, 463–472. [Google Scholar] [CrossRef]
- Chia, J.S.M.; Farouk, A.A.O.; Mohamad, T.; Sulaiman, M.R.; Zakaria, H.; Hassan, N.I.; Perimal, E.K. Zerumbone Ameliorates Neuropathic Pain Symptoms via Cannabinoid and PPAR Receptors Using In Vivo and In Silico Models. Molecules 2021, 26, 3849. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Otto, W.R.; Sanchez-Herrera, D.; Facer, P.; Yiangou, Y.; Korchev, Y.; Birch, R.; Benham, C.; Bountra, C.; Chessell, I.P.; et al. Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain 2008, 138, 667–680. [Google Scholar] [CrossRef]
- Rahn, E.J.; Hohmann, A.G. Cannabinoids as pharmacotherapies for neuropathic pain: From the bench to the bedside. Neurotherapeutics 2009, 6, 713–737. [Google Scholar] [CrossRef]
- Shohami, E.; Cohen-Yeshurun, A.; Magid, L.; Algali, M.; Mechoulam, R. Endocannabinoids and traumatic brain injury. Br. J. Pharmacol. 2011, 163, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as novel anti-inflammatory drugs. Future Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef]
- Staton, P.C.; Hatcher, J.P.; Walker, D.J.; Morrison, A.D.; Shapland, E.M.; Hughes, J.P.; Chong, E.; Mander, P.K.; Green, P.J.; Billinton, A.; et al. The putative cannabinoid receptor GPR55 plays a role in mechanical hyperalgesia associated with inflammatory and neuropathic pain. Pain 2008, 139, 225–236. [Google Scholar] [CrossRef]
- Burstein, S. Cannabidiol (CBD) and its analogs: A review of their effects on inflammation. Bioorg. Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef]
- Porter, B.E.; Jacobson, C. Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy. Epilepsy Behav. 2013, 29, 574–577. [Google Scholar] [CrossRef]
- Ng, T.K.S.; Coughlan, C.; Heyn, P.C.; Tagawa, A.; Carollo, J.J.; Kua, E.H.; Mahendran, R. Increased plasma brain-derived neurotrophic factor (BDNF) as a potential biomarker for and compensatory mechanism in mild cognitive impairment: A case-control study. Aging 2021, 13, 22666–22689. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, G.; Beiser, A.S.; Choi, S.H.; Preis, S.R.; Chen, T.C.; Vorgas, D.; Au, R.; Pikula, A.; Wolf, P.A.; DeStefano, A.L.; et al. Serum brain-derived neurotrophic factor and the risk for dementia: The Framingham Heart Study. JAMA Neurol. 2014, 71, 55–61. [Google Scholar] [CrossRef]
- Di-Bonaventura, S.; Gurdiel-Alvarez, F.; Reina-Varona, A.; Pacheco-Barrios, K.; Molina-Alvarez, M.; Fernandez-Carnero, J.; Ferrer-Pena, R. Differences in Plasma BDNF Levels Between Chronic Primary Musculoskeletal Pain, Fibromyalgia Syndrome, and Asymptomatic Subjects: A Cross-Sectional Study. Biol. Res. Nurs. 2025, 10998004251313741. [Google Scholar] [CrossRef] [PubMed]
- Hock, C.; Heese, K.; Hulette, C.; Rosenberg, C.; Otten, U. Region-specific neurotrophin imbalances in Alzheimer disease: Decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch. Neurol. 2000, 57, 846–851. [Google Scholar] [CrossRef]
- Merighi, A. Brain-Derived Neurotrophic Factor, Nociception, and Pain. Biomolecules 2024, 14, 539. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.Y.; Hendrix, J.; Schabrun, S.; Wyns, A.; Campenhout, J.V.; Nijs, J.; Polli, A. The Role of the Brain-Derived Neurotrophic Factor in Chronic Pain: Links to Central Sensitization and Neuroinflammation. Biomolecules 2024, 14, 71. [Google Scholar] [CrossRef]
- Babayeva, M.; Loewy, Z.G. Cannabis Pharmacogenomics: A Path to Personalized Medicine. Curr. Issues Mol. Biol. 2023, 45, 3479–3514. [Google Scholar] [CrossRef]
- Hryhorowicz, S.; Walczak, M.; Zakerska-Banaszak, O.; Slomski, R.; Skrzypczak-Zielinska, M. Pharmacogenetics of Cannabinoids. Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 1–12. [Google Scholar] [CrossRef]
- Huestis, M.A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef]
- Lodzki, M.; Godin, B.; Rakou, L.; Mechoulam, R.; Gallily, R.; Touitou, E. Cannabidiol-transdermal delivery and anti-inflammatory effect in a murine model. J. Control Release 2003, 93, 377–387. [Google Scholar] [CrossRef]
- Khalil, H. Medicinal cannabis: Presenting possible treatment modalities for the future. Int. J. Evid. Based Healthc. 2018, 16, 81–82. [Google Scholar] [CrossRef] [PubMed]
- Manca, A.; Chiara, F.; Mula, J.; Palermiti, A.; Maiese, D.; Zeaiter, S.; De Nicolo, A.; Imperiale, D.; De Filippis, G.; Vischia, F.; et al. A new UHPLC-MS/MS method for cannabinoids determination in human plasma: A clinical tool for therapeutic drug monitoring. Biomed. Pharmacother. 2022, 156, 113899. [Google Scholar] [CrossRef]
- Manca, A.; Valz, C.; Chiara, F.; Mula, J.; Palermiti, A.; Billi, M.; Antonucci, M.; Nicolo, A.; Luxardo, N.; Imperiale, D.; et al. Cannabinoid levels description in a cohort of patients with chronic and neuropathic pain treated with Cannabis decoction: A possible role of TDM. Biomed. Pharmacother. 2024, 175, 116686. [Google Scholar] [CrossRef]
- Joffre, J.; Yeh, C.C.; Wong, E.; Thete, M.; Xu, F.; Zlatanova, I.; Lloyd, E.; Kobzik, L.; Legrand, M.; Hellman, J. Activation of CB(1)R Promotes Lipopolysaccharide-Induced IL-10 Secretion by Monocytic Myeloid-Derived Suppressive Cells and Reduces Acute Inflammation and Organ Injury. J. Immunol. 2020, 204, 3339–3350. [Google Scholar] [CrossRef]
- Tan, K.B.C.; Alexander, H.D.; Linden, J.; Murray, E.K.; Gibson, D.S. Anti-inflammatory effects of phytocannabinoids and terpenes on inflamed Tregs and Th17 cells in vitro. Exp. Mol. Pathol. 2024, 139, 104924. [Google Scholar] [CrossRef]
- Haas, L.; Portela, L.V.; Bohmer, A.E.; Oses, J.P.; Lara, D.R. Increased plasma levels of brain derived neurotrophic factor (BDNF) in patients with fibromyalgia. Neurochem. Res. 2010, 35, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Binder, D.K.; Scharfman, H.E. Brain-derived neurotrophic factor. Growth Factors 2004, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb. Exp. Pharmacol. 2014, 220, 223–250. [Google Scholar] [CrossRef]
- Blesch, A. Neurotrophic factors in neurodegeneration. Brain Pathol. 2006, 16, 295–303. [Google Scholar] [CrossRef]
- Weis, J.; Saxena, S.; Evangelopoulos, M.E.; Kruttgen, A. Trophic factors in neurodegenerative disorders. IUBMB Life 2003, 55, 353–357. [Google Scholar] [CrossRef]
- Janakiraman, U.; Manivasagam, T.; Justin Thenmozhi, A.; Dhanalakshmi, C.; Essa, M.M.; Song, B.J.; Guillemin, G.J. Chronic mild stress augments MPTP induced neurotoxicity in a murine model of Parkinson’s disease. Physiol. Behav. 2017, 173, 132–143. [Google Scholar] [CrossRef]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.; Bromberg, E.; de Vries, E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2018, 56, 3295–3312. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Wuu, J.; Mufson, E.J.; Fahnestock, M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer’s disease. J. Neurochem. 2005, 93, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, D.; Urshansky, N.; Karni, A. Low and dysregulated BDNF secretion from immune cells of MS patients is related to reduced neuroprotection. J. Neuroimmunol. 2008, 195, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Patanella, A.K.; Zinno, M.; Quaranta, D.; Nociti, V.; Frisullo, G.; Gainotti, G.; Tonali, P.A.; Batocchi, A.P.; Marra, C. Correlations between peripheral blood mononuclear cell production of BDNF, TNF-alpha, IL-6, IL-10 and cognitive performances in multiple sclerosis patients. J. Neurosci. Res. 2010, 88, 1106–1112. [Google Scholar] [CrossRef]
- Almeida, R.D.; Manadas, B.J.; Melo, C.V.; Gomes, J.R.; Mendes, C.S.; Graos, M.M.; Carvalho, R.F.; Carvalho, A.P.; Duarte, C.B. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005, 12, 1329–1343. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Asevedo, E.; Gadelha, A.; Noto, C.; Mansur, R.B.; Zugman, A.; Belangero, S.I.; Berberian, A.A.; Scarpato, B.S.; Leclerc, E.; Teixeira, A.L.; et al. Impact of peripheral levels of chemokines, BDNF and oxidative markers on cognition in individuals with schizophrenia. J. Psychiatr. Res. 2013, 47, 1376–1382. [Google Scholar] [CrossRef]
- Carlino, D.; Leone, E.; Di Cola, F.; Baj, G.; Marin, R.; Dinelli, G.; Tongiorgi, E.; De Vanna, M. Low serum truncated-BDNF isoform correlates with higher cognitive impairment in schizophrenia. J. Psychiatr. Res. 2011, 45, 273–279. [Google Scholar] [CrossRef]
- Segal-Gavish, H.; Gazit, N.; Barhum, Y.; Ben-Zur, T.; Taler, M.; Hornfeld, S.H.; Gil-Ad, I.; Weizman, A.; Slutsky, I.; Niwa, M.; et al. BDNF overexpression prevents cognitive deficit elicited by adolescent cannabis exposure and host susceptibility interaction. Hum. Mol. Genet. 2017, 26, 2462–2471. [Google Scholar] [CrossRef]
- Butovsky, E.; Juknat, A.; Goncharov, I.; Elbaz, J.; Eilam, R.; Zangen, A.; Vogel, Z. In vivo up-regulation of brain-derived neurotrophic factor in specific brain areas by chronic exposure to Delta-tetrahydrocannabinol. J. Neurochem. 2005, 93, 802–811. [Google Scholar] [CrossRef]
- Blazquez, C.; Chiarlone, A.; Bellocchio, L.; Resel, E.; Pruunsild, P.; Garcia-Rincon, D.; Sendtner, M.; Timmusk, T.; Lutz, B.; Galve-Roperh, I.; et al. The CB(1) cannabinoid receptor signals striatal neuroprotection via a PI3K/Akt/mTORC1/BDNF pathway. Cell Death Differ. 2015, 22, 1618–1629. [Google Scholar] [CrossRef]
- Derkinderen, P.; Valjent, E.; Toutant, M.; Corvol, J.C.; Enslen, H.; Ledent, C.; Trzaskos, J.; Caboche, J.; Girault, J.A. Regulation of extracellular signal-regulated kinase by cannabinoids in hippocampus. J. Neurosci. 2003, 23, 2371–2382. [Google Scholar] [CrossRef] [PubMed]
- Hirota, T.; Eguchi, S.; Ieiri, I. Impact of genetic polymorphisms in CYP2C9 and CYP2C19 on the pharmacokinetics of clinically used drugs. Drug Metab. Pharmacokinet. 2012, 28, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Papastergiou, J.; Li, W.; Sterling, C.; van den Bemt, B. Pharmacogenetic-guided cannabis usage in the community pharmacy: Evaluation of a pilot program. J. Cannabis Res. 2020, 2, 24. [Google Scholar] [CrossRef]
- Beers, J.L.; Fu, D.; Jackson, K.D. Cytochrome P450-Catalyzed Metabolism of Cannabidiol to the Active Metabolite 7-Hydroxy-Cannabidiol. Drug Metab. Dispos. 2021, 49, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M. Predictive association of ABCB1 C3435T genetic polymorphism with the efficacy or safety of lopinavir and ritonavir in COVID-19 patients. Pharmacogenomics 2021, 22, 375–381. [Google Scholar] [CrossRef]
- Wolking, S.; Schaeffeler, E.; Lerche, H.; Schwab, M.; Nies, A.T. Impact of Genetic Polymorphisms of ABCB1 (MDR1, P-Glycoprotein) on Drug Disposition and Potential Clinical Implications: Update of the Literature. Clin. Pharmacokinet. 2015, 54, 709–735. [Google Scholar] [CrossRef]
- Yan, R.J.; Lou, T.T.; Wu, Y.F.; Chen, W.S. Single nucleotide polymorphisms of ABCB1 gene and response to etanercept treatment in patients with ankylosing spondylitis in a Chinese Han population. Medicine 2017, 96, e5929. [Google Scholar] [CrossRef]
- Benyamina, A.; Bonhomme-Faivre, L.; Picard, V.; Sabbagh, A.; Richard, D.; Blecha, L.; Rahioui, H.; Karila, L.; Lukasiewicz, M.; Farinotti, R.; et al. Association between ABCB1 C3435T polymorphism and increased risk of cannabis dependence. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 1270–1274. [Google Scholar] [CrossRef]
- Kebir, O.; Lafaye, G.; Blecha, L.; Chaumette, B.; Mouaffak, F.; Laqueille, X.; Benyamina, A. ABCB1 C3435T polymorphism is associated with tetrahydrocannabinol blood levels in heavy cannabis users. Psychiatry Res. 2017, 262, 357–358. [Google Scholar] [CrossRef] [PubMed]
- Henquet, C.; Rosa, A.; Krabbendam, L.; Papiol, S.; Fananas, L.; Drukker, M.; Ramaekers, J.G.; van Os, J. An experimental study of catechol-o-methyltransferase Val158Met moderation of delta-9-tetrahydrocannabinol-induced effects on psychosis and cognition. Neuropsychopharmacology 2006, 31, 2748–2757. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | |
|---|---|
| No. of patients | 58 |
| Cigarette smokers, n (%) | 19 (32.8%) |
| Gender (male), n (%) | 20 (34.5%) |
| Caucasian, n (%) | 100% |
| BMI (kg/m2), median (IQR) | 20.6 (17.9; 23.4) |
| Age (years), median (IQR) | 61 (52; 67) |
| Fibromyalgia, n (%) | 27 (46.6%) |
| Headache, n (%) | 5 (8.6%) |
| Cancer, n (%) | 4 (6.9%) |
| Polytraumatized patients, n (%) | 38 (65.5%) |
| Cannabis mg | Number of Patients |
|---|---|
| 0–250 mg | 25 (43.0%) |
| 300–500 mg | 23 (39.7%) |
| >500 mg | 10 (17.3%) |
| Medical Cannabis with High Levels of THC | |||
|---|---|---|---|
| Cannabinoids | Inhaled Cannabis, ng/mL Median (IQR) | Oral Cannabis (Decoction), ng/mL Median (IQR) | p-Value |
| Δ9-THC | 14.26 (5.70; 23.99) | 5.08 (4.53; 11.04) | 0.011 |
| 11-OH-THC | 0 (0; 11.34) | 0 (0; 0) | 0.017 |
| COOH-THC | 62.99 (27.85; 248.33) | 10.53 (6.62; 23.59) | 0.004 |
| COOH-THC-glucuronide | 511.35 (103.44; 1076.27) | 47.92 (7.32; 80.01) | 0.003 |
| CBD | 5.26 (1.45; 11.45) | 2.94 (0.56; 5.73) | 0.364 |
| 7-OH-CBD | 2.26 (0.79; 9.82) | 0 (0; 0) | <0.001 |
| THCA | 0 (0; 2.11) | 3.35 (0; 11.75) | 0.127 |
| CBDA | 0 (0; 0.41) | 0 (0; 0.95) | 0.546 |
| Medical Cannabis with THC and CBD Level Standardized at Concentration of 6.5% and 8% | |||
|---|---|---|---|
| Cannabinoids | Inhaled Cannabis, ng/mL Median (IQR) | Oral Cannabis (Decoction), ng/mL Median (IQR) | p-Value |
| Δ9-THC | 5.85 (4.60; /) | 4.52 (4.18; 5.48) | 0.326 |
| 11-OH-THC | 0 (0; 0) | 0 (0; 1.39) | 0.412 |
| COOH-THC | 43.76 (5.21; /) | 11.43 (4.91; 21.70) | 0.517 |
| COOH-THC-glucuronide | 197.70 (17.81; /) | 35.07 (10.35; 63.88) | 0.404 |
| CBD | 7.83 (3.44; /) | 2.12 (0; 3.72) | 0.104 |
| 7-OH-CBD | 0.96 (0; /) | 0 (0; 1.67) | 0.667 |
| THCA | 0 (0; 0) | 4.89 (0; 9.04) | 0.100 |
| CBDA | 0 (0; 0) | 1.05 (0; 5.76) | 0.118 |
| Biomarkers | Median (Interquartile Range) |
|---|---|
| Tumor necrosis factor alpha, (ng/mL) | 110.40 (98.43; 140.90) |
| Interleukin-6, (ng/mL) | 73.8 (68.1; 88.4) |
| Interleukin-10, (pg/mL) | 245.3 (222.7; 307.3) |
| Brain-derived neurotrophic factor, (pg/mL) | 1672.6 (912.4; 5384.5) |
| Neurofilament Light Chain, (pg/mL) | 6.96 (4.53; 9.72) |
| Cannabis Metabolites | ||||||||
|---|---|---|---|---|---|---|---|---|
| Δ9-THC | OH-THC | COOH-THC | COOH-THC-Glucuronide | CBD | 7-OH-CBD | THCA | CBDA | |
| CYP2D6 4180 CG/GG | 0.023 | |||||||
| CYP1A1 2794 AA | 0.045 | 0.020 | ||||||
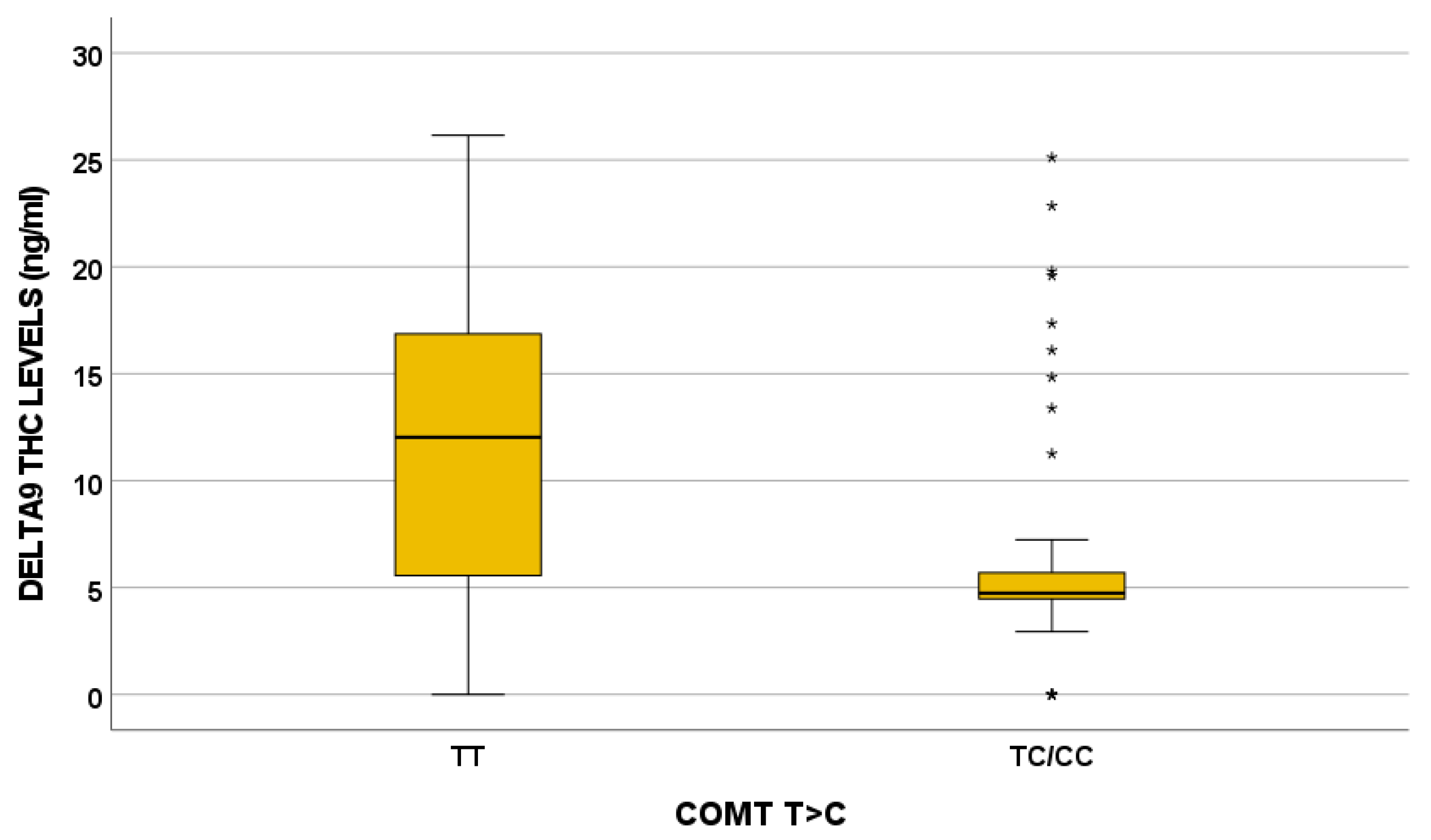
| COMT 680 TC/CC | 0.017 | 0.031 | 0.019 | 0.031 | 0.035 | |||
| BSEP TC/CC | 0.037 | |||||||
| BSEP CC | 0.047 | |||||||
| ABCB1 1236 CT/TT | 0.040 | |||||||
| CYP1A2 890 CT/TT | 0.033 | 0.033 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manca, A.; Valz, C.; Chiara, F.; Palermiti, A.; Mula, J.; Soloperto, S.; Antonucci, M.; De Nicolò, A.; Luxardo, N.; Imperiale, D.; et al. COMT Genetic Variants and BDNF Level Associations with Cannabinoid Plasma Exposure: A Preliminary Study. J. Xenobiot. 2025, 15, 66. https://doi.org/10.3390/jox15030066
Manca A, Valz C, Chiara F, Palermiti A, Mula J, Soloperto S, Antonucci M, De Nicolò A, Luxardo N, Imperiale D, et al. COMT Genetic Variants and BDNF Level Associations with Cannabinoid Plasma Exposure: A Preliminary Study. Journal of Xenobiotics. 2025; 15(3):66. https://doi.org/10.3390/jox15030066
Chicago/Turabian StyleManca, Alessandra, Cristina Valz, Francesco Chiara, Alice Palermiti, Jacopo Mula, Sara Soloperto, Miriam Antonucci, Amedeo De Nicolò, Nicola Luxardo, Daniele Imperiale, and et al. 2025. "COMT Genetic Variants and BDNF Level Associations with Cannabinoid Plasma Exposure: A Preliminary Study" Journal of Xenobiotics 15, no. 3: 66. https://doi.org/10.3390/jox15030066
APA StyleManca, A., Valz, C., Chiara, F., Palermiti, A., Mula, J., Soloperto, S., Antonucci, M., De Nicolò, A., Luxardo, N., Imperiale, D., Vischia, F., De Cori, D., Cusato, J., & D’Avolio, A. (2025). COMT Genetic Variants and BDNF Level Associations with Cannabinoid Plasma Exposure: A Preliminary Study. Journal of Xenobiotics, 15(3), 66. https://doi.org/10.3390/jox15030066








