Stability Dynamics of Plant-Based Extracellular Vesicles Drug Delivery
Abstract
1. Introduction
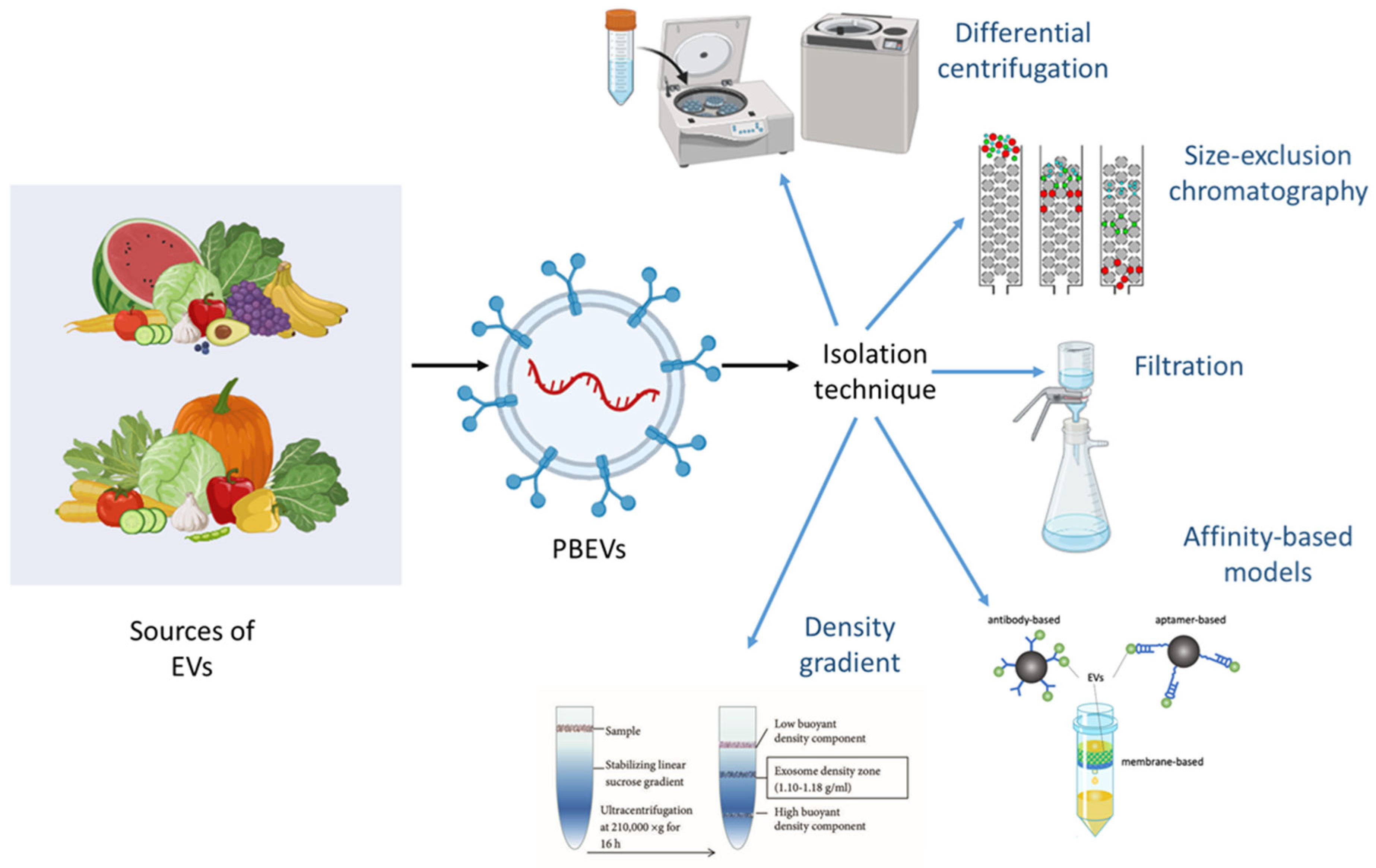
2. Sources and Isolation Techniques for PBEVs
2.1. Differential Centrifugation-Based Isolation
2.2. Density Gradient Separation
2.3. Size Exclusion Chromatography for EV Purification
2.4. Affinity-Based Isolation Methods
2.5. Membrane Filtration Approaches
2.6. Advanced Isolation Techniques for PBEVs
2.6.1. Microfluidic-Based Isolation
2.6.2. Acoustofluidics-Assisted Separation
2.6.3. Deterministic Lateral Displacement (DLD) for EV Sorting
| S. No. | Method of Extraction/Isolation and Purification | Plant Source | Disease/Application | Reference |
|---|---|---|---|---|
| 1. | Differential centrifugation and sucrose gradient ultracentrifugation | Grape-fruit | DSS-Induced colitis | [49] |
| 2. | Centrifugation and ultracentrifugation | Panax Ginseng | Cancer immunotherapy | [50] |
| 3. | Centrifugation and column filtration | Broccoli | Colitis | [51] |
| 4. | Ultracentrifugation | Apple | GI tract diseases | [52] |
| 5. | Differential Centrifugation | Strawberry | Oxidative stress in human mesenchymal stromal cells | [53] |
| 6. | Ultracentrifugation and tangential flow filtration | Aloe vera | Wound healing | [54] |
| 7. | Ultracentrifugation, electrophoresis combined with dialysis | Lemon | Gastric Cancer | [55] |
| 8. | Differential centrifugation and ultracentrifugation | Celery | Tumors | [56] |
| 9. | Ultracentrifugation and sucrose gradient centrifugation | Turmeric | Murine colitis | [57] |
| 10. | Differential centrifugation | Blueberry | Immunomodulatory Therapy | [58] |
| 11. | Ultracentrifugation | Ginger | Inflammatory bowel disease | [59] |
| 12. | Ultracentrifugation and sucrose gradient centrifugation | Garlic | Obesity-induced systemic and brain inflammatory activity | [60] |
| 13. | Centrifugation and Ultracentrifugation | Black Bean | Cancer Therapy | [61] |
| 14. | Centrifugation and sucrose gradient ultracentrifugation | Tea flower | Breast Cancer | [62] |
| 15. | Differential Ultracentrifugation | Catharanthus roseus | Anti-tumor, Anti-helmintic, anti-diabetes | [63] |
| 16. | Differential Centrifugation | Asparagus cochinchinenis | Hepatocellular carcinoma | [64] |
| 17. | Centrifugation and ultracentrifugation | Lonicera japonica | Intraepithelial neoplasia caused by Human Papillomavirus | [65] |
| 18. | Centrifugation | Centella Asiatica | Anti-proliferative activity | [66] |
| 19. | Centrifugation, ultracentrifugation, size exclusion chromatography | Sesame | Anti-inflammatory | [67] |
| 20. | Size exclusion chromatography and ultracentrifugation | Cabbage and Red cabbage | Inflammation and inhibition of apoptosis | [68] |
| 21. | Size exclusion chromatography and High-pressure homogenization (HPH) | Cucumber | Improved dermal penetration | [69] |
| 22. | PEG-based precipitation | Ginger and grapefruit | COVID-19 | [70] |
| 23. | Cold maceration technique | Terminalia chebula | Hepatocellular carcinoma | [71] |
3. Therapeutic Applications of PBEVS in Drug Delivery
4. Stability Considerations and Characterization of PBEVs
4.1. Key Factors Affecting the Stability of PBEVs
4.1.1. Impact of Environmental Conditions
4.1.2. Storage-Related Stability Challenges
4.1.3. Effect of Formulation Components
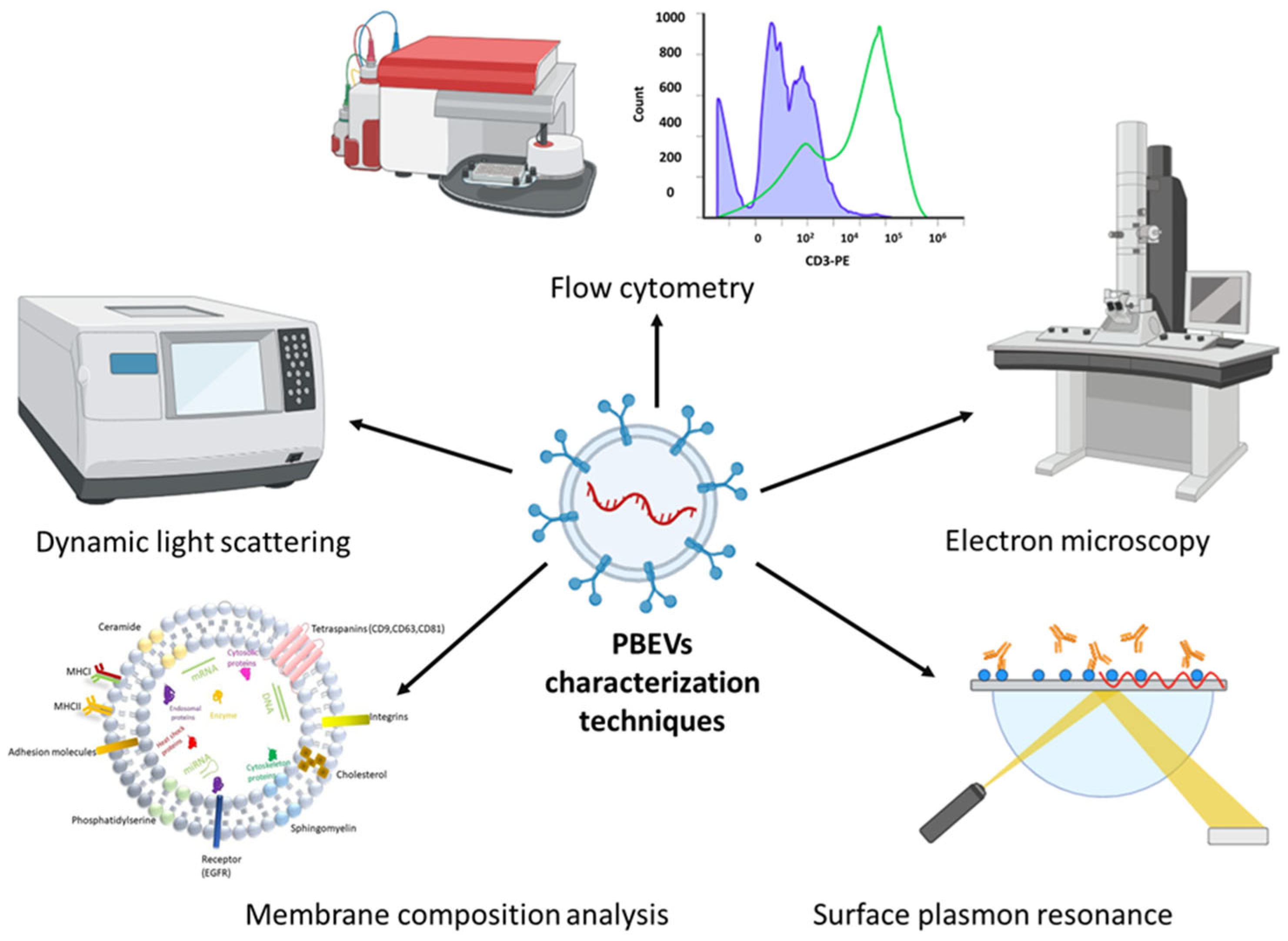
4.2. Techniques for Evaluating the Stability of PBEVs
4.2.1. Morphological Characterization Methods
EM for Structural Analysis
AFM for Surface Characterization
4.2.2. Size Distribution and Surface Charge Analysis
Dynamic Light Scattering (DLS) and Nanoparticle Tracking Analysis (NTA)
Flow Cytometry for EV Profiling
4.2.3. Emerging Technologies for PBEV Characterization
Single-Particle Analysis Techniques
Super-Resolution Microscopy for Nanoscale Imaging
Surface-Enhanced Raman Spectroscopy (SERS) for Molecular Profiling
Microfluidic-Based EV Characterization
Tunable Resistive Pulse Sensing (TRPS) for Size and Concentration Analysis
4.2.4. Study of Molecular Interactions by Surface Plasmon Resonance
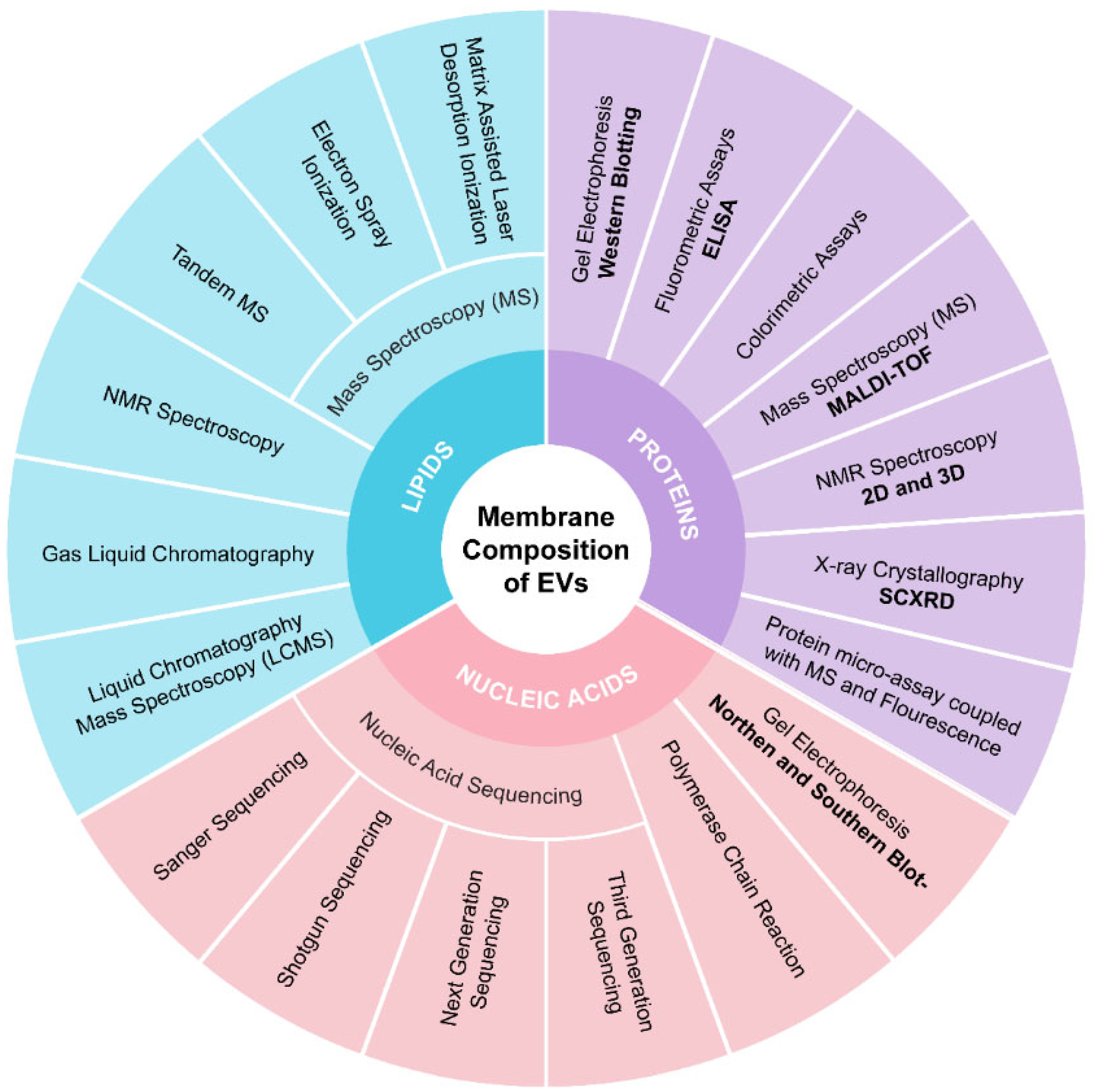
4.2.5. Membrane Composition Analysis
4.2.6. Advanced Computational Approaches in EV Stability Analysis
5. Strategies to Enhance the Stability of PBEVs
5.1. Chemical and Physical Modifications for Stability Improvement
5.2. Encapsulation Techniques for Preservation
5.3. Lyophilization and Freeze-Drying Approaches
6. Quality Control Parameters for PBEVs
6.1. Characterization of Intravesicular Contents
6.2. Membrane Composition and Structural Integrity
7. Potential of PBEVs in Nucleic Acid Delivery and Associated Stability Challenges
8. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Agrawal, A.K.; Aqil, F.; Jeyabalan, J.; Spencer, W.A.; Beck, J.; Gachuki, B.W.; Alhakeem, S.S.; Oben, K.; Munagala, R.; Bondada, S. Milk-derived exosomes for oral delivery of paclitaxel. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1627–1636. [Google Scholar] [CrossRef]
- Lee, K.W.A.; Chan, L.K.W.; Hung, L.C.; Phoebe, L.K.W.; Park, Y.; Yi, K.-H. Clinical applications of exosomes: A critical review. Int. J. Mol. Sci. 2024, 25, 7794. [Google Scholar] [CrossRef] [PubMed]
- Ageta, H.; Tsuchida, K. Post-translational modification and protein sorting to small extracellular vesicles including exosomes by ubiquitin and UBLs. Cell. Mol. Life Sci. 2019, 76, 4829–4848. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Huang, B. Safety and Effectiveness Study of Tumor Cell-Derived Microparticles to Treat Malignant Ascites and Pleural Effusion; Huazhong University of Science and Technology: Wuhan, China, 2013. [Google Scholar]
- Fawzy, M.P.; Hassan, H.A.; Amin, M.U.; Preis, E.; Bakowsky, U.; Fahmy, S.A. Deploying nucleic acids-loaded plant-derived exosomes as green nano gadget in cancer gene therapy. Mater. Adv. 2025, 6, 1230–1261. [Google Scholar] [CrossRef]
- Anjum, M.M.; Kumar, D.N.; Chaudhuri, A.; Singh, S.; Agrawal, A.K. Extracellular Vesicles for Nucleic Acid Delivery: Progress and Prospects for Safe RNA-Based Gene Therapy. Gene Deliv. 2022, 31–50. [Google Scholar]
- Sanwlani, R.; Fonseka, P.; Chitti, S.V.; Mathivanan, S. Milk-Derived Extracellular Vesicles in Inter-Organism, Cross-Species Communication and Drug Delivery. Proteomes 2020, 8, 11. [Google Scholar] [CrossRef]
- Gai, C.; Pomatto, M.A.C.; Deregibus, M.C.; Dieci, M.; Piga, A.; Camussi, G. Edible Plant-Derived Extracellular Vesicles for Oral mRNA Vaccine Delivery. Vaccines 2024, 12, 200. [Google Scholar] [CrossRef]
- Halperin, W.; Jensen, W.A. Ultrastructural changes during growth and embryogenesis in carrot cell cultures. J. Ultrastruct. Res. 1967, 18, 428–443. [Google Scholar] [CrossRef]
- An, Q.; van Bel, A.J.E.; Hückelhoven, R. Do Plant Cells Secrete Exosomes Derived from Multivesicular Bodies? Plant Signal. Behav. 2007, 2, 4–7. [Google Scholar] [CrossRef]
- Winter, V.; Hauser, M.T. Exploring the ESCRTing machinery in eukaryotes. Trends. Plant Sci. 2006, 11, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Ruf, A.; Oberkofler, L.; Robatzek, S.; Weiberg, A. Spotlight on plant RNA-containing extracellular vesicles. Curr. Opin. Plant Biol. 2022, 69, 102272. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, Y.; Wang, J.; Hillmer, S.; Miao, Y.; Lo, S.W.; Wang, X.; Robinson, D.G.; Jiang, L. EXPO, an Exocyst-Positive Organelle Distinct from Multivesicular Endosomes and Autophagosomes, Mediates Cytosol to Cell Wall Exocytosis in Arabidopsis and Tobacco Cells. Plant Cell 2010, 22, 4009–4030. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, J.; Chun Lai, J.H.; Ling Chan, V.H.; Wang, X.; Cai, Y.; Tan, X.; Bao, Y.; Xia, J.; Robinson, D.G.; et al. Exo70E2 is essential for exocyst subunit recruitment and EXPO formation in both plants and animals. Mol. Biol. Cell 2013, 25, 412–426. [Google Scholar] [CrossRef]
- Woith, E.; Guerriero, G.; Hausman, J.-F.; Renaut, J.; Leclercq, C.C.; Weise, C.; Legay, S.; Weng, A.; Melzig, M.F. Plant Extracellular Vesicles and Nanovesicles: Focus on Secondary Metabolites, Proteins and Lipids with Perspectives on Their Potential and Sources. Int. J. Mol. Sci. 2021, 22, 3719. [Google Scholar] [CrossRef]
- Woith, E.; Fuhrmann, G.; Melzig, M.F. Extracellular Vesicles—Connecting Kingdoms. Int. J. Mol. Sci. 2019, 20, 5695. [Google Scholar] [CrossRef]
- Movahed, N.; Cabanillas, D.G.; Wan, J.; Vali, H.; Laliberté, J.-F.; Zheng, H. Turnip Mosaic Virus Components Are Released into the Extracellular Space by Vesicles in Infected Leaves. Plant Physiol. 2019, 180, 1375–1388. [Google Scholar] [CrossRef]
- Rutter, B.D.; Innes, R.W. Extracellular vesicles as key mediators of plant–microbe interactions. Curr. Opin. Plant Biol. 2018, 44, 16–22. [Google Scholar] [CrossRef]
- de la Canal, L.; Pinedo, M. Extracellular vesicles: A missing component in plant cell wall remodeling. J. Exp. Bot. 2018, 69, 4655–4658. [Google Scholar] [CrossRef]
- Dryden, G.W. Study Investigating the Ability of Plant Exosomes to Deliver Curcumin to Normal and Colon Cancer Tissue; University of Louisville: Louisville, KY, USA, 2023. [Google Scholar]
- Alzahrani, F.A.; Khan, M.I.; Kameli, N.; Alsahafi, E.; Riza, Y.M. Plant-Derived Extracellular Vesicles and Their Exciting Potential as the Future of Next-Generation Drug Delivery. Biomolecules 2023, 13, 839. [Google Scholar] [CrossRef]
- Zhuang, X.; Teng, Y.; Samykutty, A.; Mu, J.; Deng, Z.; Zhang, L.; Cao, P.; Rong, Y.; Yan, J.; Miller, D.; et al. Grapefruit-derived Nanovectors Delivering Therapeutic miR17 Through an Intranasal Route Inhibit Brain Tumor Progression. Mol. Ther. 2016, 24, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Lou, K.; Luo, H.; Jiang, X.; Feng, S. Applications of emerging extracellular vesicles technologies in the treatment of inflammatory diseases. Front. Immunol. 2024, 15, 1364401. [Google Scholar] [CrossRef] [PubMed]
- Roefs, M.T.; Sluijter, J.P.G.; Vader, P. Extracellular Vesicle-Associated Proteins in Tissue Repair. Trends Cell Biol. 2020, 30, 990–1013. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; McKay, P.F.; Samnuan, K.; Najer, A.; Blakney, A.K.; Che, J.; O’Driscoll, G.; Cihova, M.; Stevens, M.M.; Shattock, R.J. Presentation of antigen on extracellular vesicles using transmembrane domains from viral glycoproteins for enhanced immunogenicity. J. Extracell Vesicles 2022, 11, e12199. [Google Scholar] [CrossRef]
- Brakhage, A.; Zimmermann, A.-K.; Rivieccio, F.; Visser, C.; Blango, M. Host-derived extracellular vesicles for antimicrobial defense. microLife 2021, 2, uqab003. [Google Scholar] [CrossRef]
- Ye, Z.W.; Yu, Z.L.; Chen, G.; Jia, J. Extracellular vesicles in tumor angiogenesis and resistance to anti-angiogenic therapy. Cancer Sci. 2023, 114, 2739–2749. [Google Scholar] [CrossRef]
- Bewicke-Copley, F.; Mulcahy, L.A.; Jacobs, L.A.; Samuel, P.; Akbar, N.; Pink, R.C.; Carter, D.R.F. Extracellular vesicles released following heat stress induce bystander effect in unstressed populations. J. Extracell Vesicles 2017, 6, 1340746. [Google Scholar] [CrossRef]
- Wu, W.C.; Song, S.J.; Zhang, Y.; Li, X. Role of Extracellular Vesicles in Autoimmune Pathogenesis. Front. Immunol. 2020, 11, 579043. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Kumar, D.N.; Chaudhuri, A.; Dehari, D.; Gamper, A.M.; Kumar, D.; Agrawal, A.K. Enhanced therapeutic efficacy against melanoma through exosomal delivery of hesperidin. Mol. Pharm. 2024, 21, 3061–3076. [Google Scholar] [CrossRef]
- Kumar, D.N.; Chaudhuri, A.; Shiromani, U.; Kumar, D.; Agrawal, A.K. An Investigation of In Vitro Anti-Cancer Efficacy of Dihydroartemisinin-Loaded Bovine Milk Exosomes Against Triple-Negative Breast Cancer. AAPS J. 2024, 26, 91. [Google Scholar] [CrossRef] [PubMed]
- Livshits, M.A.; Khomyakova, E.; Evtushenko, E.G.; Lazarev, V.N.; Kulemin, N.A.; Semina, S.E.; Generozov, E.V.; Govorun, V.M. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep. 2015, 5, 17319. [Google Scholar] [CrossRef] [PubMed]
- Bosch, S.; de Beaurepaire, L.; Allard, M.; Mosser, M.; Heichette, C.; Chrétien, D.; Jegou, D.; Bach, J.M. Trehalose prevents aggregation of exosomes and cryodamage. Sci. Rep. 2016, 6, 36162. [Google Scholar] [CrossRef]
- Nakai, W.; Yoshida, T.; Diez, D.; Miyatake, Y.; Nishibu, T.; Imawaka, N.; Naruse, K.; Sadamura, Y.; Hanayama, R. A novel affinity-based method for the isolation of highly purified extracellular vesicles. Sci. Rep. 2016, 6, 33935. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K.; Minamisawa, T.; Suga, K.; Yajima, Y.; Shiba, K. Isolation of human salivary extracellular vesicles by iodixanol density gradient ultracentrifugation and their characterizations. J. Extracell Vesicles 2016, 5, 30829. [Google Scholar] [CrossRef]
- Coskun, O. Separation techniques: Chromatography. North Clin. Istanb. 2016, 3, 156–160. [Google Scholar] [CrossRef]
- Jiawei, S.; Zhi, C.; Kewei, T.; Xiaoping, L. Magnetic bead-based adsorption strategy for exosome isolation. Front. Bioeng. Biotechnol. 2022, 10, 942077. [Google Scholar] [CrossRef]
- Ravi, R.; Khosroheidari, M.; DiStefano, J. A Modified Precipitation Method to Isolate Urinary Exosomes. J. Vis. Exp. JoVE 2015, 95. [Google Scholar] [CrossRef]
- Musante, L.; Tataruch-Weinert, D.; Kerjaschki, D.; Henry, M.; Meleady, P.; Holthofer, H. Residual urinary extracellular vesicles in ultracentrifugation supernatants after hydrostatic filtration dialysis enrichment. J. Extracell Vesicles 2017, 6, 1267896. [Google Scholar] [CrossRef]
- Abreu, C.M.; Costa-Silva, B.; Reis, R.L.; Kundu, S.C.; Caballero, D. Microfluidic platforms for extracellular vesicle isolation, analysis and therapy in cancer. Lab A Chip 2022, 22, 1093–1125. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, X.; Kong, Q.; He, H.; Sun, J.; Qiu, W.; Zhang, L.; Yang, M. Extracellular vesicle preparation and analysis: A state-of-the-art review. Adv. Sci. 2024, 11, 2401069. [Google Scholar] [CrossRef] [PubMed]
- Rabienezhad Ganji, N. Functional Characterization of Mammalian and Plant Extracellular Vesicles as Systems for Cancer Treatment; Università degli Studi di Palermo: Palermo, Italy, 2024. [Google Scholar]
- Hu, J.; Gao, D. Recent Advances in Aptamer-Based Microfluidic Biosensors for the Isolation, Signal Amplification and Detection of Exosomes. Sensors 2025, 25, 848. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, J.; Luo, Y. A Microfluidic Device for Nano-scale Extracellular Vesicles Differentiation via The Synergetic Effect of Deterministic Lateral Displacement and Dielectrophoresis. In Proceedings of the 2023 IEEE 18th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Jeju Island, Republic of Korea, 14–17 May 2023; pp. 174–177. [Google Scholar]
- He, W.; Zheng, S.; Zhang, H.; Gao, B.; Jin, J.; Zhang, M.; He, Q. Plant-derived vesicle-like nanoparticles: Clinical application exploration and challenges. Int. J. Nanomed. 2023, 18, 5671–5683. [Google Scholar] [CrossRef]
- Patel, G.; Agnihotri, T.G.; Gitte, M.; Shinde, T.; Gomte, S.S.; Goswami, R.; Jain, A. Exosomes: A potential diagnostic and treatment modality in the quest for counteracting cancer. Cell. Oncol. 2023, 46, 1159–1179. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.-B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Yan, H.; Han, X.; Weng, L.; Wei, Q.; Sun, X.; Lu, W.; Wei, Q.; Ye, J.; Cai, X.; et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J. Immunother. Cancer 2019, 7, 326. [Google Scholar] [CrossRef]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D.; et al. Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef]
- Fujita, D.; Arai, T.; Komori, H.; Shirasaki, Y.; Wakayama, T.; Nakanishi, T.; Tamai, I. Apple-Derived Nanoparticles Modulate Expression of Organic-Anion-Transporting Polypeptide (OATP) 2B1 in Caco-2 Cells. Mol. Pharm. 2018, 15, 5772–5780. [Google Scholar] [CrossRef]
- Perut, F.; Roncuzzi, L.; Avnet, S.; Massa, A.; Zini, N.; Sabbadini, S.; Giampieri, F.; Mezzetti, B.; Baldini, N. Strawberry-Derived Exosome-Like Nanoparticles Prevent Oxidative Stress in Human Mesenchymal Stromal Cells. Biomolecules 2021, 11, 87. [Google Scholar] [CrossRef]
- Kim, M.K.; Choi, Y.C.; Cho, S.H.; Choi, J.S.; Cho, Y.W. The Antioxidant Effect of Small Extracellular Vesicles Derived from Aloe vera Peels for Wound Healing. Tissue Eng. Regen. Med. 2021, 18, 561–571. [Google Scholar] [CrossRef]
- Yang, M.; Liu, X.; Luo, Q.; Xu, L.; Chen, F. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J. Nanobiotechnol. 2020, 18, 100. [Google Scholar] [CrossRef]
- Lu, X.; Han, Q.; Chen, J.; Wu, T.; Cheng, Y.; Li, F.; Xia, W. Celery (Apium graveolens L.) Exosome-like Nanovesicles as a New-Generation Chemotherapy Drug Delivery Platform against Tumor Proliferation. J. Agric. Food Chem. 2023, 71, 8413–8424. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yan, X.; Zhang, Y.; Yang, M.; Ma, Y.; Zhang, Y.; Xu, Q.; Tu, K.; Zhang, M. Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J. Nanobiotechnol. 2022, 20, 206. [Google Scholar] [CrossRef]
- Nguyen, T.N.-G.; Pham, C.V.; Chowdhury, R.; Patel, S.; Jaysawal, S.K.; Hou, Y.; Xu, H.; Jia, L.; Duan, A.; Tran, P.H.-L.; et al. Development of Blueberry-Derived Extracellular Nanovesicles for Immunomodulatory Therapy. Pharmaceutics 2023, 15, 2115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xiao, B.; Wang, H.; Han, M.K.; Zhang, Z.; Viennois, E.; Xu, C.; Merlin, D. Edible Ginger-derived Nano-lipids Loaded with Doxorubicin as a Novel Drug-delivery Approach for Colon Cancer Therapy. Mol. Ther. 2016, 24, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, K.; Mu, J.; Kumar, A.; Behera, J.; Lei, C.; Sriwastva, M.K.; Xu, F.; Dryden, G.W.; Zhang, L.; Chen, S. Garlic exosome-like nanoparticles reverse high-fat diet induced obesity via the gut/brain axis. Theranostics 2022, 12, 1220. [Google Scholar] [CrossRef]
- Donoso-Quezada, J.; Guajardo-Flores, D.; González-Valdez, J. Enhanced exosome-mediated delivery of black bean phytochemicals (Phaseolus vulgaris L.) for cancer treatment applications. Biomed. Pharmacother. 2020, 131, 110771. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Q.; Liang, Y.; Zu, M.; Chen, N.; Canup, B.S.B.; Luo, L.; Wang, C.; Zeng, L.; Xiao, B. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharm. Sin. B 2022, 12, 907–923. [Google Scholar] [CrossRef]
- Ou, X.; Wang, H.; Tie, H.; Liao, J.; Luo, Y.; Huang, W.; Yu, R.; Song, L.; Zhu, J. Novel plant-derived exosome-like nanovesicles from Catharanthus roseus: Preparation, characterization, and immunostimulatory effect via TNF-α/NF-κB/PU.1 axis. J. Nanobiotechnol. 2023, 21, 160. [Google Scholar] [CrossRef]
- Zhang, L.; He, F.; Gao, L.; Cong, M.; Sun, J.; Xu, J.; Wang, Y.; Hu, Y.; Asghar, S.; Hu, L.; et al. Engineering Exosome-Like Nanovesicles Derived from Asparagus cochinchinensis Can Inhibit the Proliferation of Hepatocellular Carcinoma Cells with Better Safety Profile. Int. J. Nanomed. 2021, 16, 1575–1586. [Google Scholar] [CrossRef]
- Chi, Y.; Shi, L.; Lu, S.; Cui, H.; Zha, W.; Shan, L.; Shen, Y. Inhibitory effect of Lonicera japonica-derived exosomal miR2911 on human papilloma virus. J. Ethnopharmacol. 2024, 318, 116969. [Google Scholar] [CrossRef]
- Huang, J.; Cao, X.; Wu, W.; Han, L.; Wang, F. Investigating the proliferative inhibition of HepG2 cells by exosome-like nanovesicles derived from Centella asiatica extract through metabolomics. Biomed. Pharmacother. 2024, 176, 116855. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Li, Z.; Liu, H.; Liu, H.; Xia, X.; Xiang, X. Plant exosome-like nanovesicles derived from sesame leaves as carriers for luteolin delivery: Molecular docking, stability and bioactivity. Food Chem. 2024, 438, 137963. [Google Scholar] [CrossRef]
- You, J.Y.; Kang, S.J.; Rhee, W.J. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact. Mater. 2021, 6, 4321–4332. [Google Scholar] [CrossRef]
- Abraham, A.M.; Wiemann, S.; Ambreen, G.; Zhou, J.; Engelhardt, K.; Brüßler, J.; Bakowsky, U.; Li, S.-M.; Mandic, R.; Pocsfalvi, G.; et al. Cucumber-Derived Exosome-like Vesicles and PlantCrystals for Improved Dermal Drug Delivery. Pharmaceutics 2022, 14, 476. [Google Scholar] [CrossRef] [PubMed]
- Kalarikkal, S.P.; Sundaram, G.M. Edible plant-derived exosomal microRNAs: Exploiting a cross-kingdom regulatory mechanism for targeting SARS-CoV-2. Toxicol. Appl. Pharmacol. 2021, 414, 115425. [Google Scholar] [CrossRef] [PubMed]
- Deb, A.; Gupta, S.; Shekhawat, G.S.; Mazumder, P.B. A study on exosome based delivery of Terminalia chebula Retz. fruit extract in hepatocellular carcinoma. Pharmacol. Res.—Nat. Prod. 2024, 3, 100053. [Google Scholar] [CrossRef]
- An, Y.; Sun, J.-X.; Ma, S.-Y.; Xu, M.-Y.; Xu, J.-Z.; Liu, C.-Q.; Wang, S.-G.; Xia, Q.-D. From Plant Based Therapy to Plant-Derived Vesicle-Like Nanoparticles for Cancer Treatment: Past, Present and Future. Int. J. Nanomed. 2025, 20, 3471–3491. [Google Scholar] [CrossRef]
- Di Raimo, R.; Mizzoni, D.; Spada, M.; Dolo, V.; Fais, S.; Logozzi, M. Oral Treatment with Plant-Derived Exosomes Restores Redox Balance in H2O2-Treated Mice. Antioxidants 2023, 12, 1169. [Google Scholar] [CrossRef]
- Kumar, D.N.; Chaudhuri, A.; Kumar, D.; Singh, S.; Agrawal, A.K. Impact of the drug loading method on the drug distribution and biological efficacy of exosomes. Aaps Pharmscitech 2023, 24, 166. [Google Scholar] [CrossRef]
- Kumar, D.N.; Chaudhuri, A.; Aqil, F.; Dehari, D.; Munagala, R.; Singh, S.; Gupta, R.C.; Agrawal, A.K. Exosomes as emerging drug delivery and diagnostic modality for breast cancer: Recent advances in isolation and application. Cancers 2022, 14, 1435. [Google Scholar] [CrossRef]
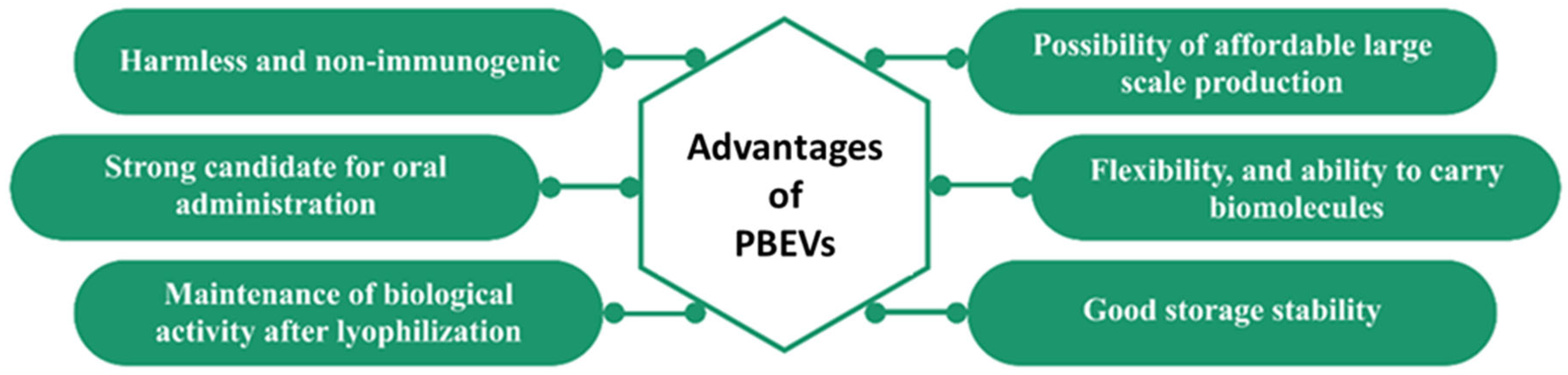
- Fang, Z.; Liu, K. Plant-derived extracellular vesicles as oral drug delivery carriers. J. Control. Release 2022, 350, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, K.B.; Gudbergsson, J.M.; Skov, M.N.; Christiansen, G.; Gurevich, L.; Moos, T.; Duroux, M. Evaluation of electroporation-induced adverse effects on adipose-derived stem cell exosomes. Cytotechnology 2016, 68, 2125–2138. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.G. A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.C.; Kim, O.Y.; Yoon, C.M.; Choi, D.-S.; Roh, T.-Y.; Park, J.; Nilsson, J.; Lötvall, J.; Kim, Y.-K.; Gho, Y.S. Bioinspired Exosome-Mimetic Nanovesicles for Targeted Delivery of Chemotherapeutics to Malignant Tumors. ACS Nano 2013, 7, 7698–7710. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Y.; Zhu, G.; Zeng, L.; Xu, S.; Cheng, H.; Ouyang, Z.; Chen, J.; Pathak, J.L.; Wu, L.; et al. The Emerging Role of Plant-Derived Exosomes-Like Nanoparticles in Immune Regulation and Periodontitis Treatment. Front. Immunol. 2022, 13, 896745. [Google Scholar] [CrossRef]
- Qin, X.; Wang, X.; Xu, K.; Zhang, Y.; Ren, X.; Qi, B.; Liang, Q.; Yang, X.; Li, L.; Li, S. Digestion of Plant Dietary miRNAs Starts in the Mouth under the Protection of Coingested Food Components and Plant-Derived Exosome-like Nanoparticles. J. Agric. Food Chem. 2022, 70, 4316–4327. [Google Scholar] [CrossRef]
- Sundaram, K.; Miller, D.P.; Kumar, A.; Teng, Y.; Sayed, M.; Mu, J.; Lei, C.; Sriwastva, M.K.; Zhang, L.; Yan, J.; et al. Plant-Derived Exosomal Nanoparticles Inhibit Pathogenicity of Porphyromonas gingivalis. iScience 2019, 21, 308–327. [Google Scholar] [CrossRef]
- Cong, M.; Tan, S.; Li, S.; Gao, L.; Huang, L.; Zhang, H.-G.; Qiao, H. Technology insight: Plant-derived vesicles—How far from the clinical biotherapeutics and therapeutic drug carriers? Adv. Drug Deliv. Rev. 2022, 182, 114108. [Google Scholar] [CrossRef]
- Liu, B.; Lu, Y.; Chen, X.; Muthuraj, P.G.; Li, X.; Pattabiraman, M.; Zempleni, J.; Kachman, S.D.; Natarajan, S.K.; Yu, J. Protective Role of Shiitake Mushroom-Derived Exosome-Like Nanoparticles in D-Galactosamine and Lipopolysaccharide-Induced Acute Liver Injury in Mice. Nutrients 2020, 12, 477. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Yin, H.; Bennett, C.; Zhang, H.-g.; Guo, P. Arrowtail RNA for Ligand Display on Ginger Exosome-like Nanovesicles to Systemic Deliver siRNA for Cancer Suppression. Sci. Rep. 2018, 8, 14644. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Li, S.; Zhang, S.; Wang, J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J. Pharm. Sci. 2022, 17, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Díez-Sainz, E.; Lorente-Cebrián, S.; Aranaz, P.; Riezu-Boj, J.I.; Martínez, J.A.; Milagro, F.I. Potential Mechanisms Linking Food-Derived MicroRNAs, Gut Microbiota and Intestinal Barrier Functions in the Context of Nutrition and Human Health. Front. Nutr. 2021, 8, 586564. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, S.; Hossain, M.N.; Conese, M. Biological properties and therapeutic effects of plant-derived nanovesicles. Open Med. 2020, 15, 1096–1122. [Google Scholar] [CrossRef]
- Zhao, W.-J.; Bian, Y.-P.; Wang, Q.-H.; Yin, F.; Yin, L.; Zhang, Y.-L.; Liu, J.-H. Blueberry-derived exosomes-like nanoparticles ameliorate nonalcoholic fatty liver disease by attenuating mitochondrial oxidative stress. Acta Pharmacol. Sin. 2022, 43, 645–658. [Google Scholar] [CrossRef]
- Sriwastva, M.K.; Deng, Z.B.; Wang, B.; Teng, Y.; Kumar, A.; Sundaram, K.; Mu, J.; Lei, C.; Dryden, G.W.; Xu, F.; et al. Exosome-like nanoparticles from Mulberry bark prevent DSS-induced colitis via the AhR/COPS8 pathway. EMBO Rep. 2022, 23, e53365. [Google Scholar] [CrossRef]
- Yin, L.; Yan, L.; Yu, Q.; Wang, J.; Liu, C.; Wang, L.; Zheng, L. Characterization of the MicroRNA Profile of Ginger Exosome-like Nanoparticles and Their Anti-Inflammatory Effects in Intestinal Caco-2 Cells. J. Agric. Food Chem. 2022, 70, 4725–4734. [Google Scholar] [CrossRef]
- Karamanidou, T.; Tsouknidas, A. Plant-Derived Extracellular Vesicles as Therapeutic Nanocarriers. Int. J. Mol. Sci. 2022, 23, 191. [Google Scholar] [CrossRef]
- Suharta, S.; Barlian, A.; Hidajah, A.C.; Notobroto, H.B.; Ana, I.D.; Indariani, S.; Wungu, T.D.K.; Wijaya, C.H. Plant-derived exosome-like nanoparticles: A concise review on its extraction methods, content, bioactivities, and potential as functional food ingredient. J. Food Sci. 2021, 86, 2838–2850. [Google Scholar] [CrossRef]
- Teng, Y.; Xu, F.; Zhang, X.; Mu, J.; Sayed, M.; Hu, X.; Lei, C.; Sriwastva, M.; Kumar, A.; Sundaram, K.; et al. Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. Mol. Ther. 2021, 29, 2424–2440. [Google Scholar] [CrossRef]
- Akao, Y.; Kuranaga, Y.; Heishima, K.; Sugito, N.; Morikawa, K.; Ito, Y.; Soga, T.; Ito, T. Plant hvu-MIR168-3p enhances expression of glucose transporter 1 (SLC2A1) in human cells by silencing genes related to mitochondrial electron transport chain complex I. J. Nutr. Biochem. 2022, 101, 108922. [Google Scholar] [CrossRef] [PubMed]
- Langellotto, M.D.; Rassu, G.; Serri, C.; Demartis, S.; Giunchedi, P.; Gavini, E. Plant-derived extracellular vesicles: A synergetic combination of a drug delivery system and a source of natural bioactive compounds. Drug Deliv. Transl. Res. 2024, 15, 1–15. [Google Scholar] [CrossRef]
- Karabay, A.Z.; Barar, J.; Hekmatshoar, Y.; Rahbar Saadat, Y. Multifaceted Therapeutic Potential of Plant-Derived Exosomes: Immunomodulation, Anticancer, Anti-Aging, Anti-Melanogenesis, Detoxification, and Drug Delivery. Biomolecules 2025, 15, 394. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, J.; Sohn, Y.; Oh, C.-E.; Park, J.-H.; Yuk, J.-M.; Yeon, J.-H. Stability of Plant Leaf-Derived Extracellular Vesicles According to Preservative and Storage Temperature. Pharmaceutics 2022, 14, 457. [Google Scholar] [CrossRef]
- Nemidkanam, V.; Chaichanawongsaroj, N. Characterizing Kaempferia parviflora extracellular vesicles, a nanomedicine candidate. PLoS ONE 2022, 17, e0262884. [Google Scholar] [CrossRef]
- Cloos, A.-S.; Ghodsi, M.; Stommen, A.; Vanderroost, J.; Dauguet, N.; Pollet, H.; D’Auria, L.; Mignolet, E.; Larondelle, Y.; Terrasi, R. Interplay between plasma membrane lipid alteration, oxidative stress and calcium-based mechanism for extracellular vesicle biogenesis from erythrocytes during blood storage. Front. Physiol. 2020, 11, 712. [Google Scholar] [CrossRef] [PubMed]
- Calzoni, E.; Bertoldi, A.; Cusumano, G.; Buratta, S.; Urbanelli, L.; Emiliani, C. Plant-Derived Extracellular Vesicles: Natural Nanocarriers for Biotechnological Drugs. Processes 2024, 12, 2938. [Google Scholar] [CrossRef]
- Fernández-Rhodes, M.; Lorca, C.; Lisa, J.; Batalla, I.; Ramos-Miguel, A.; Gallart-Palau, X.; Serra, A. New Origins of Yeast, Plant and Bacterial-Derived Extracellular Vesicles to Expand and Advance Compound Delivery. Int. J. Mol. Sci. 2024, 25, 7151. [Google Scholar] [CrossRef]
- Feng, J.; Xiu, Q.; Huang, Y.; Troyer, Z.; Li, B.; Zheng, L. Plant-derived vesicle-like nanoparticles as promising biotherapeutic tools: Present and future. Adv. Mater. 2023, 35, 2207826. [Google Scholar] [CrossRef]
- Susa, F.; Limongi, T.; Borgione, F.; Peiretti, S.; Vallino, M.; Cauda, V.; Pisano, R. Comparative studies of different preservation methods and relative freeze-drying formulations for extracellular vesicle pharmaceutical applications. ACS Biomater. Sci. Eng. 2023, 9, 5871–5885. [Google Scholar] [CrossRef]
- Guarro, M.; Suñer, F.; Lecina, M.; Borrós, S.; Fornaguera, C. Efficient extracellular vesicles freeze-dry method for direct formulations preparation and use. Colloids Surf. B Biointerfaces 2022, 218, 112745. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.A.; Davidovich, I.; Yang, Y.; Lai, A.; Goncalves, J.P.; Deliwala, V.; Busatto, S.; Shapiro, S.; Koifman, N.a.; Salomon, C. Sucrose-based cryoprotective storage of extracellular vesicles. Extracell. Vesicle 2022, 1, 100016. [Google Scholar] [CrossRef]
- Görgens, A.; Corso, G.; Hagey, D.W.; Jawad Wiklander, R.; Gustafsson, M.O.; Felldin, U.; Lee, Y.; Bostancioglu, R.B.; Sork, H.; Liang, X.; et al. Identification of storage conditions stabilizing extracellular vesicles preparations. J. Extracell Vesicles 2022, 11, e12238. [Google Scholar] [CrossRef] [PubMed]
- Garaeva, L.; Kamyshinsky, R.; Kil, Y.; Varfolomeeva, E.; Verlov, N.; Komarova, E.; Garmay, Y.; Landa, S.; Burdakov, V.; Myasnikov, A.; et al. Delivery of functional exogenous proteins by plant-derived vesicles to human cells in vitro. Sci. Rep. 2021, 11, 6489. [Google Scholar] [CrossRef]
- Chukhchin, D.G.; Bolotova, K.; Sinelnikov, I.; Churilov, D.; Novozhilov, E. Exosomes in the phloem and xylem of woody plants. Planta 2019, 251, 12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.N.; Chaudhuri, A.; Dehari, D.; Gamper, A.M.; Kumar, D.; Agrawal, A.K. Oral delivery of dihydroartemisinin for the treatment of melanoma via bovine milk exosomes. Drug Deliv. Transl. Res. 2025, 1–16. [Google Scholar] [CrossRef]
- Malenica, M.; Vukomanović, M.; Kurtjak, M.; Masciotti, V.; Dal Zilio, S.; Greco, S.; Lazzarino, M.; Krušić, V.; Perčić, M.; Jelovica Badovinac, I.; et al. Perspectives of Microscopy Methods for Morphology Characterisation of Extracellular Vesicles from Human Biofluids. Biomedicines 2021, 9, 603. [Google Scholar] [CrossRef]
- Khatun, Z.; Bhat, A.; Sharma, S.; Sharma, A. Elucidating Diversity of Exosomes: Biophysical and Molecular Characterization Methods. Nanomedicine 2016, 11, 2359–2377. [Google Scholar] [CrossRef]
- Midekessa, G.; Godakumara, K.; Ord, J.; Viil, J.; Lättekivi, F.; Dissanayake, K.; Kopanchuk, S.; Rinken, A.; Andronowska, A.; Bhattacharjee, S.; et al. Zeta Potential of Extracellular Vesicles: Toward Understanding the Attributes that Determine Colloidal Stability. ACS Omega 2020, 5, 16701–16710. [Google Scholar] [CrossRef]
- Kwon, Y.; Park, J. Methods to analyze extracellular vesicles at single particle level. Micro Nano Syst. Lett. 2022, 10, 14. [Google Scholar] [CrossRef]
- Kumar, D.N.; Chaudhuri, A.; Dehari, D.; Gamper, A.M.; Kumar, D.; Agrawal, A.K. Exosomes improved the in vitro anti-melanoma efficacy of dihydroartemisinin. J. Drug Deliv. Sci. Technol. 2024, 99, 105957. [Google Scholar] [CrossRef]
- Gul, D.-B.; Syed, F.; Khan, S.; Iqbal, A.; Ahmad, I. Characterization of extracellular vesicles by flow cytometry: Challenges and promises. Micron 2022, 161, 103341. [Google Scholar] [CrossRef] [PubMed]
- Van Der Pol, E.; Van Gemert, M.J.C.; Sturk, A.; Nieuwland, R.; Van Leeuwen, T.G. Single vs. swarm detection of microparticles and exosomes by flow cytometry. J. Thromb. Haemost. 2012, 10, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, Y.; Wang, Q.; Cai, N.; Wu, L.; Yan, X. Single-particle assessment of six different drug-loading strategies for incorporating doxorubicin into small extracellular vesicles. Anal. Bioanal. Chem. 2023, 415, 1287–1298. [Google Scholar] [CrossRef]
- Das, A.; Sonar, S.; Kalele, K.; Subramaniyan, V. Fruit exosomes: A sustainable green cancer therapeutic. Sustain. Food Technol. 2025, 3, 145–160. [Google Scholar] [CrossRef]
- Kim, K.W. In situ and ex situ imaging of plant extracellular vesicles as nanovectors for cross-domain communication. J. Phytopathol. 2021, 169, 515–524. [Google Scholar] [CrossRef]
- Yang, L.; Jia, J.; Li, S. Advances in the application of exosomes identification using surface-enhanced Raman spectroscopy for the early detection of cancers. Front. Bioeng. Biotechnol. 2022, 9, 808933. [Google Scholar] [CrossRef]
- Dad, H.A.; Gu, T.-W.; Zhu, A.-Q.; Huang, L.-Q.; Peng, L.-H. Plant exosome-like nanovesicles: Emerging therapeutics and drug delivery nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef]
- Cao, M.; Diao, N.; Cai, X.; Chen, X.; Xiao, Y.; Guo, C.; Chen, D.; Zhang, X. Plant exosome nanovesicles (PENs): Green delivery platforms. Mater. Horiz. 2023, 10, 3879–3894. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, K.; Cui, J.; Liu, H.; Bu, X.; Ma, H.; Wang, W.; Gong, H.; Lausted, C.; Hood, L.; et al. Label-Free Quantitative Detection of Tumor-Derived Exosomes through Surface Plasmon Resonance Imaging. Anal. Chem. 2014, 86, 8857–8864. [Google Scholar] [CrossRef]
- Im, H.; Shao, H.; Weissleder, R.; Castro, C.M.; Lee, H. Nano-plasmonic exosome diagnostics. Expert Rev. Mol. Diagn. 2015, 15, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Rupert, D.L.M.; Lässer, C.; Eldh, M.; Block, S.; Zhdanov, V.P.; Lotvall, J.O.; Bally, M.; Höök, F. Determination of Exosome Concentration in Solution Using Surface Plasmon Resonance Spectroscopy. Anal. Chem. 2014, 86, 5929–5936. [Google Scholar] [CrossRef]
- Hegmans, J.P.J.J.; Gerber, P.J.; Lambrecht, B.N. Exosomes. In Functional Proteomics: Methods and Protocols; Thompson, J.D., Ueffing, M., Schaeffer-Reiss, C., Eds.; Humana Press: Totowa, NJ, USA, 2008; pp. 97–109. [Google Scholar] [CrossRef]
- Mears, R.; Craven, R.A.; Hanrahan, S.; Totty, N.; Upton, C.; Young, S.L.; Patel, P.; Selby, P.J.; Banks, R.E. Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics 2004, 4, 4019–4031. [Google Scholar] [CrossRef]
- Kurien, B.T. Selecting Antibodies for Western Blotting. In Western Blotting for the Non-Expert; Kurien, B.T., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 31–34. [Google Scholar] [CrossRef]
- Conde-Vancells, J.; Rodriguez-Suarez, E.; Embade, N.; Gil, D.; Matthiesen, R.; Valle, M.; Elortza, F.; Lu, S.C.; Mato, J.M.; Falcon-Perez, J.M. Characterization and Comprehensive Proteome Profiling of Exosomes Secreted by Hepatocytes. J. Proteome Res. 2008, 7, 5157–5166. [Google Scholar] [CrossRef]
- Llorente, A.; Skotland, T.; Sylvänne, T.; Kauhanen, D.; Róg, T.; Orłowski, A.; Vattulainen, I.; Ekroos, K.; Sandvig, K. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim. Biophys. Acta 2013, 1831, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Yash, J.; Maharsh, J.; Kamble, S.S.; Guldhe, A.; Bastikar, V. In silico techniques, artificial intelligence, and machine learning for enhanced efficacy of extracellular vesicle–based diagnosis and therapeutics. In Extracellular Vesicles for Therapeutic and Diagnostic Applications; Elsevier: Amsterdam, The Netherlands, 2025; pp. 495–521. [Google Scholar]
- Shin, H.; Oh, S.; Hong, S.; Kang, M.; Kang, D.; Ji, Y.-g.; Choi, B.H.; Kang, K.-W.; Jeong, H.; Park, Y. Early-stage lung cancer diagnosis by deep learning-based spectroscopic analysis of circulating exosomes. ACS Nano 2020, 14, 5435–5444. [Google Scholar] [CrossRef]
- Greenberg, Z.F.; Graim, K.S.; He, M. Towards artificial intelligence-enabled extracellular vesicle precision drug delivery. Adv. Drug Deliv. Rev. 2023, 199, 114974. [Google Scholar] [CrossRef]
- Li, C.; Song, Q.; Yin, X.; Song, R.; Chen, G. Preparation, characterization, and in vitro anticancer activity evaluation of broccoli-derived extracellular vesicle-coated astaxanthin nanoparticles. Molecules 2022, 27, 3955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, C.; Liu, D.; Han, M.K.; Wang, L.; Merlin, D. Oral delivery of nanoparticles loaded with ginger active compound, 6-shogaol, attenuates ulcerative colitis and promotes wound healing in a murine model of ulcerative colitis. J. Crohn’s Colitis 2018, 12, 217–229. [Google Scholar] [CrossRef]
- Deng, C.; Hu, Y.; Conceição, M.; Wood, M.J.; Zhong, H.; Wang, Y.; Shao, P.; Chen, J.; Qiu, L. Oral delivery of layer-by-layer coated exosomes for colitis therapy. J. Control. Release 2023, 354, 635–650. [Google Scholar] [CrossRef]
- Che, K.; Wang, C.; Chen, H. Advancing functional foods: A systematic analysis of plant-derived exosome-like nanoparticles and their health-promoting properties. Front. Nutr. 2025, 12, 1544746. [Google Scholar] [CrossRef]
- Arora, S.; Dash, S.K.; Dhawan, D.; Sahoo, P.K.; Jindal, A.; Gugulothu, D. Freeze-drying revolution: Unleashing the potential of lyophilization in advancing drug delivery systems. Drug Deliv. Transl. Res. 2024, 14, 1111–1153. [Google Scholar] [CrossRef] [PubMed]
- Chiaradia, E.; Tancini, B.; Emiliani, C.; Delo, F.; Pellegrino, R.M.; Tognoloni, A.; Urbanelli, L.; Buratta, S. Extracellular vesicles under oxidative stress conditions: Biological properties and physiological roles. Cells 2021, 10, 1763. [Google Scholar] [CrossRef]
- Biscans, A.; Haraszti, R.A.; Echeverria, D.; Miller, R.; Didiot, M.-C.; Nikan, M.; Roux, L.; Aronin, N.; Khvorova, A. Hydrophobicity of lipid-conjugated siRNAs predicts productive loading to small extracellular vesicles. Mol. Ther. 2018, 26, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Vila, M.; Yoshioka, Y.; Ochiya, T. Biological Functions Driven by mRNAs Carried by Extracellular Vesicles in Cancer. Front. Cell Dev. Biol. 2021, 9, 620498. [Google Scholar] [CrossRef]
- Ståhl, A.L.; Johansson, K.; Mossberg, M.; Kahn, R.; Karpman, D. Exosomes and microvesicles in normal physiology, pathophysiology, and renal diseases. Pediatr. Nephrol. 2019, 34, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Aqil, F.; Jeyabalan, J.; kumar Agrawal, A.; Kyakulaga, A.-H.; Munagala, R. Milk exosomes—A “platform” nano-carrier for siRNA delivery. J. Extracell. Vesicles 2018, 7, 12. [Google Scholar]
- Skog, J.; Würdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Baj-Krzyworzeka, M.; Szatanek, R.; Węglarczyk, K.; Baran, J.; Urbanowicz, B.; Brański, P.; Ratajczak, M.Z.; Zembala, M. Tumour-derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer Immunol. Immunother. 2006, 55, 808–818. [Google Scholar] [CrossRef]
- Ingato, D.; Lee, J.U.; Sim, S.J.; Kwon, Y.J. Good things come in small packages: Overcoming challenges to harness extracellular vesicles for therapeutic delivery. J. Control. Release 2016, 241, 174–185. [Google Scholar] [CrossRef]
- Obregon, C.; Rothen-Rutishauser, B.; Gerber, P.; Gehr, P.; Nicod, L.P. Active uptake of dendritic cell-derived exovesicles by epithelial cells induces the release of inflammatory mediators through a TNF-alpha-mediated pathway. Am. J. Pathol. 2009, 175, 696–705. [Google Scholar] [CrossRef]
- Singh, G.; Mehra, A.; Arora, S.; Gugulothu, D.; Vora, L.K.; Prasad, R.; Khatri, D.K. Exosome-mediated delivery and regulation in neurological disease progression. Int. J. Biol. Macromol. 2024, 264, 130728. [Google Scholar] [CrossRef] [PubMed]
- Pomatto, M.A.C.; Gai, C.; Negro, F.; Massari, L.; Deregibus, M.C.; Grange, C.; De Rosa, F.G.; Camussi, G. Plant-Derived Extracellular Vesicles as a Delivery Platform for RNA-Based Vaccine: Feasibility Study of an Oral and Intranasal SARS-CoV-2 Vaccine. Pharmaceutics 2023, 15, 974. [Google Scholar] [CrossRef] [PubMed]
- Pomatto, M.A.C.; Gai, C.; Negro, F.; Massari, L.; Deregibus, M.C.; De Rosa, F.G.; Camussi, G. Oral Delivery of mRNA Vaccine by Plant-Derived Extracellular Vesicle Carriers. Cells 2023, 12, 1826. [Google Scholar] [CrossRef]
- Sivanantham, A.; Jin, Y. Impact of Storage Conditions on EV Integrity/Surface Markers and Cargos. Life 2022, 12, 697. [Google Scholar] [CrossRef] [PubMed]
- Baust, J.G.; Gao, D.; Baust, J.M. Cryopreservation: An emerging paradigm change. Organogenesis 2009, 5, 90–96. [Google Scholar] [CrossRef]
- Charoenviriyakul, C.; Takahashi, Y.; Nishikawa, M.; Takakura, Y. Preservation of exosomes at room temperature using lyophilizationγAuthor Names. Int. J. Pharm. 2018, 553, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Zhuang, X.; Deng, Z.-B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.-G. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.-B.; Jiang, H.; Zhang, L.; Xiang, X.; Wang, B.; Yan, J.; Miller, D.; et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat. Commun. 2013, 4, 1867. [Google Scholar] [CrossRef]
- Lee, M.; Ban, J.-J.; Im, W.; Kim, M. Influence of storage condition on exosome recovery. Biotechnol. Bioprocess Eng. 2016, 21, 299–304. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Maroto, R.; Zhao, Y.; Jamaluddin, M.; Popov, V.L.; Wang, H.; Kalubowilage, M.; Zhang, Y.; Luisi, J.; Sun, H.; Culbertson, C.T.; et al. Effects of storage temperature on airway exosome integrity for diagnostic and functional analyses. J. Extracell. Vesicles 2017, 6, 1359478. [Google Scholar] [CrossRef] [PubMed]
- Yerneni, S.S.; Yalcintas, E.P.; Smith, J.D.; Averick, S.; Campbell, P.G.; Ozdoganlar, O.B. Skin-targeted delivery of extracellular vesicle-encapsulated curcumin using dissolvable microneedle arrays. Acta Biomater. 2022, 149, 198–212. [Google Scholar] [CrossRef]

| Tissue/Disease for Targeting | Prospects | Challenges and Issues | PDENs Source | Findings | Ref. |
|---|---|---|---|---|---|
| Periodontitis | PBEVs have the potential to transport drugs for oral mucosal delivery to regulate oral immunity to periodontopathogen | PBEVs are limited to carry minimal amounts of drugs, with an unclear mechanism of cellular uptake as it may differ with each extraction batch |
|
| [80,81,82] |
| Colitis, tumors, liver diseases, skin diseases. | PBEVs have the potential to act as a drug-delivery system for the delivery of RNAs and lipids to inhibit the inflammation genes, as well as bacterial and tumor growth | There is a lack of transparency on quality control and evaluation systems, stability, biomarker confirmation, and biochemical characterization |
|
| [83,84,85] |
| Unspecified | Simplified large-scale mass production for their large biodiversity with minimal cytotoxicity for drug-delivery system | The internalization mechanisms remain elusive, and there is a lack of clarity regarding their specific receptors and ligands for PBEVs. Biosafety and toxicity of genetic transfer are unclear |
|
| [81,85,86] |
| Intestine | miRNAs derived from PBEVs have the potential to modulate gut microbiome, intestinal permeability, and mucosal immunity | The results of studies exhibit significant variability due to the lack of consensus regarding PBEVs derived miRNAs |
|
| [49,87] |
| Inflammatory bowel disease, liver disease, cancer | PBEVs have the potential to mediate interspecies communications to exert their anti-oxidant, anti-inflammatory, and regenerative activities | The quantities of proteins derived from PBEVs are lower, and their types differ when compared to MSC-derived EXOs |
|
| [84,88,89] |
| Colitis | PBEVs can transport both exogenous drugs and endogenous cargo to epithelial and bacterial cells for their stability in intestinal fluid. | Standardization on mass producing and purification techniques |
|
| [90,91,92] |
| Unspecified | The stability of PBEVs in the digestive system suggests its capability as a functional food to alleviate inflammation. | Instability during isolation and processing with unclear proteomic profiling |
|
| [93,94,95] |
| Source | Encapsulated API/Bioactive Compound | Stability Conditions | Conclusion | Ref. |
|---|---|---|---|---|
| PBEVs | ||||
| Ginger | Doxorubicin | Stored for 25 days at 4 °C | The experiments showed that Ginger-derived Nano vectors (GDNVs) were still stable and detectable up to 48 h after intravenous injection. This longer residence time provides a longer time window for GDNVs to accumulate at the tumor site. | [59] |
| Ginger | Shogaols | Obtained negative zeta potential value ranging from 76.2 to 33.5 mV | The magnitude of the measured zeta potential was used to predict the long-term stability of the product. | [156] |
| Grape-fruit | Exogenous proteins: Exogenous Alexa Fluor 647 labeled bovine serum albumin (BSA) and heat shock protein 70 (HSP70) | The resulting pellet was gently resuspended in 500 μL of PBS with continuous shaking for at least 1 h at 4 °C. The final grapefruit-derived nanovesicle samples were then aliquoted, flash-frozen in liquid nitrogen, and stored at −80 °C until further analysis. | Native PBEVs might be safe and effective carriers of exogenous proteins in human cells | [108] |
| Grape-fruit | Methotrexate | 37 °C for 30 min | Grapefruit-derived nanovesicles were very stable at physiologic temperature (37 °C) | [49] |
| Grape-fruit | Curcumin | 4 °C for more than one month | Grapefruit-derived nano vectors were very stable and did not lose their ability to carry curcumin as well as maintain the biological activity of curcumin. | [157] |
| Animal-derived EVs | ||||
| HEK 293 cell | Penicillin-Streptomycin in medium | Short-term storage conditions: 4 to 90 °C for 30 min Long-term storage conditions: From −70 °C to room temperature (RT) for ten days | EXOs incubated at 60 °C for 30 min showed a slight decrease in HSP70, while 90 °C caused complete protein degradation, indicating that temperatures above 37 °C compromise EXO stability. Markers remained intact at −20 °C and −70 °C, whereas CD63 was lost at 4 °C and RT, with HSP70 partially reduced at RT. EXOs stored at RT showed greater protein and RNA loss compared to those at colder temperatures. Thus, freezing below −20 °C is ideal for long-term storage, and CD63 and HSP70 are more heat-sensitive than CD9. | [158] |
| Macrophages | Paclitaxel | 4 °C, RT, and 37 °C over a period of one month | The remarkable stability of EXOs in aqueous solution was demonstrated over a one-month period at three different temperatures: 4 °C, room temperature (RT), and 37 °C. | [159] |
| Mouse bronchoalveolar lavage fluid (BALF) | +4 °C and −80 °C | The diameter of BALF-EXOs increased by approximately 10% when stored at +4 °C and by 25% at −80 °C, likely due to the formation of multilamellar structures. These findings suggest that storage conditions can compromise the morphological integrity, surface characteristics, and protein composition of BALF EXOs. | [160] | |
| J774A.1 cells | Curcumin | Short-term storage conditions: Stored in PBS at 37 °C for 3 h Long-term storage conditions: 7 days in PBS at 37 °C | Only 5% of the naked curcumin remained stable. Free curcumin degraded completely within a single day, while only about 8% of albumin-bound or EV-encapsulated curcumin remained stable by day 7. In contrast, 45% of the curcumin in Curcumin Albumin-EVs was stable by day 7. These results highlight that embedding curcumin within CA-EVs significantly enhances its stability, outperforming both albumin-curcumin and EV-loaded curcumin formulations. | [161] |
| Milk | Paclitaxel | pH 5.0 for 2 h pH 5.8 for 4 h | Incubation in FeSSGF (pH 5.0) for 2 h had no effect on EXO and ExoPAC size, while a slight size increase was observed after 4 h in FeSSIF (pH 5.8). | [1] |
| EL-4 | Curcumin | 37 °C for 150 min | Free curcumin degraded rapidly in PBS, with only 25% remaining after 150 min, whereas exosomal curcumin retained over 80% under the same conditions at pH 7.4. | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rawat, S.; Arora, S.; Dhondale, M.R.; Khadilkar, M.; Kumar, S.; Agrawal, A.K. Stability Dynamics of Plant-Based Extracellular Vesicles Drug Delivery. J. Xenobiot. 2025, 15, 55. https://doi.org/10.3390/jox15020055
Rawat S, Arora S, Dhondale MR, Khadilkar M, Kumar S, Agrawal AK. Stability Dynamics of Plant-Based Extracellular Vesicles Drug Delivery. Journal of Xenobiotics. 2025; 15(2):55. https://doi.org/10.3390/jox15020055
Chicago/Turabian StyleRawat, Satyavati, Sanchit Arora, Madhukiran R. Dhondale, Mansi Khadilkar, Sanjeev Kumar, and Ashish Kumar Agrawal. 2025. "Stability Dynamics of Plant-Based Extracellular Vesicles Drug Delivery" Journal of Xenobiotics 15, no. 2: 55. https://doi.org/10.3390/jox15020055
APA StyleRawat, S., Arora, S., Dhondale, M. R., Khadilkar, M., Kumar, S., & Agrawal, A. K. (2025). Stability Dynamics of Plant-Based Extracellular Vesicles Drug Delivery. Journal of Xenobiotics, 15(2), 55. https://doi.org/10.3390/jox15020055






