The Role of Xenobiotic Caffeine on Cardiovascular Health: Promises and Challenges
Abstract
1. Introduction
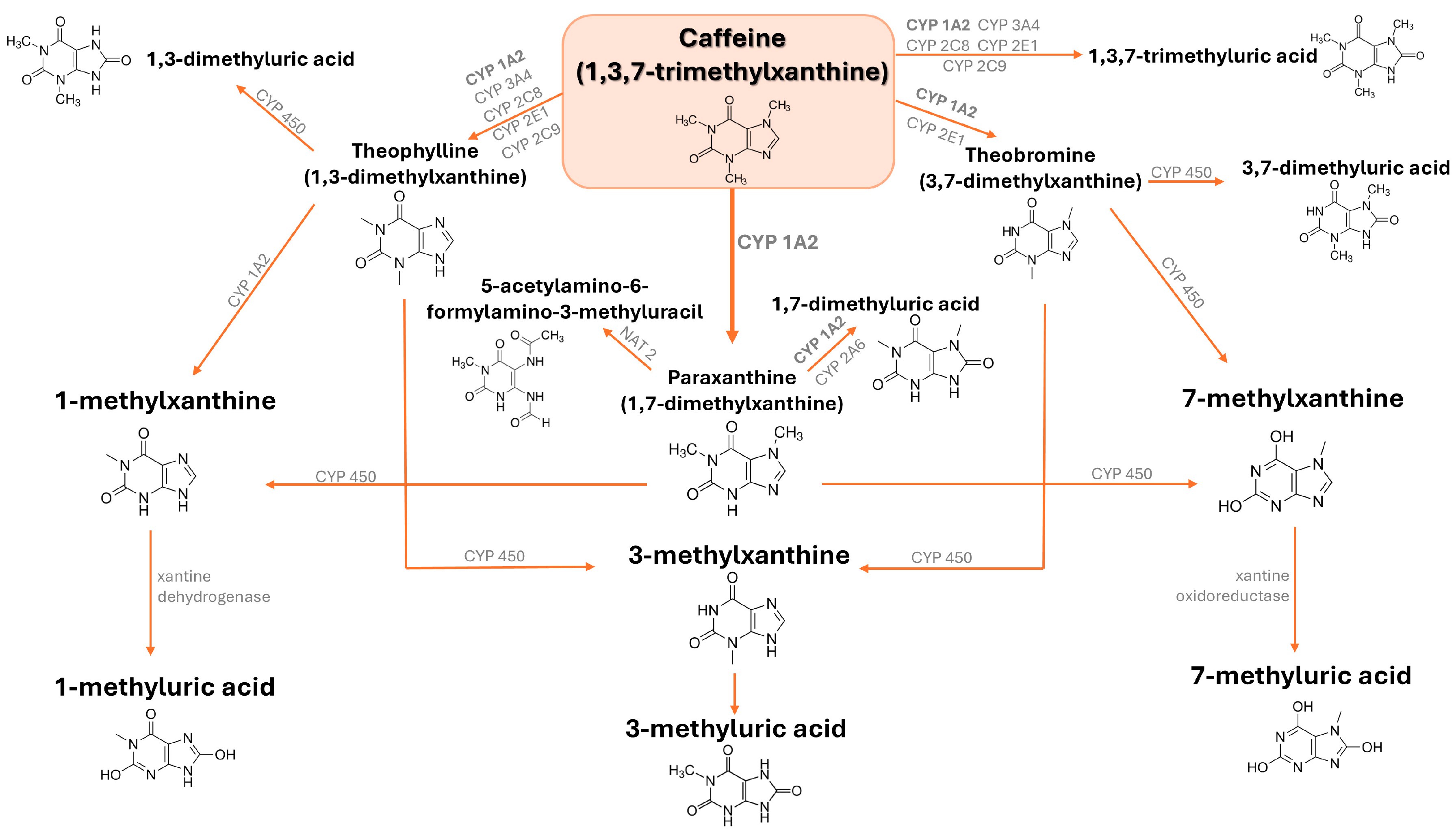
2. Metabolism of Caffeine
2.1. Sources, Metabolism, and Pharmacokinetics of Caffeine
2.2. Genetic Modifiers of Caffeine Metabolism
3. Cardiovascular Benefits of Caffeine
3.1. Hypertension
| Type of CVD | Subjects of the Study | Source of CAF | Main Findings | Limitations | Year | Reference |
|---|---|---|---|---|---|---|
| Hypertension | 11 groups of 6 rats | CAF (5–25 mg/kg) and caffeic acid (5–25 mg/kg) administrated orally by gavage | Anti-hypertensive effect of CAF. Systolic blood pressure ↓ ACE activity ↓ Arginase activity ↓ NO levels ↑ MDA levels ↓ | Small size group of rats | 2021 | [75] |
| Hypertension | 1010 human subjects (meta-analysis) | Coffee (725 mL/day) or CAF (410 mg/day) | Systolic pressure ↑ | None | 2005 | [76] |
| Hypertension | 63,257 human subjects (aged 45–74 years) | Daily coffee and caffeinated products consumption (food-frequency questionnaire) | 1 cup of coffe/week or more than 2 cups/day reduced risk of hypertension compared to consuming 1 cup of coffee per day | Other ingredients in coffee may offset the effect of CAF | 2018 | [77] |
| Hypertension | 196,256 human subjects (meta-analysis) | Daily coffee and caffeinated products consumption (food-frequency questionnaire) | Moderate coffee intake is not associated with higher risk of hypertension | A cause-effect relationship between coffee consumption and risk of hypertension cannot be stated based on the evidence available | 2019 | [78] |
| Hypertension | 48 human subjects (men aged 20–39 years) | CAF (3.3 mg/kg) | Diastolic blood pressure ↑ in patients with borderline hypertension | Small-size cohort groups. Gender biased. | 1996 | [79] |
| Hypertension | 182 human subjects (men aged 25–40 years) | CAF (3.3 mg/kg, average 260 mg/person or fixed dose of 250 mg) | No impact on blood pressure in non-hypertensive subjects;Blood pressure in patients at stage 1 hypertension ↑ | Gender biased | 2000 | [80] |
| Hypertension | Human subjects (46,395 men and 64,190 women aged 40 to 79 years) | Daily coffee and caffeinated products consumption (food-frequency questionnaire) | CVD mortality in patients with grade 2–3 hypertension ↑ | Other ingredients in coffee/tea may offset the effect of CAF | 2023 | [81] |
| Hypertension | 6076 humans (elderly hypertensive patients aged aged ≥65 years) | Daily coffee and caffeinated products consumption (food-frequency questionnaire) | Risk of all-cause and cardiovascular mortality in patients with moderate CAF intake ↓ | Cohort limited to US country. | 2022 | [82] |
| Hypertension | 29,985 human subjects (post-menopausal normotensive women aged 50–79 years old) | Daily coffee and caffeinated products consumption (food-frequency questionnaire) | Caffeinated coffee, decaffeinated coffee, and CAF are not associated with risk of incident hypertension | Other ingredients in coffee/tea may offset the effect of CAF | 2016 | [83] |
| Hypertension | 31 human subjects (men): 20 subjects at low risk and 11 at high risk for hypertension | CAF (3.3-mg/kg) | CAF + stress increased cortisol levels and blood pressure; CAF intake increased mean systolic blood pressure in 46% of high risk subjects | Small-size cohort groups. Gender biased. | 2000 | [85] |
| Hypertension | 52 human subjects (26 men and 26 women aged 18–29) with family history of hypertension | CAF (3.3-mg/kg) | Systolic blood pressure during stress response ↑; Stress interacted with CAF and sex altering cortisolm fibrinogen, systolic blood pressure | Small-size cohort groups. | 2013 | [86] |
| Hypertension | 8780 human subjects | Daily coffee and caffeinated products consumption (food-frequency questionnaire) | Risk of hypertention was lower for subjects consuming 1–3 cups/day compared to non-coffee drinkers | Self-reported data on dietary intake and coffee consumption may have resulted in some misclassification and residual confounding | 2021 | [87] |
| Hypertension | 19,133 human subjects (Taiwanese adults aged 30–70) | Daily coffee and caffeinated products consumption (food-frequency questionnaire) | Subject carrying cytochrome P450 1°2 rs762551 AC + CC genotype was associated with lower risk of hypertension | Cohort limited to Taiwan country | 2021 | [88] |
| Hypertension | 98,765 human subjects (aged 40–69) | Daily coffee and caffeinated products consumption (food-frequency questionnaire) | Moderate consumption of unsweetened was associated with a lower risk of hypertension | Comorbidities that accompany a new diagnosis of hypertension were not considered | 2024 | [89] |
| Hypertension | 453,913 human subjects (207,324 men and 246,589 women aged 39–74) | Daily coffee and caffeinated products consumption (food-frequency questionnaire); assumption of 75 mg of CAF/cup of coffee and 40 mg of CAF/cup of tea | Hypertensive patients who drank 0.5–1 cup/day displayed the lower risk of dementia | Self-report of coffee and tea consumption at baseline may be subject to information bias | 2024 | [90] |
| Hypertension | 3010 human subjects (aged 13–17 with equal distribution between male and female) | Daily coffee and caffeinated products consumption (food-frequency questionnaire) | CAF consumption was not associated with alterations in blood pressure, dyslipidemia, glomerular hyperfiltration, albuminuria, or insulin resistance | The study was based on single-day recall questionnaires. Lack of longitudinal data on the participants in the study. No differentiation between different sources of CAF intake. | 2024 | [91] |
| Hypertension | 18,914 human subjects (with pre-diabetes and diabetes, aged >20; average age 54.8 years; 9746 men, 9168 women) | 24-h dietary recall interviews | Pre-diabetic and diabetic subjects: increased CAF intake → reduced all-cause mortality; Pre-diabetic individuals: significant negative correlation between CAF intake and CVD events. | CAF intake levels were self-reported at baseline, which may result in a different level over long-term follow-up | 2024 | [92] |
| Hypertension | 30 humans (athletic and non-athletic women, aged 18–30 years) | CAF (80–120 mg) | CAF consumption does not significantly affect blood pressure in either athletic or non-athletic women | Small-size cohort groups. Gender biased. | 2024 | [93] |
| Hypertension | 12,093 human subjects (5687 men, 6406 women) | 24-h dietary recall interviews; CAF (100–400 mg/day) | Moderate CAF intake may be beneficial for hypertensive patients | CAF intake was assessed based on a single day of the interview, which may not accurately represent the long-term intake patterns | 2024 | [94] |
3.2. Arrhythmia
3.3. Dyslipidemia
3.4. Acute Coronary Syndrome
3.5. Angina Pectoris
3.6. Heart Failure
3.7. Other CVDs
| Type of CVD | Subjects of the Study | Source of CAF | Main Findings | Limitations | Year | Reference |
|---|---|---|---|---|---|---|
| Arrhythmia | 13 dogs | CAF | Dose-dependent arrhythmogenecity of CAF | Small-size group To be confirmed in human clinical trial. | 1997 | [95] |
| Arrhythmia | 14 male wistar rats | CAF-sodium salicylate (15 mg/kg/min) | CAF triggered sinus tachycardia and ectopic beats of heart resulting in fatal ventricular fibrillation | Small-size group To be confirmed in human clinical trial | 1999 | [96] |
| Arrhythmia | 34 adult male Japanese white rabbits | CAF (0.3 or 1.0 mg/kg per min) | CAF administration was correlated with an increased risk of ventricular tachycardia | To be confirmed in human clinical trial | 1996 | [97] |
| Arrhythmia | 33,638 women | Daily coffee and caffeinated products consumption (median caffeine intakes across increasing quintiles of caffeine intake were 22, 135, 285, 402, and 656 mg/d, respectively) | High caffeine consumption was not associated with an increased risk of incident atrial fibrillation | Only two measures over years may miss short-term effects; limited to middle-aged, white, female health professionals, affecting generalizability to men or other female populations; limited statistical power to find associations in small beverage subgroups; no ECG screening: possibly missing undetected AF cases; difficult to accurately defining AF onset, potentially introducing small bias if the time of incidence is incorrectly specified | 2010 | [98] |
| Arrhythmia | 57,053 Danish subjects (27,178 males and 29,875 females) aged 50–64 years | Daily coffee and caffeinated products consumption (food-frequency questionnaire) | Coffee consumption was inversely correlated with atrial fibrillation occurrence | No distinction between impacts of caffeinated or decaffeinated coffee; No information on brewing method or genetic polymorphism; Limited to cases with recorded hospitalizations or deaths for AF | 2016 | [99] |
| Arrhythmia | 41,881 men and 34,594 women | Daily coffee and caffeinated products consumption (food-frequency questionnaire) | Coffee intake was not associated with atrial fibrillation incidence | AF cases in the cohort are symptomatic, possible bias introduced if patients with with first episode of less seious AF reduced coffe consumption; Assessment of coffee consumption can show some measurement error because it was assessed with self-administered questionnaire and only at baseline: no information on the type of coffee and preparation method | 2015 | [100] |
| Arrhythmia | 130,054 human subjects | Daily coffee and caffeinated products consumption (food-frequency questionnaire) | Inverse association between coffee and CAF intake and hospitalization for arrhythmias | No data about follow-up coffee use; Incomplete caffeine data: no data about circumstances leading to hospitalization, coffee preparation method, cup size, and time of day for the coffee intake | 2011 | [101] |
| Arrhythmia | 1416 human subjects (44.1% men, 55.9% women) | Daily coffee and caffeinated products consumption (food-frequency questionnaire) | No relationship between chronic consumption of caffeinated products and ectopy | No evidence of a clinically large effect; Patients self-reporting; absence of total caffeine quantification; no discrimination among amounts of daily consumption | 2016 | [102] |
| Arrhythmia | 51 patients with moderate-to-severe left ventricular systolic dysfunction (37 men; 14 women, mean age 60.6 years) | CAF (total of 500 mg during a 5-h protocol) | No significant association between CAF intake and the incidence of ventricular and supraventricular premature beats | Small-size cohort groups. | 2016 | [103] |
| Arrhythmia | 101 patients presenting for regadenoson stress myocardial perfusion imaging | Daily coffee and caffeinated products consumption (food-frequency questionnaire) | No significant association between use of CAF and arrhythmia was detected | Patient self-reporting | 2020 | [104] |
| Arrhythmia | 47,949 human subjects aged 50–64 years (22,533 men and 25,416 women) | Daily coffee and caffeinated products consumption (food-frequency questionnaire) | No significant association between CAF intake with the incident of atrial fibrillation or atrial flutter was detected | Self-reported data on the consumption of caffeine; caffeine content change across brands | 2005 | [105] |
| Arrhythmia | Animals and humans (meta-analysis) | CAF | Increased risk of ventricular premature beats in humans. Mean change of −2.15 mA in ventricular fibrillation threshold was detected in studies involving animals | Effects found in animal studies areprobably the result of excessive caffeine doses that are not consumed on regular daily basis in humans (35 mg/kg) | 2016 | [106] |
| Arrhythmia | 18,983 and 6479 human subjects (meta-analysis) | Daily coffee and caffeinated products consumption (food-frequency questionnaire) | No association was identified for higher levels of caffeinated coffee intake (>1 cup per day). Average levels (1–7 cups/week) were found to be associated with a reduction in atrial fibrillation risk | Possibility of reverse causality in the association between coffee consumption and AF cannot be excluded | 2021 | [107] |
| Arrhythmia | 449,563 human subjects(median 58 years, 55.3% females) | Daily coffee and caffeinated products consumption(0, <1, 1, 2–3, 4–5, and >5 cups/day) | Coffee consumption reduced CVD risk and reduction of all-cause death. Decaffeinated coffee decreased arrhythmia incidence. | Participant self-reporting; outcome assessment relied on ICD-10 codes; detection of certain arrhythmias may be missed; predominantly Caucasian population | 2022 | [108] |
| Arrhythmia | 3835 human patients (Swiss-AF, n = 2387; Beat-AF, n = 1507) | “daily” and “not-daily” coffee consumers | Daily coffee intake was associated with a 23% lower hazard for major cardiovascular events | The main population of study was of European origin; coffee consumption was self-assessed by the patients; male patients are overrepresented | 2024 | [109] |
| Arrhythmia | 449,563 human subjects; median age 58 years; 55.3% females, of which 100,510 (22.35%) were controls (non-coffee drinkers) | Daily coffee and caffeinated products consumption (food-frequency questionnaire) | 4–5 cups/day of ground coffee was associated with a significant reduction in incident arrhythmia including atrial fibrillation | Coffee consumption was self-reported; ICD-10 codes is susceptible to measurement and reporting errors; population of the study is predominantly Caucasian therefore study conclusions may not be entirely applicable to populations of other ethnicities. | 2023 | [110] |
| Dyslipidemia | 96 4-week old male, Sprague-Dawley rats | Coffee (average amount of coffee intakes per rat: 0.12 g freeze-dried instant coffee/100 g body weight/d). | Triglycerides ↑ HDL cholesterol ↓ | Applying study results to athletes performance is difficult | 2013 | [115] |
| Dyslipidemia | 60 humans (obese women aged 30–50 years) | Green coffee bean extract (500 mg) | Levels of total cholesterol ↓ | Small-size cohort groups. Short duration of the study. Gender biased. | 2019 | [116] |
| Dyslipidemia | 1182 human subjects (meta-analysis, aged 18–70 years) | Daily coffee consumption | Serum levels of triglycerides ↑ Total cholesterol ↑ LDL ↑ | Small sample size; Different type and concentration of coffee; no enough evidence to assess effect of coffee consumption on HDL-C | 2020 | [117] |
| Dyslipidemia | 1017 human subjects (meta-analysis) | Daily coffee consumption | Total cholesterol ↑ LDL ↑ Triglycerides ↑ Individuals affected by hyperlipidemia were more inclined to coffee-induced dyslipidemia effects | Bias in the meta-analysis of total cholesterol | 2012 | [118] |
| Dyslipidemia | Humans (1987 subjects from Belgium and 900 subjects from Swiss; in both studies 53% of subjects were females) | CAF-derived metabolites plasma levels (methylxanthines) | Total cholesterol ↑ LDL ↑ Triglyceride levels ↑ | Population biased. No information about source of plasma methylxanthines source | 2021 | [119] |
| Dyslipidemia | Human subjects (2527 men and 2371 women) | Daily coffee consumption | Female subjects: minor alleles of ADORA1 rs10800901, ADORA2B rs2779212, and ADORA3 rs2786967 → higher protective effects from coffee intake against dyslipidemia Male subjects: minor allele ADORA3A rs3393 → lower risk | Small-size cohort group; No information regarding amount of CAF consumed; Physical activity could influence the blood lipid profile | 2020 | [120] |
| Dyslipidemia | 9876 human subjects | Daily coffee consumption | High plasma CAF levels may decrease adiposity and type-2 diabetes risk | Two-sample Mendelian randomization design; causal findings may not be applied to clinical or public health interventions; use of only two SNPs reduced analysis power; results may not be generalisable to non-European populations | 2023 | [121] |
| Acute Coronary Syndrome | 103 human subjects with acute STEMI (males) | Coffee or decaffeinated coffee (352.5 ± 90 mg (4.7 ± 1.1 cups) of CAF per day and 4.5 ± 1.3 cups per day of decaffeinated beverage) | CAF can be considered to be safe regarding cardiovascular adverse effects | Small-size cohort group; post-STEMI dysrhythmias did not allow for the interpretation of 24-h HRV analyses | 2009 | [125] |
| Acute Coronary Syndrome | 928 human subjects with acute coronary syndrome | Daily coffee and caffeinated products consumption | Moderate coffee intake was inversely correlated with total mortality | No information about brewing process or coffee type | 2021 | [126] |
| Acute Coronary Syndrome | 1369 and 1902 human subjects (meta-analysis) | Daily coffee consumption | Intake > 2 cups/day was associated with a risk ratio of 0.54 | No information about brewing process or coffee type Publication bias | 2016 | [127] |
| Angina Pectoris | 17 male rats | CAF (ranging 1 µg–1 mg) | CAF exposure boosts the release of prostacyclin (PGI2) which could be responsible for the beneficial effect of CAF in angina | Small-size group. To be confirmed in human clinical trial | 1994 | [133] |
| Angina Pectoris | 17 male subjects with coronary artery disease | Daily coffee and caffeinated products consumption | 2 cups/day intake increased the exercise duration of 12% until angina onset | Small-size cohort groups. Gender biased. | 1985 | [134] |
| Heart Failure | 14 male Sprague-Dawley rats | CAF 10 µg/kg/min | CAFenhances basal renin secretion by blocking intrarenal adenosine receptors and, in case of increased sympathetic activity, CAF boosts renin release in part by blockade of brain adenosine receptors, which results in enhanced central sympathetic tone | Small-size cohort groups. | 1996 | [138,139] |
| Heart Failure | Seven male, 9-month-old SHHF/Mcc-facp rats; Seven 9-month-old SHRs eight 9-month-old normotensive WKY rats; Fifteen aged (14-month-old) male lean SHHF/Mcc-facp rats | CAF (10 mg/kg + 150 μg/min over 40 min) | 10 mg/kg CAF followed by 150 g/min over 40 min enhanced both heart rate and left-ventricular peak systolic pressure and enhanced plasmatic norepinephrine, epinephrine, and renin activity levels | Small-size cohort group Long-term studies are needed | 1999 | [140] |
| Heart Failure | 10 human subjects (7 men, 3 women) | CAF (4 mg/kg) intravenously | CAF intake enhanced both exercise duration and performance | Small-size cohort group | 2006 | [141] |
| Heart Failure | 20,433 men (mean age 66.4) | Daily coffee and caffeinated products consumption (food-frequency questionnaire) | No significant correlation between coffee and CAF intake and the risk of HF | CAF intake was based on participant self-reporting, with the attendant risk of reporting bias | 2020 | [142] |
| Heart Failure | 140,220 human subjects (meta-analysis) | Daily coffee and caffeinated products consumption | 4 cups/day intake of coffee seems to be protective against HF | Other ingredients in coffee may offset the effect of caffeine | 2012 | [144] |
| Heart Failure | 1668 human subjects (751 men, 917 women, aged 58.8–80.5) | Daily coffee and caffeinated products consumption (food-frequency questionnaire) | Introducing > 230 mg/day CAF present a reduced risk of heart failure, while CAF intake > 280 mg/day can reduce risk of cerebrovascular events and arrhythmic events | CAF intake was based on participant self-reporting, with the attendant risk of reporting bias | 2023 | [145] |
| Hypothyroidism | 60 female Wistar albino rats | CAF 10 mg/kg/day in water via gavage for 2 months | CAF administration could improve thyroid and cardiac irregularities | Lack of dose dependent observations due to the use of a single concentration | 2025 | [146] |
| Systemic vascular resistance | 77 infants (<32 weeks gestational age; 39 male and 38 female) | CAF citrate 5 mg/kg | CAF administration was associated with increased systemic vascular resistance and more negative tissue oxygenation-heart rate reactivity index values | Lack of continuous capnography monitoring | 2024 | [147] |
| Cardiovascular Health | 11 women (mean age 24) | CAF 4 mg/kg | CAF intake did not induce any negative effects on the cardiovascular system | Small-size cohort group | 2023 | [148] |
| Abdominal aortic calcification | 2548 adult subjects (age mean 58.71) | 24-h dietary recall interviews | Heavy coffee consumption (>390 g/day) was associated with higher abdominal aortic calcification scores among individuals with hypertension, diabetes, and CVDs | A causal relationship between coffee consumption and abdominal aortic calcification could not be considered due to the crosssectional study design | 2023 | [149] |
| Liver sinusoidal endothelial cells defenestration | Primary rat LSECs | CAF (8 and 150 μg/mL) | LSECs porosity and fenestration distribution was affected by high CAF amounts. A dose-dependent rise in fenestration number was detected upon CAF treatment. | Very high doses of CAF | 2023 | [151] |
4. Caffeine Intoxication
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noale, M.; Limongi, F.; Maggi, S. Epidemiology of Cardiovascular Diseases in the Elderly. Adv. Exp. Med. Biol. 2020, 1216, 29–38. [Google Scholar]
- Gaidai, O.; Cao, Y.; Loginov, S. Global Cardiovascular Diseases Death Rate Prediction. Curr. Probl. Cardiol. 2023, 48, 101622. [Google Scholar] [PubMed]
- Paneni, F.; Diaz Canestro, C.; Libby, P.; Luscher, T.F.; Camici, G.G. The Aging Cardiovascular System: Understanding It at the Cellular and Clinical Levels. J. Am. Coll. Cardiol. 2017, 69, 1952–1967. [Google Scholar] [PubMed]
- Campagna, R.; Vignini, A. NAD+ Homeostasis and NAD+-Consuming Enzymes: Implications for Vascular Health. Antioxidants 2023, 12, 376. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Liu, S.; Sun, Y.; Chen, C.; Yang, S.; Lin, M.; Long, J.; Yao, J.; Lin, Y.; Yi, F.; et al. Targeting oxidative stress as a preventive and therapeutic approach for cardiovascular disease. J. Transl. Med. 2023, 21, 519. [Google Scholar]
- Campagna, R.; Mazzanti, L.; Pompei, V.; Alia, S.; Vignini, A.; Emanuelli, M. The Multifaceted Role of Endothelial Sirt1 in Vascular Aging: An Update. Cells 2024, 13, 1469. [Google Scholar] [CrossRef]
- Szczesny-Malysiak, E.; Stojak, M.; Campagna, R.; Grosicki, M.; Jamrozik, M.; Kaczara, P.; Chlopicki, S. Bardoxolone Methyl Displays Detrimental Effects on Endothelial Bioenergetics, Suppresses Endothelial ET-1 Release, and Increases Endothelial Permeability in Human Microvascular Endothelium. Oxid. Med. Cell. Longev. 2020, 2020, 4678252. [Google Scholar]
- Piani, F.; Tossetta, G.; Cara-Fuentes, G.; Agnoletti, D.; Marzioni, D.; Borghi, C. Diagnostic and Prognostic Role of CD93 in Cardiovascular Disease: A Systematic Review. Biomolecules 2023, 13, 910. [Google Scholar] [CrossRef]
- Tossetta, G.; Piani, F.; Borghi, C.; Marzioni, D. Role of CD93 in Health and Disease. Cells 2023, 12, 1778. [Google Scholar] [CrossRef]
- Mateuszuk, L.; Campagna, R.; Kutryb-Zajac, B.; Kus, K.; Slominska, E.M.; Smolenski, R.T.; Chlopicki, S. Reversal of endothelial dysfunction by nicotinamide mononucleotide via extracellular conversion to nicotinamide riboside. Biochem. Pharmacol. 2020, 178, 114019. [Google Scholar]
- Campagna, R.; Mateuszuk, L.; Wojnar-Lason, K.; Kaczara, P.; Tworzydlo, A.; Kij, A.; Bujok, R.; Mlynarski, J.; Wang, Y.; Sartini, D.; et al. Nicotinamide N-methyltransferase in endothelium protects against oxidant stress-induced endothelial injury. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119082. [Google Scholar] [CrossRef] [PubMed]
- Nedkoff, L.; Briffa, T.; Zemedikun, D.; Herrington, S.; Wright, F.L. Global Trends in Atherosclerotic Cardiovascular Disease. Clin. Ther. 2023, 45, 1087–1091. [Google Scholar] [CrossRef]
- Schiavoni, V.; Emanuelli, M.; Sartini, D.; Salvolini, E.; Pozzi, V.; Campagna, R. Curcumin and its Analogues in Oral Squamous Cell Carcinoma: State-of-the-art and Therapeutic Potential. Anticancer Agents Med. Chem. 2024, 25, 313–329. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Togni, L.; Santarelli, A.; Olivieri, F.; Marzioni, D.; Rippo, M.R. Modulation of NRF2/KEAP1 Signaling by Phytotherapeutics in Periodontitis. Antioxidants 2024, 13, 1270. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Honey and Its Phenolic Compounds as an Effective Natural Medicine for Cardiovascular Diseases in Humans? Nutrients 2020, 12, 283. [Google Scholar] [CrossRef]
- Campagna, R.; Cecati, M.; Vignini, A. The Multifaceted Role of the Polyphenol Curcumin: A Focus on Type 2 Diabetes Mellitus. Curr. Diabetes Rev. 2024, 21, e15733998313402. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Fantone, S.; Marzioni, D.; Mazzucchelli, R. Role of Natural and Synthetic Compounds in Modulating NRF2/KEAP1 Signaling Pathway in Prostate Cancer. Cancers 2023, 15, 3037. [Google Scholar] [CrossRef]
- Bacchetti, T.; Campagna, R.; Sartini, D.; Cecati, M.; Morresi, C.; Bellachioma, L.; Martinelli, E.; Rocchetti, G.; Lucini, L.; Ferretti, G.; et al. C. spinosa L. subsp. rupestris Phytochemical Profile and Effect on Oxidative Stress in Normal and Cancer Cells. Molecules 2022, 27, 6488. [Google Scholar]
- Fantone, S.; Marzioni, D.; Tossetta, G. NRF2/KEAP1 signaling inhibitors in gynecologic cancers. Expert Rev. Anticancer Ther. 2024, 24, 1191–1194. [Google Scholar] [CrossRef]
- Gal, R.; Halmosi, R.; Gallyas, F., Jr.; Tschida, M.; Mutirangura, P.; Toth, K.; Alexy, T.; Czopf, L. Resveratrol and beyond: The Effect of Natural Polyphenols on the Cardiovascular System: A Narrative Review. Biomedicines 2023, 11, 2888. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Busilacchi, E.M.; Di Simone, N.; Giannubilo, S.R.; Scambia, G.; Giordano, A.; Marzioni, D. Modulation of matrix metalloproteases by ciliary neurotrophic factor in human placental development. Cell Tissue Res. 2022, 390, 113–129. [Google Scholar] [PubMed]
- Gorecki, M.; Hallmann, E. The Antioxidant Content of Coffee and Its In Vitro Activity as an Effect of Its Production Method and Roasting and Brewing Time. Antioxidants 2020, 9, 308. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; He, B.; Wang, J.; Liu, J.; Hu, X. Risks of caffeine residues in the environment: Necessity for a targeted ecopharmacovigilance program. Chemosphere 2020, 243, 125343. [Google Scholar] [PubMed]
- Quadra, G.R.; Paranaiba, J.R.; Vilas-Boas, J.; Roland, F.; Amado, A.M.; Barros, N.; Dias, R.J.P.; Cardoso, S.J. A global trend of caffeine consumption over time and related-environmental impacts. Environ. Pollut. 2020, 256, 113343. [Google Scholar]
- Li, S.; Wen, J.; He, B.; Wang, J.; Hu, X.; Liu, J. Occurrence of caffeine in the freshwater environment: Implications for ecopharmacovigilance. Environ. Pollut. 2020, 263, 114371. [Google Scholar]
- Vieira, L.R.; Soares, A.; Freitas, R. Caffeine as a contaminant of concern: A review on concentrations and impacts in marine coastal systems. Chemosphere 2022, 286, 131675. [Google Scholar] [CrossRef]
- Barone, J.J.; Roberts, H.R. Caffeine consumption. Food Chem. Toxicol. 1996, 34, 119–129. [Google Scholar] [PubMed]
- Osz, B.E.; Jitca, G.; Stefanescu, R.E.; Puscas, A.; Tero-Vescan, A.; Vari, C.E. Caffeine and Its Antioxidant Properties-It Is All about Dose and Source. Int. J. Mol. Sci. 2022, 23, 13074. [Google Scholar] [CrossRef]
- Lippi, G.; Cervellin, G.; Sanchis-Gomar, F. Energy Drinks and Myocardial Ischemia: A Review of Case Reports. Cardiovasc. Toxicol. 2016, 16, 207–212. [Google Scholar]
- Milovanovic, D.D.; Jakovljevic, M.; Scekic, M.; Djordjevic, N. Caffeine consumption patterns and determinants among adolescents in Serbia. Int. J. Adolesc. Med. Health 2018, 30, 20160076. [Google Scholar] [CrossRef]
- Nawrot, P.; Jordan, S.; Eastwood, J.; Rotstein, J.; Hugenholtz, A.; Feeley, M. Effects of caffeine on human health. Food Addit. Contam. 2003, 20, 1–30. [Google Scholar]
- Safranow, K.; Machoy, Z. Methylated purines in urinary stones. Clin. Chem. 2005, 51, 1493–1498. [Google Scholar]
- Kaplan, G.B.; Greenblatt, D.J.; Ehrenberg, B.L.; Goddard, J.E.; Cotreau, M.M.; Harmatz, J.S.; Shader, R.I. Dose-dependent pharmacokinetics and psychomotor effects of caffeine in humans. J. Clin. Pharmacol. 1997, 37, 693–703. [Google Scholar]
- Bonati, M.; Latini, R.; Galletti, F.; Young, J.F.; Tognoni, G.; Garattini, S. Caffeine disposition after oral doses. Clin. Pharmacol. Ther. 1982, 32, 98–106. [Google Scholar]
- Mandel, H.G. Update on caffeine consumption, disposition and action. Food Chem. Toxicol. 2002, 40, 1231–1234. [Google Scholar]
- Willson, C. The clinical toxicology of caffeine: A review and case study. Toxicol. Rep. 2018, 5, 1140–1152. [Google Scholar]
- Ngueta, G. Caffeine and caffeine metabolites in relation to hypertension in U.S. adults. Eur. J. Clin. Nutr. 2020, 74, 77–86. [Google Scholar]
- Bhardwaj, I.; Ansari, A.H.; Rai, S.P.; Singh, S.; Singh, D. Molecular targets of caffeine in the central nervous system. Prog. Brain Res. 2024, 288, 35–58. [Google Scholar]
- Pettenuzzo, L.F.; Noschang, C.; von Pozzer Toigo, E.; Fachin, A.; Vendite, D.; Dalmaz, C. Effects of chronic administration of caffeine and stress on feeding behavior of rats. Physiol. Behav. 2008, 95, 295–301. [Google Scholar]
- Jacobson, K.A.; Gao, Z.G.; Matricon, P.; Eddy, M.T.; Carlsson, J. Adenosine A(2A) receptor antagonists: From caffeine to selective non-xanthines. Br. J. Pharmacol. 2022, 179, 3496–3511. [Google Scholar] [PubMed]
- Liu, Y.; Burger, S.K.; Ayers, P.W.; Vohringer-Martinez, E. Computational study of the binding modes of caffeine to the adenosine A2A receptor. J. Phys. Chem. B 2011, 115, 13880–13890. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Sebastiao, A.M. Caffeine and adenosine. J. Alzheimer’s Dis. 2010, 20 (Suppl. S1), S3–S15. [Google Scholar] [CrossRef]
- Ferre, S.; Ciruela, F.; Borycz, J.; Solinas, M.; Quarta, D.; Antoniou, K.; Quiroz, C.; Justinova, Z.; Lluis, C.; Franco, R.; et al. Adenosine A1-A2A receptor heteromers: New targets for caffeine in the brain. Front. Biosci. 2008, 13, 2391–2399. [Google Scholar] [CrossRef]
- Costenla, A.R.; Cunha, R.A.; de Mendonca, A. Caffeine, adenosine receptors, and synaptic plasticity. J. Alzheimer’s Dis. 2010, 20 (Suppl. S1), S25–S34. [Google Scholar] [CrossRef]
- Nehlig, A. Interindividual Differences in Caffeine Metabolism and Factors Driving Caffeine Consumption. Pharmacol. Rev. 2018, 70, 384–411. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Zhi, L.; Zhou, G.; Wang, H.; Zhang, X.; Hao, B.; Zhu, Y.; Cheng, Z.; He, F. The G-113A polymorphism in CYP1A2 affects the caffeine metabolic ratio in a Chinese population. Clin. Pharmacol. Ther. 2005, 78, 249–259. [Google Scholar]
- Womack, C.J.; Saunders, M.J.; Bechtel, M.K.; Bolton, D.J.; Martin, M.; Luden, N.D.; Dunham, W.; Hancock, M. The influence of a CYP1A2 polymorphism on the ergogenic effects of caffeine. J. Int. Soc. Sports Nutr. 2012, 9, 7. [Google Scholar] [CrossRef]
- Thomas, R.M.; Algrain, H.A.; Ryan, E.J.; Popojas, A.; Carrigan, P.; Abdulrahman, A.; Carrillo, A.E. Influence of a CYP1A2 polymorphism on post-exercise heart rate variability in response to caffeine intake: A double-blind, placebo-controlled trial. Ir. J. Med. Sci. 2017, 186, 285–291. [Google Scholar] [CrossRef]
- Cornelis, M.C.; El-Sohemy, A.; Kabagambe, E.K.; Campos, H. Coffee, CYP1A2 genotype, and risk of myocardial infarction. JAMA 2006, 295, 1135–1141. [Google Scholar] [CrossRef]
- Castorena-Torres, F.; Mendoza-Cantu, A.; de Leon, M.B.; Cisneros, B.; Zapata-Perez, O.; Lopez-Carrillo, L.; Salinas, J.E.; Albores, A. CYP1A2 phenotype and genotype in a population from the Carboniferous Region of Coahuila, Mexico. Toxicol. Lett. 2005, 156, 331–339. [Google Scholar] [CrossRef]
- Sachse, C.; Brockmoller, J.; Bauer, S.; Roots, I. Functional significance of a C-->A polymorphism in intron 1 of the cytochrome P450 CYP1A2 gene tested with caffeine. Br. J. Clin. Pharmacol. 1999, 47, 445–449. [Google Scholar] [CrossRef]
- Han, X.M.; Ou-Yang, D.S.; Lu, P.X.; Jiang, C.H.; Shu, Y.; Chen, X.P.; Tan, Z.R.; Zhou, H.H. Plasma caffeine metabolite ratio (17X/137X) in vivo associated with G-2964A and C734A polymorphisms of human CYP1A2. Pharmacogenetics 2001, 11, 429–435. [Google Scholar] [CrossRef]
- Minaei, S.; Rahimi, M.R.; Mohammadi, H.; Jourkesh, M.; Kreider, R.B.; Forbes, S.C.; Souza-Junior, T.P.; McAnulty, S.R.; Kalman, D. CYP1A2 Genotype Polymorphism Influences the Effect of Caffeine on Anaerobic Performance in Trained Males. Int. J. Sport Nutr. Exerc. Metab. 2022, 32, 16–21. [Google Scholar] [CrossRef]
- Guest, N.; Corey, P.; Vescovi, J.; El-Sohemy, A. Caffeine, CYP1A2 Genotype, and Endurance Performance in Athletes. Med. Sci. Sports Exerc. 2018, 50, 1570–1578. [Google Scholar] [CrossRef]
- Uwishema, O.; Nazir, A.; Munyangaju, I.; Shariff, S.; Al Komi, O.; Chibueze, N.; Wojtara, M. The pulse of sleep: Novel interventions in understanding the sleep-cardiovascular connection: A literature review. Ann. Med. Surg. 2024, 86, 5283–5291. [Google Scholar] [CrossRef]
- Contrada, R.J. Stress and Cardiovascular Disease: The Role of Affective Traits and Mental Disorders. Annu. Rev. Clin. Psychol. 2025. [Google Scholar] [CrossRef]
- Feng, L.S.; Wang, Y.M.; Liu, H.; Ning, B.; Yu, H.B.; Li, S.L.; Wang, Y.T.; Zhao, M.J.; Ma, J. Hyperactivity in the Hypothalamic-Pituitary-Adrenal Axis: An Invisible Killer for Anxiety and/or Depression in Coronary Artherosclerotic Heart Disease. J. Integr. Neurosci. 2024, 23, 222. [Google Scholar] [CrossRef]
- Shahid, I.; Zakaria, F.; Chang, R.; Javed, U.; Amin, Z.M.; Al-Kindi, S.; Nasir, K.; Javed, Z. Obesity and Atherosclerotic Cardiovascular Disease: A Review of Social and Biobehavioral Pathways. Methodist Debakey Cardiovasc. J. 2025, 21, 23–34. [Google Scholar] [CrossRef]
- Huang, Z.L.; Qu, W.M.; Eguchi, N.; Chen, J.F.; Schwarzschild, M.A.; Fredholm, B.B.; Urade, Y.; Hayaishi, O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat. Neurosci. 2005, 8, 858–859. [Google Scholar] [CrossRef]
- Retey, J.V.; Adam, M.; Khatami, R.; Luhmann, U.F.; Jung, H.H.; Berger, W.; Landolt, H.P. A genetic variation in the adenosine A2A receptor gene (ADORA2A) contributes to individual sensitivity to caffeine effects on sleep. Clin. Pharmacol. Ther. 2007, 81, 692–698. [Google Scholar] [CrossRef]
- Merica, H.; Blois, R.; Gaillard, J.M. Spectral characteristics of sleep EEG in chronic insomnia. Eur. J. Neurosci. 1998, 10, 1826–1834. [Google Scholar]
- Perlis, M.L.; Merica, H.; Smith, M.T.; Giles, D.E. Beta EEG activity and insomnia. Sleep Med. Rev. 2001, 5, 363–374. [Google Scholar] [CrossRef]
- Alsene, K.; Deckert, J.; Sand, P.; de Wit, H. Association between A2a receptor gene polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology 2003, 28, 1694–1702. [Google Scholar]
- Childs, E.; Hohoff, C.; Deckert, J.; Xu, K.; Badner, J.; de Wit, H. Association between ADORA2A and DRD2 polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology 2008, 33, 2791–2800. [Google Scholar] [PubMed]
- Hohoff, C.; McDonald, J.M.; Baune, B.T.; Cook, E.H.; Deckert, J.; de Wit, H. Interindividual variation in anxiety response to amphetamine: Possible role for adenosine A2A receptor gene variants. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005, 139B, 42–44. [Google Scholar] [PubMed]
- Deckert, J.; Nothen, M.M.; Franke, P.; Delmo, C.; Fritze, J.; Knapp, M.; Maier, W.; Beckmann, H.; Propping, P. Systematic mutation screening and association study of the A1 and A2a adenosine receptor genes in panic disorder suggest a contribution of the A2a gene to the development of disease. Mol. Psychiatry 1998, 3, 81–85. [Google Scholar] [PubMed]
- Hamilton, S.P.; Slager, S.L.; De Leon, A.B.; Heiman, G.A.; Klein, D.F.; Hodge, S.E.; Weissman, M.M.; Fyer, A.J.; Knowles, J.A. Evidence for genetic linkage between a polymorphism in the adenosine 2A receptor and panic disorder. Neuropsychopharmacology 2004, 29, 558–565. [Google Scholar]
- Yamada, K.; Hattori, E.; Shimizu, M.; Sugaya, A.; Shibuya, H.; Yoshikawa, T. Association studies of the cholecystokinin B receptor and A2a adenosine receptor genes in panic disorder. J. Neural Transm 2001, 108, 837–848. [Google Scholar]
- Lam, P.; Hong, C.J.; Tsai, S.J. Association study of A2a adenosine receptor genetic polymorphism in panic disorder. Neurosci. Lett. 2005, 378, 98–101. [Google Scholar]
- Nardi, A.E.; Lopes, F.L.; Freire, R.C.; Veras, A.B.; Nascimento, I.; Valenca, A.M.; de-Melo-Neto, V.L.; Soares-Filho, G.L.; King, A.L.; Araujo, D.M.; et al. Panic disorder and social anxiety disorder subtypes in a caffeine challenge test. Psychiatry Res. 2009, 169, 149–153. [Google Scholar]
- El Yacoubi, M.; Ledent, C.; Parmentier, M.; Costentin, J.; Vaugeois, J.M. Reduced appetite for caffeine in adenosine A(2A) receptor knockout mice. Eur. J. Pharmacol. 2005, 519, 290–291. [Google Scholar] [PubMed]
- Arias Horcajadas, F.; Sanchez Romero, S.; Padin Calo, J.; Fernandez-Rojo, S.; Fernandez Martin, G. Psychoactive drugs use in patients with panic disorder. Actas Esp. Psiquiatr. 2005, 33, 160–164. [Google Scholar] [PubMed]
- Fuchs, F.D.; Fuchs, S.C.; Berwanger, O.; Whelton, P.K. Clinical Trials in Hypertension: A Mathematical Endorsement for Diagnosis and Treatment. Hypertension 2025, 82, 411–418. [Google Scholar] [PubMed]
- Elliott, W.J. Systemic hypertension. Curr. Probl. Cardiol. 2007, 32, 201–259. [Google Scholar]
- Oboh, G.; Ojueromi, O.O.; Ademosun, A.O.; Omayone, T.P.; Oyagbemi, A.A.; Ajibade, T.O.; Adedapo, A.A. Effects of caffeine and caffeic acid on selected biochemical parameters in L-NAME-induced hypertensive rats. J. Food Biochem. 2021, 45, e13384. [Google Scholar]
- Noordzij, M.; Uiterwaal, C.S.; Arends, L.R.; Kok, F.J.; Grobbee, D.E.; Geleijnse, J.M. Blood pressure response to chronic intake of coffee and caffeine: A meta-analysis of randomized controlled trials. J. Hypertens. 2005, 23, 921–928. [Google Scholar]
- Chei, C.L.; Loh, J.K.; Soh, A.; Yuan, J.M.; Koh, W.P. Coffee, tea, caffeine, and risk of hypertension: The Singapore Chinese Health Study. Eur. J. Nutr. 2018, 57, 1333–1342. [Google Scholar]
- D’Elia, L.; La Fata, E.; Galletti, F.; Scalfi, L.; Strazzullo, P. Coffee consumption and risk of hypertension: A dose-response meta-analysis of prospective studies. Eur. J. Nutr. 2019, 58, 271–280. [Google Scholar] [CrossRef]
- Pincomb, G.A.; Lovallo, W.R.; McKey, B.S.; Sung, B.H.; Passey, R.B.; Everson, S.A.; Wilson, M.F. Acute blood pressure elevations with caffeine in men with borderline systemic hypertension. Am. J. Cardiol. 1996, 77, 270–274. [Google Scholar]
- Hartley, T.R.; Sung, B.H.; Pincomb, G.A.; Whitsett, T.L.; Wilson, M.F.; Lovallo, W.R. Hypertension risk status and effect of caffeine on blood pressure. Hypertension 2000, 36, 137–141. [Google Scholar]
- Teramoto, M.; Yamagishi, K.; Muraki, I.; Tamakoshi, A.; Iso, H. Coffee and Green Tea Consumption and Cardiovascular Disease Mortality Among People With and Without Hypertension. J. Am. Heart Assoc. 2023, 12, e026477. [Google Scholar] [PubMed]
- Chen, S.; Li, J.; Gao, M.; Li, D.; Shen, R.; Lyu, L.; Shen, J.; Shen, X.; Fu, G.; Wei, T.; et al. Association of caffeine intake with all-cause and cardiovascular mortality in elderly patients with hypertension. Front. Nutr. 2022, 9, 1023345. [Google Scholar]
- Rhee, J.J.; Qin, F.; Hedlin, H.K.; Chang, T.I.; Bird, C.E.; Zaslavsky, O.; Manson, J.E.; Stefanick, M.L.; Winkelmayer, W.C. Coffee and caffeine consumption and the risk of hypertension in postmenopausal women. Am. J. Clin. Nutr. 2016, 103, 210–217. [Google Scholar]
- Liu, M.Y.; Li, N.; Li, W.A.; Khan, H. Association between psychosocial stress and hypertension: A systematic review and meta-analysis. Neurol. Res. 2017, 39, 573–580. [Google Scholar] [PubMed]
- Shepard, J.D.; al’Absi, M.; Whitsett, T.L.; Passey, R.B.; Lovallo, W.R. Additive pressor effects of caffeine and stress in male medical students at risk for hypertension. Am. J. Hypertens. 2000, 13, 475–481. [Google Scholar]
- Bennett, J.M.; Rodrigues, I.M.; Klein, L.C. Effects of caffeine and stress on biomarkers of cardiovascular disease in healthy men and women with a family history of hypertension. Stress Health 2013, 29, 401–409. [Google Scholar]
- Miranda, A.M.; Goulart, A.C.; Bensenor, I.M.; Lotufo, P.A.; Marchioni, D.M. Coffee consumption and risk of hypertension: A prospective analysis in the cohort study. Clin. Nutr. 2021, 40, 542–549. [Google Scholar]
- Hou, C.C.; Tantoh, D.M.; Lin, C.C.; Chen, P.H.; Yang, H.J.; Liaw, Y.P. Association between hypertension and coffee drinking based on CYP1A2 rs762551 single nucleotide polymorphism in Taiwanese. Nutr. Metab. 2021, 18, 78. [Google Scholar]
- Liu, M.; Zhang, Y.; Ye, Z.; Yang, S.; Zhang, Y.; He, P.; Zhou, C.; Qin, X. Evaluation of the Association Between Coffee Consumption, Including Type (Instant, Ground), and Addition of Milk or Sweeteners and New-Onset Hypertension and Potential Modifiers. J. Acad. Nutr. Diet. 2024, in press. [Google Scholar]
- Wang, B.; Ma, T.; Yang, L.; He, S.; Li, J.; Sun, X. Association between coffee and tea consumption and the risk of dementia in individuals with hypertension: A prospective cohort study. Sci. Rep. 2024, 14, 21063. [Google Scholar]
- Sturm, H.; Basalely, A.; Singer, P.; Castellanos, L.; Frank, R.; Sethna, C.B. Caffeine intake and cardiometabolic risk factors in adolescents in the United States. Pediatr. Res. 2024. [Google Scholar] [CrossRef]
- Yao, H.; Li, L.; Wang, X.; Wang, Z. Association of caffeine intake with all-cause and cardiovascular mortality in diabetes and prediabetes. Diabetol. Metab. Syndr. 2024, 16, 177. [Google Scholar] [PubMed]
- Al-Shebel, D.; Al-Sowayan, N. The effects of caffeine ingestion on blood pressure levels in athletic and non-athletic women. Afr. J. Reprod. Health 2024, 28, 84–89. [Google Scholar]
- Wang, K.; Li, Z.; He, J. Association of caffeine consumption with all-cause and cause-specific mortality in adult Americans with hypertension. Food Sci. Nutr. 2024, 12, 4185–4195. [Google Scholar] [PubMed]
- Mehta, A.; Jain, A.C.; Mehta, M.C.; Billie, M. Caffeine and cardiac arrhythmias. An experimental study in dogs with review of literature. Acta Cardiol. 1997, 52, 273–283. [Google Scholar] [PubMed]
- Strubelt, O.; Diederich, K.W. Experimental treatment of the acute cardiovascular toxicity of caffeine. J. Toxicol. Clin. Toxicol. 1999, 37, 29–33. [Google Scholar]
- Ishida, S.; Ito, M.; Takahashi, N.; Fujino, T.; Akimitsu, T.; Saikawa, T. Caffeine induces ventricular tachyarrhythmias possibly due to triggered activity in rabbits in vivo. Jpn. Circ. J. 1996, 60, 157–165. [Google Scholar]
- Conen, D.; Chiuve, S.E.; Everett, B.M.; Zhang, S.M.; Buring, J.E.; Albert, C.M. Caffeine consumption and incident atrial fibrillation in women. Am. J. Clin. Nutr. 2010, 92, 509–514. [Google Scholar]
- Mostofsky, E.; Johansen, M.B.; Lundbye-Christensen, S.; Tjonneland, A.; Mittleman, M.A.; Overvad, K. Risk of atrial fibrillation associated with coffee intake: Findings from the Danish Diet, Cancer, and Health study. Eur. J. Prev. Cardiol. 2016, 23, 922–930. [Google Scholar]
- Larsson, S.C.; Drca, N.; Jensen-Urstad, M.; Wolk, A. Coffee consumption is not associated with increased risk of atrial fibrillation: Results from two prospective cohorts and a meta-analysis. BMC Med. 2015, 13, 207. [Google Scholar]
- Klatsky, A.L.; Hasan, A.S.; Armstrong, M.A.; Udaltsova, N.; Morton, C. Coffee, caffeine, and risk of hospitalization for arrhythmias. Perm. J. 2011, 15, 19–25. [Google Scholar] [PubMed]
- Dixit, S.; Stein, P.K.; Dewland, T.A.; Dukes, J.W.; Vittinghoff, E.; Heckbert, S.R.; Marcus, G.M. Consumption of Caffeinated Products and Cardiac Ectopy. J. Am. Heart Assoc. 2016, 5, e002503. [Google Scholar]
- Zuchinali, P.; Souza, G.C.; Pimentel, M.; Chemello, D.; Zimerman, A.; Giaretta, V.; Salamoni, J.; Fracasso, B.; Zimerman, L.I.; Rohde, L.E. Short-term Effects of High-Dose Caffeine on Cardiac Arrhythmias in Patients With Heart Failure: A Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 1752–1759. [Google Scholar]
- Posch, M.I.; Kay, M.D.; Harhash, A.A.; Huang, J.J.; Krupinski, E.A.; Abidov, A.; McMillan, N.A.; Kuo, P.H. Daily Caffeine Consumption Is Associated with Decreased Incidence of Symptoms and Hemodynamic Changes During Pharmacologic Stress with Regadenoson. J. Nucl. Med. Technol. 2020, 48, 73–76. [Google Scholar] [PubMed]
- Frost, L.; Vestergaard, P. Caffeine and risk of atrial fibrillation or flutter: The Danish Diet, Cancer, and Health Study. Am. J. Clin. Nutr. 2005, 81, 578–582. [Google Scholar] [PubMed]
- Zuchinali, P.; Ribeiro, P.A.; Pimentel, M.; da Rosa, P.R.; Zimerman, L.I.; Rohde, L.E. Effect of caffeine on ventricular arrhythmia: A systematic review and meta-analysis of experimental and clinical studies. Europace 2016, 18, 257–266. [Google Scholar]
- Bazal, P.; Gea, A.; Navarro, A.M.; Salas-Salvado, J.; Corella, D.; Alonso-Gomez, A.; Fito, M.; Munoz-Bravo, C.; Estruch, R.; Fiol, M.; et al. Caffeinated coffee consumption and risk of atrial fibrillation in two Spanish cohorts. Eur. J. Prev. Cardiol. 2021, 28, 648–657. [Google Scholar]
- Chieng, D.; Canovas, R.; Segan, L.; Sugumar, H.; Voskoboinik, A.; Prabhu, S.; Ling, L.H.; Lee, G.; Morton, J.B.; Kaye, D.M.; et al. The impact of coffee subtypes on incident cardiovascular disease, arrhythmias, and mortality: Long-term outcomes from the UK Biobank. Eur. J. Prev. Cardiol. 2022, 29, 2240–2249. [Google Scholar]
- Iten, V.; Herber, E.; Coslovsky, M.; Hennings, E.; Paladini, R.E.; Reichlin, T.; Rodondi, N.; Muller, A.S.; Stauber, A.; Beer, J.H.; et al. Coffee consumption and adverse cardiovascular events in patients with atrial fibrillation. BMC Med. 2024, 22, 593. [Google Scholar]
- Susy, K. Long-term outcomes from the UK Biobank on the impact of coffee on cardiovascular disease, arrhythmias, and mortality: Does the future hold coffee prescriptions? Glob. Cardiol. Sci. Pract. 2023, 2023, e202313. [Google Scholar]
- Ballard-Hernandez, J.; Sall, J. Dyslipidemia Update. Nurs. Clin. N. Am. 2023, 58, 295–308. [Google Scholar]
- Berberich, A.J.; Hegele, R.A. A Modern Approach to Dyslipidemia. Endocr. Rev. 2022, 43, 611–653. [Google Scholar] [PubMed]
- Pirillo, A.; Casula, M.; Olmastroni, E.; Norata, G.D.; Catapano, A.L. Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 2021, 18, 689–700. [Google Scholar]
- Le Master, E.; Levitan, I. Endothelial stiffening in dyslipidemia. Aging 2019, 11, 299–300. [Google Scholar]
- Choi, E.Y.; Cho, Y.O. Interaction of physical trainings and coffee intakes in fuel utilization during exercise in rats. Nutr. Res. Pract. 2013, 7, 178–184. [Google Scholar]
- Banitalebi, E.; Rahimi, A.; Faramarzi, M.; Mardaniyan Ghahfarrokhi, M. The effects of elastic resistance band training and green coffee bean extract supplement on novel combined indices of cardiometabolic risk in obese women. Res. Pharm. Sci. 2019, 14, 414–423. [Google Scholar]
- Du, Y.; Lv, Y.; Zha, W.; Hong, X.; Luo, Q. Effect of coffee consumption on dyslipidemia: A meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 2159–2170. [Google Scholar] [PubMed]
- Cai, L.; Ma, D.; Zhang, Y.; Liu, Z.; Wang, P. The effect of coffee consumption on serum lipids: A meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2012, 66, 872–877. [Google Scholar] [CrossRef]
- Petrovic, D.; Pruijm, M.; Ponte, B.; Dhayat, N.A.; Ackermann, D.; Ehret, G.; Ansermot, N.; Vogt, B.; Martin, P.Y.; Stringhini, S.; et al. Investigating the Relations Between Caffeine-Derived Metabolites and Plasma Lipids in 2 Population-Based Studies. Mayo Clin. Proc. 2021, 96, 3071–3085. [Google Scholar] [CrossRef]
- Han, J.; Shon, J.; Hwang, J.Y.; Park, Y.J. Effects of Coffee Intake on Dyslipidemia Risk According to Genetic Variants in the ADORA Gene Family among Korean Adults. Nutrients 2020, 12, 493. [Google Scholar] [CrossRef]
- Larsson, S.C.; Woolf, B.; Gill, D. Appraisal of the causal effect of plasma caffeine on adiposity, type 2 diabetes, and cardiovascular disease: Two sample mendelian randomisation study. BMJ Med. 2023, 2, 1–8. [Google Scholar] [CrossRef]
- Atwood, J. Management of Acute Coronary Syndrome. Emerg. Med. Clin. N. Am. 2022, 40, 693–706. [Google Scholar]
- Pollard, T.J. The acute myocardial infarction. Prim. Care 2000, 27, 631–649. [Google Scholar] [CrossRef]
- Braunwald, E.; Morrow, D.A. Unstable angina: Is it time for a requiem? Circulation 2013, 127, 2452–2457. [Google Scholar] [CrossRef] [PubMed]
- Richardson, T.; Baker, J.; Thomas, P.W.; Meckes, C.; Rozkovec, A.; Kerr, D. Randomized control trial investigating the influence of coffee on heart rate variability in patients with ST-segment elevation myocardial infarction. QJM 2009, 102, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.M.; Goulart, A.C.; Bensenor, I.M.; Lotufo, P.A.; Marchioni, D.M. Moderate coffee consumption is associated with lower risk of mortality in prior Acute Coronary Syndrome patients: A prospective analysis in the ERICO cohort. Int. J. Food Sci. Nutr. 2021, 72, 794–804. [Google Scholar]
- Brown, O.I.; Allgar, V.; Wong, K.Y. Coffee reduces the risk of death after acute myocardial infarction: A meta-analysis. Coron. Artery Dis. 2016, 27, 566–572. [Google Scholar] [CrossRef]
- Lindholm, D.; Storey, R.F.; Christersson, C.; Halvorsen, S.; Grove, E.L.; Braun, O.O.; Varenhorst, C.; James, S.K. Design and rationale of TROCADERO: A TRial Of Caffeine to Alleviate DyspnEa Related to ticagrelOr. Am. Heart J. 2015, 170, 465–470. [Google Scholar]
- Surma, S.; Sahebkar, A.; Banach, M. Coffee or tea: Anti-inflammatory properties in the context of atherosclerotic cardiovascular disease prevention. Pharmacol. Res. 2023, 187, 106596. [Google Scholar]
- Chieng, D.; Kistler, P.M. Coffee and tea on cardiovascular disease (CVD) prevention. Trends Cardiovasc. Med. 2022, 32, 399–405. [Google Scholar]
- Joshi, P.H.; de Lemos, J.A. Diagnosis and Management of Stable Angina: A Review. JAMA 2021, 325, 1765–1778. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, R.; Verdoia, M.; Compagnucci, P.; Barbarossa, A.; Stronati, G.; Casella, M.; Dello Russo, A.; Guerra, F.; Ciliberti, G. Angina in 2022: Current Perspectives. J. Clin. Med. 2022, 11, 6891. [Google Scholar] [CrossRef] [PubMed]
- Naderali, E.K.; Poyser, N.L. The effect of caffeine on prostaglandin output from the perfused mesenteric vascular bed of the rat. Prostaglandins Leukot. Essent. Fatty Acids 1994, 51, 415–418. [Google Scholar] [PubMed]
- Piters, K.M.; Colombo, A.; Olson, H.G.; Butman, S.M. Effect of coffee on exercise-induced angina pectoris due to coronary artery disease in habitual coffee drinkers. Am. J. Cardiol. 1985, 55, 277–280. [Google Scholar]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar]
- Ziaeian, B.; Fonarow, G.C. Epidemiology and aetiology of heart failure. Nat. Rev. Cardiol. 2016, 13, 368–378. [Google Scholar]
- Murphy, S.P.; Ibrahim, N.E.; Januzzi, J.L., Jr. Heart Failure With Reduced Ejection Fraction: A Review. JAMA 2020, 324, 488–504. [Google Scholar] [CrossRef]
- Tofovic, S.P.; Branch, K.R.; Oliver, R.D.; Magee, W.D.; Jackson, E.K. Caffeine potentiates vasodilator-induced renin release. J. Pharmacol. Exp. Ther. 1991, 256, 850–860. [Google Scholar]
- Tofovic, S.P.; Kusaka, H.; Pfeifer, C.A.; Jackson, E.K. Central effects of caffeine on renal renin secretion and norepinephrine spillover. J. Cardiovasc. Pharmacol. 1996, 28, 302–313. [Google Scholar]
- Tofovic, S.P.; Kusaka, H.; Rominski, B.; Jackson, E.K. Caffeine increases renal renin secretion in a rat model of genetic heart failure. J. Cardiovasc. Pharmacol. 1999, 33, 440–450. [Google Scholar] [CrossRef]
- Notarius, C.F.; Morris, B.; Floras, J.S. Caffeine prolongs exercise duration in heart failure. J. Card. Fail. 2006, 12, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Bodar, V.; Chen, J.; Sesso, H.D.; Gaziano, J.M.; Djousse, L. Coffee consumption and risk of heart failure in the Physicians’ Health Study. Clin. Nutr. ESPEN 2020, 40, 133–137. [Google Scholar]
- van Oort, S.; Beulens, J.W.J.; van Ballegooijen, A.J.; Handoko, M.L.; Larsson, S.C. Modifiable lifestyle factors and heart failure: A Mendelian randomization study. Am. Heart J. 2020, 227, 64–73. [Google Scholar]
- Mostofsky, E.; Rice, M.S.; Levitan, E.B.; Mittleman, M.A. Habitual coffee consumption and risk of heart failure: A dose-response meta-analysis. Circ. Heart Fail. 2012, 5, 401–405. [Google Scholar]
- Tikhonoff, V.; Casiglia, E. Prognostic cardiovascular cut-off values of dietary caffeine in a cohort of unselected men and women from general population. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 2160–2168. [Google Scholar]
- Yuksel, D.; Ozmen, O. Cardiovascular Findings and Effects of Caffeine on Experimental Hypothyroidism. Endocr. Metab. Immune Disord. Drug Targets 2025, online ahead of print. [Google Scholar] [CrossRef]
- Parladori, R.; Austin, T.; Smielewski, P.; Czosnyka, M.; Paoletti, V.; Vitali, F.; Corvaglia, L.; Martini, S. Cardiovascular and cerebrovascular effects of caffeine maintenance in preterm infants during the transitional period. Pediatr. Res. 2024, 96, 1267–1274. [Google Scholar]
- Parks, J.C.; Joplin, M.C.P.; Marshall, E.M.; Kearney, S.G.; Humm, S.M.; Kern, M.A.; Pinzone, A.G.; Erb, E.K.; Smith, T.M.; Kingsley, J.D. Effects of resistance exercise alone or with caffeine on hemodynamics, autonomic modulation and arterial stiffness in resistance-trained women. Eur. J. Appl. Physiol. 2023, 123, 2711–2721. [Google Scholar]
- Fan, H.; Xiong, Y.; Huang, Y.; Li, W.; Xu, C.; Feng, X.; Hua, R.; Yang, Y.; Wang, Z.; Yuan, Z.; et al. Coffee consumption and abdominal aortic calcification among adults with and without hypertension, diabetes, and cardiovascular diseases. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1960–1968. [Google Scholar]
- Zapotoczny, B.; Braet, F.; Kus, E.; Ginda-Makela, K.; Klejevskaja, B.; Campagna, R.; Chlopicki, S.; Szymonski, M. Actin-spectrin scaffold supports open fenestrae in liver sinusoidal endothelial cells. Traffic 2019, 20, 932–942. [Google Scholar]
- Mao, H.; Szafranska, K.; Kruse, L.; Holte, C.; Wolfson, D.L.; Ahluwalia, B.S.; Whitchurch, C.B.; Cole, L.; Lockwood, G.P.; Diekmann, R.; et al. Effect of caffeine and other xanthines on liver sinusoidal endothelial cell ultrastructure. Sci. Rep. 2023, 13, 13390. [Google Scholar]
- Evans, J.; Richards, J.R.; Battisti, A.S. Caffeine. In StatPearls; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Wikoff, D.; Welsh, B.T.; Henderson, R.; Brorby, G.P.; Britt, J.; Myers, E.; Goldberger, J.; Lieberman, H.R.; O’Brien, C.; Peck, J.; et al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem. Toxicol. 2017, 109, 585–648. [Google Scholar] [PubMed]
- Pina Cabral, J.; Sousa, D.L.; Carvalho, C.; Girao, A.; Pacheco Mendes, A.; Pina, R. Caffeine Intoxication: Unregulated, Over-the-Counter Sale of Potentially Deadly Supplements. Cureus 2022, 14, e21045. [Google Scholar] [PubMed]
- Gil, Y.E.; Lee, M.J.; Cho, S.; Chung, C.S. Effect of caffeine and caffeine cessation on cerebrovascular reactivity in patients with migraine. Headache 2022, 62, 169–175. [Google Scholar]
- de Souza, J.G.; Del Coso, J.; Fonseca, F.S.; Silva, B.V.C.; de Souza, D.B.; da Silva Gianoni, R.L.; Filip-Stachnik, A.; Serrao, J.C.; Claudino, J.G. Risk or benefit? Side effects of caffeine supplementation in sport: A systematic review. Eur. J. Nutr. 2022, 61, 3823–3834. [Google Scholar]
- Boison, D. Methylxanthines, seizures, and excitotoxicity. In Methylxanthines; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 251–266. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campagna, R.; Vignini, A. The Role of Xenobiotic Caffeine on Cardiovascular Health: Promises and Challenges. J. Xenobiot. 2025, 15, 51. https://doi.org/10.3390/jox15020051
Campagna R, Vignini A. The Role of Xenobiotic Caffeine on Cardiovascular Health: Promises and Challenges. Journal of Xenobiotics. 2025; 15(2):51. https://doi.org/10.3390/jox15020051
Chicago/Turabian StyleCampagna, Roberto, and Arianna Vignini. 2025. "The Role of Xenobiotic Caffeine on Cardiovascular Health: Promises and Challenges" Journal of Xenobiotics 15, no. 2: 51. https://doi.org/10.3390/jox15020051
APA StyleCampagna, R., & Vignini, A. (2025). The Role of Xenobiotic Caffeine on Cardiovascular Health: Promises and Challenges. Journal of Xenobiotics, 15(2), 51. https://doi.org/10.3390/jox15020051







