Abstract
Infertility affects 8–12% of couples worldwide, and 30–75% of preclinical pregnancy losses are due to a failure during the implantation process. Exposure to endocrine disruptors, like bisphenols, among others, has been associated with the increase in infertility observed in the past decades. An increase in infertility has correlated with exposure to endocrine disruptors like bisphenols. The uterus harbors its own microbiota, and changes in this microbiota have been linked to several gynecological conditions, including reproductive failure. There are no studies on the effects of bisphenols on the uterine–microbiota composition, but some inferences can be gleaned by looking at the gut. Bisphenols can alter the gut microbiota, and the molecular mechanism by which gut microbiota regulates intestinal permeability involves Toll-like receptors (TLRs) and tight junction (TJ) proteins. TJs participate in embryo implantation in the uterus, but bisphenol exposure disrupts the expression and localization of TJ proteins. The aim of this review is to summarize the current knowledge on the microbiota of the female reproductive tract (FRT), its association with different reproductive diseases—particularly reproductive failure—the effects of bisphenols on microbiota composition and reproductive health, and the molecular mechanisms regulating uterine–microbiota interactions crucial for embryo implantation. This review also highlights existing knowledge gaps and outlines research needs for future risk assessments regarding the effects of bisphenols on reproduction.
1. Introduction
Environmental pollution, as a consequence of urbanization and industrialization, has raised the incidence of health issues caused by endocrine disruptors (EDs), which are xenobiotics that can interact with hormone receptors, altering the endocrine system in the exposed individual. One of the most studied EDs is bisphenol A (BPA), a compound used as an important intermediate in the production of epoxy resins and polymers. This ED is used to provide desirable properties to a wide range of products, including bottles, liners, pipes, dental sealants, food packaging, children’s toys, nail polish, fire retardant materials, medical and electronic equipment, thermal paper, etc. [1]. As a result, humans are continuously exposed to this ED, which has been detected in urine, amniotic fluid, blood of adults and neonates, placenta, umbilical cord blood, and human breast milk at a range of levels known to be biologically active (>10 μg/L) [2].
Due to growing concerns about the detrimental effects of BPA on reproductive and metabolic health, its use was banned in the manufacturing of baby bottles, sippy cups, and infant formula packaging in the European Union (EU), the United States, and Canada [3]. In Denmark, Belgium, Sweden, and France, more bans have been established on the use of BPA in food contact materials and coatings [4]. Consequently, BPA analogs, like bisphenol S (BPS), bisphenol F (BPF), bisphenol AF (BPAF), and tetrabromobisphenol A (TBBPA) (Figure 1), have gradually replaced BPA in many consumer products labeled as “BPA-free”, resulting in a significant increase in human exposure to these substances. Unfortunately, little or no research was conducted to determine the safety of these BPA-free products before they were marketed to the public as a healthier alternative. These analogs are used in the production of everyday use products. BPS is used in paper currency and cashier’s receipts; BPF in tank and pipe linings, dental sealants, and food packaging materials; BPAF in the plastics industry; and TBBPA is applied in plastics, paper, textiles, and circuit boards as a flame retardant. As a consequence of their unrestricted use, all these BPA substitutes have been detected in human samples around the world: BPF, BPAF, and BPS were detected in urine samples with a concentration of 0.15 to 0.54 μg/L, below the detection level to 3.93 μg/L, and 0.654 ng/mL (which was comparable to BPA), respectively. Meanwhile, TBBPA was identified in human serum and breast milk samples at levels of 480 ng/L and between 50 and 350 pg/kg/day, respectively [5,6,7,8,9,10,11]. BPA and its chemical structure analogs are similar to steroid hormones, so they can bind to membrane and nuclear receptors, such as estrogen, androgen, and thyroid hormone receptors. In doing so, they may produce endocrine disruption, tumors, adverse reproductive outcomes, and transgenerational effects, posing a threat to human health [12].
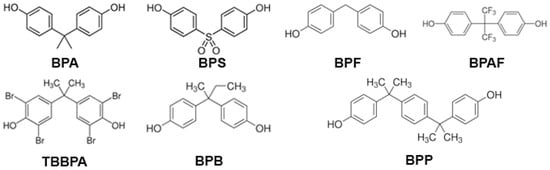
Figure 1.
Chemical structure of bisphenols: BPA, bisphenol A; BPAF, bisphenol AF; BPB, bisphenol B; BPF, bisphenol F; BPP, bisphenol P; BPS, bisphenol S; and TBBPA, tetrabromobisphenol A.
Microbiota, the diverse community of commensal, symbiotic, and pathogenic microorganisms that colonize different parts of the body of all animals [13,14], plays a key role in the host’s health and disease by regulating many diverse and complex biological processes such as brain development and behavior, metabolism, immune response, and reproduction [15,16,17]. Having over 3 million unique genes, the human microbiota is often referred to as a “second genome”. Its composition can vary or be disrupted due to various intrinsic and extrinsic factors, including host genetics, body location, diet, and exposure to xenobiotics [17,18]. Indeed, bisphenols (mainly BPA, BPS, and BPF) can accumulate at bacterial membranes due to their lipophilicity, disrupting and disturbing membrane permeability and cell function and unchaining cell destruction [19,20]. Importantly, bisphenols have also been shown to alter microbial composition in both soil and gut microbiomes [13,21]. Research has shown that microbiota and xenobiotics, including endocrine disruptors like bisphenols, may potentially modulate adverse health effects via microbial-xenobiotic interactions [22].
For nearly a century, it was believed that the uterus was a sterile environment, with any kind of colonization of the upper reproductive tract linked solely to infections, diseases, or health problems. However, in 2008, the Human Microbiome Project revealed the presence of microbiota in body sites previously considered sterile, such as the female upper reproductive tract [23]. Since then, several studies have attempted to establish a “baseline” or “core” microbiome of the healthy endometrium; however, due to limitations in these studies, this has not been fully achieved. Nevertheless, identifying microbial communities in the reproductive tract of healthy women and those with gynecological diseases has shown that microbial dysbiosis may be associated with different gynecological disorders and with treatment success in assisted reproductive technology (ART).
This review aims to summarize information about the microbiota in the female reproductive tract (FRT), its association with different reproductive diseases, especially reproduction failure, the impact of bisphenols on microbiota composition and reproduction, and the molecular mechanisms that may regulate interactions between the uterus and microbiota, which are important for embryo implantation. Additionally, we identify knowledge gaps and research needs for future risk assessments concerning the effects of bisphenols on reproduction.
2. The Cervicovaginal Microbiota Impacts on Reproduction
Given the crucial role of the microbiome in human physiology, humans have been described as holobionts or communities composed of the host and its symbiotic microbes rather than individuals [24]. Interestingly, the combination of the host genome and microbiome enhances genetic variation and phenotypic plasticity, allowing the holobiont to improve its overall fitness. Consequently, bacteria might arguably play an essential role in producing reproductively fit and healthy offspring in addition to influencing the overall health of an individual.
The FRT can be divided into two connected parts: the upper and lower reproductive tracts. The former includes the ovaries, fallopian tubes, and uterus, while the latter comprises the cervix and vagina.
The cervicovaginal microbiome impacts several important reproductive outcomes, including preterm birth, fertility, cervicitis, and risk of sexually transmitted infections (STIs) [25]. Twenty different genera of bacteria have been recognized in the vagina [26]. Although Lactobacilli (L.) are most often the dominant species in the vagina (99.97%), significant changes in the vaginal microbiota have been reported between individuals and even in the same person under different circumstances, like the follicular phase, the secretory phase, during menstruation, and sexual intercourse [27,28,29,30,31,32]. In the cervix, the microbiota composition is similar to the one in the vagina, although in a lower amount (vagina: 1010–1011; cervix: 107–108) [28,33]. In particular, it has been proposed that L. crispatus and L. gasseri species are related to the preservation of a simple vaginal microbiome, dominating their respective vaginal communities and providing a barrier against opportunistic pathogens. This can be explained by the production of bacteriostatic and bactericidal compounds (e.g., lactic acid and hydrogen peroxide) that preserve a low pH (≤4.5). Furthermore, L. crispatus is probably involved in a successful pregnancy [34,35]. Even though L. iners has been related to health-promoting effects, it is also able to increase the vaginal pH, producing microbiome perturbation and species-specific virulence factors [35]. Moreover, it has been reported that a L. iners-dominated cervicovaginal microbiota at gestation is associated with an increased risk of a short cervix or preterm birth [36,37].
However, not all women have Lactobacillus-dominant vaginal flora. Women with a low percentage of Lactobacillus in their vaginal sample are less likely to have a successful embryo implantation [38,39]. Preterm birth and a short cervix are also associated with a community characterized by a scarcity of Lactobacillus species and a wide array of anaerobic bacteria [40], which are also typically found in common vaginal infections, such as bacterial vaginosis, where the overgrowth of typically non-Lactobacillus anaerobic bacteria, including Gardnerella vaginalis, Mobiluncus spp., and Atopobium vaginae, leads to a disruption of the ecological vaginal balance [32]. Furthermore, for patients undergoing in vitro fertilization (IVF), Lactobacillus, Akkermansia, Desulfovibrio, Atopobium, Prevotella, and Gardnerella showed differential abundance between pregnant and non-pregnant women. Among them, L. iners was the predominant group in the non-pregnant IVF patients and was negatively correlated with the other genera and positively correlated with serum estradiol (E2) levels. Meanwhile, in the pregnant IVF patients, L. crispatus was the predominant group and vaginal progesterone (P4) did not appear to impact the vaginal microbiota during pregnancy [37,41,42].
While it is known that there is a connection between an optimal Lactobacillus-dominant microbiota and favorable reproductive health outcomes, the mechanisms through which this occurs are still unknown [43].
The mucosal surface of the FRT is the primary site of entry for many STIs, including HIV. It is composed of a non-keratinized stratified squamous epithelium that forms a substantial physical and immunological barrier against pathogens [44,45,46]. Interactions between the FRT epithelial cells are mediated by intercellular junctional molecules comprising tight junction (TJ) complexes, which are a hallmark of the vaginal mucosa. Under specific conditions, the permeability of these complexes is altered to permit passage of innate immune effector molecules secreted by the vaginal epithelial cells [45].
It has been reported that colonization of cell multilayer cultures from the vaginal epithelium by common vaginal commensals, including L. crispatus, L. jensenii, and L. rhamnosus, led to an intimate association of Lactobacillus with the epithelial cells. This exclusively occurs on the apical surface and protects it from Staphylococcus epidermidis colonization, which can trigger cytokine secretion and produce inflammation [45]. One mechanism by which cervicovaginal Lactobacilli improve the integrity of the FRT epithelial barrier may involve the metabolite lactic acid, which directly strengthens the barrier by upregulating the expression of TJ proteins claudin-1 and claudin-4 in vitro [43]. It has been demonstrated that L. crispatus and soluble factors from this species accelerated the re-epithelialization of vaginal epithelial cells and augmented vascular endometrial growth factor secretion [47].
3. Microbiota in the Uterus
Since 1900, the uterus was considered a sterile environment for microorganisms until the mid-1980s, when it was demonstrated that the cervical mucus plug was not entirely impermeable to bacterial ascension from the vagina [48]. Due to the challenges of simulating living organ conditions with culture methods, colonization in the upper reproductive tract has only been associated with infections, diseases, or health issues such as endometriosis or preterm birth [49,50,51]. Furthermore, several IVF clinical studies indicated that bacterial contamination of the embryo transfer catheter significantly reduces clinical pregnancy rates [52,53,54,55]. Thus, eradication of endocervical microorganisms with the administration of prophylactic antibiotics has been attempted to improve implantation [52,53]. In 2008, the Human Microbiome Project laid the groundwork for research on human microbiota, revealing the presence of microbiota in body sites once thought to be sterile, thanks to advances in sequencing techniques [23]. Short-read 16S rRNA sequencing-based analysis of microbiome profiling generally provides information on microbial compositions at the genus level.
Common applications include identifying microbiome composition across various sample groups to investigate microbial differences between groups and conditions. Chen et al. identified distinct microbial communities in the vagina, cervical canal, uterus, fallopian tube, and peritoneal fluid, demonstrating that the FRT is not sterile [28]. These findings challenged the dogma that a healthy uterine cavity was sterile and that the presence of microbes was a sign of pathology (ascension of bacteria through the cervix, through blood, or as a result of gynecological procedures like ART or insertion/removal of intrauterine devices). In addition, it was demonstrated that the endometrial microbiota (EM) detected was not due to contamination of the samples by vaginal microbiota since some bacteria genera present in the endometrium were absent in the vagina of the same subject, and vice versa [26]. The Human Microbiome Project has revealed that approximately 9% of the total human microbiome is found in the FRT [56,57].
Recent studies that analyzed the EM helped to identify microbial communities in healthy women, revealing that microbial dysbiosis could be associated with different gynecological disorders and with treatment success in ART [26,58]. The predominant phyla identified in the endometrium of healthy women were Firmicutes (mainly Lactobacillus spp.), Bacteroidetes (mainly Flavobacterium spp., Bacteroides spp., Prevotella spp.), Proteobacteria (mainly Pseudomonas spp., Acinetobacter spp.), and Actinobacteria (mainly Gardnerella spp., Bifidobacterium spp.) [24,26,48,59,60,61,62]. The endometrium possesses a higher bacterial diversity with a lower bacterial biomass as compared with those in the vagina (endometrium biomass: 106–107; vagina biomass: 1010–1011) [28].
The endometrium proliferates and dies during the menstrual cycle in order to provide an adequate environment for implantation and pregnancy. These changes are driven by variations in ovarian steroid hormones [26]. However, it is still unclear if the changes in ovarian steroid hormones induce alterations in the uterine microbiome during the menstrual cycle. Studies in endometrial samples showed a higher abundance of Prevotella spp. during the proliferative phase, while increased Sneathia spp. were observed during the secretory phase [28,63,64]. It was reported that some bacteria were exclusively detected in specific phases. Bacteria genera such as Actinobaculum, Mobiluncus, and Porphyromonas were only discovered in the proliferative phase, while other genera like Aerococcus, Delftia, and Sneathia were only identified in the secretory phase. Moreover, predominant species were different between phases as well. The most frequent bacteria genera in both phases were Bifidobacterium, Burkholderia, Gardnerella, and Lactobacillus. Meanwhile, Escherichia and Prevotella were the most common genera in the proliferative phase, while Atopobium and Streptococcus were in the secretory phase [28,64,65]. Low levels of Lactobacillus were detected after menstruation, and they gradually increased during the proliferative phase, with a peak during the secretory phase [66]. In contrast, other investigations showed stable microbiota profiles in >80% of patients analyzed during two different time points of the secretory phase of the menstrual cycle (LH+2 and LH+7), suggesting that the EM is not hormonally regulated during the acquisition of endometrial receptivity [26]. Moreover, the EM may be altered by exogenous hormones, including those used for ovarian stimulation, progesterone (P4) supplementation, and various types of ovulation induction and luteal support used during IVF [63]. For instance, progestin administration has been associated with a loss of Lactobacillus spp. diversity and the emergence of L. crispatus as the dominant phylotype in the endometrium of women with menorrhagia and dysmenorrhea [64]. Additionally, ovarian stimulation and P4 luteal supplementation were associated with a slight decrease in the proportion of Lactobacilli and an increase in Prevotella and Atopobium in the endometrium of women undergoing IVF [67].
While it is still unclear how the uterus is colonized, the ascension of bacteria from the vagina is one of the most possible paths [23,51]. Similar to vaginal microbiota, the endometrium is mainly dominated by Lactobacillus spp. and its depletion is associated with preterm birth and infertility too [24]. However, despite the similarities, it has been observed that bacterial composition in the vagina greatly differs from the intrauterine microbiome [48,68]. The divergence in microbiomes could be due to the differences between the tissues since the upper reproductive tract is lined by a monolayer of columnar epithelial cells while the vagina is lined by a layer of non-keratinized squamous epithelium and has a lower pH, which would produce a different environment for the growth of microorganisms [69,70]. Others suggested that there may be other routes of uterus colonization, like the hematogenous transfer of microbiota from another site, such as the gut or oral microbiota to the uterine cavity; the transfer of microbes through the fallopian tubes; via insertion of intrauterine devices; the possible transportation of microbiota in the external environment or the lower genital tract into the uterine cavity by sperm (spread with sperm); and gynecological procedures related to ART [51,71].
The functions of the EM include: (a) participation in the proliferation and apoptosis of endometrial cells; (b) improving the anti-infection capacity of the endometrium by preventing the proliferation and attachment of pathogenic microorganisms to the endometrial surface; (c) regulation of the uterine immune response as a result of the production of inflammatory cytokines, chemokines and antibacterial substances which are induced by the binding of microbial ligands to host receptors; and (d) participation in blastocyst implantation and pregnancy maintenance [71].
4. Uterine Microbiota in Gynecological Diseases
Studies on EM in gynecological diseases have shown that women with chronic endometritis (CE) (persistent inflammation of the endometrial lining, characterized by the presence of edema, increased stromal cell density, and dissociated maturation of the stroma and epithelium throughout the menstrual cycle) had microbiomes with significantly higher proportions of Firmicutes and lower proportions of Proteobacteria than healthy women [68]. Moreover, it has been reported that the abundance of Acinetobacter, Actinobacteria, Anaerococcus, Bifidobacterium, Dialister, Enterobacteriaceae, Enterococcus, Fusobacteria, Gardnerella, Klebsiella pneumoniae, Neisseria, Phyllobacterium, Prevotella, Sphingomonas, Staphylococcus, and Streptococcus was significantly increased in chronic endometritis patients [72]. These bacteria may regulate an increase in immune cells, producing a decrease in the receptivity of the endometrium. Thus, this disease has been related to pregnancy failure of both spontaneous and ART conceptions since 14–41% of CE patients presented recurrent implantation failure (RIF), and 8–28% had repeated pregnancy loss (RPL) [73,74]. Infertile patients with CE who underwent IVF had a significantly lower clinical pregnancy rate (32%) as compared to infertile non-chronic endometritis patients (59.4%) [68,73,74,75,76,77]. The prevalence of CE has been estimated at 2.8–39% in infertile patients but can be as high as 60% or 66% in patients with inexplicable RPL or RIF, respectively [78].
In women with endometriosis (growth of endometrial tissues (glands and stroma) outside the uterine cavity), Lactobacillacae were significantly decreased, while Staphylococcus, Streptococcaceae, Gardnerella, Enterococcus, Alishewanella, Prevotella, Acinetobacter, Vagococcus, Comamonas, Escherichia coli, Pseudomonas, and Moraxellaceae were significantly increased, as compared to healthy women [79,80,81,82,83]. It has been proposed that the EM contributes to the incidence and progression of endometriosis by regulating the immune system [71].
In women with adenomyosis (presence in the myometrium of endometrial tissue), the endometrium was enriched by Comamonadaceae, Enterobacteriaceae, Prevotella copri, Citrobacter freundii, Weissella confusa, Burkholderia cepacia, Lactobacillus zeae, Delftia spp., Acinetobacter spp., Shewanella spp., Peptoniphilus spp., Pseudomonas viridiflava, Tissierellaceae (1–68 spp.), Pseudomonas spp. and Corynebacterium spp., as compared to control women [28,84].
The endometrium of women with hysteromyoma (benign tumors in the uterus) was enriched with Ruminococcaceae, Alcaligenaceae, and Blastomonas natatoria as compared to healthy women [28].
In patients with endometrial hyperplasia (increased gland-to-stroma ratio in the endometrium compared with the normal proliferative endometrium), an increase in the relative quantity of Actinobacteria, Bacteroides, E.coli, Firmicutes, Fusobacteria, and Proteobacteria has been reported, while Lactobacillus abundance was reduced [71,85]. The increase in these microorganisms may be related to the elevation in estrogen levels and the production of inflammatory cytokines (IL-6, IL-1β, and TNF-α) observed in these patients, factors that are implicated in the development, promotion, and progression of this pathology [85].
Several investigations have proposed that a dysfunction of the immune system, as well as an imbalance of the genital tract microbiota, may be involved in the incidence, development, and metastasis of gynecological malignancies. It has been reported that in endometrial cancer patients, the proportion of Anaerococcus, Anaerostipes, Anaerotruncus, Arthrospira, Atopobium vaginae, Bacillus cereus, Bacillus pseudofirmus, Bacteroides, Bacteroides fragilis, Clostridium botulinum, Dialister, Micrococcus, Muribaculum, Mycoplasma hyopneumoniae, Nocardioides, Pasteurella multocida, Pelomonas, Peptoniphilus, Porphyromonas spp., Prevotella, Pseudomonas uter, Ruminococcus, Treponema, and Stenotrophomonas rhizophila was higher than in healthy people, while the abundance of Lactobacillus and Oscillibacter was decreased [86,87,88,89,90,91,92,93].
Therefore, the microbiota components change in the different types of endometrial diseases. However, it is important to note that there are common bacteria in these disorders: Bifidobacterium, Bacteroides, E. coli, Gardnerella, Lactobacillus, Prevotella, Pseudomonas, Staphylococcus, and Streptococcus. In endometrial diseases, the proportion of Lactobacillus and Firmicutes declined, while the abundance of Actinobacteria (like Gardnerella and Bifidobacteria), Bacteroidetes (like Bacteroides fragilis, Prevotella, and Bacteroides), and Proteobacteria (like Staphylococcus and E.coli) augmented, as compared with healthy endometria. Thus, the changes in EM composition play a key role in endometrial pathology, making the microbiota a candidate to become a target for the prevention and treatment of various endometrial illnesses [71].
5. Uterine Microbiota and Reproduction
Infertility affects about 8–12% of couples worldwide and remains a major global concern both socially and economically. Its prevalence has increased in recent decades [94]. In couples afflicted by infertility, 26–30% of cases are produced by male factors, while 45–60% are generated by female factors [95]. While the underlying origin of human infertility has been potentially challenged by the successful utilization of IVF and embryo transfer techniques, the success rate of implantation remains frustratingly low. This is considered primarily due to the poor understanding of uterine receptivity during embryo transfer. Other factors that may impact the embryo–endometrium coordination, which affects implantation, are the maternal immune system, the reproductive tract microbiome, anatomical issues, hematological aspects, the endocrine environment, and embryo and parental genetics. Recurrent implantation failure (RIF) is described as the incapability to accomplish a clinical pregnancy after the transfer of at least four good-quality embryos in no less than three fresh or frozen cycles in a woman under 40 years of age [96]. In order to have a successful implantation, there has to be correct timing between the development of high-quality embryos and a receptive endometrium. Sixty-six percent of implantation failures are attributed to inadequate endometrial receptivity, while embryo quality is only responsible for 33% [97]. Therefore, it is essential to resolve the complex interplay between embryo development and endometrium receptivity to optimize success. Recent studies have indicated that the microbiota may participate in the interaction between hormones, immune cells, and physiological adaptations required for a favorable pregnancy [98]. Microbial dysbiosis, which is a change in the conformation, allocation, or functioning of the normal microbiota [96], may play a role in IVF failure, and the restoration of an adequate uterine microbiota could make available new clinical treatments for infertile couples [74]. However, although microbiota dysbiosis in the uterus appears to be related to unsuccessful implantation and birth, it is not yet clear whether it is a determining factor due to the limitations of the studies. In particular, hormonal and physiological changes within the menstrual cycle, the lack of ethnic diversity, differences in socioeconomic status and lifestyle, environmental factors and the challenge of collecting uncontaminated uterine samples, lacking vaginal or cervical bacteria and bacterial DNA found in the air and in laboratory reagents and equipment, have hindered progress in the identification of a “baseline” or “core” microbiome of the healthy endometrium [58,99]. However, in order to avoid contamination from vagina and cervical canal microbiota, some studies have employed a double-lumen catheter to obtain endometrial microbiota samples [62,72,96,100,101].
During early pregnancy, it has been described that Lactobacillus, the most common microbe identified in the endometrium, was associated with defense mechanisms in the reproductive tract, such as maintaining the pH balance, preventing prolonged colonization by harmful bacteria through adhesion to epithelial cells, producing lactic acid, hydrogen peroxide, and bacteriocins, and regulating the local immune system (Table 1) [102,103]. Proteobacteria, Cupriavidus, Finegoldia, Microbacterium, Achromobacter, and Tepidimonas are also beneficial bacteria since it has been reported that their relative abundance was significantly higher in successful pregnancy groups [72,74,104]. Studies in women undergoing IVF have suggested that a non-Lactobacillus-dominated EM (NLDM) (<90% Lactobacilli with >10% of other bacteria) was associated with infertility, with a significantly decreased implantation (NLDM 23.1% vs. Lactobacillus-dominated EM (LDM) 60.7%), significantly decreased pregnancy (NLDM 33.3% vs. LDM 70.6%), significantly decreased ongoing pregnancy (NLDM 13.3% vs. LDM 58.8%), and significantly decreased live births (NLDM 6.7% vs. LDM 58.8%) (Table 1) [26,62,96,100,101,102]. Another study suggested that the amount, rather than the proportion, of Lactobacillus in the endometrium plays a role in developing endometrial receptivity, as patients with extremely low microbial biomass were strongly linked to a pre-receptive endometrium (Table 1) [105]. In contrast, other authors reported that endometrial bacterial colonization was found to consist of a polymicrobial environment, with Lactobacilli being uniquely present in the group that experienced unsuccessful IVF outcomes. This microbiota may originate from the vagina due to a failure of the barriers that typically block such migration (Table 1) [106]. Moreover, other IVF studies indicated that the pregnancy, implantation, and miscarriage rates were comparable between infertile patients with an eubiotic endometrium (≥80% Lactobacillus + Bifidobacterium spp.) and dysbiotic endometrium (<80% Lactobacillus + Bifidobacterium spp. with ≥20% of other bacteria) (Table 1) [101,107]. Furthermore, some patients achieved pregnancies despite having 0% Lactobacillus and 95.5% Streptococcus, or 0% Lactobacillus, 60.8% Atopobium, and 21.9% Gardnerella; these pregnancies were ongoing beyond 16 weeks at the time the article was published (Table 1) [107]. Consequently, the normal span of endometrial Lactobacillus levels should be reevaluated in fertile women when assessing its use as a biomarker for RIF.
Moreno et al. proposed that the primary factor affecting fertility may be the occurrence of pathogens in the uterine cavity rather than the necessity of a specific commensal taxon [72]. The absence of bacteria, including Lactobacillus, does not hinder implantation, further highlighting the role of pathogenic bacteria as a risk factor in reproduction. Indeed, the presence of pathogenic bacteria, such as Actinobacteria, Atopobium, Bacillus halosaccharovorans, Bacillus simplex, Bifidobacterium, Burkholderia, Chryseobacterium, Corynebacterium coyleae, Delftia, Dialister, Dietzia, Enterococcus, E. coli, Gardnerella vaginalis, Glutamicibacter spp., Haemophilus, Hydrogenophaga, Klebsiella, Kocuria dechangensis, Leucobacter, Megasphaera, Microbacterium maritypicum, Micrococcus, Neisseria, Paenibacillus glucanolyticus, Prevotella, Pseudomonas, Psychrobacter, Ralstonia, Romboutsia, Roseiflexaceae, Schlegelella, Serratia marcescens, Sphingobacterium, Staphylococcus aureus, and Streptococci, that would constitute part of a more diverse microbiota compared to their corresponding controls, was suggested to be related to reproductive failure (Table 1) [23,24,38,72,96,100,104,106,108,109,110,111,112,113,114,115]. Of all these pathogenic bacteria, Actinobacteria, Fusobacteria, Streptococcus agalactiae, Klebsiella pneumoniae, Enterococcus faecalis, Neisseria gonorrhoeae, Gardnerella vaginalis, and Staphylococcus are the major pathogens in the endometrium of patients with CE (Table 1) [72,74,116]. As it has been previously mentioned, this disease has been related to pregnancy failure since 14–41% of CE patients presented RIF, and 8–28% had RPL [74]. However, there is no consensus about which pathogenic bacteria(s) are undoubtedly correlated with infertility since the studies that report them do not completely agree [23,24,34,72,74,96,100,106,108,109,110,111,112,113,114,115]. Therefore, it has been postulated that the primary role of Lactobacillus spp. in reproduction is to prevent the establishment of pathogenic bacteria in the uterine cavity [72]. Further research is required to explore the mechanism through which pathogenic bacteria may influence embryo implantation. Also, it may be necessary to analyze microbiota at the species-level resolution to identify the true pathogenic bacteria of the endometrium since pathogenicity can vary between bacterial species; for example, Streptococcus agalactiae and Streptococcus anginosus belong to the same genus but may exhibit distinct behaviors in the endometrium [107]. Moreover, although Lactobacillus has been suggested as the dominant microbiome species that benefits and supports endometrial receptivity, the majority of the reports do not specify which Lactobacillus species may be most beneficial for pregnancy. However, L. iners has been described as offering a notable improvement in achieving favorable pregnancy outcomes [106].
In conclusion, a dysbiotic endometrial microbiome has been linked to implantation failure. This imbalance may trigger excessive immune stimulation, leading to inflammation and local tissue damage, which can result in transplantation failure, spontaneous preterm birth, and other adverse obstetric outcomes, such as growth restriction and stillbirth [115,117,118,119,120]. Nevertheless, the precise regulatory mechanisms remain unclear.
These data suggest that the EM may be regarded as an emerging factor contributing to implantation failure and/or pregnancy loss. As a result, microbial interventions such as antibiotics, probiotics, prebiotics, and microbial transplantation have been explored as strategies to modify the EM composition prior to a subsequent conception attempt in order to improve infertility treatment outcomes and IVF success [74]. Probiotics are living microorganisms that benefit the host by promoting a healthy balance of the microbiota, including preparations of Lactobacillus and Bifidobacterium. In contrast, prebiotics are nondigestible nutritive substances acting as substrates for protective endogenous bacteria to stimulate their growth and metabolism [66,121]. Lactoferrin (LF), an iron-binding glycoprotein found in human external secretions like breast milk, is one of the prebiotics described as being effective against numerous infectious diseases. LF exerts bacteriostatic effects due to its capacity to bind iron, making it unavailable to bacteria. LF effectively prevented preterm delivery in patients with a history of multiple miscarriages or early preterm delivery produced by refractory bacterial vaginitis by promoting the growth of Lactobacilli in their vaginal flora [122]. In the infertility clinical setting, antibiotic therapy, followed by a combination of prebiotics and/or probiotics, has been used to change the NLDM into LDM. Kadogami and cols. notified that in RIF patients, the combined use of a vaginal probiotic suppository (L. gasseri, L. fermentum, and L. plantarum) and vaginal antibiotics (metronidazole) transformed the NLDM into LDM (>90% Lactobacillus + Bifidobacterium) in 78.6% of the patients treated and that the therapeutic effects were unaffected by the prevailing bacteria present prior to the intervention [66]. Likewise, it has been reported in RIF patients that vaginal antibiotic (metronidazole) and vaginal probiotic formulations (L. acidophilus La-14 and L. rhamnosus HN001) significantly enhanced Lactobacillus presence in the uterine microflora compared to oral formulations. Furthermore, it was observed that the Lactobacillus species that proliferated in the uterus after treatment was not limited to the dispensed species since L. crispatus, L. jensenii, L. fermentum, and L. iners were also detected. This indicates that the dispensed Lactobacillus species may have facilitated the creation of an intrauterine environment leading to Lactobacillus growth, allowing a subsequent proliferation of the originally existing Lactobacillus species in the uterine cavity [123]. Treatment with broad-spectrum antibiotics (amoxicillin or levofloxacin) in combination with LF and vaginal probiotics transformed the uterine NLDM into LDM. This change significantly increased pregnancy rates among LDM patients (61.3%) compared to the NLDM group (40%), with LDM defined as having ≥80% Lactobacillus spp. (Table 1) [124]. Similarly, other work reported that reproductive results in the immediate subsequent vitrified–warmed blastocyst transfer cycle were better in RIF women who resolved NLDM after LF supplementation compared to those whose local microbiota remained unchanged. Notably, the clinical pregnancy rate and the live birth rate for RIF women who increased the proportion of Lactobacillus species by at least 10% in EF samples were significantly higher (71.4% and 57.1%, respectively) than for RIF patients who did not overcome NLDM (22.2% and 11.1%, respectively) (Table 1) [121]. A similar increase in clinical pregnancy rate, ongoing pregnant rate, and live birth rate was observed in RIF patients who were treated with antibiotics (amoxicillin and clavulanic acid, or metronidazole) followed by vaginal probiotics to overcome NLDM, as compared to the non-treated RIF patients (Table 1) [125]. Additionally, Wei et al. informed that transvaginal Lactobacillus treatment significantly improved the clinical pregnancy rate in women with prior failed cycles and a low initial proportion of Lactobacillus (Table 1) [104].

Table 1.
Microbiota composition in endometrium of patients undergoing assisted reproductive technology (ART), and positive or negative outcomes of the treatment.
Table 1.
Microbiota composition in endometrium of patients undergoing assisted reproductive technology (ART), and positive or negative outcomes of the treatment.
| Ref. | Patient Profile and Treatment Approach for Correcting the NLDM (the Latter, When Indicated) | Detected Microbiota Composition | Positive Outcomes (Ongoing Pregnancy Included) | Negative Outcomes (Non-Pregnant or Decreased Clinical Pregnancy Rate) |
|---|---|---|---|---|
| [26] | 35 infertile subjects undergoing IVF 35 fertile women at pre-receptive (LH+2) and receptive phases (LH+7) LDM (>90% Lactobacillus spp.) NLDM (<90% Lactobacillus spp. with >10% other bacteria) | 166 different OTUs Lactobacillus 71.7%, Gardnerella 12.6%, Bifidobacterium 3.7%, Streptococcus 3.2%, Prevotella 0.866%. Others: Bacillus, Bacteroides, Bifidobacterium, Blautia, Clostridiales, Clostridium, Escherichia, Faecalibacterium, Gardnerella, Lachnospiraceae, Propionibacterium, Pseudomonas, Roseburia, Ruminococcus, Veillonella. EM was not hormonally regulated during the acquisition of endometrial receptivity. | LDM was associated with significant increases in implantation rate: 60.7% *; pregnancy rate: 70.6% *; ongoing pregnancy rate: 58.8% *; and live birth rate: 58.8% *. | NLDM was linked to significant reductions in implantation rate: 23.1%; pregnancy rate: 33.3%; ongoing pregnancy rate: 13.3%; and live birth rate: 6.7%. |
| [60] | 33 IVF patients 26 (79%) Caucasian 5 (15%) Asian 1 (3%) African American 1 (3%) Hispanic | 278 different genera Flavobacterium and Lactobacillus constitute the predominant bacterial genera observed in both groups; Other detected bacterium were Acidovorax, Acinetobacter, Bdellovibrio, Blvii28, Candidatus aquiluna, Cellvibrio, Chryseobacterium, Clostridium, Curvibacter, Delftia, Fluviicola, Janthinobacterium, Limnohabitans, Methylotenera, Microbacterium, Paucibacter, Paudibacter, Pedobacter, Polosinus, Polynucleobacter, Pseudomonas, Salinibacterium, Shuttleworthia, Spirocheta, Streptococcus, Sulfospirilum, Sulfuricurvum | 18 patients had ongoing pregnancies. | 15 non-pregnant patients. Certain major species seemed to differ based on the outcome, although these differences were not statistically significant. |
| [62] | 40 reproductive-aged Chinese women, 10 infertile patients. Participants with an IUD, vaginal inflammation, acute inflammation, suspected cervical or endometrial neoplasia, or endocrine or autoimmune disorders were excluded. Additionally, participants had no recent use of hormones, antibiotics, or vaginal medications; no cervical treatment, endometrial biopsy, IUD removal, or hysteroscopy within the past week; no douching within 5 days; and no sexual intercourse within 48 h. None of the participants were pregnant, lactating, or menstruating at the time of sampling. | Genera with the highest abundance: Bacteroides, Elizabethkingia, Lactobacillus, Methylotenera, Porphyrobacter, Prevotella, Pseudochrobactrum, Rheinheimera, Streptophyta. Differences between endometrial and vaginal microbiota The uterine cavity microbiota may help to distinguish infertile patients from healthy individuals and could play a role in infertility. | L. iners and L. crispatus showed a significant reduction in the uterine cavity of 10 infertile patients. | |
| [72] | A total of 342 infertile patients undergoing ART were enrolled across 13 centers on three continents, with the following demographics: Caucasian (57.3%), East Asian (14.0%), Hispanic (11.4%), and other ethnicities (17.3%). Personalized assessment of window of implantation (and optimal time frame for embryo transfer) by ERA test. No antibiotics in the last 3 months before sample collection, no uterine pathologies, no women with serious or uncontrolled bacterial, fungal, or viral infections were included. | Identified microbiota composition in EF partially reflected that in endometrial biopsy. But association with clinical outcome was consistent. | Lactobacillus was consistently enriched in patients who achieved live birth. Some commensal bacteria, including Cupriavidus, Finegoldia, Microbacterium, and Tepidimonas were positively correlated with live birth outcomes. | Reduced levels of Lactobacillus spp. accompanied by an increased presence of Anaerococcus, Atopobium, Bifidobacterium, Chryseobacterium, Escherichia, Bacillus, Gardnerella, Haemophilus, Klebsiella, Neisseria, Propionibacterium, Staphylococcus, and Streptococcus were linked to either no pregnancy or clinical miscarriage. |
| [74] | 94 IVF Asian patients 25 patients with chronic endometritis (CE) 69 patients with non-chronic endometritis (NCE) | Ten most abundant phyla: Acidobacteria, Actinobacteria, Bacteroidetes, Chloroflexi, Deinococcus-Thermus, Firmicutes, Fusobacteria, Gemmatimonadetes, Patescibacteria, Proteobacteria. Ten most abundant genera: Chelativorans, Gardnerella, Halomonas, Lactobacillus, Lysobacter, Mitochondria, Pelagibacterium, Pseudomonas, Sneathia, Sphingomonas. | CE group with clinical pregnancy n = 8 (32%) NCE group with clinical pregnancy n = 41 (59.4%) * Relative abundance of Proteobacteria * and Acidobacteria * significantly higher in pregnant NCE group vs. non-pregnant CE group. | CE group with pregnancy failure n = 17 (68%) NCE group with pregnancy failure n = 28 (40.6%) * Relative abundance of Actinobacteria * significantly higher in non-pregnant CE group vs. pregnant and non-pregnant NCE groups. Relative abundance of Fusobacteria * significantly higher in pregnant CE group vs. pregnant and non-pregnant NCE groups. Relative abundance of Gardnerella * significantly increased in CE group vs. NCE and was elevated in non-pregnant groups vs. pregnant group in both CE and NCE groups. |
| [96] | 45 Caucasian females who underwent ART: 27 women RIF and 18 women without RIF (control). | Most frequent genera in control patients: Anaerobacillus (0.22%), Bacillus (0.02%), Bifidobacterium (0.11%), Burkholderia (0.11%), Citrobacter (0.01%), Delftia (0.05%), Dialister (0.06%), Gardnerella (1.07%), Lactobacillus (97.96%), Lysinibacillus (0.03%), Prevotella (0.00%), Ralstonia (0.45%), Streptococcus (0.05%). | Most frequent genera in RIF patients: Anaerobacillus (0.63%), Bacillus (0.03%), Bifidobacterium (0.00%) *, Burkholderia (0.45%), Citrobacter (0.07%), Delftia (0.23%), Dialister (0.15%) *, Gardnerella (2.18%), Lactobacillus (92.27%) *, Lysinibacillus (0.03%), Prevotella (2.19%) *, Ralstonia (1.16%), Streptococcus (0.18%) *. | |
| [102] | 102 Japanese infertile patients (79 IVF and 23 non-IVF) 7 healthy volunteers | Major taxonomies present in samples: Aerococcus, Atopobium, Bifidobacterium, Enterococcus, Escherichia, Gardnerella, Lactobacillus, Prevotella, Sneathia, Staphylococcus, Streptococcus, Ureaplasma. Endometrial microbiome of the healthy women exhibited high stability both between and within cycles. Percentage of patients with LDM (>90% Lactobacillus spp.): IVF 38% (30/79) * Non-IVF 73.9% (17/23) Healthy 85.7% (6/7) Median percentage of the endometrial Lactobacilli in patients: IVF 63.90 ± 41.43% of Lactobacilli * Non-IVF 96.20 ± 34.61% of Lactobacilli Healthy 99.50 ± 15.85% of Lactobacilli | 18 patients pregnant: 3 natural conception, 15 FBT. Median percentage of the endometrial Lactobacilli in pregnant individuals: 96.45% ± 33.61%. LDM endometrium might favor implantation. 7 NLDM cases pregnant (6 IVF, 1 non-IVF): 5 cases are ongoing, 1 early miscarriage, 1 was lost to follow-up. | |
| [104] | 60 Chinese patients with previous failed cycles: control group (n = 30) and treatment group (n = 30). A live Lactobacillus was given intravaginally for 30 consecutive days prior to the initiation of the FET cycle. | A small percentage of Lactobacillus (2.7%). Three most dominant microbiota: Rhodococcus (23.7%), Pseudomonas (4.9%), and Achromobacter (4.1%) No significant modifications in the EM conformation were recorded among the clinical pregnant, miscarriage, and non-pregnant groups. | Clinical pregnancy rate: treated 66.7% (20/30) * Transvaginal Lactobacillus supplementation significantly increased the clinical pregnancy rate. Associated with clinical pregnancy: Achromobacter | Clinical pregnancy rate: Control 36.7% (11/30): The miscarriage frequency presented no variance amongst the two groups. Associated with miscarriage: Corynebacterium, Enterobacter, Nocardioides, Roseifexaceae. Negative correlations with clinical pregnancy: Chryseobacterium, Psychrobacter, Romboutsia, Roseifexaceae. |
| [101] | 48 women undergoing IVF with FET with no antibiotic treatment in the 3 months preceding the fertility treatment. | Clear dominance of Lactobacillus genus in the endometrial microbiome. | 21 women pregnant, 5 (23.81%) women with RIF. Greater abundance of Anaerobacillus spp., Burkholderia spp., Gardnerella spp., Lactobacillus spp., although the difference was not significant. Greater abundance of L. iners, L. jensenii, and Ralstonia spp. in women without RIF. Women with NLDM, characterized by a relative abundance of over 80% Lactobacillus spp. in the endometrium, presented favorable pregnancy outcomes. | 27 women not pregnant, 18 (66.66%) women with RIF. Greater abundance of Delftia spp., Prevotella spp., Ralstonia spp., and Streptococcus spp., although the difference was not significant. Greater abundance of Prevotella spp., L. helveticus and Sneathia amnii in women with RIF. |
| [100] | 93 infertile women IVF. Exclusion criteria included pelvic inflammatory disease, fibroids, endometrial polyps or septate uterus, endometrial hyperplasia or cancer, failure in oocyte recovery, poor quality blastocytes, cervicovaginal infections, sexually transmitted disease, and antimicrobial treatment in the last 4 weeks. | Microbiota phyla detected (species): 87.76% Firmicutes (Bacillus halosaccharovans, Bacillus simplex, Enterococcus faecalis, Escherichia coli, L. crispatus, L. fermentum, L. gasseri, L. iners, L. jensenii, L. jonsonii, L. paracasei, L. rhamnosus, Paenibacillus glucanolyticus, Paenibacillus spp., S. aureus, S. capitis, S.epidermidis, S. hominis, S. pasteuri, S. warneri; Se. agalactiae, Se. anginosus, Se. mitis, Se. oralis, Se. salivarius, Se. urinalis, Se. vestibularis). 27.94% Proteobacteria (Alcaligenes faecalis, Citrobacter koseri, Enterobacter kobei, Haemophilus haemolyticus, Klebsiella pneumoniae, Neisseria subflava). 10.29% Actinobacteria (Bifidobacterium scardovii, Corynebacterium coyleae, Corynebacterium spp., Gardnerella vaginalis, Microbacterium maritypicum) 8.82% Ascomycota (C. albicans, C. glabrata, C. krusei, C. lusitaniae, C. parapsilosis) | 35 patients (37.6%) achieved clinical pregnancy. 27 patients (29%) presented endometrial bacterial colonization and 8 (8.6%) showed no microbial development. No differences in reproductive outcome between these two group of patients. Positive impact of Lactobacillus spp. on ongoing pregnancy rate. | 58 patients (62.4%) non-pregnant. 41 patients (44.1%) presented endometrial bacterial colonization and 17 (18.3%) showed no microbial development. Higher proportion of the following families in non-pregnant patients: Enterobacteriaceae and Staphylococcaceae. The Phylum Actinobacteria was exclusively found in non-pregnant patients. Specific species found in non-pregnant patients: Bacillus halosaccharovorans, Bacillus simplex, Bifidobacteria scardovii, Corynebacterium coyleae, Gardnerella vaginalis, Haemophilus haemolyticus, Microbacterium maritypicum, Paenibacillus glucanolyticus. |
| [105] | 185 infertile Japanese patients. EMMA and ALICE evaluated 40 patients pattern 1 (Lactobacillus > 90%), 8 patients pattern 2 (Lactobacillus < 90% and negative for bacterial pathogens producing CE), 32 patients pattern 3 (Lactobacillus < 90% and positive for bacterial pathogens producing CE), 49 patients pattern 4 (minor dysbiotic microbiome profile), 56 patients pattern 5 (ultralow biomass microbiome). | Pathogens causing CE included: Chlamydia, Enterococcus, Escherichia Klebsiella, Mycoplasma, Staphylococcus, Streptococcus, Ureaplasma. | 111 patients receptive ERA group. Normal microbiome (pattern 1) was significantly associated with receptive endometrium. | 74 patients pre-receptive ERA group. Aging and ultralow biomass EM (pattern 5) were both significantly linked to a pre-receptive endometrium. |
| [106] | 34 Caucasian women personalized hormonal stimulation, IVF. Infertility was attributed to tubal occlusion (7/34), endometriosis (3/34), ovulatory disorder (9/34), or idiopathic infertility (13/34) persisting for at least 1 year. | 319 bacterial species identified: Actinobacteria, Bacteroidetes, Cyanobacteria, FBP, Firmicutes, Proteobacteria, Thermi, Verrucomicrobia. | 4/34 pregnant. Predominant presence of Lachnospiraceae and Enterobacteriaceae with a significant reduction in bacterial richness. | 30/34 non-pregnant. Lactobacilli were identified only in the group with failed in vitro fertilization outcomes. Kocuria dechangensis was the only endometrial species with a significantly increased relative proportion in non-pregnant women. |
| [110] | 70 IVF patients: 43 (61%) Caucasian, 12 (17%) Asian, 1 (1.4%) African American, 4 (5.6%) Hispanic, 11 (15%) unknown. | 50 different genera: Achromobacter, Acinetobacter, Actinomyces, Aerococcus, Alloscardovia, Anaerococcus, Bacillus, Bdelovrio, Bifidobacterium, Bosea, Brevundimonas, Brochothrix, Burkholderia, Caloramator, Clostridium, Comamonas, Corynebacterium, Enterococcus, Escherichia, Facklamia, Finegoldia, Fusobacterium, Gardnerella, Herbaspirilum, Hydrogenophylus, Jonquetella, Kouleothrix, Lactobacillus, Lysinbacilus, Methylobacterium, Moraxella, Moritella, Moryella, Paenibacillus, Peptoniphilus, Petrobacter, Photobacterium, Prevotella, Pseudomonas, Raistonia, Serratia, Sphingomonas, Staphylococcus, Stenotrophomonas, Streptococcus, Thermicanus, Varibacterium, Veillonella, Vogesella 33 patients > 90% Lactobacillus abundance 50 patients > 70% Lactobacillus abundance | Not evaluated | Not evaluated |
| [111] | All Japanese population: 28 infertile patients with RIF history and 18 infertile patients undertaking their first IVF cycle (control group). CE was detected in 6 (21.4%) RIF patients and in 2 (11.1%) control. | 26,725 OTUs Aerococcus, Atopobium, Bacillus, Bifidobacterium, Burkholderia, Corynebacterium, Dialister, Enhydrobacter, Enterococcus, Exiguobacterium, Finegoldia, Fusobacterium, Gardnerella, Lactobacillus, Leucobacter, Megasphaera, Mobiluncus, Mycoplasma, Nesterenkonia, Peptoniphilus, Prevotella, Pseudoalteromonas, Shewanella, Sneathia, Staphylococcus, Streptococcus, Ureaplasma, Variovorax, Vibrio. EF microbiota significantly differed between the RIF and the control group (p = 0.0089). Percentage of patients with LDM (>90% Lactobacillus spp.): RIF 64.3% (18/28) Control 38.9% (7/18) | Burkholderia was absent from all EF microbiota samples in the control group. | Burkholderia was detected in 7 of 28 (25%) * RIF patients. |
| [112] | 145 patients with RIF 21 controls RIF patients without endometrial polyps, submucosal myomas, intrauterine adhesions, thrombophilia, endocrinologic abnormalities, collagen disease, recent antibiotic treatment or parental chromosomal imbalances, or translocations. | 131 bacterial species detected in endometrial samples. Relative quantity of endometrial Lactobacillus did not change significantly between the RIF and control groups (51.2 ± 37.5% and 51.6 ± 38.3%, respectively). | Bacterial abundance: Atopobium (0.1 ± 0.2), Burkholderia (0.1 ± 0.2), Delftia (0 ± 0.1), Dietzia (0 ± 0), Enterococcus (0 ± 0), Gardnerella (0.6 ± 1.6), Hydrogenophaga (0 ± 0), Leucobacter (0.1 ± 0.2), Megasphaera (0 ± 0), Micrococcus (0 ± 0), Prevotella (0 ± 0.1), Ralstonia (0 ± 0.1), Schlegelella (0 ± 0), Sphingobacterium (0 ± 0) | Bacterial abundance: Atopobium (2.1 ± 9.4) *, Burkholderia (0.5 ± 1.3) *, Delftia (0.2 ± 0.3) *, Dietzia (0.1 ± 0.5) *, Enterococcus (0.1 ± 0.3) *, Gardnerella (5.3 ± 16.3) *, Hydrogenophaga (0.1 ± 0.3) *, Leucobacter (0.2 ± 0.6) *, Megasphaera (0.8 ± 3.2) *, Micrococcus (0.1 ± 0.7) *, Prevotella (0.7 ± 2.6) *, Ralstonia (0.3 ± 1.2) *, Schlegelella (0.4 ± 1.1) *, Sphingobacterium (0.3 ± 1.1) *. |
| [113] | 130 infertile patients. Group I: 39 women with the first IVF attempt with ovarian stimulation. Group II: 27 RIF patients with ovarian stimulation and embryo transfer. Group III: 64 RIF patients with frozen-thawed embryo transfer in natural cycle. | Lactobacilli (14 species) were dominant across all groups, with L. crispatus, L. jensenii, L. vaginalis being the most prevalent. | The pregnancy rate per embryo transfer was 51.3% in group I, higher than 29.6% in group II, and 35.9% in group III, although the differences were not statistically significant. Group I showed a significantly higher isolation frequency of obligate anaerobic microorganisms and G. vaginalis than group III. | Enterobacteria and Staphylococci were more frequently observed in patients from group III compared to those in groups I and II. Streptococci were more commonly detected in patients from groups II and III than in those from group I. |
| [114] | 177 Caucasian infertile patients. Participants had not used hormonal contraceptives, antibiotics, or probiotic, or prebiotic, or synbiotic formulations for at least 3 months prior to the examination. No malformations of the uterus and fallopian tubes, no endometriosis, no vaginal infections. | 105 strains of bacteria. 10 bacteria most common in patients: Bifidobacterium longum, Escherichia coli, Gardnerella vaginalis, L. gasseri, L. helveticus, L. iners, L. jensenii, L. paracasei, L. reuteri, Staphylococcus aureus. | 67 women were pregnant based on β-hCG levels 14 days after embryo implantation. In 65 patients (97%), the pregnancy ended in childbirth, while the remaining two suffered a miscarriage. | E. coli and Gardnerella vaginalis reduced the protective effect of Lactobacilli before, during, and after embryo implantation. |
| [115] | 30 infertile patients undergoing IVF. No recent history of inflammatory disease, chronic endometritis, antibiotic treatment, moderate to severe endometriosis, adenomyosis, uterine hyperplasia, or endometrial polyps. | 2168 OTUs were identified. Lactobacillus genus was not significantly different between pregnant and non-pregnant groups. | Pregnant women (n = 16). 39 (14.39%) unique species found. Bosea spp. was detected frequently in more than 30% of the samples. More frequent genera: Ralstonia genus (28.89%), Lactobacillus spp. (14.44%), Pseudomonas spp. (0.77%), Delftia spp. (0.21%). | Non-pregnant women (n = 14). 62 (22.88%) unique species found. Bacteria that occur frequently: Acetomicrobium spp., Bacteroides spp., Cutibacterium granulosum, Isoptericola spp., Marivivens spp., Syntrophomonas spp. More frequent genera: Ralstonia genus (33.88%), Lactobacillus spp. (10.16%), Ureaplasma spp (1.27%), Faecalibacterium spp. (0.89%), Pseudomonas spp. (0.87%), Delftia spp. (0.72%). Significantly enriched Delftia spp., Glutamicibacter spp., Serratia marcescens, Staphyloccocus spp. |
| [116] | 80 asymptomatic Chinese women with RIF: 40 patients non-CE and 40 patients CE. CE patients were treated with doxycycline (100 mg twice daily for 14 days). After treatment, the 40 CE patients were CD138-negative by immunohistochemistry. | Lactobacillus is non-predominant genera of EM Top microbiota phylum: Acinetobacter, Lactobacillus, Pseudomonas, Rhodococcus. Associated with CE: Aminicenantales, Chloroflexaceae, Proteobacteria. Associated with non-CE Acinetobacter, Herbaspirillum, Lactobacillus, Micrococcaceae, Ralstonia, Shewanela. | Clinical pregnancy rate Non-CE group 62.5% (25/40) * Associated with clinical pregnancy: Achromobacter, Acinetobacter, Lactobacillus, Proteobacteria. | Clinical pregnancy rate CE group 37.5% (15/40) There was no variation in the miscarriage rate between the two groups. Correlated with miscarriage: Enterococus, Gardnerella, Phyllobacterium, Pseudomonas. Correlated with non-pregnancy: Clostridium, Prevotella, Romboutsia, Streptococcus. |
| [121] | 117 RIF women and 55 infertile women without RIF LDM (>90% Lactobacillus-dominant microbiota) and NLDM (≤90% Lactobacillus microbiota). Patients with NLDM EF treated with oral lactoferrin supplementation (700 mg/day for a minimum of 28 consecutive days). Improved EF microbiotas are patients who increased 10% or more the proportion of Lactobacillus species in EF samples after lactoferrin treatment. | No identification of single microorganisms or characterization of the local microbiota associated with NLDM. | RIF group with improved EF microbiota: Clinical pregnancy rate: 71.4%(10/14) * Live birth rate: 57.1% (8/14) * | RIF group with unimproved EF microbiota: Clinical pregnancy rate: 22.2% (2/9) Live birth rate: 11.1% (1/9) |
| [124] | 92 Asian IVF patients (90 Japanese, 1 Korean, and 1 Chinese). Nine NLDM patients treated with amoxicillin or levofloxacin, followed by combination of prebiotics (lactoferrin) and/or probiotics. | Major taxonomies present in samples: Aerococcus, Atopobium, Bifidobacterium, Enterococcus, Escherichia, Gardnerella, Lactobacillus, Prevotella, Sneathia, Staphylococcus, Streptococcus, Ureaplasma. 62 patients with LDM (≥80% Lactobacillus spp.) 30 patients with NLDM (< 80% Lactobacillus spp.) | Pregnancy rate per patient: LDM 38 patients (61.3%) * NLDM 12 patients (40%) All nine NLDM patients became LDM, and five patients achieved pregnancies (three ongoing and two miscarriages). LDM endometrium might benefit implantation | Non-pregnant patients: LDM 32 patients (47.1%) NLDM 14 patients (45.2%) In these patients, the median percentage of Lactobacilli was 14.75% (range 0–78.6%), Gardnerella (11.0–98.8%), Atopobium (3.8–97.3%), Streptococcus (65.4–81.5%). |
| [125] | 195 Japanese RIF patients: 131 EMMA evaluated (initially, 67 patients LDM, 64 patients NLDM) and 64 not evaluated (control group). Patients were excluded if they had intrauterine lesions, untreated hydrosalpinx, an allergy to antibiotics or inability to adhere to antibiotic treatment, or received antibiotic treatments within 3 months prior to sample collection. Antibiotics were chosen based on the pathogens identified in the EMMA test and the clinical profile of each patient. Metronidazole (500 mg twice a day for 7 days) when Gardnerella was detected. Amoxicillin and clavulanic acid (500–125 mg every 8 h for 8 days) when Streptococcus > 10% of EM. Vaginal suppositories containing Lactobacillus strains administered after antibiotic treatment for 7–10 days or for 10–17 days from day 5 of their FET cycle. All control group patients were given intravaginal probiotic treatment for 7–10 days, beginning on the 5th day of their FET cycle. Al 64 NLDM patients treated achieved LDM. Median percentage Lactobacillus spp: before treatment 25.8%, after treatment 90.8%. | Lactobacillus spp. detected in all patients. More frequently detected genera: Atopobium, Bifidobacterium, Gardnerella, Streptococcus. | Patients with LDM Clinical pregnancy rate: 64.5% (79/131) * Ongoing pregnant rate: 48.9% (64/131) * Live birth rate: 48.9% (64/131) * Weeks of gestation for single births: 38.8 ± 1.71 * | Patients not evaluated with EMMA (control group): Clinical pregnancy rate: 33.3% (25/64); Ongoing pregnant rate: 32.8% (21/64); Live birth rate: 31.2% (20/64); and Weeks of gestation for single births: 37.6 ± 3.42. |
ALICE, analysis of infectious chronic endometritis; ART, assisted reproductive technology; βhCG: β-human chorionic gonadotropin; C, Candida; CE, chronic endometritis; EM, endometrial microbiota; EMMA, endometrial microbiome metagenomics analysis; ERA, endometrial receptivity analysis; EF, endometrial fluid; ET, embryo transfer; FET, frozen embryo transfer; IVF, in vitro fertilization; IUD, intrauterine device; L, Lactobacillus; LDM, Lactobacillus-dominated microbiota; LH+2, pre-receptive phase, two days after the luteinizing hormone surge; LH+7 receptive phase, seven days after the luteinizing hormone surge; NLDM, non-Lactobacillus-dominated microbiome; OTU, operational taxonomic units; RIF, recurrent implantation failure; RPL, repeated pregnancy loss; S, Staphylococcus; and Se, Streptococcus. * p < 0.05 between groups.
In conclusion, maintaining an adequate EM is essential for improving pregnancy results in RIF patients. Personalized treatment strategies guided by microbial 16S rRNA gene sequencing can help establish an optimal intrauterine environment and enhance IVF success rates in RIF cases. Additionally, the use of microbial 16S rRNA gene sequencing can minimize the need for broad-spectrum antibiotics, thereby reducing the physical, psychological and economic loads on patients. However, further studies are necessary to analyze the mechanisms by which pathogenic bacteria affect embryo implantation.
6. Effect of Bisphenols on Reproduction
Human exposure to BPA and its analogs is a public health concern because bisphenols have the ability to bind to membrane and nuclear receptors such as androgen, estrogen, and thyroid receptors, causing endocrine disruption, tumors, adverse reproductive outcomes, and transgenerational effects [12]. BPA has been associated with alterations in hormonal levels, with elevated levels of testosterone (T), and E2 and P4 detected in the urine of adolescent women with impaired reproductive functions or with polycystic ovary syndrome (PCOS) [126,127]. Studies demonstrating impaired ovarian and uterine function indicated that elevated urinary BPA levels are linked to a higher possibility of developing PCOS and a decreased antral follicle count [127,128]. Additionally, urinary BPA levels were negatively correlated with both the number of oocytes recovered in women undertaking IVF and the serum E2 levels [129]. Also, an association between high urinary BPA concentration and increased serum T, E2, and pregnenolone levels was reported in girls diagnosed with precocious puberty [130]. Moreover, increased urine BPA concentrations were associated with reduced fecundity in Chinese women attempting to conceive [131]. Other studies have demonstrated that elevated serum and urinary BPA levels are linked to a higher risk of miscarriage [132,133]. Furthermore, high BPA levels in maternal blood, urine, or amniotic fluid have been related to reduced weight gain throughout pregnancy and low birth weight [134]. Likewise, BPA was consistently linked to preeclampsia in several studies [135]. Regarding uterine morphology, BPA has been associated with non-ovarian pelvic endometriosis, as an increment in urinary concentrations of the endocrine disruptor was found in these patients [136,137]. Taken together, these results suggest a link between BPA exposure and impaired reproductive function in women.
BPA’s effects on reproduction and, specifically, on uterine physiology have been the focus of numerous studies [137]. Research in rodents has shown that exposure to BPA throughout the critical phase of blastocyst implantation interferes with pregnancy. This may be linked to disturbance in various markers of uterine implantation, particularly those regulated by ovarian steroid hormones, leading to fewer implantation sites and lower pregnancy rates. These effects could result from a discrepancy between the timing of blastocyst formation and the uterine receptivity window or from the direct interference with uterine receptivity due to the estrogenic properties of BPA [137,138,139,140]. The expression of blastocyst implantation markers like HOXA10, Mucin 1, E-cadherin, and TJ proteins (occludin) have been reported to be altered by BPA [140,141]. Moreover, BPA exposure has been demonstrated to impact ovarian function. Prenatal exposure to BPA prevented germ cell nest breakdown in the ovaries of F1 generation in mice, reduced the number of primordial, primary, preantral, and total healthy follicle at post-natal day 21, and reduced E2 levels in female rats exposed for 1 year, indicating that BPA directly targets the ovaries [142,143]. Furthermore, studies have shown that BPA disturbs the hypothalamic–pituitary–gonadal (HPG) axis in mice, rats, and zebrafish [144,145,146].
Recent studies have concentrated on the effects of compounds structurally similar to BPA on endocrine disruption [147]. For instance, exposure to bisphenol analogs negatively impacts ovarian steroidogenesis. In zebrafish, BPS, BPF, BPB, and BPAF have been shown to disrupt the normal function of the HPG axis, leading to an aberrant production of the luteinizing hormone (LH) and the follicle-stimulating hormone (FSH) [148]. In rodents, exposure to BPS, BPF, and BPB adversely affected the secretion of hormones such as E2, P4, and T [149]. Additionally, BPE, BPS, BPF, and BPAF negatively impact the transcription of several genes critical for ovarian steroidogenesis, including StAR, Cyp17a1, 3β-HSD, and Cyp19a1 [150]. Prenatal or prepubertal exposure to BPB, BPF, and BPS may reduce the number of antral follicles by activating apoptosis and autophagy pathways, ultimately lowering the production of E2 and P4, and increasing the number of atretic and cystic follicles [149]. Bisphenol analogs can mimic E2 by interacting with the estrogen receptor (ER) to form complexes that bind to the nuclear DNA response element. This binding activates downstream transcription factors, initiating estrogenic effects. BPA, BPF, and BPS behave as partial agonists for human ERα (hERα) and full agonists for hERβ, with BPA being the most potent, followed by BPF and then BPS for both receptors [151]. Regarding androgenic activity, BPA and BPF function as full androgen receptor antagonists, with BPA showing stronger affinity [151]. Both BPA and BPS act as weak androgen receptor agonists [151]. In rats, exposure to BPS and BPF stimulated uterine growth, demonstrating estrogenic activity [152,153]. Additionally, neonatal exposure to BPS has been associated with delayed puberty onset, disrupted estrous cycles, increased body weight, and significantly reduced absolute and relative uterine weights. A decrease in plasma P4, LH and FSH concentrations was also observed, while T and E2 plasma concentrations were significantly increased. An increase in the number of cystic and atretic follicles in the ovaries was also reported [154]. In mice, a PCOS-like condition after exposure to BPS has been reported [155]. PCOS has been known to cause infertility in humans. BPS and BPE triggered follicular development problems in mice. Along the same line, a significant decline in pregnancy rates was observed, along with a reduced number of live births, an increase in deceased pups, and increased complications during childbirth [150]. Additionally, studies have reported that BPS and BPF can impair the receptivity of human endometrial epithelial cell in vitro by modulating steroid hormone receptor function, ultimately disrupting embryo implantation. These EDs suppressed spheroid (blastocyst surrogate) attachment to human endometrial epithelial cells through the regulation of genes that control endometrial receptivity, such as progesterone receptor (PR), olfactomedin 1, and thrombospondin 1 [156]. There are also several epidemiological studies suggesting that BPA alternatives (BPS or the mixture of BPS, BPF, and BPA) can alter the duration of pregnancy leading to preterm births [157,158]. In addition, in rat models, more than 80% of pregnant dams exposed to BPF during gestation experienced spontaneous abortions [159].
Collectively, these studies indicate that bisphenols detrimentally affect the reproductive tract and fertility of animals and humans. However, there are no reports that investigate the effects of bisphenols on the uterine microbiota, but some inferences can be gleaned by looking at the gut.
7. Bisphenols and the Gut Microbiota
The gut microbiota represents the largest microbial community of the human body. As a vital “microbial organ”, gut microbiota plays a role in host health, in nutrient digestion and absorption, growth, development, and disease prevention [160]. In addition, host phenotypic status and/or resident microbiota may influence the pharmacokinetics of EDs, including uptake, absorption, distribution, and metabolism [161]. In humans, the gut microbiota is an abundant ecosystem of highly diverse microorganisms where Firmicutes and Bacteroidetes are the dominant phyla (representing at least 90%), followed by Proteobacteria and Spirochaetae [162]. In rodents, the five dominant phyla—Verrucomicrobia, Bacteroidetes, Firmicutes, Proteobacteria, and Actinobacteria—collectively represent at least 99% of all detected phyla [163]. The Bacteroidetes phylum primarily generates acetate and propionate, whereas the Firmicutes phylum is the main producer of butyrate.
Studies in rodents have shown evidence that BPA exposure (either by direct ingestion or through the pregnant mother to the offspring) affects the gut microbiota by decreasing the diversity [13,164,165,166,167]. In these animals, the gut microbiota was similar to the one found in animals fed a high-fat diet, presenting a significant increase in microbial dysbiosis indicators, such as Proteobacteria (mainly Epsilonproteobacteria, Parasutterella, and Helicobacteriaceae (Helicobacter ganmani)) (Table 2) [13,164]. Also, in BPA-exposed rodents, a significant decrease in Actinobacteria (Bifidobacterium spp.), Bacteroidetes (Prevotellaceae NK3B31 group, Rikenellaceae RC9 gut group, Prevotella 1, Prevotella 2, Prevotella 9, Parabacteroides, Muribaculum, and Alloprevotella), Firmicutes (Lactobacillus intestinalis, Tenericutes, Allobaculum, Marvinbryantia, Christensenellaceae_R_7_group, Streptococcus, and Clostridia (Clostridium viridae, Eubacterium dolichum, Coprococcus comes, Ruminococcaceae NK4A214 group, Ruminococcaceae UCG 002, Ruminococcaceae UCG 010, Eubacterium coprostanoligenes group, Oscillospira, Clostridium butyricum, and Clostridium Cluster XIVa)), and Verrucomicrobia (Akkermancia), which are indicators of a healthy intestinal barrier function, has been reported, as compared to the control animals [13,166,167,168,169,170,171,172,173] (Table 2).

Table 2.
Changes in the abundance of intestinal bacteria on different model systems treated with bisphenols.
In contrast, experiments with other rodents have shown no changes in gut microbiota diversity and even increases in proportions of Firmicutes (Lactobacillaceae, Lactobacillus spp., L. reuteri, Subdoligranulum, Blautia, Veillonella, Bacillales, Faecalibaculum, and Clostridia (Ruminococcaceae, Oscillospira spp., Oscillibacter, Ruminococcaceae NK4A214 group, Ruminococcaceae UGG 009, Ruminococcaceae UCG-010, Clostridium perfringens, and Clostridium ruminantiums)), Verrucomicrobia (Akkermancia), Bacteroidetes (Rikenellaceae, Prevotella, and Rikenellaceae RC9 gut group), and Bifidobacterium when treated with BPA, as compared to the control animals (Table 2) [163,166,167,169,170,171,172,173,174,175,176,177]. These discrepancies are probably due to differences in the window of exposure, exposure methods, sex of the individual, the examined host species, as well as the dose and extent of bisphenol exposure (Table 2).
Moreover, effects like changes in microbial diversity, the prevalence of Proteobacteria, and the increase in intestinal permeability have also been reported in fish and rabbit models (Table 2) [17,165,178,179,180].
Regarding BPA analogs, few studies have been carried out using mice, zebrafish, and human gut cultures as model systems to examine their effects on gut microbiota. Results showed that in perinatally treated mice, BPS had the most prominent effect on microbiota diversity as compared to TBBPA [18]. Early life exposure to BPS or TBBPA led to a downregulation of most species within the Firmicutes phylum, while less abundant taxa in the Bacteroidetes phylum showed consistent regulation by these endocrine disruptors. Notably, S24-7, the most abundant taxon in the adult fecal microbiome from the Bacteroidetes phylum, was significantly upregulated following early life exposure to BPS (Table 2). BPS and TBBPA perinatal animal exposure presented specific changes in some microbiota, but the most distinct microbial biomarkers were Rikenellaceae for TBBPA and Lactobacillus for BPS (Table 2) [18]. The direct exposure of female mice to BPS also induced gut microbiota dysbiosis, presenting a significant reduction in Acidobacteria and a large increase in Actinobacteria in comparison with the control group (Table 2) [163]. While Bacillales were significantly induced, Sphingomonadales and Caldilineales were markedly reduced in BPS-treated female mice (Table 2) [163]. Male mice exposed to bisphenol P (BPP) also presented decreased microbiota diversity and dysbiosis. Animals exposed to BPP exhibited a higher proportion of Firmicutes, a lower proportion of Bacteroidetes, and an increased Firmicutes/Bacteroidetes ratio compared to the control animals (Table 2) [181]. A similar increase in the Firmicutes/Bacteroidetes ratio was also observed in mice treated with BPS [163] or BPF [171]. An elevated Firmicutes/Bacteroidetes ratio, leading to a reduction in the total short-chain fatty acids (SCFAs), is associated with LPS-induced inflammatory chemocytokines release, metabolic endotoxemia, and an intensified risk of metabolic disorders such as obesity and type 2 diabetes mellitus [182]. In addition, Proteobacteria relative abundance was also significantly increased by BPP. Proteobacteria was linked to fecal LPS levels and included pathogenic bacteria capable of causing several illnesses. At the genus level, BPP treatment decreased the relative abundance of Oscillospira, Prevotella, Bacteroides, and Lactobacillus, while Helicobacter increased significantly in relative abundance [181] (Table 2). Male mice treated with BPF also presented a significant decrease in several bacteria of the Firmicutes phylum, such as Lactobacillaceae, Streptococcus spp., and Ruminococcaceae UCG-014, while others increased (Lachnospiraceae, Ruminococcaceae UCG-010, Oscillibacter, and Roseburia). Bacteroidetes, including Butrycimonas, Prevotellaceae UCG-001E, and Alistipes, increased, while Prevotellaceae UCG-011 and Prevotella 9 decreased as compared to the control group [171] (Table 2).
In zebrafish, BPS was identified as the least potent analog compared to BPA and BPF in a toxicological essay. However, it affected the microbial community at different concentrations by increasing the quantity of potentially pathogenic bacteria, including Flavobacterium, Pseudomonas, and Stenotrophomonas, that could cause oxidative damage and inflammatory effects (Table 2) [17,182,183]. BPAF and bisphenol B (BPB) did not appear to alter the microbial community structure [183] (Table 2). Furthermore, simplified human intestinal microbiota showed no differences after BPS exposure [19].
Altogether, experimental evidence indicates that direct or indirect exposure to bisphenols significantly alters the structure of the gut microbiota. Identifying specific features or species within the gut microbiota may help establish potential biomarkers for assessing the risks associated with bisphenol exposure.
8. Gut Microbiota Regulates Intestinal Permeability
Changes in microbiota diversity have been associated with increased intestinal permeability. The intestinal barrier consists of (1) a TJ complex that connects adjacent intestinal epithelial cells (IECs) at their apical surface, forming a polarized monolayer with distinct apical and basolateral domains; (2) a mucus layer covering the surface of IECs; and (3) the intestinal microbiota [184]. Proper operation of the intestinal barrier function is essential to ensure selective permeability of the intestine. Disruption of the barrier and permeability functions has severe consequences, including bacterial translocation to the intestine, which may lead to immune activation and inflammation, often involved in many intestinal diseases, including celiac disease, colorectal cancer, inflammatory bowel disease, and irritable bowel syndrome [185]. Disruption of intestinal flora homeostasis can alter intestinal permeability. An overgrowth of Proteobacteria may modify the structure and conformation of the intestinal TJs, resulting in increased intestinal permeability and absorption of lipopolysaccharides (LPS), which then enter the bloodstream (Table 2) [164,165,173,186]. Therefore, an excess of Proteobacteria has been associated with the onset of intestinal inflammation and intestinal diseases [187,188].
In contrast, Akkermansia is recognized for its ability to augment the thickness of the intestinal mucus. This mucus consists of mucins (mucin 2 being the most abundant mucin covering the intestinal epithelial cells), digestive enzymes, antimicrobial peptides, and immunoglobulins, and it improves gut barrier function, resulting in a beneficial immune response [166,189]. A decrease in mucin 2 mRNA and protein has been reported in the gut of BPA-treated animals [166,167]. In addition, dietary intake of BPA significantly reduced the number of colonic goblet cells, which produce and secrete the mucus [166]. Therefore, in animals exposed to dietary BPA, the reduced number of goblet cells and the reduced expression of mucin 2 in the colonic epithelium suggest damage to the intestinal chemical barrier.
Ruminococcaceae, Parabacteroides, Alloprevotella, Allobaculum, Ruminiclostridium 9, Clostridium, Odoribacter spp., and Oscillospira spp., which are important producers of SCFAs, are significantly altered by exposure to bisphenols [18,165,167,170,172,173,177,188,190,191,192]. SCFAs are the final products of food fermentation by the intestinal microbiota, with acetic, propionic, and butyric acid accounting for 95% of SCFAs in the human gut [193,194]. Several studies have shown that bisphenol-induced alteration of the intestinal microbiota is accompanied by a decrease in the concentration of SCFAs (acetic acid, propionic acid, butyric acid, isobutyric acid, and caproic acid) in the gut (Table 2) [18,165,167,172,173].
These SCFAs provide energy for the host and play a role in various metabolic processes in the body, including energy metabolism, the inflammatory response, adipose tissue development, and liver metabolism [193,195,196]. SCFAs help in the maintenance of intestinal homeostasis by stimulating mucus production, inducing epithelial cells to synthesize antimicrobial peptides (such as β-defensins and REG3γ), increasing the expression of intestinal TJ proteins, and preserving the integrity of the intestinal epithelial barrier. A healthy gut epithelial barrier prevents bacterial and LPS translocation into the bloodstream. Among the various SCFAs, butyric acid is considered the most prominent, as several studies have shown that it effectively regulates the apoptotic pathway in cells, prevents colon cancer, reduces bacterial translocation and the inflammatory response, and enhances intestinal barrier function by boosting TJ protein expression, thereby improving gut defense barriers [197]. Accordingly, a decrease in the expression of TJ mRNA and proteins (occludin, claudin-1, claudin-4, and ZO-1) has been reported in the gut of animals exposed to bisphenol (Table 2) [165,166,167,173,179,181]. The TJs are crucial components of the intestinal physical barrier, as they regulate the selective permeability of the intestinal epithelium by controlling paracellular pathways [198]. The function of TJs is to permit the passage of ions and small soluble molecules while blocking toxic substances and microorganisms, thereby playing a vital role in maintaining the intestinal barrier [199]. Occludin and claudins are integral TJ proteins that play a role in regulating epithelial permeability and are key components of the filamentous structures of TJs detected by freeze–fracture microscopy [200]. ZO-1 is a peripheral membrane protein of TJs, serving as a scaffold that brings together structurally diverse yet functionally related proteins in the near vicinity of the TJs. Thus, ZO-1 connects occludin and the claudins to the actin cytoskeleton. The levels of occludin and ZO-1 in tissue are inversely associated with its permeability and directly related to the maintenance of intestinal epithelium integrity [201]. Bisphenols markedly decreased the expression of ZO-1, claudin-1, claudin-4, and occludin and significantly disturbed the TJs amongst intestinal epithelial cells, affecting the colonic epithelial physical barrier function. Moreover, it has been shown that exposure of human colon mucosal epithelial cells (NCM460) to BPS and BPF increased the permeability of intestinal mucosa by down-regulating the expression of TJ proteins claudin-1 and ZO-1 [202,203]. In addition, the concentrations of serum LPS, endotoxins, zonulin, DAO, and D-lactate (biomarkers of gut permeability) [204], were also increased along with the breakdown of TJs (Table 2) [164,165,166,172,198], indicating that bisphenol exposure impaired the intestinal barrier function, increased the intestinal permeability, and enhanced serum LPS levels.
There are several important receptors in the cells of the intestinal barrier that monitor the environment of the intestinal lumen. These pattern-recognition receptors (PRRs) are present in various cell types in diverse proportions, depending on the cell’s function, and they can detect a broad range of pathogen-associated molecular patterns (PAMPs), including viral and bacterial components. PRRs encompass several super-families like Toll-like receptors (TLRs), NOD-like receptors (NLRs), RIG-I-like receptors (RLRs), and C-type lectin receptors (CLRs). TLRs are capable of detecting a wide array of molecules, including those from fungi, viruses, bacteria, and protozoa. In mammals, 13 distinct TLRs have been described (human: TLR1-11; mouse: TLR1-9, TLR11-13), with only minor functional modifications noted between humans and mice [205]. TLRs are transmembrane proteins featuring an extracellular domain with leucine-rich repeats and an intracellular domain similar to the IL-1R, known as the Toll/IL-1R (TIR) domain. When activated, the TIR domain binds to a homologous domain in the protein Myeloid Differentiation Primary Response 88 (MyD88), initiating a signaling cascade that activates the nuclear factor-κB (NF-κB). NFκB-dependent gene transcription controls a range of genes, including those involved in the immune response, such as cytokines and chemokines, as well as cell adhesion molecules, growth factors and their receptors, and apoptosis-related genes [206]. TLR expressly differentiate between self- and microbial non-self by recognizing widely conserved molecular patterns. They are crucial in microbial recognition, the regulation of adaptive immune responses, and the activation of antimicrobial effector pathways, which contribute to the effective elimination of pathogens that threaten host health. Particular TLRs are expressed along the apical and basolateral domains of the IEC membranes, which has led researchers to speculate that microbial ligands regulate TJ proteins through their contact with TLRs. Indeed, TLRs play a role in maintaining IEC homeostasis by controlling the host’s responses to intestinal microbes. Some of the receptors that have been involved in this function are TLR2 and TLR4. In mice, TLR expression changes significantly along the length of the intestinal tract. Colonic IECs show a high expression of TLR2 and TLR4, whereas small intestinal IECs have very low quantities of these receptors [205]. This difference may be due to the fact that, under healthy conditions, the small intestine harbors a less concentrated microbiota compared to the colon [207]. Healthy human colon tissues contain low levels of TLR2 and TLR4 in the IECs [205]. Where detected, TLR2 and TLR4 are located on the apical surface of the epithelial cells. This apical localization allows TLRs to be stimulated by substances in the intestinal lumen, including both commensal bacteria and harmful pathogens [207]. TLR2 and TLR4 recognize PAMPs from Gram-positive and Gram-negative bacteria, respectively. For effective LPS-induced signaling, TLR4 must associate with MD-2, a secreted glycoprotein, and CD14 [205]. Another TLR family member, TLR2, is activated by various molecules, including peptidoglycan, zymosan, and bacterial lipopeptides, to trigger innate immune responses. TLR2 forms a functional heterodimeric complex with either TLR1 or TLR6 on the surface of enterocyte and immune cells [208]. The TLR1 and TLR6 subunits are crucial for recognizing microbe-associated molecular patterns (MAMPs). Bacterial cell wall components, such as triacyl and diacyl lipopeptides, are detected by TLR2/TLR1 and TLR2/TLR6 heterodimers, respectively [208]. Stimulation of two different IEC lines (HT-29, Caco-2) with TLR2 ligand Pam3CysSK4 (which is a synthetic bacterial lipopeptide) resulted in significant activation (phosphorylation, enzymatic activity, and translocation) of specific PKC isoforms (PKCα and PKCδ). The activation of TLR2 by ligands significantly increased transepithelial resistance in IECs, an effect that was blocked by pretreatment with PKC-selective antagonists. This response was associated with the tightening and sealing of apical TJ-associated ZO-1, mediated by PKC in response to TLR2 ligands, while no noticeable morphological changes were observed in occludin, claudin-1, or the actin cytoskeleton [209]. In addition, specific Lactobacillus species mitigated barrier disruption by upregulating TJ proteins. L. acidophilus LA1 and L. plantarum MB452 were shown to augment occludin protein expression in vivo and in vitro models, respectively, through stimulation of TLR2 [208,210]. In addition, L. plantarum increased occludin protein expression and induced apical relocalization of ZO-1 and occludin.
Interestingly, TLR activation does not always lead to improved barrier function. LPSs, key components of the Gram-negative bacterial cell wall, play a crucial role in driving intestinal inflammatory responses associated with inflammatory bowel disease. In vitro and in vivo studies have demonstrated that physiologically relevant concentrations of LPS increase intestinal epithelial TJs permeability through the TLR4/MyD88 signal-transduction pathway, leading to up-regulation of myosin light chain kinase (MLCK) expression and activity. MLCK facilitates the opening of TJs by contracting the perijunctional ring of actin–myosin filaments. This process generates contractile tension and causes centripetal retraction of the perijunctional plasma membrane and the TJ complex, ultimately resulting in the functional opening of the TJ barrier [211].
Therefore, the changes in gut microbiota diversity produced by bisphenols have been related to the decrease in the expression of TJ proteins in the gut [166,167], leading to increased intestinal permeability, presence of endotoxins in serum and imbalance in the immune system by gut and liver inflammation [167,168].
These results can guide future research and policies regarding the safety of bisphenols, emphasizing the necessity for stricter regulations and limitations on the use of these compounds in consumer products.
9. Uterine Microbiota May Regulate Implantation Through Toll-like-Receptors (TLRs)-Tight Junctions (TJs)
As previously mentioned, changes in the composition of resident microbiota present in the uterus can affect implantation success. There are no studies on the impact of bisphenols on the EM, but as it has been reviewed, bisphenols influence the intestinal microbiota species. These changes produce alterations in the intestine permeability, which is partly regulated through TLR and TJs.
The genital epithelial cells express a wide range of PRRs that promote the ability to recognize and differentially respond to various pathogens. The PRRs found in the FRT include TLRs and NOD-like receptors, which play crucial roles in defending against pathogenic invasion, supporting tissue adaptation, and ensuring successful reproduction. In human endometrial tissues, the expression of TLR1-10 has been reported, and it has been proposed that these receptors are involved in microbial recognition and immune defense within the reproductive tract [212]. Studies have demonstrated that TLR mRNA and protein levels vary through the menstrual cycle [212,213,214,215,216,217]. Specifically, the expression of TLRs 1–10 in endometrial cells is low during the proliferative phase and increases during the secretory phase. Additionally, research indicates that the activation of TLRs by their specific ligands around the time of embryo implantation negatively impacts implantation outcomes both in vivo [215] and in vitro [216,217]. Most of these studies evaluate the immune response produced by the activation of TLRs. For instance, it has been reported in mice that the activation of TLR2 and 2/6 reduced embryo implantation chances and increased levels of proinflammatory cytokines, including the interleukin (IL)-1β and monocyte chemotactic protein (MCP)-1, in uterine horn flushing on the preimplantation day, along with increased IL-1 receptor antagonist on the implantation day [215]. In vitro, the stimulation of TLR3 in endometrial cells resulted in a decreased proportion of trophoblasts adhering to endometrial cells and a significant reduction in CD98 expression. These alterations were shown to be regulated via MYD88-MAPK pathways [216]. A similar decrease in the proportion of trophoblasts adhered to endometrial cells was reported for TLR5, with an increased expression of IL-8 and MCP-1 [217]. The immune response during implantation and early pregnancy has been widely studied, and providing a detailed review lies outside the scope of this work. Recent reviews address this subject [71,218,219].
However, there is scarce information about the effects of microbiota-activated TLRs on the regulation of TJ proteins in the endometrium during implantation. Some in vivo and in vitro studies with human endometrial epithelial cell lines have reported the effect of diverse microorganisms on the expression of TJ proteins. For example, in ECC-1 cells, a human endometrial epithelial cell line originating from endometrial adenocarcinoma, live Chlamydia trachomatis (C. trachomatis) but not heat-killed forms, has been shown to reduce transepithelial resistance (TER), indicating compromised TJ barrier integrity, and decrease the expression of TJ genes, including occludin, claudin-2, claudin-3, and ZO-1 [220]. This indicates that an active infection with live bacteria is necessary to compromise the integrity of the epithelial cell barrier, as exposure to factors on the C. trachomatis surface alone is insufficient. Interestingly, live C. trachomatis did not affect the TER of polarized rat endometrial epithelial cells [221], suggesting species-specific variations in endometrial responses to infection. C. trachomatis, the most prevalent sexually transmitted bacteria pathogen worldwide [222], has been associated with miscarriage, stillbirth, and preterm premature rupture of membranes [223,224,225]. Similarly, Neisseria gonorrhea (N. gonorrhea), a Gram-negative bacterium and the second most common sexually transmitted bacterial pathogen globally, can ascend through the cervix, producing pelvic inflammatory disease linked to tubal infertility, stillbirth, and preterm birth [226]. Infection of the polarized human endometrial epithelial cell line HEC-1B with live but not gentamicin-killed N. gonorrhea disrupted cell junctional complexes, redistributing ZO-1, occludin, E-cadherin, and β-catenin from the apical surface to the cytoplasm without disturbing total protein levels [227]. Similarly, in polarized Ishikawa cells, N. gonorrhea provoked the rearrangement of E-cadherin and β-catenin from the apical membrane to the cytoplasm but did not alter the expression or localization of the TJ proteins occludin and ZO-1 [228]. N. gonorrhea infection enhanced TLR2 and TLR4 expression and p65 NF-κB levels and reduced nuclear factor erythroid 2-related factor 2 (Nrf2), driving the inflammatory response [229]. Staphylococcus aureus (S. aureus), a Gram-positive bacterium commonly part of the normal human microbiota, can act as an opportunistic pathogen associated with infertility in both sexes [230,231]. Elevated endometrial levels of S. aureus have also been linked to poor IVF outcomes [232]. In mice uterus, S. aureus reduced the expression of the TJ proteins ZO-1, occludin, and claudin-3 [233] while inducing uterine structural disruption, luminal dilation, and fluid accumulation [234]. In both rats and mice, S. aureus increased Tlr2 expression and activated IκB and p65 NF-κB phosphorylation [235,236]. Additionally, S. aureus elevated uterine Tlr4 expression [237] and downstream TLR signaling mediators, including MyD88, IRAK1/4, and TRAF6 [236]. In vitro studies further showed that S. aureus increased TLR2 levels and enhanced IκB and p65 NF-κB phosphorylation in mouse epithelial cells [238].
10. Tight Junctions (TJs) Participation in Embryo Implantation
In the uterus, TJs join the uterine epithelial cells (UECs) lining the lumen and regulate the passage of ions and molecules through the paracellular path. Protein composition of TJs present in the luminal UECs changes during the different days of the menstrual/estrous cycle and of pregnancy, which suggests that the expression of TJ proteins participates in providing an adequate environment for successful fertilization and implantation [239,240,241,242,243]. Indeed, TJs are implicated in the transformation of the plasma membrane that renders UECs receptive to the attachment of trophoblastic cells and control the composition and volume of the luminal fluid, which serves multiple purposes, such as aiding in the maturation of the ovum and spermatozoa and providing nutrients and signaling molecules for the implanting blastocyst [244]. The movement of luminal fluid is regulated in the UECs by TJs. While these early embryo–maternal interactions cannot be studied directly in humans, extensive research has been carried out in various animal models. On gestational day (GD) 1, the edema observed in the rat endometrium relies on the movement of fluid from the stroma into the uterine lumen via the paracellular pathway, facilitated by leaky TJs consisting of parallel strands located at the apical region of the plasma membrane [245,246]. During this GD, ZO-1 and claudin-1 and -3 were detected in the lateral membrane, whereas claudin-7, associated with cell-to-extracellular matrix attachment [247], was also observed [243,244,246,247,248,249,250,251]. Claudin-1 and -3 establish heterotypical interactions with each other [252], and claudin-1 produces linear, non-branching arrays of strands [250] similar to those observed during this GD.
During blastocyst implantation, marked by the irreversible adhesion of the blastocyst to the luminal epithelium on GD 6 in the rat, the TJ network expands threefold in depth along the lateral plasma membrane. It also develops additional branches and interconnections with adjacent strands, as revealed by freeze–fracture microscopy [245,246,251]. On this GD, ZO-1 was predominantly localized in the upper third of the lateral plasma membrane, claudin-1 in the lower half, claudin-3 throughout the lateral membrane, and claudin-7 in the lower half [243]. For the first time during this GD, claudin-4 was detected at the basolateral membrane of the UECs [243,249]. Additionally, increased claudin-4 mRNA expression has been described during the implantation window in humans [253,254], indicating a potential functional role in this process. Claudin-4 overexpression in cultured cells is known to increase the complexity of the TJ strand network [255], suggesting that it enhances the number of branches and interconnections observed by freeze–fracture microscopy on GD 6. Meanwhile, claudin-7 expression decreased, and its localization shifted from the basolateral region seen on GD 1 to the lower half of the basolateral membrane [246,247]. Claudin-4 reduces paracellular Na+ permeability [255], whereas claudin-7 acts as a paracellular Na+ channel and a Cl− barrier [256]. At the implantation stage, epithelial Na+ channels (ENaC) are induced in rat UECs, reducing luminal Na+ levels [257,258]. The concurrent presence of ENaC and claudin-4, along with the absence of claudin-7 from the TJ region, ensures efficient transcellular Na+ movement from the apical to the basal epithelial surface without paracellular backflow. This Na+ transport, combined with water reabsorption from the luminal fluid via aquaporin 5 [259], enables the blastocyst to closely align with the luminal epithelium, facilitating surface contact [258,260]. The reduced expression of claudin-7 on GD 6 may also weaken cell-to-extracellular matrix adhesions, contributing to the loosely adhered epithelial monolayer characteristic of implantation day [261]. Claudin-7 forms a stable protein complex with claudin-1 and integrin α2, suggesting that it stimulates cell–matrix adhesion by stabilizing integrin α2 proteins, which mediate connection with extracellular matrix components [247].
In rodents, during the blastocyst’s penetration of the UECs into the stromal layer (GD 7), the presence of ZO-1, occludin and claudin-1, -3, and -4 has been detected in stromal cells [243,262,263]. These proteins may create a barrier within stromal cells to shield the blastocysts from maternal immunoglobulins.
11. Impact of Bisphenols on Uterine Epithelial Cells (UECs) Tight Junctions (TJs)
There is scarce information on the effects of bisphenols on UEC TJ proteins during pregnancy. Rats treated with BPA during the perinatal period were mated at 3 months of age. In these animals, BPA treatment did not induce changes in the ovulation rate, but it reduced the implantation rate [243].
The most important alteration observed in the expression of UEC TJ proteins was the presence of claudin-4 from GD 1, whereas, in the control group, this claudin was only expressed at a high level on GD 6. The premature expression of claudin-4 could reduce paracellular Na+ permeability in UEC, potentially altering the composition of the luminal fluid, which could hinder the implantation process [242].
BPA exposure also induced an alteration in the localization of ZO-1 on GD3. In the control group, ZO-1 protein was highly expressed along the basolateral membrane in UECs, whereas in BPA-treated groups, its expression was reduced and confined to the uppermost portion of the lateral membrane [242]. Given that ZO-1 serves as a scaffold for claudin polymerization [264], BPA treatment may hinder the increased depth of TJs typically observed on GD 3 [244,245].
Claudin-7 also showed important differences between the BPA-treated group and control rats. In the control group, this protein was located in the basolateral membrane of UECs on GD 1 and shifted to the lower half of the lateral plasma membrane by GD 6 [242]. In the BPA-treated group, claudin-7 expression decreased in UECs on GD 1, with the protein localized to the lower half of the lateral plasma membrane. This change overlapped with a rise in claudin-4 protein expression, supporting the opinion that BPA treatment promotes the formation of a cation-impermeable TJ on GD 1. Furthermore, as claudin-7 enhances cell–extracellular matrix adhesion [246], its reduced expression during the non-receptive phase may hamper the conservation of an intact uterine epithelial barrier.
On GD 7, perinatal BPA treatment produced the loss of claudin-3 and -4 in stromal cells. The absence of these proteins may disrupt the formation of a barrier between stromal cells, which normally prevents maternal immunoglobulins from reaching the embryo [242].
In conclusion, BPA exposure creates an intrauterine environment that hinders successful embryo implantation during early pregnancy.
12. Conclusions and Future Directions
The dogma that the uterus was a sterile environment has come to an end. The female upper reproductive tract has its own microbiota, but the “baseline” or “core” microbiome of the healthy endometrium has not been completely established due to limitations in the studies, such as the challenging collection of uncontaminated uterine samples, free from vaginal or cervical bacteria and bacterial DNA found in the air and laboratory reagents and equipment. Other limiting factors are fluctuations due to hormonal changes within the menstrual cycle, the lack of ethnic diversity, differences in socioeconomic status, lifestyle, and environmental factors. However, it has become clear that the predominant phyla identified in the endometrium of healthy women are Firmicutes (mainly Lactobacillus spp.), Bacteroidetes (mainly Flavobacterium spp., Bacteroides spp., Prevotella spp.), Proteobacteria (mainly Pseudomonas spp. and Acinetobacter spp.), and Actinobacteria (mainly Gardnerella spp., Bifidobacterium spp.) [24,26,48,59,60,61,62]. It has been proposed that several gynecological diseases, such as chronic endometritis, endometriosis, adenomyosis, hysteromyoma, endometrial hyperplasia, cancer, and infertility, may be related to dysbiosis of the microbial community present in the endometrium. Studies in women undergoing IVF have suggested that a NLDM (<90% Lactobacilli with >10% of other bacteria) was associated with infertility, with significantly decreased implantations, pregnancies, and live births. However, some patients achieved pregnancies despite having 0% Lactobacillus. Furthermore, other studies proposed that the primary factor affecting fertility may be the occurrence of pathogens in the uterine cavity rather than the necessity of a specific commensal taxon [24,38,72,96,102,104,106,108,109,110,111,112,113,114,115]. Therefore, it has been postulated that the primary role of Lactobacillus spp. in reproduction is to prevent the establishment of pathogenic bacteria in the uterine cavity [72]. These data suggest that the EM may be regarded as an emerging factor contributing to implantation failure and/or pregnancy loss. As a result, microbial interventions with antibiotic therapy, followed by the combination of prebiotics and/or probiotics, have been used to change the NLDM into LDM prior to subsequent conception attempts in order to improve infertility treatment outcomes and IVF success [74].
Bisphenols are one of the environmental factors that may contribute to EM dysbiosis, similar to what has been reported in the gut microbiota. In the human gut, Firmicutes and Bacteroidetes are the dominant phyla (representing at least 90%), followed by Proteobacteria and Spirochaetae [162]. The gut microbiota produces SCFAs that contribute to the maintenance of intestinal homeostasis by stimulating mucus production, inducing epithelial cells to synthesize antimicrobial peptides, increasing the expression of intestinal TJ proteins, and preserving the integrity of the intestinal epithelial barrier. In several animal models, bisphenol exposure reduced microbiota diversity and significantly altered the structure of the gut microbiota. This microbiota dysbiosis is detected by TLRs, which induce changes in the expression/localization of TJ proteins, the same that regulate the selective permeability of the intestinal epithelium by controlling paracellular pathways [198]. TJ proteins are composed, among others, of occludin and claudins, which are integral TJ proteins, and ZO proteins that connect occludin and claudins to the actin cytoskeleton. Bisphenols markedly decreased the expression of ZO-1, claudin-1, claudin-4, and occludin and significantly disturbed the TJs amongst intestinal epithelial cells, affecting the colonic epithelial physical barrier function, which results in the absorption of LPS, endotoxins, zonulin, DAO, and D-lactate, which then enter into the bloodstream. In the endometrium, TJ proteins are important during embryo implantation, and their expression and localization are affected by exposure to bisphenols. TJs are implicated in the transformation of the plasma membrane that renders UECs receptive to the attachment of trophoblastic cells and control the composition and volume of the luminal fluid, which serves multiple purposes, such as participation in the maturation of the ovum and spermatozoa and providing nutrients and signaling molecules for the implanting blastocyst [244]. BPA exposure, by altering the expression/localization of TJ proteins during early pregnancy, creates an intrauterine environment that hinders successful embryo implantation. The knowledge that the microbiota may regulate TJs in the endometrium via TLRs, as in the gut, opens new treatment options for RIF patients. However, it is imperative to investigate the effect of bisphenols on the uterine microbiota and to establish their participation in gynecological diseases, including infertility, for future risk assessments regarding the effects of bisphenols on reproduction.
Author Contributions
Conceptualization, S.I.P.-C. and C.A.M.-R.; investigation, D.C.-R., J.G.O.-B., O.V.R.-G., A.A.M.-P. and C.A.M.-R.; data curation, D.C.-R., J.G.O.-B., O.V.R.-G., S.I.P.-C. and C.A.M.-R.; writing—original draft preparation, D.C.-R., J.G.O.-B., O.V.R.-G. and C.A.M.-R.; writing—review and editing, A.A.M.-P., M.E.I.-R., M.G.-R. and C.A.M.-R.; supervision, M.E.I.-R., M.G.-R. and C.A.M.-R.; funding acquisition, M.E.I.-R., M.G.-R. and C.A.M.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by CONAHCyT (Project number 319631), Mexico. Sheila I. Peña-Corona acknowledges the Postdoctoral Scholarship from DGAPA-UNAM (October 2021–August 2022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was created.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
ART, assisted reproductive technology; BPA, bisphenol A; BPAF, bisphenol AF; BPF, bisphenol F; BPS, bisphenol S; EDs, endocrine disruptors; EM, endometrial microbiota; FET, frozen embryo transfer; FRT, female reproductive tract; GD, gestational day; IVF, in vitro fertilization; L., Lactobacillus; LDM, Lactobacillus-dominated endometrium microbiota; LPS, lipopolysaccharides; NLDM, non-Lactobacillus-dominated endometrium microbiota; PCOS, polycystic ovary syndrome; RIF, recurrent implantation failure; RPL, repeated pregnancy loss; SCFAs, short-chain fatty acids; TBBPA, tetrabromobisphenol A; TJs, tight junctions; TLRs, Toll-like receptors; UECs, uterine epithelial cells.
References
- Ma, Y.; Liu, H.; Wu, J.; Yuan, L.; Wang, Y.; Du, X.; Wang, R.; Marwa, P.W.; Petlulu, P.; Chen, X.; et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; García-Arévalo, M.; Quesada, I.; Nadal, Á. Bisphenol-A treatment during pregnancy in mice: A new window of susceptibility for the development of diabetes in mothers later in life. Endocrinology 2015, 156, 1659–1670. [Google Scholar] [CrossRef] [PubMed]
- Commission Directive 2011/8/EU of 28 January 2011 Amending Directive 2002/72/EC as Regards the Restriction of Use of Bisphenol A in Plastic Infant Feeding Bottles Text with EEA relevance. OJ L Jan 28. 2011. Available online: http://data.europa.eu/eli/dir/2011/8/oj/eng (accessed on 8 May 2023).
- Björnsdotter, M.K.; de Boer, J.; Ballesteros-Gómez, A. Bisphenol A and replacements in thermal paper: A review. Chemosphere 2017, 182, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Liu, F.; Alomirah, H.; Loi, V.D.; Mohd, M.A.; Moon, H.-B.; Nakata, H.; Kannan, K. Bisphenol S in urine from the United States and seven Asian countries: Occurrence and human exposures. Environ. Sci. Technol. 2012, 46, 6860–6866. [Google Scholar] [CrossRef]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wong, L.Y.; Kramer, J.; Zhou, X.; Jia, T.; Calafat, A.M. Urinary concentrations of bisphenol A and three other bisphenols in convenience samples of U.S. adults during 2000–2014. Environ. Sci. Technol. 2015, 49, 11834–11839. [Google Scholar] [CrossRef]
- Asimakopoulos, A.G.; Xue, J.; De Carvalho, B.P.; Iyer, A.; Abualnaja, K.O.; Yaghmoor, S.S.; Kumosani, T.A.; Kannan, K. Urinary biomarkers of exposure to 57 xenobiotics and its association with oxidative stress in a population in Jeddah, Saudi Arabia. Environ. Res. 2016, 150, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guan, J.; Yin, J.; Shao, B.; Li, H. Urinary levels of bisphenol analogues in residents living near a manufacturing plant in south China. Chemosphere 2014, 112, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Dallaire, R.; Ayotte, P.; Pereg, D.; Déry, S.; Dumas, P.; Langlois, E.; Dewailly, E. Determinants of plasma concentrations of perfluorooctanesulfonate and brominated organic compounds in Nunavik Inuit adults (Canada). Environ. Sci. Technol. 2009, 43, 5130–5136. [Google Scholar] [CrossRef] [PubMed]
- Carignan, C.C.; Abdallah, M.A.-E.; Wu, N.; Heiger-Bernays, W.; McClean, M.D.; Harrad, S.; Webster, T.F. Predictors of tetrabromobisphenol-A (TBBP-A) and hexabromocyclododecanes (HBCD) in milk from Boston mothers. Environ. Sci. Technol. 2012, 46, 12146–12153. [Google Scholar] [CrossRef] [PubMed]
- Gonsioroski, A.; Mourikes, V.E.; Flaws, J.A. Endocrine disruptors in water and their effects on the reproductive system. Int. J. Mol. Sci. 2020, 21, 1929. [Google Scholar] [CrossRef]
- Lai, K.-P.; Chung, Y.-T.; Li, R.; Wan, H.-T.; Wong, C.K.-C. Bisphenol A alters gut microbiome: Comparative metagenomics analysis. Environ. Pollut. 2016, 218, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Balbi, T.; Vezzulli, L.; Lasa, A.; Pallavicini, A.; Canesi, L. Insight into the microbial communities associated with first larval stages of Mytilus galloprovincialis: Possible interference by estrogenic compounds. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2020, 237, 108833. [Google Scholar] [CrossRef]
- Diaz Heijtz, R.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Catron, T.R.; Keely, S.P.; E Brinkman, N.; Zurlinden, T.J.; E Wood, C.; Wright, J.R.; Phelps, D.; Wheaton, E.; Kvasnicka, A.; Gaballah, S.; et al. Host developmental toxicity of BPA and BPA alternatives is inversely related to microbiota disruption in zebrafish. Toxicol. Sci. 2019, 167, 468–483. [Google Scholar] [CrossRef]
- Gomez, M.V.; Dutta, M.; Suvorov, A.; Shi, X.; Gu, H.; Mani, S.; Cui, J.Y. Early life exposure to environmental contaminants (BDE-47, TBBPA, and BPS) produced persistent alterations in fecal microbiome in adult male mice. Toxicol. Sci. 2021, 179, 14–30. [Google Scholar] [CrossRef]
- Schäpe, S.S.; Krause, J.L.; Masanetz, R.K.; Riesbeck, S.; Starke, R.; Rolle-Kampczyk, U.; Eberlein, C.; Heipieper, H.-J.; Herberth, G.; von Bergen, M.; et al. Environmentally relevant concentration of bisphenol S shows slight effects on SIHUMIx. Microorganisms 2020, 8, 1436. [Google Scholar] [CrossRef] [PubMed]
- Wyżga, B.; Połeć, K.; Olechowska, K.; Hąc-Wydro, K. The impact of toxic bisphenols on model human erythrocyte membranes. Colloids Surf. B Biointerfaces 2020, 186, 110670. [Google Scholar] [CrossRef]
- Tong, T.; Li, R.; Chai, M.; Wang, Q.; Yang, Y.; Xie, S. Metagenomic analysis of microbial communities continuously exposed to Bisphenol A in mangrove rhizosphere and non-rhizosphere soils. Sci. Total Environ. 2021, 792, 148486. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Godoy-Vitorino, F.; Knight, R.; Blaser, M.J. Role of the microbiome in human development. Gut 2019, 68, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Grau, I.; Simon, C.; Moreno, I. Uterine microbiome-low biomass and high expectations. Biol. Reprod. 2019, 101, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Younes, J.A.; Lievens, E.; Hummelen, R.; van der Westen, R.; Reid, G.; Petrova, M.I. Women and their microbes: The unexpected friendship. Trends Microbiol. 2018, 26, 16–32. [Google Scholar] [CrossRef]
- Munoz, A.; Hayward, M.R.; Bloom, S.M.; Rocafort, M.; Ngcapu, S.; Mafunda, N.A.; Xu, J.; Xulu, N.; Dong, M.; Dong, K.L.; et al. Modeling the temporal dynamics of cervicovaginal microbiota identifies targets that may promote reproductive health. Microbiome 2021, 9, 163. [Google Scholar]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4680–4687. [Google Scholar] [CrossRef]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef]
- Borovkova, N.; Korrovits, P.; Ausmees, K.; Türk, S.; Jõers, K.; Punab, M.; Mändar, R. Influence of sexual intercourse on genital tract microbiota in infertile couples. Anaerobe 2011, 17, 414–418. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O.C. The vaginal microenvironment: The physiologic role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bian, G.; Zheng, M.; Lu, G.; Chan, W.-Y.; Li, W.; Yang, K.; Chen, Z.-J.; Du, Y. Fertility factors affect the vaginal microbiome in women of reproductive age. Am. J. Reprod. Immunol. 2020, 83, e13220. [Google Scholar] [CrossRef]
- Singer, M.; Koedooder, R.; Bos, M.P.; Poort, L.; Schoenmakers, S.; Savelkoul, P.H.M.; Laven, J.S.E.; de Jonge, J.D.; Morré, S.A.; Budding, A.E. The profiling of microbiota in vaginal swab samples using 16S rRNA gene sequencing and IS-pro analysis. BMC Microbiol. 2021, 21, 100. [Google Scholar] [CrossRef] [PubMed]
- Brandão, P.; Gonçalves-Henriques, M. The impact of female genital microbiota on fertility and assisted reproductive treatments. J. Fam. Reprod. Health 2020, 14, 131–149. [Google Scholar] [CrossRef]
- Koedooder, R.; Mackens, S.; Budding, A.; Fares, D.; Blockeel, C.; Laven, J.; Schoenmakers, S. Identification and evaluation of the microbiome in the female and male reproductive tracts. Hum. Reprod. Update 2019, 25, 298–325. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.; LaMere, B.; Weihe, C.; Wandro, S.; Lindsay, K.L.; Wadhwa, P.D.; Mills, D.A.; Pride, D.T.; Fiehn, O.; Northen, T.; et al. Cervicovaginal microbiome composition is associated with metabolic profiles in healthy pregnancy. mBio 2020, 11, e01851-20. [Google Scholar] [CrossRef]
- Kacerovsky, M.; Pliskova, L.; Bolehovska, R.; Gerychova, R.; Janku, P.; Matlak, P.; Simetka, O.; Faist, T.; Mls, J.; Vescicik, P.; et al. Lactobacilli-dominated cervical microbiota in women with preterm prelabor rupture of membranes. Pediatr. Res. 2020, 87, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Kindinger, L.M.; Bennett, P.R.; Lee, Y.S.; Marchesi, J.R.; Smith, A.; Cacciatore, S.; Holmes, E.; Nicholson, J.K.; Teoh, T.G.; MacIntyre, D.A. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome 2017, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Koedooder, R.; Singer, M.; Schoenmakers, S.; Savelkoul, P.H.M.; Morré, S.A.; de Jonge, J.D.; Poort, L.; Cuypers, W.J.S.S.; Beckers, N.G.M.; Broekmans, F.J.M.; et al. The vaginal microbiome as a predictor for outcome of in vitro fertilization with or without intracytoplasmic sperm injection: A prospective study. Hum. Reprod. 2019, 34, 1042–1054. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Zhang, X.; Liang, Y.; Lin, S.; Qian, W.; Fan, S. Alterations in Vaginal Microbiota and Associated Metabolome in Women with Recurrent Implantation Failure. mBio 2020, 11, e03242-19. [Google Scholar] [CrossRef] [PubMed]
- Gerson, K.D.; McCarthy, C.; Elovitz, M.A.; Ravel, J.; Sammel, M.D.; Burris, H.H. Cervicovaginal microbial communities deficient in Lactobacillus species are associated with second trimester short cervix. Am. J. Obstet. Gynecol. 2020, 222, 491.e1–491.e8. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.F.; Zhang, Y.X.; Chen, S.; Zhu, F.F.; Huang, Y.X.; Liu, X.J.; Luo, S.P.; Deng, G.P.; Gao, J. Non-Lactobacillus-Dominated vaginal microbiota is associated with a tubal pregnancy in symptomatic Chinese women in the early stage of pregnancy: A nested case-control study. Front. Cell. Infect. Microbiol. 2021, 11, 659505. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Li, P.; Wu, S.; Tan, J. Association of the cervical microbiota with pregnancy outcome in a subfertile population undergoing in vitro fertilization: A case-control study. Front. Cell. Infect. Microbiol. 2021, 11, 654202. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Diaz, D.J.; Jesaveluk, B.; Hayward, J.A.; Tyssen, D.; Alisoltani, A.; Potgieter, M.; Bell, L.; Ross, E.; Iranzadeh, A.; Allali, I.; et al. Lactic acid from vaginal microbiota enhances cervicovaginal epithelial barrier integrity by promoting tight junction protein expression. Microbiome 2022, 10, 141. [Google Scholar] [CrossRef]
- Farage, M.A.; Stadler, A. Risk factors for recurrent vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 2005, 192, 981–982; author reply 982–983. [Google Scholar] [CrossRef] [PubMed]
- Rose, W.A.; McGowin, C.L.; Spagnuolo, R.A.; Eaves-Pyles, T.D.; Popov, V.L.; Pyles, R.B. Commensal bacteria modulate innate immune responses of vaginal epithelial cell multilayer cultures. PLoS ONE 2012, 7, e32728. [Google Scholar] [CrossRef] [PubMed]
- Eschenbach, D.A.; Thwin, S.S.; Patton, D.L.; Hooton, T.M.; Stapleton, A.E.; Agnew, K.; Winter, C.; Meier, A.; Stamm, W.E. Influence of the normal menstrual cycle on vaginal tissue, discharge, and microflora. Clin. Infect. Dis. 2000, 30, 901–907. [Google Scholar] [CrossRef]
- Takada, K.; Komine-Aizawa, S.; Kuramochi, T.; Ito, S.; Trinh, Q.D.; Pham, N.T.K.; Sasano, M.; Hayakawa, S. Lactobacillus crispatus accelerates re-epithelialization in vaginal epithelial cell line MS74. Am. J. Reprod. Immunol. 2018, 80, e13027. [Google Scholar] [CrossRef]
- Baker, J.M.; Chase, D.M.; Herbst-Kralovetz, M.M. Uterine microbiota: Residents, tourists, or invaders? Front. Immunol. 2018, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.C.; McAndrew, T.; Chen, Z.; Harari, A.; Barris, D.M.; Viswanathan, S.; Rodriguez, A.C.; Castle, P.; Herrero, R.; Schiffman, M.; et al. The cervical microbiome over 7 years and a comparison of methodologies for its characterization. PLoS ONE 2012, 7, e40425. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Simon, C. Relevance of assessing the uterine microbiota in infertility. Fertil. Steril. 2018, 110, 337–343. [Google Scholar] [CrossRef]
- Payne, M.S.; Bayatibojakhi, S. Exploring preterm birth as a polymicrobial disease: An overview of the uterine microbiome. Front. Immunol. 2014, 5, 595. [Google Scholar] [CrossRef] [PubMed]
- Salim, R.; Zafran, N.; Nachum, Z.; Edelstein, S.; Shalev, E. Employing nifedipine as a tocolytic agent prior to external cephalic version. Acta Obstet. Gynecol. Scand. 2008, 87, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Egbase, P.E.; Udo, E.E.; Al-Sharhan, M.; Grudzinskas, J.G. Prophylactic antibiotics and endocervical microbial inoculation of the endometrium at embryo transfer. Lancet 1999, 354, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Fanchin, R.; Harmas, A.; Benaoudia, F.; Lundkvist, U.; Olivennes, F.; Frydman, R. Microbial flora of the cervix assessed at the time of embryo transfer adversely affects in vitro fertilization outcome. Fertil. Steril. 1998, 70, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Egbase, P.E.; Al-Sharhan, M.; Al-Othman, S.; Al-Mutawa, M.; Udo, E.E.; Grudzinskas, J.G. Incidence of microbial growth from the tip of the embryo transfer catheter after embryo transfer in relation to clinical pregnancy rate following in-vitro fertilization and embryo transfer. Hum. Reprod. 1996, 11, 1687–1689. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Peterson, J.; Garges, S.; Giovanni, M.; McInnes, P.; Wang, L.; Schloss, J.A.; Bonazzi, V.; McEwen, J.E.; Wetterstrand, K.A.; Deal, C.; et al. The NIH Human Microbiome Project. Genome Res. 2009, 19, 2317–2323. [Google Scholar]
- Molina, N.M.; Sola-Leyva, A.; Saez-Lara, M.J.; Plaza-Diaz, J.; Tubić-Pavlović, A.; Romero, B.; Clavero, A.; Mozas-Moreno, J.; Fontes, J.; Altmäe, S. New opportunities for endometrial health by modifying uterine microbial composition: Present or future? Biomolecules 2020, 10, 593. [Google Scholar] [CrossRef]
- Mitchell, C.M.; Haick, A.; Nkpara, E.; Gacria, R.; Rendi, M.; Agnew, K.; Fredricks, D.N.; Eschenbach, D. Colonization of the upper genital tract by vaginal bacterial species in non-pregnant women. Am. J. Obstet. Gynecol. 2015, 212, 611.e1–611.e9. [Google Scholar] [CrossRef]
- Franasiak, J.M.; Werner, M.D.; Juneau, C.R.; Tao, X.; Landis, J.; Zhan, Y.; Treff, N.R.; Scott, R.T. Endometrial microbiome at the time of embryo transfer: Next-generation sequencing of the 16S ribosomal subunit. J. Assist. Reprod. Genet. 2016, 33, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Verstraelen, H.; Vilchez-Vargas, R.; Desimpel, F.; Jauregui, R.; Vankeirsbilck, N.; Weyers, S.; Verhelst, R.; De Sutter, P.; Pieper, D.H.; Van De Wiele, T. Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1-2 region of the 16S rRNA gene. PeerJ 2016, 4, e1602. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Ding, H.; Yu, H.; Ji, Y.; Xifang, X.; Pang, W.; Wang, X.; Zhang, Q.; Li, W. Comprehensive characterization of microbial community in the female genital tract of reproductive-aged women in China. Front. Cell. Infect. Microbiol. 2021, 11, 649067. [Google Scholar] [CrossRef] [PubMed]
- Vomstein, K.; Reider, S.; Böttcher, B.; Watschinger, C.; Kyvelidou, C.; Tilg, H.; Moschen, A.R.; Toth, B. Uterine microbiota plasticity during the menstrual cycle: Differences between healthy controls and patients with recurrent miscarriage or implantation failure. J. Reprod. Immunol. 2022, 151, 103634. [Google Scholar] [CrossRef] [PubMed]
- Pelzer, E.S.; Willner, D.; Buttini, M.; Huygens, F. A role for the endometrial microbiome in dysfunctional menstrual bleeding. Antonie Leeuwenhoek 2018, 111, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Odawara, K.; Akino, R.; Sekizawa, A.; Sakamoto, M.; Yuriko, S.; Tanaka, K.; Mikashima, M.; Suzuki, M.; Odawara, Y. Examination of clinical factors affecting intrauterine microbiota. Reprod. Fertil. 2021, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kadogami, D.; Nakaoka, Y.; Morimoto, Y. Use of a vaginal probiotic suppository and antibiotics to influence the composition of the endometrial microbiota. Reprod. Biol. 2020, 20, 307–314. [Google Scholar] [CrossRef]
- Carosso, A.; Revelli, A.; Gennarelli, G.; Canosa, S.; Cosma, S.; Borella, F.; Tancredi, A.; Paschero, C.; Boatti, L.; Zanotto, E.; et al. Controlled ovarian stimulation and progesterone supplementation affect vaginal and endometrial microbiota in IVF cycles: A pilot study. J. Assist. Reprod. Genet. 2020, 37, 2315–2326. [Google Scholar] [CrossRef]
- Fang, R.L.; Chen, L.X.; Shu, W.S.; Yao, S.Z.; Wang, S.W.; Chen, Y.Q. Barcoded sequencing reveals diverse intrauterine microbiomes in patients suffering with endometrial polyps. Am. J. Transl. Res. 2016, 8, 1581. [Google Scholar] [PubMed]
- Monin, L.; Whettlock, E.M.; Male, V. Immune responses in the human female reproductive tract. Immunology 2020, 160, 106–115. [Google Scholar] [CrossRef]
- Tomaiuolo, R.; Veneruso, I.; Cariati, F.; D’Argenio, V. Microbiota and Human Reproduction: The Case of Female Infertility. High-Throughput 2020, 9, 12. [Google Scholar] [CrossRef]
- Zhu, N.; Yang, X.; Liu, Q.; Chen, Y.; Wang, X.; Li, H.; Gao, H. “Iron triangle” of regulating the uterine microecology: Endometrial microbiota, immunity and endometrium. Front. Immunol. 2022, 13, 928475. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Garcia-Grau, I.; Perez-Villaroya, D.; Gonzalez-Monfort, M.; Bahçeci, M.; Barrionuevo, M.J.; Taguchi, S.; Puente, E.; Dimattina, M.; Lim, M.W.; et al. Endometrial microbiota composition is associated with reproductive outcome in infertile patients. Microbiome 2022, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, P.; Guo, Y.; Fang, C.; Li, T. Interaction between chronic endometritis caused endometrial microbiota disorder and endometrial immune environment change in recurrent implantation failure. Front. Immunol. 2021, 12, 748447. [Google Scholar] [CrossRef]
- Chen, W.; Wei, K.; He, X.; Wei, J.; Yang, L.; Li, L.; Chen, T.; Tan, B. Identification of uterine microbiota in infertile women receiving in vitro fertilization with and without chronic endometritis. Front. Cell Dev. Biol. 2021, 9, 693267. [Google Scholar] [CrossRef] [PubMed]
- Kimura, F.; Takebayashi, A.; Ishida, M.; Nakamura, A.; Kitazawa, J.; Morimune, A.; Hirata, K.; Takahashi, A.; Tsuji, S.; Takashima, A.; et al. Review: Chronic endometritis and its effect on reproduction. J. Obstet. Gynaecol. Res. 2019, 45, 951–960. [Google Scholar] [CrossRef]
- Kitaya, K.; Takeuchi, T.; Mizuta, S.; Matsubayashi, H.; Ishikawa, T. Endometritis: New time, new concepts. Fertil. Steril. 2018, 110, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ko, E.Y.-L.; Wong, K.K.-W.; Chen, X.; Cheung, W.-C.; Law, T.S.-M.; Chung, J.P.-W.; Tsui, S.K.-W.; Li, T.-C.; Chim, S.S.-C. Endometrial microbiota in infertile women with and without chronic endometritis as diagnosed using a quantitative and reference range-based method. Fertil. Steril. 2019, 112, 707–717.e1. [Google Scholar] [CrossRef]
- Moreno, I.; Cicinelli, E.; Garcia-Grau, I.; Gonzalez-Monfort, M.; Bau, D.; Vilella, F.; De Ziegler, D.; Resta, L.; Valbuena, D.; Simon, C. The diagnosis of chronic endometritis in infertile asymptomatic women: A comparative study of histology, microbial cultures, hysteroscopy, and molecular microbiology. Am. J. Obstet. Gynecol. 2018, 218, 602.e1–602.e16. [Google Scholar] [CrossRef]
- Khan, K.N.; Fujishita, A.; Kitajima, M.; Hiraki, K.; Nakashima, M.; Masuzaki, H. Intra-uterine microbial colonization and occurrence of endometritis in women with endometriosis. Hum. Reprod. 2014, 29, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Fujishita, A.; Masumoto, H.; Muto, H.; Kitajima, M.; Masuzaki, H.; Kitawaki, J. Molecular detection of intrauterine microbial colonization in women with endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 199, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, C.; Silveira, P.; Rodrigues Sereia, A.F.; Christoff, A.P.; Mendes, H.; Valter de Oliveira, L.F.; Podgaec, S. Microbiome profile of deep endometriosis patients: Comparison of vaginal fluid, endometrium and lesion. Diagnostics 2020, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhang, X.; Tang, H.; Zeng, L.; Wu, R. Microbiota composition and distribution along the female reproductive tract of women with endometriosis. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 15. [Google Scholar] [CrossRef]
- Khan, K.N.; Fujishita, A.; Muto, H.; Masumoto, H.; Ogawa, K.; Koshiba, A.; Mori, T.; Itoh, K.; Teramukai, S.; Matsuda, K.; et al. Levofloxacin or gonadotropin releasing hormone agonist treatment decreases intrauterine microbial colonization in human endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 264, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Duan, H.; Wang, S.; Guo, Z.; Wang, S.; Chang, Y.; Chen, C.; Shen, M.; Shou, H.; Zhou, C. Endometrial microbiota in women with and without adenomyosis: A pilot study. Front. Microbiol. 2023, 14, 1075900. [Google Scholar] [CrossRef]
- Kubyshkin, A.V.; Aliev, L.L.; Fomochkina, I.I.; Kovalenko, Y.P.; Litvinova, S.V.; Filonenko, T.G.; Lomakin, N.V.; Kubyshkin, V.A.; Karapetian, O.V. Endometrial hyperplasia-related inflammation: Its role in the development and progression of endometrial hyperplasia. Inflamm. Res. 2016, 65, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Walther-António, M.R.S.; Chen, J.; Multinu, F.; Hokenstad, A.; Distad, T.J.; Cheek, E.H.; Keeney, G.L.; Creedon, D.J.; Nelson, H.; Mariani, A.; et al. Potential contribution of the uterine microbiome in the development of endometrial cancer. Genome Med. 2016, 8, 122. [Google Scholar] [CrossRef]
- Walsh, D.M.; Hokenstad, A.N.; Chen, J.; Sung, J.; Jenkins, G.D.; Chia, N.; Nelson, H.; Mariani, A.; Walther-Antonio, M.R.S. Postmenopause as a key factor in the composition of the Endometrial Cancer Microbiome (ECbiome). Sci. Rep. 2019, 9, 19213. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; He, F.; Lin, Z.; Liu, S.; Tang, L.; Huang, Y.; Hu, Z. Dysbiosis of the endometrial microbiota and its association with inflammatory cytokines in endometrial cancer. Int. J. Cancer 2021, 148, 1708–1716. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Guo, Y.; Jia, L.; Wan, J.; He, T.; Fang, C.; Li, T. Interaction between functionally activate endometrial microbiota and host gene regulation in endometrial cancer. Front. Cell Dev. Biol. 2021, 9, 727286. [Google Scholar] [CrossRef]
- Gressel, G.M.; Usyk, M.; Frimer, M.; Kuo, D.Y.S.; Burk, R.D. Characterization of the endometrial, cervicovaginal and anorectal microbiota in post-menopausal women with endometrioid and serous endometrial cancers. PLoS ONE 2021, 16, e0259188. [Google Scholar] [CrossRef]
- Li, C.; Gu, Y.; He, Q.; Huang, J.; Song, Y.; Wan, X.; Li, Y. Integrated analysis of microbiome and transcriptome data reveals the interplay between commensal bacteria and fibrin degradation in endometrial cancer. Front. Cell. Infect. Microbiol. 2021, 11, 748558. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Chao, A.S.; Lin, C.Y.; Weng, C.H.; Wu, R.C.; Yeh, Y.M.; Huang, S.S.; Lee, Y.S.; Lai, C.H.; Huang, H.J.; et al. Analysis of endometrial lavage microbiota reveals an increased relative abundance of the plastic-degrading bacteria Bacillus pseudofirmus and Stenotrophomonas rhizophila in women with endometrial cancer/endometrial hyperplasia. Front. Cell. Infect. Microbiol. 2022, 12, 1031967. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Su, H.; Shi, L.; Chen, B.; Zhang, S. Endometrial microbiota from endometrial cancer and paired pericancer tissues in postmenopausal women: Differences and clinical relevance. Menopause 2022, 29, 1168–1175. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, A.K. Trends of male factor infertility, an important cause of infertility: A review of literature. J. Hum. Reprod. Sci. 2015, 8, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, T.J.; Vitrikas, K.R. Evaluation and treatment of infertility. Am. Fam. Physician 2015, 91, 308–314. [Google Scholar]
- Lozano, F.M.; Lledó, B.; Morales, R.; Cascales, A.; Hortal, M.; Bernabeu, A.; Bernabeu, R. Characterization of the endometrial microbiome in patients with recurrent implantation failure. Microorganisms 2023, 11, 741. [Google Scholar] [CrossRef] [PubMed]
- Idelevich, A.; Vilella, F. Mother and embryo cross-communication. Genes 2020, 11, 376. [Google Scholar] [CrossRef]
- Wang, W.; Feng, D.; Ling, B. Biologia Futura: Endometrial microbiome affects endometrial receptivity from the perspective of the endometrial immune microenvironment. Biol. Futur. 2022, 73, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Elnashar, A.M. Impact of endometrial microbiome on fertility. Middle East Fertil. Soc. J. 2021, 26, 4. [Google Scholar] [CrossRef]
- Cariati, F.; Carotenuto, C.; Bagnulo, F.; Pacella, D.; Marrone, V.; Paolillo, R.; Catania, M.R.; Di Girolamo, R.; Conforti, A.; Strina, I.; et al. Endometrial microbiota profile in in-vitro fertilization (IVF) patients by culturomics-based analysis. Front. Endocrinol. 2023, 14, 1204729. [Google Scholar] [CrossRef]
- Diaz-Martínez, M.D.C.; Bernabeu, A.; Lledó, B.; Carratalá-Munuera, C.; Quesada, J.A.; Lozano, F.M.; Ruiz, V.; Morales, R.; Llácer, J.; Ten, J.; et al. Impact of the vaginal and endometrial microbiome pattern on assisted reproduction outcomes. J. Clin. Med. 2021, 10, 4063. [Google Scholar] [CrossRef] [PubMed]
- Kyono, K.; Hashimoto, T.; Nagai, Y.; Sakuraba, Y. Analysis of endometrial microbiota by 16S ribosomal RNA gene sequencing among infertile patients: A single-center pilot study. Reprod. Med. Biol. 2018, 17, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Garcia-Grau, I.; Bau, D.; Perez-Villaroya, D.; Gonzalez-Monfort, M.; Vilella, F.; Romero, R.; Simón, C. The first glimpse of the endometrial microbiota in early pregnancy. Am. J. Obstet. Gynecol. 2020, 222, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Chen, H.; Zou, H.; Zhang, H.; Liu, S.; Zheng, J.; Zhang, S.; Hu, L. Impact of vaginal microecological differences on pregnancy outcomes and endometrial microbiota in frozen embryo transfer cycles. J. Assist. Reprod. Genet. 2024, 41, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Oguchi, T. Age- and endometrial microbiota-related delay in development of endometrial receptivity. Reprod. Med. Biol. 2023, 22, e12523. [Google Scholar] [CrossRef] [PubMed]
- Riganelli, L.; Iebba, V.; Piccioni, M.; Illuminati, I.; Bonfiglio, G.; Neroni, B.; Calvo, L.; Gagliardi, A.; Levrero, M.; Merlino, L.; et al. Structural variations of vaginal and endometrial microbiota: Hints on female infertility. Front. Cell. Infect. Microbiol. 2020, 10, 350. [Google Scholar] [CrossRef]
- Hashimoto, T.; Kyono, K. Does dysbiotic endometrium affect blastocyst implantation in IVF patients? J. Assist. Reprod. Genet. 2019, 36, 2471–2479. [Google Scholar] [CrossRef]
- Fotouh, I.A.; Al-Inany, M.G. The levels of bacterial contamination of the embryo transfer catheter relate negatively to the outcome of embryo transfer. Middle East Fertil. Soc. J. 2008, 13, 39–43. [Google Scholar]
- Kuon, R.; Togawa, R.; Vomstein, K.; Weber, M.; Goeggl, T.; Strowitzki, T.; Markert, U.; Zimmermann, S.; Daniel, V.; Dalpke, A.; et al. Higher prevalence of colonization with Gardnerella vaginalis and gram-negative anaerobes in patients with recurrent miscarriage and elevated peripheral natural killer cells. J. Reprod. Immunol. 2017, 120, 15–19. [Google Scholar] [CrossRef]
- Tao, X.; Franasiak, J.M.; Zhan, Y.; Scott, R.T.; Rajchel, J.; Bedard, J.; Newby, R.; Scott, R.T.; Treff, N.R.; Chu, T. Characterizing the endometrial microbiome by analyzing the ultra-low bacteria from embryo transfer catheter tips in IVF cycles: Next generation sequencing (NGS) analysis of the 16S ribosomal gene. Hum. Microbiome J. 2017, 3, 15–21. [Google Scholar] [CrossRef]
- Kitaya, K.; Nagai, Y.; Arai, W.; Sakuraba, Y.; Ishikawa, T. Characterization of microbiota in endometrial fluid and vaginal secretions in infertile women with repeated implantation failure. Mediat. Inflamm. 2019, 2019, 4893437. [Google Scholar] [CrossRef] [PubMed]
- Ichiyama, T.; Kuroda, K.; Nagai, Y.; Urushiyama, D.; Ohno, M.; Yamaguchi, T.; Nagayoshi, M.; Sakuraba, Y.; Yamasaki, F.; Hata, K.; et al. Analysis of vaginal and endometrial microbiota communities in infertile women with a history of repeated implantation failure. Reprod. Med. Biol. 2021, 20, 334–344. [Google Scholar] [CrossRef]
- Keburiya, L.K.; Smolnikova, V.Y.; Priputnevich, T.V.; Muravieva, V.V.; Gordeev, A.B.; Trofimov, D.Y.; Shubina, E.S.; Kochetkova, T.O.; Rogacheva, M.S.; Kalinina, E.A.; et al. Does the uterine microbiota affect the reproductive outcomes in women with recurrent implantation failures? BMC Women’s Health 2022, 22, 168. [Google Scholar] [CrossRef] [PubMed]
- Bednarska-Czerwińska, A.; Morawiec, E.; Zmarzły, N.; Szapski, M.; Jendrysek, J.; Pecyna, A.; Zapletał-Pudełko, K.; Małysiak, W.; Sirek, T.; Ossowski, P.; et al. Dynamics of microbiome changes in the endometrium and uterine cervix during embryo implantation: A comparative analysis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2023, 29, e941289. [Google Scholar] [CrossRef] [PubMed]
- Parvanov, D.; Ganeva, R.; Ruseva, M.; Handzhiyska, M.; Tihomirova, T.; Chapanova, S.; Staneva, R.; Rukova, B.; Pancheva, M.; Serafimova, M.; et al. Association between endometrial microbiome and implantation success in women with frozen embryo transfer: Results of a prospective cohort study. Biotechnol. Biotechnol. Equip. 2023, 37, 2250007. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, H.; Zhang, C.; Zhang, S. Chronic endometritis and the endometrial microbiota: Implications for reproductive success in patients with recurrent implantation failure. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 49. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.; Eichelberger, K. Maternal microbiome and pregnancy outcomes. Fertil. Steril. 2015, 104, 1358–1363. [Google Scholar] [CrossRef]
- Liu, Y.; Wong, K.K.W.; Ko, E.Y.L.; Chen, X.; Huang, J.; Tsui, S.K.W.; Li, T.C.; Chim, S.S.C. Systematic comparison of bacterial colonization of endometrial tissue and fluid samples in recurrent miscarriage patients: Implications for future endometrial microbiome studies. Clin. Chem. 2018, 64, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Makrigiannakis, A.; Makrygiannakis, F.; Vrekoussis, T. Approaches to improve endometrial receptivity in case of repeated implantation failures. Front. Cell Dev. Biol. 2021, 9, 613277. [Google Scholar] [CrossRef] [PubMed]
- Sola-Leyva, A.; Andrés-León, E.; Molina, N.M.; Terron-Camero, L.C.; Plaza-Díaz, J.; Sáez-Lara, M.J.; Gonzalvo, M.C.; Sánchez, R.; Ruíz, S.; Martínez, L.; et al. Mapping the entire functionally active endometrial microbiota. Hum. Reprod. 2021, 36, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Kitaya, K.; Ishikawa, T. Genital tract dysbiosis in infertile women with a history of repeated implantation failure and pilot study for reproductive outcomes following oral enteric coating lactoferrin supplementation. Arch. Gynecol. Obstet. 2022, 306, 1761–1769. [Google Scholar] [CrossRef]
- Otsuki, K.; Tokunaka, M.; Oba, T.; Nakamura, M.; Shirato, N.; Okai, T. Administration of oral and vaginal prebiotic lactoferrin for a woman with a refractory vaginitis recurring preterm delivery: Appearance of lactobacillus in vaginal flora followed by term delivery. J. Obstet. Gynaecol. Res. 2014, 40, 583–585. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Tanaka, H.; Enomoto, S.; Takeuchi, H.; Nishioka, M.; Tanaka, K.; Takayama, E.; Maezawa, T.; Kondo, E.; Ikeda, T. The efficacy of vaginal treatment for non-Lactobacillus dominant endometrial microbiota-A case-control study. J. Obstet. Gynaecol. Res. 2024, 50, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Kyono, K.; Hashimoto, T.; Kikuchi, S.; Nagai, Y.; Sakuraba, Y. A pilot study and case reports on endometrial microbiota and pregnancy outcome: An analysis using 16S rRNA gene sequencing among IVF patients, and trial therapeutic intervention for dysbiotic endometrium. Reprod. Med. Biol. 2019, 18, 72–82. [Google Scholar] [CrossRef]
- Iwami, N.; Kawamata, M.; Ozawa, N.; Yamamoto, T.; Watanabe, E.; Mizuuchi, M.; Moriwaka, O.; Kamiya, H. Therapeutic intervention based on gene sequencing analysis of microbial 16S ribosomal RNA of the intrauterine microbiome improves pregnancy outcomes in IVF patients: A prospective cohort study. J. Assist. Reprod. Genet. 2023, 40, 125–135. [Google Scholar] [CrossRef]
- Scinicariello, F.; Buser, M.C. Serum testosterone concentrations and urinary bisphenol A, benzophenone-3, triclosan, and paraben levels in male and female children and adolescents: NHANES 2011–2012. Environ. Health Perspect. 2016, 124, 1898–1904. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Z.; Xia, M.; Zhuang, S.; Gong, X.; Pan, J.; Li, C.; Fan, R.; Pang, Q.; Lu, S. Neurotoxicity of low bisphenol A (BPA) exposure for young male mice: Implications for children exposed to environmental levels of BPA. Environ. Pollut. 2017, 229, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Akın, L.; Kendirci, M.; Narin, F.; Kurtoglu, S.; Saraymen, R.; Kondolot, M.; Koçak, S.; Elmali, F. The endocrine disruptor bisphenol A may play a role in the aetiopathogenesis of polycystic ovary syndrome in adolescent girls. Acta Paediatr. 2015, 104, e171–e177. [Google Scholar] [CrossRef] [PubMed]
- Mok-Lin, E.; Ehrlich, S.; Williams, P.L.; Petrozza, J.; Wright, D.L.; Calafat, A.M.; Ye, X.; Hauser, R. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int. J. Androl. 2010, 33, 385–393. [Google Scholar] [CrossRef]
- Lee, S.H.; Kang, S.M.; Choi, M.H.; Lee, J.; Park, M.J.; Kim, S.H.; Lee, W.-Y.; Hong, J.; Chung, B.C. Changes in steroid metabolism among girls with precocious puberty may not be associated with urinary levels of bisphenol A. Reprod. Toxicol. 2014, 44, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhou, W.; Zhu, W.; Chen, L.; Wang, W.; Tian, Y.; Shen, L.; Zhang, J.; Shanghai Birth Cohort Study. Associations of female exposure to bisphenol A with fecundability: Evidence from a preconception cohort study. Environ. Int. 2018, 117, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Lathi, R.B.; Liebert, C.A.; Brookfield, K.F.; Taylor, J.A.; vom Saal, F.S.; Fujimoto, V.Y.; Baker, V.L. Conjugated bisphenol A in maternal serum in relation to miscarriage risk. Fertil. Steril. 2014, 102, 123–128. [Google Scholar] [CrossRef]
- Shen, Y.; Zheng, Y.; Jiang, J.; Liu, Y.; Luo, X.; Shen, Z.; Chen, X.; Wang, Y.; Dai, Y.; Zhao, J.; et al. Higher urinary bisphenol A concentration is associated with unexplained recurrent miscarriage risk: Evidence from a case-control study in eastern China. PLoS ONE 2015, 10, e0127886. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.; Xia, W.; Wan, Y.; Zhang, B.; Zhou, A.; Zhang, Y.; Huang, K.; Zhu, Y.; Wu, C.; Peng, Y.; et al. Maternal urinary bisphenol A levels and infant low birth weight: A nested case-control study of the Health Baby Cohort in China. Environ. Int. 2015, 85, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Cantonwine, D.E.; Meeker, J.D.; Ferguson, K.K.; Mukherjee, B.; Hauser, R.; McElrath, T.F. Urinary concentrations of bisphenol A and phthalate metabolites measured during pregnancy and risk of preeclampsia. Environ. Health Perspect. 2016, 124, 1651–1655. [Google Scholar] [CrossRef]
- Upson, K.; Sathyanarayana, S.; De Roos, A.J.; Koch, H.M.; Scholes, D.; Holt, V.L. A population-based case-control study of urinary bisphenol A concentrations and risk of endometriosis. Hum. Reprod. 2014, 29, 2457–2464. [Google Scholar] [CrossRef] [PubMed]
- Varayoud, J.; Ramos, J.G.; Bosquiazzo, V.L.; Lower, M.; Muñoz-de-Toro, M.; Luque, E.H. Neonatal exposure to bisphenol A alters rat uterine implantation-associated gene expression and reduces the number of implantation sites. Endocrinology 2011, 152, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.G.; Hancock, T.; deCatanzaro, D. Influence of oral and subcutaneous bisphenol-A on intrauterine implantation of fertilized ova in inseminated female mice. Reprod. Toxicol. 2007, 23, 138–144. [Google Scholar] [CrossRef]
- Machtinger, R.; Orvieto, R. Bisphenol A, oocyte maturation, implantation, and IVF outcome: Review of animal and human data. Reprod. Biomed. Online 2014, 29, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Peña, A.A.; Peña-Castillo, A.; Parra-Forero, L.Y.; Hernández-Ochoa, I.; Hernández-Barrientos, L.R.; Morimoto, S.; Mendoza-Rodríguez, C.A. Parental perinatal exposure to bisphenol A reduces the threshold to disrupt blastocyst implantation via decreasing talin, occudin and E-cadherin levels. Reprod. Toxicol. 2019, 86, 86–97. [Google Scholar] [CrossRef]
- Caserta, D.; Costanzi, F.; De Marco, M.P.; Di Benedetto, L.; Matteucci, E.; Assorgi, C.; Pacilli, M.C.; Besharat, A.R.; Bellati, F.; Ruscito, I. Effects of endocrine-disrupting chemicals on endometrial receptivity and embryo implantation: A systematic review of 34 mouse model studies. Int. J. Environ. Res. Public. Health 2021, 18, 6840. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Ziv-Gal, A.; Cudiamat, J.; Wang, W.; Zhou, C.; Flaws, J.A. The effects of in utero bisphenol A exposure on the ovaries in multiple generations of mice. Reprod. Toxicol. 2016, 60, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Brehm, E.; Gao, L.; Rattan, S.; Ziv-Gal, A.; Flaws, J.A. Bisphenol A exposure, ovarian follicle numbers, and female sex steroid hormone levels: Results from a CLARITY-BPA study. Endocrinology 2017, 158, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Lee, C.K.F.; Yeung, W.S.B.; Giesy, J.P.; Wong, M.H.; Zhang, X.; Hecker, M.; Wong, C.K.C. Effect of perinatal and postnatal bisphenol A exposure to the regulatory circuits at the hypothalamus-pituitary-gonadal axis of CD-1 mice. Reprod. Toxicol. 2011, 31, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.; Abril, N.; Morales-Prieto, N.; Monterde, J.; Ayala, N.; Lora, A.; Moyano, R. Hypothalamic-pituitary-ovarian axis perturbation in the basis of bisphenol A (BPA) reproductive toxicity in female zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2018, 156, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Han, A.; Xu, N.; Su, P. Effects of maternal exposure to bisphenol A during pregnancy on puberty in advance and hypothalamo-pituitary gonadal axis hormones level in female offspring. J. Hyg. Res. 2018, 47, 425–431. [Google Scholar]
- Wu, X.; Tian, Y.; Zhu, H.; Xu, P.; Zhang, J.; Hu, Y.; Ji, X.; Yan, R.; Yue, H.; Sang, N. Invisible hand behind female reproductive disorders: Bisphenols, recent evidence and future perspectives. Toxics 2023, 11, 1000. [Google Scholar] [CrossRef]
- Qiu, W.; Liu, S.; Chen, H.; Luo, S.; Xiong, Y.; Wang, X.; Xu, B.; Zheng, C.; Wang, K.-J. The comparative toxicities of BPA, BPB, BPS, BPF, and BPAF on the reproductive neuroendocrine system of zebrafish embryos and its mechanisms. J. Hazard. Mater. 2021, 406, 124303. [Google Scholar] [CrossRef]
- Ijaz, S.; Ullah, A.; Shaheen, G.; Jahan, S. Exposure of BPA and its alternatives like BPB, BPF, and BPS impair subsequent reproductive potentials in adult female Sprague Dawley rats. Toxicol. Mech. Methods 2020, 30, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Sekulovski, N.; MacLean, J.A.; Whorton, A.; Hayashi, K. Prenatal exposure to bisphenol A analogues on female reproductive functions in mice. Toxicol. Sci. 2019, 168, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Molina-Molina, J.-M.; Amaya, E.; Grimaldi, M.; Sáenz, J.-M.; Real, M.; Fernández, M.F.; Balaguer, P.; Olea, N. In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicol. Appl. Pharmacol. 2013, 272, 127–136. [Google Scholar] [CrossRef]
- Stroheker, T.; Chagnon, M.-C.; Pinnert, M.-F.; Berges, R.; Canivenc-Lavier, M.-C. Estrogenic effects of food wrap packaging xenoestrogens and flavonoids in female Wistar rats: A comparative study. Reprod. Toxicol. 2003, 17, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Noda, S.; Imatanaka, N.; Yakabe, Y. Comparative study of the uterotrophic potency of 14 chemicals in a uterotrophic assay and their receptor-binding affinity. Toxicol. Lett. 2004, 146, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, N.; Ullah, H.; Ullah, W.; Jahan, S. Comparative effects of Bisphenol S and Bisphenol A on the development of female reproductive system in rats; a neonatal exposure study. Chemosphere 2018, 197, 336–343. [Google Scholar] [CrossRef]
- Demacopulo, B.; Kreimann, E.L. Bisphenol S increases EZRIN expression and the detrimental effects induced by dehydroepiandrosterone in rat endometrium. Mol. Cell. Endocrinol. 2019, 483, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Fernando, S.R.; Jiang, L.; Wang, Z.; Kodithuwakku, S.P.; Wong, C.K.C.; Ng, E.H.Y.; Yeung, W.S.B.; Lee, K.-F. Bisphenol A analogues suppress spheroid attachment on human endometrial epithelial cells through modulation of steroid hormone receptors signaling pathway. Cells 2021, 10, 2882. [Google Scholar] [CrossRef]
- Wan, Y.; Huo, W.; Xu, S.; Zheng, T.; Zhang, B.; Li, Y.; Zhou, A.; Zhang, Y.; Hu, J.; Zhu, Y.; et al. Relationship between maternal exposure to bisphenol S and pregnancy duration. Environ. Pollut. 2018, 238, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yang, C.; Liu, T.; Tan, H.J.J.; Sheng, Y.; Wei, L.; Tang, P.; Huang, H.; Zeng, X.; Liu, S.; et al. Prenatal exposure to bisphenols and risk of preterm birth: Findings from Guangxi Zhuang birth cohort in China. Ecotoxicol. Environ. Saf. 2021, 228, 112960. [Google Scholar] [CrossRef] [PubMed]
- Kaimal, A.; Al Mansi, M.H.; Dagher, J.B.; Pope, C.; Varghese, M.G.; Rudi, T.B.; Almond, A.E.; Cagle, L.A.; Beyene, H.K.; Bradford, W.T.; et al. Prenatal exposure to bisphenols affects pregnancy outcomes and offspring development in rats. Chemosphere 2021, 276, 130118. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhou, D.D.; Gan, R.Y.; Huang, S.Y.; Zhao, C.N.; Shang, A.; Xu, X.-Y.; Li, H.-B. Effects and mechanisms of probiotics, prebiotics, synbiotics, and postbiotics on metabolic diseases targeting gut microbiota: A narrative review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef] [PubMed]
- Weitekamp, C.A.; Phelps, D.; Swank, A.; McCord, J.; Sobus, J.R.; Catron, T.; Keely, S.; Brinkman, N.; Zurlinden, T.; Wheaton, E.; et al. Triclosan-selected host-associated microbiota perform xenobiotic biotransformations in larval zebrafish. Toxicol. Sci. 2019, 172, 109–122. [Google Scholar] [CrossRef]
- Feng, W.; Ao, H.; Peng, C.; Yan, D. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol. Res. 2019, 142, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Zhu, L.; Wang, Y.; Peng, C.; Lin, Y.; Ji, S.; Wei, J. Long-term Bisphenol S exposure induced gut microbiota dysbiosis, obesity, hepatic lipid accumulation, intestinal lesions and dyslipidemia in mice. Toxicology 2024, 504, 153798. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Zhang, H.; Jiang, X.; Zou, J.; Li, Q.; Mai, H.; Su, D.; Ling, W.; Feng, X. Bisphenol A exposure induces gut microbiota dysbiosis and consequent activation of gut-liver axis leading to hepatic steatosis in CD-1 mice. Environ. Pollut. 2020, 265, 114880. [Google Scholar] [CrossRef]
- Reddivari, L.; Veeramachaneni, D.N.R.; Walters, W.A.; Lozupone, C.; Palmer, J.; Hewage, M.K.K.; Bhatnagar, R.; Amir, A.; Kennett, M.J.; Knight, R.; et al. Perinatal bisphenol A exposure induces chronic inflammation in rabbit offspring via modulation of gut bacteria and their metabolites. mSystems 2017, 2, e00093-17. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, S.; Zhang, L.; Qu, W.; Chen, Z. Bisphenol A increases intestinal permeability through disrupting intestinal barrier function in mice. Environ. Pollut. 2019, 254, 112960. [Google Scholar] [CrossRef]
- Ni, Y.; Hu, L.; Yang, S.; Ni, L.; Ma, L.; Zhao, Y.; Zheng, A.; Jin, Y.; Fu, Z. Bisphenol A impairs cognitive function and 5-HT metabolism in adult male mice by modulating the microbiota-gut-brain axis. Chemosphere 2021, 282, 130952. [Google Scholar] [CrossRef] [PubMed]
- Malaisé, Y.; Ménard, S.; Cartier, C.; Lencina, C.; Sommer, C.; Gaultier, E.; Houdeau, E.; Guzylack-Piriou, L. Consequences of bisphenol a perinatal exposure on immune responses and gut barrier function in mice. Arch. Toxicol. 2018, 92, 347–358. [Google Scholar] [CrossRef]
- Diamante, G.; Cely, I.; Zamora, Z.; Ding, J.; Blencowe, M.; Lang, J.; Bline, A.; Singh, M.; Lusis, A.J.; Yang, X. Systems toxicogenomics of prenatal low-dose BPA exposure on liver metabolic pathways, gut microbiota, and metabolic health in mice. Environ. Int. 2021, 146, 106260. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.X.; Chen, Y.W.; Shih, M.K.; Tain, Y.L.; Yeh, Y.T.; Chiu, M.H.; Chang, S.K.C.; Hou, C.Y. Resveratrol butyrate esters inhibit BPA-induced liver damage in male offspring rats by modulating antioxidant capacity and gut microbiota. Int. J. Mol. Sci. 2021, 22, 5273. [Google Scholar] [CrossRef]
- Meng, L.Y.; Tao, W.F.; Li, J.; Zhu, M.; Zhong, D.B.; Zhou, J.; Qin, X.; Wei, R.G. Effects of bisphenol A and its substitute, bisphenol F, on the gut microbiota in mice. Biomed. Environ. Sci. 2024, 37, 19–30. [Google Scholar]
- Zhang, H.; Zha, X.; Zhang, B.; Zheng, Y.; Elsabagh, M.; Wang, H.; Wang, M. Gut microbiota contributes to bisphenol A-induced maternal intestinal and placental apoptosis, oxidative stress, and fetal growth restriction in pregnant ewe model by regulating gut-placental axis. Microbiome 2024, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhou, T.; Shen, H.; Jiang, Y.; Yang, Q.; Su, S.; Wu, L.; Fan, X.; Gao, M.; Wu, Y.; et al. Mixed probiotics modulated gut microbiota to improve spermatogenesis in bisphenol A-exposed male mice. Ecotoxicol. Environ. Saf. 2024, 270, 115922. [Google Scholar] [CrossRef]
- Javurek, A.B.; Spollen, W.G.; Johnson, S.A.; Bivens, N.J.; Bromert, K.H.; Givan, S.A.; Rosenfeld, C.S. Effects of exposure to bisphenol A and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model. Gut Microbes 2016, 7, 471–485. [Google Scholar] [CrossRef]
- Xu, J.; Huang, G.; Nagy, T.; Guo, T.L. Bisphenol A alteration of type 1 diabetes in non-obese diabetic (NOD) female mice is dependent on window of exposure. Arch. Toxicol. 2019, 93, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Sarma, S.J.; Marshall, B.L.; Liu, Y.; Kinkade, J.A.; Bellamy, M.M.; Mao, J.; Helferich, W.G.; Schenk, A.K.; Bivens, N.J.; et al. Developmental exposure of California mice to endocrine disrupting chemicals and potential effects on the microbiome-gut-brain axis at adulthood. Sci. Rep. 2020, 10, 10902. [Google Scholar]
- Shih, M.-K.; Tain, Y.-L.; Chen, Y.-W.; Hsu, W.-H.; Yeh, Y.-T.; Chang, S.K.C.; Liao, J.-X.; Hou, C.-Y. Resveratrol butyrate esters inhibit obesity caused by perinatal exposure to bisphenol A in female offspring rats. Molecules 2021, 26, 4010. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, Y.; Li, H.; Qiao, F.; Wu, J.; Du, Z.-Y.; Zhang, M. Influence of endogenous and exogenous estrogenic endocrine on intestinal microbiota in zebrafish. PLoS ONE 2016, 11, e0163895. [Google Scholar] [CrossRef]
- Chen, L.; Guo, Y.; Hu, C.; Lam, P.K.S.; Lam, J.C.W.; Zhou, B. Dysbiosis of gut microbiota by chronic coexposure to titanium dioxide nanoparticles and bisphenol A: Implications for host health in zebrafish. Environ. Pollut. 2018, 234, 307–317. [Google Scholar] [CrossRef]
- Zheng, Q.; Cui, L.; Liao, H.; Junaid, M.; Li, Z.; Liu, S.; Gao, D.; Zheng, Y.; Lu, S.; Qiu, J.; et al. Combined exposure to polystyrene nanoplastics and bisphenol A induces hepato- and intestinal-toxicity and disturbs gut microbiota in channel catfish (Ictalurus punctatus). Sci. Total Environ. 2023, 891, 164319. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Ma, D.; Liu, X.; Zhao, L.; Ma, L.; Ma, D.; Dong, S. Bisphenol P exposure in C57BL/6 mice caused gut microbiota dysbiosis and induced intestinal barrier disruption via LPS/TLR4/NF-κB signaling pathway. Environ. Int. 2023, 175, 107949. [Google Scholar] [CrossRef]
- Wang, H.; Qi, S.; Mu, X.; Yuan, L.; Li, Y.; Qiu, J. Bisphenol F induces liver-gut alteration in zebrafish. Sci. Total Environ. 2022, 851, 157974. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, B.; Wang, Q.; Liu, Y.; Liu, X.; Wu, B.; Lu, G. Intestinal toxicity and microbial community disorder induced by bisphenol F and bisphenol S in zebrafish. Chemosphere 2021, 280, 130711. [Google Scholar] [CrossRef] [PubMed]
- González-Castro, A.M.; Martínez, C.; Salvo-Romero, E.; Fortea, M.; Pardo-Camacho, C.; Pérez-Berezo, T.; Alonso-Cotoner, C.; Santos, J.; Vicario, M. Mucosal pathobiology and molecular signature of epithelial barrier dysfunction in the small intestine in irritable bowel syndrome. J. Gastroenterol. Hepatol. 2017, 32, 53–63. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Tulkens, J.; Vergauwen, G.; Van Deun, J.; Geeurickx, E.; Dhondt, B.; Lippens, L.; De Scheerder, M.-A.; Miinalainen, I.; Rappu, P.; De Geest, B.G.; et al. Increased levels of systemic LPS-positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction. Gut 2020, 69, 191–193. [Google Scholar] [CrossRef]
- Zhu, W.; Winter, M.G.; Byndloss, M.X.; Spiga, L.; Duerkop, B.A.; Hughes, E.R.; Büttner, L.; de Lima Romão, E.; Behrendt, C.L.; Lopez, C.A.; et al. Precision editing of the gut microbiota ameliorates colitis. Nature 2018, 553, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, M.; Chen, X.; Li, M. Edible fungus degrade bisphenol A with no harmful effect on its fatty acid composition. Ecotoxicol. Environ. Saf. 2015, 118, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Ottman, N.; Geerlings, S.Y.; Aalvink, S.; de Vos, W.M.; Belzer, C. Action and function of Akkermansia muciniphila in microbiome ecology, health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, A.; Nara, M.; Sugiyama, Y.; Sakanaka, M.; Yachi, H.; Kitakata, A.; Nakagawa, A.; Minami, H.; Okuda, S.; Katoh, T.; et al. Use of Gifu Anaerobic Medium for culturing 32 dominant species of human gut microbes and its evaluation based on short-chain fatty acids fermentation profiles. Biosci. Biotechnol. Biochem. 2017, 81, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Han, X.; Zhan, J.; You, Y.; Huang, W. Vanillin alleviates high fat diet-induced obesity and improves the gut microbiota composition. Front. Microbiol. 2018, 9, 2733. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.A.; Montag, D. Exploring the relationship between gut microbiota and exercise: Short-chain fatty acids and their role in metabolism. BMJ Open Sport Exerc. Med. 2021, 7, e000930. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, Y.; Heidari, H.R.; Khosroushahi, A.Y. Review of short-chain fatty acids effects on the immune system and cancer. Food Biosci. 2020, 38, 100793. [Google Scholar] [CrossRef]
- Iván, J.; Major, E.; Sipos, A.; Kovács, K.; Horváth, D.; Tamás, I.; Bay, P.; Dombrádi, V.; Lontay, B. The short-chain fatty acid propionate inhibits adipogenic differentiation of human chorion-derived mesenchymal stem cells through the free fatty acid receptor 2. Stem Cells Dev. 2017, 26, 1724–1733. [Google Scholar] [CrossRef]
- Behary, J.; Amorim, N.; Jiang, X.-T.; Raposo, A.; Gong, L.; McGovern, E.; Ibrahim, R.; Chu, F.; Stephens, C.; Jebeili, H.; et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 2021, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.W.; de Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Wu, T.-K.; Lim, P.-S.; Jin, J.-S.; Wu, M.-Y.; Chen, C.-H. Impaired gut epithelial tight junction expression in hemodialysis patients complicated with intradialytic hypotension. BioMed Res. Int. 2018, 2018, 2670312. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, H.; Li, W.; He, Q.; Liang, J.; Yan, X.; Yuan, Y.; Yue, T. Selenium-containing tea polysaccharides ameliorate DSS-induced ulcerative colitis via enhancing the intestinal barrier and regulating the gut microbiota. Int. J. Biol. Macromol. 2022, 209, 356–366. [Google Scholar] [CrossRef]
- Tsukita, S.; Furuse, M.; Itoh, M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001, 2, 285–293. [Google Scholar] [CrossRef]
- González-Mariscal, L.; Betanzos, A.; Avila-Flores, A. MAGUK proteins: Structure and role in the tight junction. Semin. Cell Dev. Biol. 2000, 11, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Ao, J.; Liu, Y.; Tang, W.; Zhang, J. Bisphenol S exposure induces intestinal inflammation: An integrated metabolomic and transcriptomic study. Chemosphere 2022, 292, 133510. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, W.; Ao, J.; Zhang, J.; Feng, L. Transcriptomics integrated with metabolomics reveals the effect of Bisphenol F (BPF) exposure on intestinal inflammation. Sci. Total Environ. 2022, 816, 151644. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.R.; Goel, R.; Seungbum, K.; Richards, E.M.; Holbert, R.C.; Pepine, C.J.; Raizada, M.K. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 2018, 67, 1555–1557. [Google Scholar] [CrossRef] [PubMed]
- Hug, H.; Mohajeri, M.H.; La Fata, G. Toll-like receptors: Regulators of the immune response in the human gut. Nutrients 2018, 10, 203. [Google Scholar] [CrossRef]
- Peterson, J.; Balogh Sivars, K.; Bianco, A.; Röper, K. Toll-like receptor signalling via IRAK4 affects epithelial integrity and tightness through regulation of junctional tension. Development 2023, 150, dev201893. [Google Scholar] [CrossRef] [PubMed]
- Rose, E.C.; Odle, J.; Blikslager, A.T.; Ziegler, A.L. Probiotics, prebiotics and epithelial tight junctions: A promising approach to modulate intestinal barrier function. Int. J. Mol. Sci. 2021, 22, 6729. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Nighot, P.; Nighot, M.; Haque, M.; Rawat, M.; Ma, T.Y. Lactobacillus acidophilus induces a strain-specific and Toll-like receptor 2-dependent enhancement of intestinal epithelial tight junction barrier and protection against intestinal inflammation. Am. J. Pathol. 2021, 191, 872–884. [Google Scholar] [CrossRef]
- Cario, E.; Gerken, G.; Podolsky, D.K. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology 2004, 127, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C.; Cookson, A.L.; McNabb, W.C.; Park, Z.; McCann, M.J.; Kelly, W.J.; Roy, N.C. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- He, W.-Q.; Wang, J.; Sheng, J.-Y.; Zha, J.-M.; Graham, W.V.; Turner, J.R. Contributions of myosin light chain kinase to regulation of epithelial paracellular permeability and mucosal homeostasis. Int. J. Mol. Sci. 2020, 21, 993. [Google Scholar] [CrossRef]
- Aflatoonian, R.; Tuckerman, E.; Elliott, S.L.; Bruce, C.; Aflatoonian, A.; Li, T.C.; Fazeli, A. Menstrual cycle-dependent changes of Toll-like receptors in endometrium. Hum. Reprod. 2007, 22, 586–593. [Google Scholar] [CrossRef]
- Jorgenson, R.L.; Young, S.L.; Lesmeister, M.J.; Lyddon, T.D.; Misfeldt, M.L. Human endometrial epithelial cells cyclically express Toll-like receptor 3 (TLR3) and exhibit TLR3-dependent responses to dsRNA. Hum. Immunol. 2005, 66, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Osuga, Y.; Hamasaki, K.; Hirota, Y.; Nose, E.; Morimoto, C.; Harada, M.; Takemura, Y.; Koga, K.; Yoshino, O.; et al. Expression of toll-like receptors 2, 3, 4, and 9 genes in the human endometrium during the menstrual cycle. J. Reprod. Immunol. 2007, 74, 53–60. [Google Scholar] [CrossRef]
- Sanchez-Lopez, J.A.; Caballero, I.; Montazeri, M.; Maslehat, N.; Elliott, S.; Fernandez-Gonzalez, R.; Calle, A.; Gutierrez-Adan, A.; Fazeli, A. Local activation of uterine Toll-like receptor 2 and 2/6 decreases embryo implantation and affects uterine receptivity in mice. Biol. Reprod. 2014, 90, 87. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, M.; Sanchez-Lopez, J.A.; Caballero, I.; Maslehat Lay, N.; Elliott, S.; López-Martín, S.; Yáñez-Mó, M.; Fazeli, A. Activation of Toll-like receptor 3 reduces actin polymerization and adhesion molecule expression in endometrial cells, a potential mechanism for viral-induced implantation failure. Hum. Reprod. 2015, 30, 893–905. [Google Scholar] [CrossRef]
- Aboussahoud, W.; Bruce, C.; Elliott, S.; Fazeli, A. Activation of Toll-like receptor 5 decreases the attachment of human trophoblast cells to endometrial cells in vitro. Hum. Reprod. 2010, 25, 2217–2228. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Al-Nasiry, S.; Ambrosino, E.; Schlaepfer, M.; Morré, S.A.; Wieten, L.; Voncken, J.W.; Spinelli, M.; Mueller, M.; Kramer, B.W. The interplay between reproductive tract microbiota and immunological system in human reproduction. Front. Immunol. 2020, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Sehring, J.; Beltsos, A.; Jeelani, R. Human implantation: The complex interplay between endometrial receptivity, inflammation, and the microbiome. Placenta 2022, 117, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Mukura, L.R.; Hickey, D.K.; Rodriguez-Garcia, M.; Fahey, J.V.; Wira, C.R. Chlamydia trachomatis regulates innate immune barrier integrity and mediates cytokine and antimicrobial responses in human uterine ECC-1 epithelial cells. Am. J. Reprod. Immunol. 2017, 78, e12764. [Google Scholar] [CrossRef]
- Kaushic, C.; Grant, K.; Crane, M.; Wira, C.R. Infection of polarized primary epithelial cells from rat uterus with Chlamydia trachomatis: Cell-cell interaction and cytokine secretion. Am. J. Reprod. Immunol. 2000, 44, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Howie, S.E.M.; Horner, P.J.; Horne, A.W. Chlamydia trachomatis infection during pregnancy: Known unknowns. Discov. Med. 2011, 12, 57–64. [Google Scholar] [PubMed]
- Baud, D.; Regan, L.; Greub, G. Emerging role of Chlamydia and Chlamydia-like organisms in adverse pregnancy outcomes. Curr. Opin. Infect. Dis. 2008, 21, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Giakoumelou, S.; Wheelhouse, N.; Cuschieri, K.; Entrican, G.; Howie, S.E.M.; Horne, A.W. The role of infection in miscarriage. Hum. Reprod. Update 2016, 22, 116–133. [Google Scholar] [CrossRef]
- Tang, W.; Mao, J.; Li, K.T.; Walker, J.S.; Chou, R.; Fu, R.; Chen, W.; Darville, T.; Klausner, J.; Tucker, J.D. Pregnancy and fertility-related adverse outcomes associated with Chlamydia trachomatis infection: A global systematic review and meta-analysis. Sex. Transm. Infect. 2020, 96, 322–329. [Google Scholar] [CrossRef]
- Vallely, L.M.; Egli-Gany, D.; Wand, H.; Pomat, W.S.; Homer, C.S.E.; Guy, R.; Silver, B.; Rumbold, A.R.; Kaldor, J.M.; Vallely, A.J.; et al. Adverse pregnancy and neonatal outcomes associated with Neisseria gonorrhoeae: Systematic review and meta-analysis. Sex. Transm. Infect. 2021, 97, 104–111. [Google Scholar] [CrossRef]
- Edwards, V.L.; Wang, L.-C.; Dawson, V.; Stein, D.C.; Song, W. Neisseria gonorrhoeae breaches the apical junction of polarized epithelial cells for transmigration by activating EGFR. Cell. Microbiol. 2013, 15, 1042–1057. [Google Scholar] [CrossRef][Green Version]
- Rodríguez-Tirado, C.; Maisey, K.; Rodríguez, F.E.; Reyes-Cerpa, S.; Reyes-López, F.E.; Imarai, M. Neisseria gonorrhoeae induced disruption of cell junction complexes in epithelial cells of the human genital tract. Microbes Infect. 2012, 14, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, S.; Liu, J.; Ta, N. Inhibition of TLR2/TLR4 alleviates the Neisseria gonorrhoeae infection damage in human endometrial epithelial cells via Nrf2 and NF-Kβsignaling. J. Reprod. Immunol. 2020, 142, 103192. [Google Scholar] [CrossRef]
- Akhi, M.T.; Esmailkhani, A.; Sadeghi, J.; Niknafs, B.; Farzadi, L.; Akhi, A.; Nasab, E.N. The frequency of Staphylococcus aureus isolated from endocervix of infertile women in northwest Iran. Int. J. Fertil. Steril. 2017, 11, 28–32. [Google Scholar] [PubMed]
- Dutta, S.; Sengupta, P.; Izuka, E.; Menuba, I.; Jegasothy, R.; Nwagha, U. Staphylococcal infections and infertility: Mechanisms and management. Mol. Cell. Biochem. 2020, 474, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.M.; Moreno, L.Z.; Poor, A.P.; Matajira, C.E.C.; Moreno, M.; Gomes, V.T.d.M.; da Silva, G.F.R.; Takeuti, K.L.; Barcellos, D.E. Antimicrobial resistance profile of Staphylococcus hyicus strains isolated from Brazilian swine herds. Antibiotics 2022, 11, 205. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Guo, J.; Xu, M.; Jiang, P.; Yuan, X.; Zhao, C.; Maimai, T.; Cao, Y.; Zhang, N.; Fu, Y. Clostridium tyrobutyricum alleviates Staphylococcus aureus-induced endometritis in mice by inhibiting endometrial barrier disruption and inflammatory response. Food Funct. 2019, 10, 6699–6710. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, C.V.; Potter, J.A.; Grimshaw, A.A.; Abrahams, V.M.; Tong, M. Endometrial responses to bacterial and viral infection: A scoping review. Hum. Reprod. Update 2023, 29, 675–693. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, C.; Li, W.; Mu, W.; Li, C.; Guo, M. Selenium plays a protective role in Staphylococcus aureus-induced endometritis in the uterine tissue of rats. Biol. Trace Elem. Res. 2016, 173, 345–353. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, X.; Liu, Z.; Zhang, W.; Fang, J.; Xue, J.; Bao, H. Hydroxysafflor yellow A inhibits Staphylococcus aureus-induced mouse endometrial inflammation via TLR2-mediated NF-kB and MAPK pathway. Inflammation 2021, 44, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.-J.; Zhang, Z.-B.; Xu, N.-N.; Guo, Y.-F.; Qiu, C.; Li, C.-Y.; Deng, G.-Z.; Guo, M.-Y. Piperine plays an anti-inflammatory role in Staphylococcus aureus endometritis by inhibiting activation of NF-κB and MAPK pathways in mice. Evid.-Based Complement. Altern. Med. 2016, 2016, 8597208. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Chen, X.; Zhao, G.; Wu, H.; Mi, J.; Qiu, C.; Peng, X.; Deng, G. IFN-τ plays an anti-inflammatory role in Staphylococcus aureus-induced endometritis in mice through the suppression of NF-κB pathway and MMP9 expression. J. Interferon Cytokine Res. 2017, 37, 81–89. [Google Scholar] [CrossRef]
- Murphy, C.R.; Swift, J.G.; Need, J.A.; Mukherjee, T.M.; Rogers, A.W. A freeze-fracture electron microscopic study of tight junctions of epithelial cells in the human uterus. Anat. Embryol. 1982, 163, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.R.; Rogers, P.A.W.; Hosie, M.J.; Leeton, J.; Beaton, L. Tight junctions of human uterine epithelial cells change during the menstrual cycle: A morphometric study. Acta Anat. 2008, 144, 36–38. [Google Scholar] [CrossRef]
- Iwanaga, S.; Inokuchi, T.; Notohara, A.; Higashi, R.; Murakami, M.; Kato, T. Alterations in tight junctions of human endometrial epithelial cells during normal menstrual cycle--freeze-fracture electron microscopic study. Nihon Sanka Fujinka Gakkai Zasshi 1985, 37, 2847–2852. [Google Scholar] [PubMed]
- Mendoza-Rodríguez, C.A.; González-Mariscal, L.; Cerbón, M. Changes in the distribution of ZO-1, occludin, and claudins in the rat uterine epithelium during the estrous cycle. Cell Tissue Res. 2005, 319, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Peña, A.A.; Rivera-Baños, J.; Méndez-Carrillo, L.L.; Ramírez-Solano, M.I.; Galindo-Bustamante, A.; Páez-Franco, J.C.; Morimoto, S.; González-Mariscal, L.; Cruz, M.E.; Mendoza-Rodríguez, C.A. Perinatal administration of bisphenol A alters the expression of tight junction proteins in the uterus and reduces the implantation rate. Reprod. Toxicol. 2017, 69, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Poon, C.E.; Madawala, R.J.; Day, M.L.; Murphy, C.R. Claudin 7 is reduced in uterine epithelial cells during early pregnancy in the rat. Histochem. Cell Biol. 2013, 139, 583–593. [Google Scholar] [CrossRef]
- Murphy, C.R.; Swift, J.G.; Mukherjee, T.M.; Rogers, A.W. Effects of ovarian hormones on cell membranes in the rat uterus. II. Freeze-fracture studies on tight junctions of the lateral plasma membrane of the luminal epithelium. Cell Biophys. 1981, 3, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.R.; Swift, J.G.; Mukherjee, T.M.; Rogers, A.W. The structure of tight junctions between uterine luminal epithelial cells at different stages of pregnancy in the rat. Cell Tissue Res. 1982, 223, 281–286. [Google Scholar] [CrossRef]
- Ding, L.; Lu, Z.; Foreman, O.; Tatum, R.; Lu, Q.; Renegar, R.; Cao, J.; Chen, Y.-H. Inflammation and disruption of the mucosal architecture in claudin-7-deficient mice. Gastroenterology 2012, 142, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Orchard, M.D.; Murphy, C.R. Alterations in tight junction molecules of uterine epithelial cells during early pregnancy in the rat. Acta Histochem. 2002, 104, 149–155. [Google Scholar] [CrossRef]
- Nicholson, M.D.O.; Lindsay, L.A.; Murphy, C.R. Ovarian hormones control the changing expression of claudins and occludin in rat uterine epithelial cells during early pregnancy. Acta Histochem. 2010, 112, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Ikenouchi, J.; Sasaki, H.; Tsukita, S.; Furuse, M.; Tsukita, S. Loss of occludin affects tricellular localization of tricellulin. Mol. Biol. Cell 2008, 19, 4687–4693. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.R. The plasma membrane transformation of uterine epithelial cells during pregnancy. J. Reprod. Fertil. Suppl. 2000, 55, 23–28. [Google Scholar]
- Van Itallie, C.M.; Anderson, J.M. Claudin interactions in and out of the tight junction. Tissue Barriers 2013, 1, e25247. [Google Scholar] [CrossRef] [PubMed]
- Carson, D.D.; Lagow, E.; Thathiah, A.; Al-Shami, R.; Farach-Carson, M.C.; Vernon, M.; Yuan, L.; Fritz, M.A.; Lessey, B. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol. Hum. Reprod. 2002, 8, 871–879. [Google Scholar] [CrossRef]
- Riesewijk, A.; Martín, J.; van Os, R.; Horcajadas, J.A.; Polman, J.; Pellicer, A.; Mosselman, S.; Simón, C. Gene expression profiling of human endometrial receptivity on days LH+2 versus LH+7 by microarray technology. Mol. Hum. Reprod. 2003, 9, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Van Itallie, C.; Rahner, C.; Anderson, J.M. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J. Clin. Investig. 2001, 107, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, M.D.; Lu, Q.; Chen, Y.-H. Overexpression of claudin-7 decreases the paracellular Cl- conductance and increases the paracellular Na+ conductance in LLC-PK1 cells. J. Cell Sci. 2005, 118 Pt 12, 2683–2693. [Google Scholar] [CrossRef]
- Tsang, L.L.; Chan, L.N.; Wang, X.F.; So, S.C.; Yuen, J.P.; Fiscus, R.R.; Chan, H.C. Enhanced epithelial Na(+) channel (ENaC) activity in mouse endometrial epithelium by upregulation of gammaENaC subunit. Jpn. J. Physiol. 2001, 51, 539–543. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salleh, N.; Baines, D.L.; Naftalin, R.J.; Milligan, S.R. The hormonal control of uterine luminal fluid secretion and absorption. J. Membr. Biol. 2005, 206, 17–28. [Google Scholar] [CrossRef]
- Lindsay, L.A.; Murphy, C.R. Redistribution of aquaporins in uterine epithelial cells at the time of implantation in the rat. Acta Histochem. 2004, 106, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Png, F.Y.; Murphy, C.R. Closure of the uterine lumen and the plasma membrane transformation do not require blastocyst implantation. Eur. J. Morphol. 2000, 38, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Lindsay, L.A.; Murphy, C.R. Focal adhesions disassemble during early pregnancy in rat uterine epithelial cells. Reprod. Fertil. Dev. 2008, 20, 892–899. [Google Scholar] [CrossRef]
- Paria, B.C.; Zhao, X.; Das, S.K.; Dey, S.K.; Yoshinaga, K. Zonula occludens-1 and E-cadherin are coordinately expressed in the mouse uterus with the initiation of implantation and decidualization. Dev. Biol. 1999, 208, 488–501. [Google Scholar] [CrossRef]
- Wang, X.; Matsumoto, H.; Zhao, X.; Das, S.K.; Paria, B.C. Embryonic signals direct the formation of tight junctional permeability barrier in the decidualizing stroma during embryo implantation. J. Cell Sci. 2004, 117, 53–62. [Google Scholar] [CrossRef]
- Umeda, K.; Ikenouchi, J.; Katahira-Tayama, S.; Furuse, K.; Sasaki, H.; Nakayama, M.; Matsui, T.; Tsukita, S.; Furuse, M.; Tsukita, S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell 2006, 126, 741–754. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).