Air-Pollution-Mediated Microbial Dysbiosis in Health and Disease: Lung–Gut Axis and Beyond
Abstract
1. Introduction
2. Respiratory Microbiome
2.1. Characteristics of the Respiratory Microbiome
2.2. Air Pollution and Respiratory Microbiome
| Study Population | Exposure | Sample Type | Results Summary |
|---|---|---|---|
| BALB/c mice, male [76] | PM2.5 | Bronchoalveolar lavage | Shannon ↓, observed ASV ↓, Fisher ↓, weighted UniFrac (+) |
| C57BL/6 mice, male [77] | PM2.5 | Lung tissue | Shannon ↓, Simpson ↓ |
| C57BL/6N mice, male [81] | PM2.5 | Bronchoalveolar lavage | (+) Serum and BALF: IL-1B, IL-6, IL-17, TNF-a, Simpson ↑, Shannon ↑, ACE ↑, Chao1 ↑, metabolic pathway alteration (+) |
| C57BL/6 mice, male [82] | Carbon black, ozone, CB + O3 | Lung tissue | Neutrophils ↑, eosinophils ↑, Shannon ↓, total bacterial load ↓ |
| Fischer 344 rat, male [83] | TRAP | Lung tissue | Lung function ↓: PEF, FVC, FEV |
| Sprague Dawley rats, male [80] | Biomass fuel, motor vehicle exhaust | Bronchoalveolar lavage | BALF macrophage ↑, IgA ↑, IgG ↓, Out ↑, Chao1 ↑, PD whole tree ↑, observed species ↑ |
| C57BL/6 mice, male [78] | Diesel exhaust particle | Bronchoalveolar lavage | ↑ BALF: IgA, IgG; ↑ lung: TNF-a, IL-10 |
3. Gut Microbiome
Air Pollution and Gut Microbiome
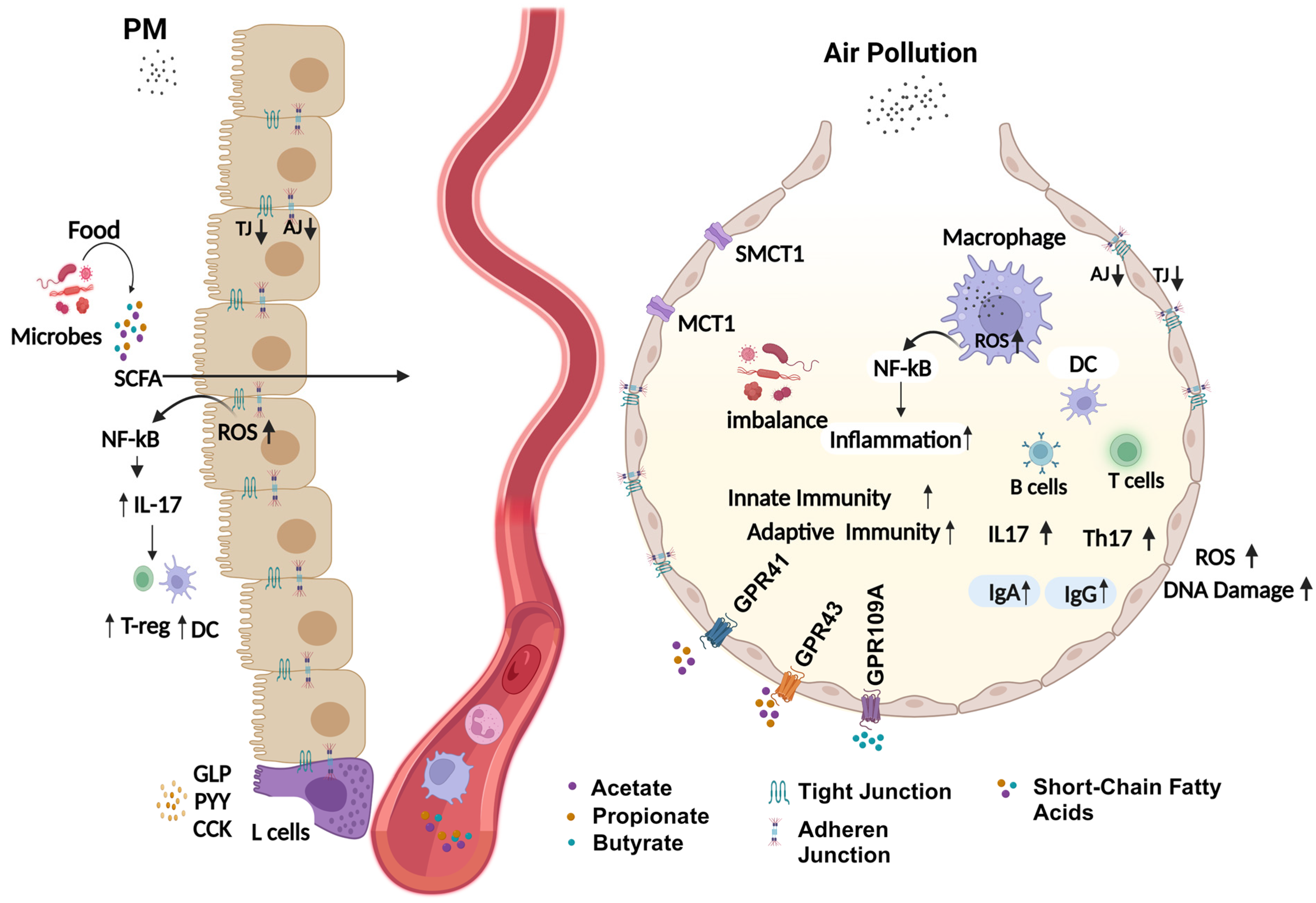
4. Potential Mechanism of Air-Pollution-Induced Respiratory Microbiome Dysbiosis
4.1. Alteration in Airway Physiological Environment
4.2. Oxidative Stress
4.3. Disrupted Barrier Integrity
4.4. Disrupted Lipid Homeostasis and Systemic Inflammation
5. Lung–Gut–Liver Axis
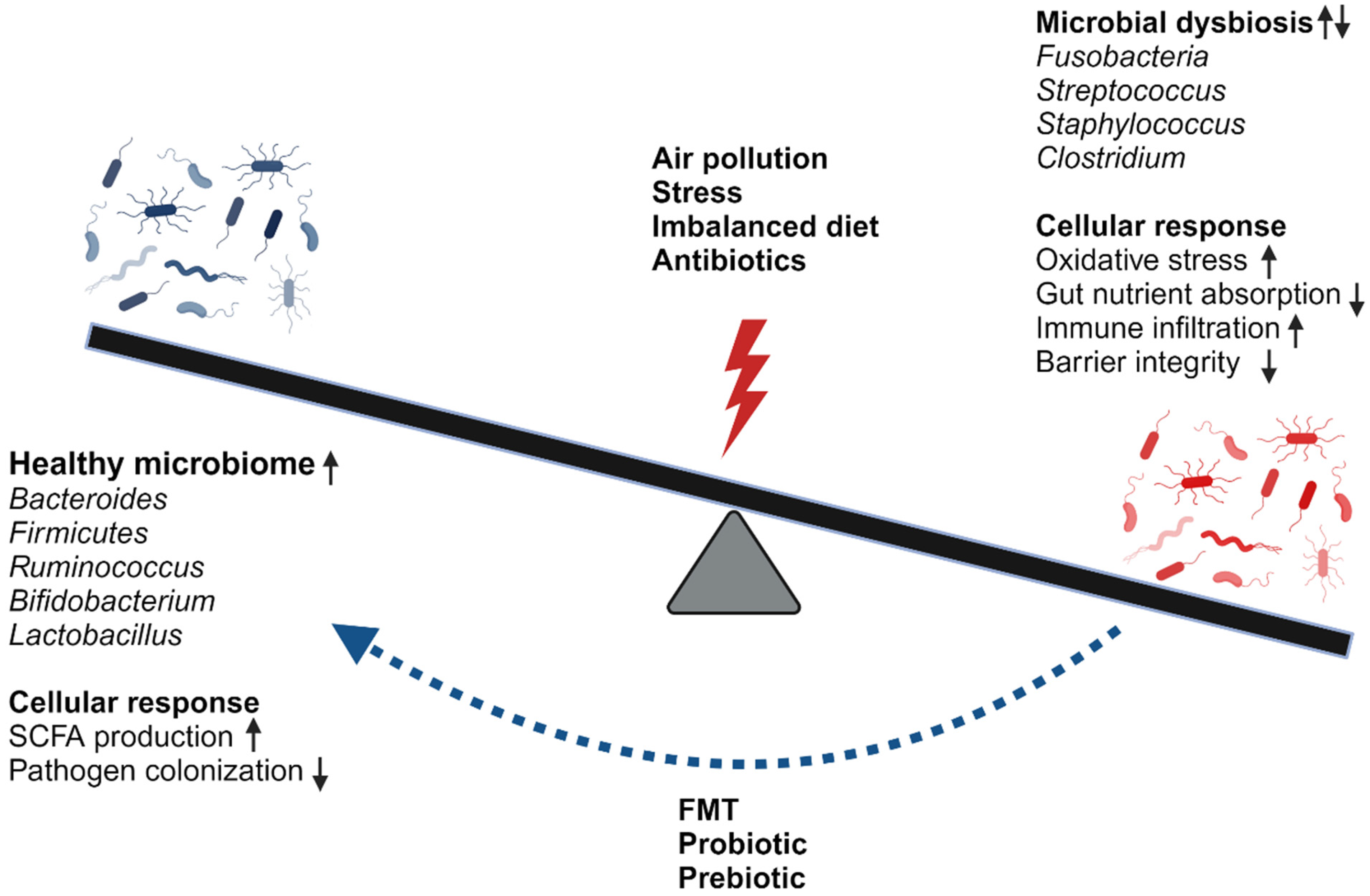
6. Microbiome as a Therapeutic Approach and Target
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kelly, F.J.; Fussell, J.C. Air Pollution and Public Health: Emerging Hazards and Improved Understanding of Risk. Environ. Geochem. Health 2015, 37, 631–649. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.R. Air Pollution and Mortality: A History. Atmos. Environ. 2009, 43, 142–152. [Google Scholar] [CrossRef]
- Harrison, R.M. Airborne Particulate Matter. Philos. Trans. R. Soc. A 2020, 378, 20190319. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Cai, Y.; Lyu, B.; Zhang, D.; Chu, S.; Jing, H.; Rahimi, K.; Tong, Z. Air Pollution and Hospitalization of Patients with Idiopathic Pulmonary Fibrosis in Beijing: A Time-Series Study. Respir. Res. 2022, 23, 81. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T.; Devlin, R.B.; Wilson, S.J.; Frew, A.J. Health Effects of Acute Exposure to Air Pollution. Part II: Healthy Subjects Exposed to Concentrated Ambient Particles. Res. Rep. Health Eff. Inst. 2003, 112, 31–50; discussion 51–67. [Google Scholar]
- Garshick, E. Effects of Short- and Long-Term Exposures to Ambient Air Pollution on COPD. Eur. Respir. J. 2014, 44, 558–561. [Google Scholar] [CrossRef]
- Chen, J.; Dan, L.; Sun, Y.; Yuan, S.; Liu, W.; Chen, X.; Jiang, F.; Fu, T.; Zhang, H.; Deng, M.; et al. Ambient Air Pollution and Risk of Enterotomy, Gastrointestinal Cancer, and All-Cause Mortality among 4,708 Individuals with Inflammatory Bowel Disease: A Prospective Cohort Study. Environ. Health Perspect. 2023, 131, 77010. [Google Scholar] [CrossRef]
- Raftery, A.L.; Tsantikos, E.; Harris, N.L.; Hibbs, M.L. Links between Inflammatory Bowel Disease and Chronic Obstructive Pulmonary Disease. Front. Immunol. 2020, 11, 2144. [Google Scholar] [CrossRef]
- Korten, I.; Ramsey, K.; Latzin, P. Air Pollution during Pregnancy and Lung Development in the Child. Paediatr. Respir. Rev. 2017, 21, 38–46. [Google Scholar] [CrossRef]
- Veras, M.M.; de Oliveira Alves, N.; Fajersztajn, L.; Saldiva, P. Before the First Breath: Prenatal Exposures to Air Pollution and Lung Development. Cell Tissue Res. 2017, 367, 445–455. [Google Scholar] [CrossRef]
- Zielinska, M.A.; Hamulka, J. Protective Effect of Breastfeeding on the Adverse Health Effects Induced by Air Pollution: Current Evidence and Possible Mechanisms. Int. J. Environ. Res. Public Health 2019, 16, 4181. [Google Scholar] [CrossRef] [PubMed]
- Brunekreef, B.; Holgate, S.T. Air Pollution and Health. Lancet 2002, 360, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Rajagopalan, S. Particulate Matter, Air Pollution, and Blood Pressure. J. Am. Soc. Hypertens. 2009, 3, 332–350. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Castanas, E. Human Health Effects of Air Pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef]
- Lodovici, M.; Bigagli, E. Oxidative Stress and Air Pollution Exposure. J. Toxicol. 2011, 2011, 487074. [Google Scholar] [CrossRef]
- Perera, F.P. Multiple Threats to Child Health from Fossil Fuel Combustion: Impacts of Air Pollution and Climate Change. Environ. Health Perspect. 2017, 125, 141–148. [Google Scholar] [CrossRef]
- Sofia, D.; Gioiella, F.; Lotrecchiano, N.; Giuliano, A. Mitigation Strategies for Reducing Air Pollution. Environ. Sci. Pollut. Res. 2020, 27, 19226–19235. [Google Scholar] [CrossRef]
- Giannadaki, D.; Giannakis, E.; Pozzer, A.; Lelieveld, J. Estimating Health and Economic Benefits of Reductions in Air Pollution from Agriculture. Sci. Total Environ. 2018, 622, 1304–1316. [Google Scholar] [CrossRef]
- Anenberg, S.C.; West, J.J.; Fiore, A.M.; Jaffe, D.A.; Prather, M.J.; Bergmann, D.; Cuvelier, K.; Dentener, F.J.; Duncan, B.N.; Gauss, M. Intercontinental Impacts of Ozone Pollution on Human Mortality; ACS Publications: Washington, DC, USA, 2009; ISBN 0013-936X. [Google Scholar]
- Pope, C.A., III; Coleman, N.; Pond, Z.A.; Burnett, R.T. Fine Particulate Air Pollution and Human Mortality: 25+ Years of Cohort Studies. Environ. Res. 2020, 183, 108924. [Google Scholar] [CrossRef]
- Samek, L. Overall Human Mortality and Morbidity Due to Exposure to Air Pollution. Int. J. Occup. Med. Environ. Health 2016, 29, 417. [Google Scholar] [CrossRef]
- Liu, C.-X.; Liu, Y.-B.; Peng, Y.; Peng, J.; Ma, Q.-L. Causal Effect of Air Pollution on the Risk of Cardiovascular and Metabolic Diseases and Potential Mediation by Gut Microbiota. Sci. Total Environ. 2024, 912, 169418. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Fang, J.; Tang, S.; Deng, F.; Liu, X.; Shen, Y.; Liu, Y.; Kong, F.; Du, Y.; Cui, L.; et al. PM2.5 and Serum Metabolome and Insulin Resistance, Potential Mediation by the Gut Microbiome: A Population-Based Panel Study of Older Adults in China. Environ. Health Perspect. 2022, 130, 27007. [Google Scholar] [CrossRef] [PubMed]
- Carré, J.; Gatimel, N.; Moreau, J.; Parinaud, J.; Léandri, R. Does Air Pollution Play a Role in Infertility?: A Systematic Review. Environ. Health 2017, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Deng, F.; Wu, S.; Lu, H.; Hao, Y.; Guo, X. The Impacts of Short-Term Exposure to Noise and Traffic-Related Air Pollution on Heart Rate Variability in Young Healthy Adults. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Blaser, M.J. The Human Microbiome: At the Interface of Health and Disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the Human Microbiome. Nutr. Rev. 2012, 70, S38–S44. [Google Scholar] [CrossRef]
- Pflughoeft, K.J.; Versalovic, J. Human Microbiome in Health and Disease. Annu. Rev. Pathol. Mech. Dis. 2012, 7, 99–122. [Google Scholar] [CrossRef]
- Hooks, K.B.; O’Malley, M.A. Dysbiosis and Its Discontents. MBio 2017, 8, e01492-17. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the Immune System. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Whiteside, S.A.; McGinniss, J.E.; Collman, R.G. The Lung Microbiome: Progress and Promise. J. Clin. Investig. 2021, 131, e150473. [Google Scholar] [CrossRef]
- Ichinohe, T.; Pang, I.K.; Kumamoto, Y.; Peaper, D.R.; Ho, J.H.; Murray, T.S.; Iwasaki, A. Microbiota Regulates Immune Defense against Respiratory Tract Influenza A Virus Infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5354–5359. [Google Scholar] [CrossRef] [PubMed]
- Tanes, C.; Bittinger, K.; Gao, Y.; Friedman, E.S.; Nessel, L.; Paladhi, U.R.; Chau, L.; Panfen, E.; Fischbach, M.A.; Braun, J.; et al. Role of Dietary Fiber in the Recovery of the Human Gut Microbiome and Its Metabolome. Cell Host Microbe 2021, 29, 394–407.e5. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, H.; Li, Y.; Huang, S.; Zhang, L.; Cao, C.; Baker, P.N.; Tong, C.; Zheng, P.; Qi, H. Altered Gut Bacterial and Metabolic Signatures and Their Interaction in Gestational Diabetes Mellitus. Gut Microbes 2020, 12, 1840765. [Google Scholar] [CrossRef] [PubMed]
- Pirola, C.J.; Salatino, A.; Quintanilla, M.F.; Castaño, G.O.; Garaycoechea, M.; Sookoian, S. The Influence of Host Genetics on Liver Microbiome Composition in Patients with NAFLD. eBioMedicine 2022, 76, 103858. [Google Scholar] [CrossRef] [PubMed]
- Le, H.H.; Lee, M.-T.; Besler, K.R.; Johnson, E.L. Host Hepatic Metabolism Is Modulated by Gut Microbiota-Derived Sphingolipids. Cell Host Microbe 2022, 30, 798–808.e7. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.T.; Marsland, B.J. Microbes, Metabolites, and the Gut–Lung Axis. Mucosal Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef]
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieërs, G.; Guery, B.; Delhaes, L. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front. Cell. Infect. Microbiol. 2020, 10, 9. [Google Scholar] [CrossRef]
- Peterson, J.; Garges, S.; Giovanni, M.; McInnes, P.; Wang, L.; Schloss, J.A.; Bonazzi, V.; McEwen, J.E.; Wetterstrand, K.A.; Deal, C. The NIH Human Microbiome Project. Genome Res. 2009, 19, 2317–2323. [Google Scholar]
- Dickson, R.P.; Erb-Downward, J.R.; Freeman, C.M.; McCloskey, L.; Falkowski, N.R.; Huffnagle, G.B.; Curtis, J.L. Bacterial Topography of the Healthy Human Lower Respiratory Tract. MBio 2017, 8, e02287-16. [Google Scholar] [CrossRef]
- Yadav, B.; Bhattacharya, S.S.; Rosen, L.; Nagpal, R.; Yadav, H.; Yadav, J.S. Oro-Respiratory Dysbiosis and Its Modulatory Effect on Lung Mucosal Toxicity during Exposure or Co-Exposure to Carbon Nanotubes and Cigarette Smoke. Nanomaterials 2024, 14, 314. [Google Scholar] [CrossRef]
- Mathieu, E.; Escribano-Vazquez, U.; Descamps, D.; Cherbuy, C.; Langella, P.; Riffault, S.; Remot, A.; Thomas, M. Paradigms of Lung Microbiota Functions in Health and Disease, Particularly, in Asthma. Front. Physiol. 2018, 9, 1168. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Deurenberg, R.H.; Bathoorn, E.; Chlebowicz, M.A.; Couto, N.; Ferdous, M.; García-Cobos, S.; Kooistra-Smid, A.M.; Raangs, E.C.; Rosema, S.; Veloo, A.C. Application of next Generation Sequencing in Clinical Microbiology and Infection Prevention. J. Biotechnol. 2017, 243, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The Human Oral Microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Wos-Oxley, M.L.; Chaves-Moreno, D.; Jáuregui, R.; Oxley, A.P.; Kaspar, U.; Plumeier, I.; Kahl, S.; Rudack, C.; Becker, K.; Pieper, D.H. Exploring the Bacterial Assemblages along the Human Nasal Passage. Environ. Microbiol. 2016, 18, 2259–2271. [Google Scholar] [CrossRef]
- Zhou, Y.; Mihindukulasuriya, K.A.; Gao, H.; La Rosa, P.S.; Wylie, K.M.; Martin, J.C.; Kota, K.; Shannon, W.D.; Mitreva, M.; Sodergren, E. Exploration of Bacterial Community Classes in Major Human Habitats. Genome Biol. 2014, 15, R66. [Google Scholar] [CrossRef]
- Segal, L.N.; Clemente, J.C.; Tsay, J.-C.J.; Koralov, S.B.; Keller, B.C.; Wu, B.G.; Li, Y.; Shen, N.; Ghedin, E.; Morris, A. Enrichment of the Lung Microbiome with Oral Taxa Is Associated with Lung Inflammation of a Th17 Phenotype. Nat. Microbiol. 2016, 1, 16031. [Google Scholar] [CrossRef]
- Dickson, R.P.; Erb-Downward, J.R.; Freeman, C.M.; McCloskey, L.; Beck, J.M.; Huffnagle, G.B.; Curtis, J.L. Spatial Variation in the Healthy Human Lung Microbiome and the Adapted Island Model of Lung Biogeography. Ann. Am. Thorac. Soc. 2015, 12, 821–830. [Google Scholar] [CrossRef]
- Dickson, R.P.; Martinez, F.J.; Huffnagle, G.B. The Role of the Microbiome in Exacerbations of Chronic Lung Diseases. Lancet 2014, 384, 691–702. [Google Scholar] [CrossRef]
- Marsland, B.J.; Yadava, K.; Nicod, L.P. The Airway Microbiome and Disease. Chest 2013, 144, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Wypych, T.P.; Wickramasinghe, L.C.; Marsland, B.J. The Influence of the Microbiome on Respiratory Health. Nat. Immunol. 2019, 20, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Hufnagl, K.; Pali-Schöll, I.; Roth-Walter, F.; Jensen-Jarolim, E. Dysbiosis of the Gut and Lung Microbiome Has a Role in Asthma. In Proceedings of the Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2020; Volume 42, pp. 75–93. [Google Scholar]
- Erb-Downward, J.R.; Thompson, D.L.; Han, M.K.; Freeman, C.M.; McCloskey, L.; Schmidt, L.A.; Young, V.B.; Toews, G.B.; Curtis, J.L.; Sundaram, B. Analysis of the Lung Microbiome in the “Healthy” Smoker and in COPD. PLoS ONE 2011, 6, e16384. [Google Scholar] [CrossRef]
- Sze, M.A.; Dimitriu, P.A.; Suzuki, M.; McDonough, J.E.; Campbell, J.D.; Brothers, J.F.; Erb-Downward, J.R.; Huffnagle, G.B.; Hayashi, S.; Elliott, W.M. Host Response to the Lung Microbiome in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2015, 192, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Jiang, F.; Yin, R.; Wang, J.; Xia, W.; Dong, G.; Ma, W.; Yang, Y.; Xu, L.; Hu, J. Interplay between the Lung Microbiome and Lung Cancer. Cancer Lett. 2018, 415, 40–48. [Google Scholar] [CrossRef]
- Yan, X.; Yang, M.; Liu, J.; Gao, R.; Hu, J.; Li, J.; Zhang, L.; Shi, Y.; Guo, H.; Cheng, J. Discovery and Validation of Potential Bacterial Biomarkers for Lung Cancer. Am. J. Cancer Res. 2015, 5, 3111. [Google Scholar]
- Caverly, L.J.; Zhao, J.; LiPuma, J.J. Cystic fibrosis lung microbiome: Opportunities to Reconsider Management of Airway Infection. Pediatr. Pulmonol. 2015, 50, S31–S38. [Google Scholar] [CrossRef]
- Tunney, M.M.; Field, T.R.; Moriarty, T.F.; Patrick, S.; Doering, G.; Muhlebach, M.S.; Wolfgang, M.C.; Boucher, R.; Gilpin, D.F.; McDowell, A. Detection of Anaerobic Bacteria in High Numbers in Sputum from Patients with Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2008, 177, 995–1001. [Google Scholar] [CrossRef]
- Molyneaux, P.L.; Cox, M.J.; Wells, A.U.; Kim, H.C.; Ji, W.; Cookson, W.O.; Moffatt, M.F.; Kim, D.S.; Maher, T.M. Changes in the Respiratory Microbiome during Acute Exacerbations of Idiopathic Pulmonary Fibrosis. Respir. Res. 2017, 18, 29. [Google Scholar] [CrossRef]
- Wang, J.; Lesko, M.; Badri, M.H.; Kapoor, B.C.; Wu, B.G.; Li, Y.; Smaldone, G.C.; Bonneau, R.; Kurtz, Z.D.; Condos, R. Lung Microbiome and Host Immune Tone in Subjects with Idiopathic Pulmonary Fibrosis Treated with Inhaled Interferon-γ. ERJ Open Res. 2017, 3, 8. [Google Scholar] [CrossRef]
- Klimkaite, L.; Liveikis, T.; Kaspute, G.; Armalyte, J.; Aldonyte, R. Air Pollution-Associated Shifts in the Human Airway Microbiome and Exposure-Associated Molecular Events. Future Microbiol. 2023, 18, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Niemeier-Walsh, C.; Ryan, P.H.; Meller, J.; Ollberding, N.J.; Adhikari, A.; Reponen, T. Exposure to Traffic-Related Air Pollution and Bacterial Diversity in the Lower Respiratory Tract of Children. PLoS ONE 2021, 16, e0244341. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, Y.; An, Y.; Wang, R.; Lin, H.; Liu, M.; Li, S.; Ma, M.; Xiao, C. Air Pollution during the Winter Period and Respiratory Tract Microbial Imbalance in a Healthy Young Population in Northeastern China. Environ. Pollut. 2019, 246, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Rylance, J.; Kankwatira, A.; Nelson, D.E.; Toh, E.; Day, R.B.; Lin, H.; Gao, X.; Dong, Q.; Sodergren, E.; Weinstock, G.M. Household Air Pollution and the Lung Microbiome of Healthy Adults in Malawi: A Cross-Sectional Study. BMC Microbiol. 2016, 16, 182. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, H.D., III; Mongodin, E.F.; Wan, Y.; Hua, X.; Rothman, N.; Hu, W.; Vermeulen, R.; Seow, W.J.; Rohan, T.; Xu, J. The Respiratory Tract Microbiome and Its Relationship to Lung Cancer and Environmental Exposures Found in Rural China. Environ. Mol. Mutagen. 2019, 60, 617–623. [Google Scholar] [CrossRef]
- Niu, Y.; Chen, R.; Wang, C.; Wang, W.; Jiang, J.; Wu, W.; Cai, J.; Zhao, Z.; Xu, X.; Kan, H. Ozone Exposure Leads to Changes in Airway Permeability, Microbiota and Metabolome: A randomised, double-blind, crossover trial. Eur. Respir. J. 2020, 56, 2000165. [Google Scholar] [CrossRef]
- Qin, T.; Zhang, F.; Zhou, H.; Ren, H.; Du, Y.; Liang, S.; Wang, F.; Cheng, L.; Xie, X.; Jin, A. High-level PM2. 5/PM10 Exposure is Associated with Alterations in the Human Pharyngeal Microbiota Composition. Front. Microbiol. 2019, 10, 54. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, H.; Wang, D.; Zhao, B.; Zhang, J.; Cheng, L.; Yao, P.; Di Narzo, A.; Shen, Y.; Yu, J. Airway Microbiome is Associated with Respiratory Functions and Responses to Ambient Particulate Matter Exposure. Ecotoxicol. Environ. Saf. 2019, 167, 269–277. [Google Scholar] [CrossRef]
- Mariani, J.; Favero, C.; Spinazzè, A.; Cavallo, D.M.; Carugno, M.; Motta, V.; Bonzini, M.; Cattaneo, A.; Pesatori, A.C.; Bollati, V. Short-Term Particulate Matter Exposure Influences Nasal Microbiota in a Population of Healthy Subjects. Environ. Res. 2018, 162, 119–126. [Google Scholar] [CrossRef]
- Yu, G.; Gail, M.H.; Consonni, D.; Carugno, M.; Humphrys, M.; Pesatori, A.C.; Caporaso, N.E.; Goedert, J.J.; Ravel, J.; Landi, M.T. Characterizing Human Lung Tissue Microbiota and Its Relationship to Epidemiological and Clinical Features. Genome Biol. 2016, 17, 163. [Google Scholar] [CrossRef]
- Residential Greenness and Air Pollution’s Association with Nasal Microbiota among Asthmatic Children—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0013935122024227 (accessed on 3 July 2024).
- Association of Short-Term PM2.5 Exposure with Airway Innate Immune Response, Microbiota and Metabolism Alterations in Human Airways—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0269749124001490 (accessed on 3 July 2024).
- Enrichment of Human Nasopharyngeal Bacteriome with Bacteria from Dust after Short-Term Exposure to Indoor Environment: A Pilot Study|BMC Microbiology. Available online: https://link.springer.com/article/10.1186/s12866-023-02951-5 (accessed on 3 July 2024).
- Chen, Y.-W.; Li, S.-W.; Lin, C.-D.; Huang, M.-Z.; Lin, H.-J.; Chin, C.-Y.; Lai, Y.-R.; Chiu, C.-H.; Yang, C.-Y.; Lai, C.-H. Fine Particulate Matter Exposure Alters Pulmonary Microbiota Composition and Aggravates Pneumococcus-Induced Lung Pathogenesis. Front. Cell Dev. Biol. 2020, 8, 570484. [Google Scholar] [CrossRef] [PubMed]
- The Effect of Real-Ambient PM2.5 Exposure on the Lung and Gut Microbiomes and the Regulation of Nrf2—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0147651323002063 (accessed on 3 July 2024).
- Daniel, S.; Phillippi, D.; Schneider, L.J.; Nguyen, K.N.; Mirpuri, J.; Lund, A.K. Exposure to Diesel Exhaust Particles Results in Altered Lung Microbial Profiles, Associated with Increased Reactive Oxygen Species/Reactive Nitrogen Species and Inflammation, in C57Bl/6 Wildtype Mice on A High-Fat Diet. Part. Fibre Toxicol. 2021, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Phillippi, D.T.; Daniel, S.; Pusadkar, V.; Youngblood, V.L.; Nguyen, K.N.; Azad, R.K.; McFarlin, B.K.; Lund, A.K. Inhaled Diesel Exhaust Particles Result in Microbiome-Related Systemic Inflammation and Altered Cardiovascular Disease Biomarkers in C57Bl/6 Male Mice. Part. Fibre Toxicol. 2022, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; He, F.; Liao, B.; Zhou, Y.; Li, B.; Ran, P. Exposure to Ambient Particulate Matter alters the Microbial Composition and Induces Immune Changes in Rat Lung. Respir. Res. 2017, 18, 143. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Liu, L.; Wang, Q.; Zeng, J.; Chen, C. PM2. 5 Exposure Perturbs Lung Microbiome and Its Metabolic Profile in Mice. Sci. Total Environ. 2020, 721, 137432. [Google Scholar] [CrossRef]
- Mazumder, M.H.H.; Gandhi, J.; Majumder, N.; Wang, L.; Cumming, R.I.; Stradtman, S.; Velayutham, M.; Hathaway, Q.A.; Shannahan, J.; Hu, G. Lung-Gut Axis of Microbiome Alterations Following Co-Exposure to Ultrafine Carbon Black and Ozone. Part. Fibre Toxicol. 2023, 20, 15. [Google Scholar] [CrossRef]
- Laiman, V.; Lo, Y.-C.; Chen, H.-C.; Yuan, T.-H.; Hsiao, T.-C.; Chen, J.-K.; Chang, C.-W.; Lin, T.-C.; Li, S.-J.; Chen, Y.-Y.; et al. Effects of Antibiotics and Metals on Lung and Intestinal Microbiome Dysbiosis after Sub-Chronic Lower-Level Exposure of Air Pollution in Ageing Rats. Ecotoxicol. Environ. Saf. 2022, 246, 114164. [Google Scholar] [CrossRef]
- Harris, M.A.; Reddy, C.A.; Carter, G.R. Anaerobic Bacteria from the Large Intestine of Mice. Appl. Environ. Microbiol. 1976, 31, 907–912. [Google Scholar] [CrossRef]
- Schloss, P.D.; Handelsman, J. Status of the Microbial Census. Microbiol. Mol. Biol. Rev. 2004, 68, 686–691. [Google Scholar] [CrossRef]
- Swidsinski, A.; Loening-Baucke, V.; Lochs, H.; Hale, L.P. Spatial Organization of Bacterial Flora in Normal and Inflamed Intestine: A Fluorescence in Situ Hybridization Study in Mice. World J. Gastroenterol. WJG 2005, 11, 1131. [Google Scholar] [CrossRef]
- Bartolomaeus, H.; Balogh, A.; Yakoub, M.; Homann, S.; Markó, L.; Höges, S.; Tsvetkov, D.; Krannich, A.; Wundersitz, S.; Avery, E.G. Short-Chain Fatty Acid Propionate Protects from Hypertensive Cardiovascular Damage. Circulation 2019, 139, 1407–1421. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, X.; Xu, Y.; Wu, W.; Tang, W.; Chen, Z.; Ji, G.; Peng, J.; Jiang, Q.; Xiao, J. Gut Microbiota Partially Mediates the Effects of Fine Particulate Matter on Type 2 Diabetes: Evidence from a Population-Based Epidemiological Study. Environ. Int. 2019, 130, 104882. [Google Scholar] [CrossRef] [PubMed]
- Van Pee, T.; Nawrot, T.S.; van Leeuwen, R.; Hogervorst, J. Ambient Particulate Air Pollution and the Intestinal Microbiome; a Systematic Review of Epidemiological, in Vivo and, in Vitro Studies. Sci. Total Environ. 2023, 878, 162769. [Google Scholar] [CrossRef] [PubMed]
- Roslund, M.I.; Rantala, S.; Oikarinen, S.; Puhakka, R.; Hui, N.; Parajuli, A.; Laitinen, O.H.; Hyöty, H.; Rantalainen, A.-L.; Sinkkonen, A. Endocrine Disruption and Commensal Bacteria Alteration Associated with Gaseous and Soil PAH Contamination among Daycare Children. Environ. Int. 2019, 130, 104894. [Google Scholar] [CrossRef] [PubMed]
- Fouladi, F.; Bailey, M.J.; Patterson, W.B.; Sioda, M.; Blakley, I.C.; Fodor, A.A.; Jones, R.B.; Chen, Z.; Kim, J.S.; Lurmann, F. Air Pollution Exposure Is Associated with the Gut Microbiome as Revealed by Shotgun Metagenomic Sequencing. Environ. Int. 2020, 138, 105604. [Google Scholar] [CrossRef]
- Zheng, P.; Zhang, B.; Zhang, K.; Lv, X.; Wang, Q.; Bai, X. The Impact of Air Pollution on Intestinal Microbiome of Asthmatic Children: A Panel Study. BioMed Res. Int. 2020, 2020, 5753427. [Google Scholar] [CrossRef]
- Alderete, T.L.; Jones, R.B.; Chen, Z.; Kim, J.S.; Habre, R.; Lurmann, F.; Gilliland, F.D.; Goran, M.I. Exposure to Traffic-Related Air Pollution and the Composition of the Gut Microbiota in Overweight and Obese Adolescents. Environ. Res. 2018, 161, 472–478. [Google Scholar] [CrossRef]
- Association of Long-Term Exposure to Ambient PM2.5 and Its Constituents with Gut Microbiota: Evidence from a China Cohort—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0048969723021964 (accessed on 3 July 2024).
- Liu, Y.; Wang, T.; Si, B.; Du, H.; Liu, Y.; Waqas, A.; Huang, S.; Zhao, G.; Chen, S.; Xu, A. Intratracheally Instillated Diesel PM2. 5 Significantly Altered the Structure and Composition of Indigenous Murine Gut Microbiota. Ecotoxicol. Environ. Saf. 2021, 210, 111903. [Google Scholar] [CrossRef]
- Mutlu, E.A.; Comba, I.Y.; Cho, T.; Engen, P.A.; Yazıcı, C.; Soberanes, S.; Hamanaka, R.B.; Niğdelioğlu, R.; Meliton, A.Y.; Ghio, A.J. Inhalational Exposure to Particulate Matter Air Pollution Alters the Composition of the Gut Microbiome. Environ. Pollut. 2018, 240, 817–830. [Google Scholar] [CrossRef]
- Dong, X.; Yao, S.; Deng, L.; Li, H.; Zhang, F.; Xu, J.; Li, Z.; Zhang, L.; Jiang, J.; Wu, W. Alterations in the Gut Microbiota and Its Metabolic Profile of PM2. 5 Exposure-Induced Thyroid Dysfunction Rats. Sci. Total Environ. 2022, 838, 156402. [Google Scholar] [CrossRef]
- Dai, S.; Wang, Z.; Yang, Y.; Du, P.; Li, X. PM2. 5 Induced Weight Loss of Mice through Altering the Intestinal Microenvironment: Mucus Barrier, Gut Microbiota, and Metabolic Profiling. J. Hazard. Mater. 2022, 431, 128653. [Google Scholar] [CrossRef] [PubMed]
- van den Brule, S.; Rappe, M.; Ambroise, J.; Bouzin, C.; Dessy, C.; Paquot, A.; Muccioli, G.G.; Lison, D. Diesel Exhaust Particles Alter the Profile and Function of the Gut Microbiota upon Subchronic Oral Administration in Mice. Part. Fibre Toxicol. 2021, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, J.; Chen, M.; Huang, X.; Xie, X.; Li, W.; Cao, Q.; Kan, H.; Xu, Y.; Ying, Z. Exposure to Concentrated Ambient PM2. 5 alters the Composition of Gut Microbiota in a Murine Model. Part. Fibre Toxicol. 2018, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ma, Y.; Ye, S.; Guo, R.; Su, Y.; Du, Q.; Yin, S.; Xiao, F. Ambient NO2 Exposure Induced Cardiotoxicity Associated with Gut Microbiome Dysregulation and Glycerophospholipid Metabolism Disruption. Ecotoxicol. Environ. Saf. 2022, 238, 113583. [Google Scholar] [CrossRef] [PubMed]
- Subchronic Inhalation Exposure to Ultrafine Particulate Matter Alters the Intestinal Microbiome in Various Mouse Models—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0013935124001464 (accessed on 3 July 2024).
- Li, R.; Yang, J.; Saffari, A.; Jacobs, J.; Baek, K.I.; Hough, G.; Larauche, M.H.; Ma, J.; Jen, N.; Moussaoui, N. Ambient Ultrafine Particle Ingestion Alters Gut Microbiota in Association with Increased Atherogenic Lipid Metabolites. Sci. Rep. 2017, 7, 42906. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, K.E.; Demoor, T.; Rauch, M.; Faruqi, A.A.; Jang, S.; Johnson, C.C.; Boushey, H.A.; Zoratti, E.; Ownby, D.; Lukacs, N.W. House Dust Exposure Mediates Gut Microbiome Lactobacillus Enrichment and Airway Immune Defense against Allergens and Virus Infection. Proc. Natl. Acad. Sci. 2014, 111, 805–810. [Google Scholar] [CrossRef]
- Lo Sasso, G.; Phillips, B.W.; Sewer, A.; Battey, J.N.; Kondylis, A.; Talikka, M.; Titz, B.; Guedj, E.; Peric, D.; Bornand, D. The Reduction of DSS-Induced Colitis Severity in Mice Exposed to Cigarette Smoke Is Linked to Immune Modulation and Microbial Shifts. Sci. Rep. 2020, 10, 3829. [Google Scholar] [CrossRef]
- Bhattacharya, S.S.; Yadav, B.; Rosen, L.; Nagpal, R.; Yadav, H.; Yadav, J.S. Crosstalk between Gut Microbiota and Lung Inflammation in Murine Toxicity Models of Respiratory Exposure or Co-Exposure to Carbon Nanotube Particles and Cigarette Smoke Extract. Toxicol. Appl. Pharmacol. 2022, 447, 116066. [Google Scholar] [CrossRef]
- Ghio, A.J.; Carraway, M.S.; Madden, M.C. Composition of Air Pollution Particles and Oxidative Stress in Cells, Tissues, and Living Systems. J. Toxicol. Environ. Health Part B 2012, 15, 1–21. [Google Scholar] [CrossRef]
- Glencross, D.A.; Ho, T.-R.; Camina, N.; Hawrylowicz, C.M.; Pfeffer, P.E. Air Pollution and Its Effects on the Immune System. Free Radic. Biol. Med. 2020, 151, 56–68. [Google Scholar] [CrossRef]
- Rao, X.; Zhong, J.; Brook, R.D.; Rajagopalan, S. Effect of Particulate Matter Air Pollution on Cardiovascular Oxidative Stress Pathways. Antioxid. Redox Signal. 2018, 28, 797–818. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Lakey, P.S.; Weber, R.J.; Shiraiwa, M. Oxidative Potential of Particulate Matter and Generation of Reactive Oxygen Species in Epithelial Lining Fluid. Environ. Sci. Technol. 2019, 53, 12784–12792. [Google Scholar] [CrossRef] [PubMed]
- Leni, Z.; Künzi, L.; Geiser, M. Air Pollution Causing Oxidative Stress. Curr. Opin. Toxicol. 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Shishodia, S.; Potdar, P.; Gairola, C.G.; Aggarwal, B.B. Curcumin (Diferuloylmethane) down-Regulates Cigarette Smoke-Induced NF-kappaB Activation through Inhibition of IkappaBalpha Kinase in Human Lung Epithelial Cells: Correlation with Suppression of COX-2, MMP-9 and Cyclin D1. Carcinogenesis 2003, 24, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Milara, J.; Peiró, T.; Serrano, A.; Cortijo, J. Epithelial to Mesenchymal Transition Is Increased in Patients with COPD and Induced by Cigarette Smoke. Thorax 2013, 68, 410–420. [Google Scholar] [CrossRef]
- Caraballo, J.C.; Yshii, C.; Westphal, W.; Moninger, T.; Comellas, A.P. Ambient Particulate Matter Affects Occludin Distribution and Increases Alveolar Transepithelial Electrical Conductance. Respirology 2011, 16, 340–349. [Google Scholar] [CrossRef]
- Kish, L.; Hotte, N.; Kaplan, G.G.; Vincent, R.; Tso, R.; Gänzle, M.; Rioux, K.P.; Thiesen, A.; Barkema, H.W.; Wine, E. Environmental Particulate Matter Induces Murine Intestinal Inflammatory Responses and Alters the Gut Microbiome. PLoS ONE 2013, 8, e62220. [Google Scholar] [CrossRef]
- Invernizzi, R.; Lloyd, C.M.; Molyneaux, P.L. Respiratory Microbiome and Epithelial Interactions Shape Immunity in the Lungs. Immunology 2020, 160, 171–182. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Moreno-Vinasco, L.; Lang, G.D.; Siegler, J.H.; Mathew, B.; Usatyuk, P.V.; Samet, J.M.; Geyh, A.S.; Breysse, P.N. Particulate Matter Air Pollution Disrupts Endothelial Cell Barrier via Calpain-Mediated Tight Junction Protein Degradation. Part. Fibre Toxicol. 2012, 9, 35. [Google Scholar] [CrossRef]
- Woodby, B.; Schiavone, M.L.; Pambianchi, E.; Mastaloudis, A.N.; Hester, S.; M. Wood, S.; Pecorelli, A.; Valacchi, G. Particulate Matter Decreases Intestinal Barrier-Associated Proteins Levels in 3D Human Intestinal Model. Int. J. Environ. Res. Public Health 2020, 17, 3234. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Dou, M.; He, H.; Ju, M.; Ji, S.; Zhou, J.; Chen, C.; Zhang, D.; Miao, C. Particulate Matter Disrupts Airway Epithelial Barrier via Oxidative Stress to Promote Pseudomonas Aeruginosa Infection. J. Thorac. Dis. 2019, 11, 2617. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cui, J.; Yang, H.; Sun, H.; Lu, R.; Gao, N.; Meng, Q.; Wu, S.; Wu, J.; Aschner, M. Colonic Injuries Induced by Inhalational Exposure to Particulate-Matter Air Pollution. Adv. Sci. 2019, 6, 1900180. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Chen, G.; Subramani, D.B.; Wolf, M.; Gilmore, R.C.; Kato, T.; Radicioni, G.; Kesimer, M.; Chua, M.; Dang, H. Localization of Secretory Mucins MUC5AC and MUC5B in Normal/Healthy Human Airways. Am. J. Respir. Crit. Care Med. 2019, 199, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Min, H.J.; Kim, J.-H.; Yoo, J.E.; Oh, J.-H.; Kim, K.S.; Yoon, J.-H.; Kim, C.-H. ROS-Dependent HMGB1 Secretion Upregulates IL-8 in upper Airway Epithelial Cells under Hypoxic Condition. Mucosal Immunol. 2017, 10, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Aghapour, M.; Raee, P.; Moghaddam, S.J.; Hiemstra, P.S.; Heijink, I.H. Airway Epithelial Barrier Dysfunction in Chronic Obstructive Pulmonary Disease: Role of Cigarette Smoke Exposure. Am. J. Respir. Cell Mol. Biol. 2018, 58, 157–169. [Google Scholar] [CrossRef]
- Wang, T.; Han, Y.; Li, H.; Wang, Y.; Xue, T.; Chen, X.; Chen, W.; Fan, Y.; Qiu, X.; Gong, J. Changes in Bioactive Lipid Mediators in Response to Short-Term Exposure to Ambient Air Particulate Matter: A Targeted Lipidomic Analysis of Oxylipin Signaling Pathways. Environ. Int. 2021, 147, 106314. [Google Scholar] [CrossRef]
- Zhivaki, D.; Kagan, J.C. Innate Immune Detection of Lipid Oxidation as a Threat Assessment Strategy. Nat. Rev. Immunol. 2022, 22, 322–330. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of Polystyrene Microplastic on the Gut Barrier, Microbiota and Metabolism of Mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Franco-Lira, M.; Torres-Jardón, R.; Henriquez-Roldán, C.; Barragán-Mejía, G.; Valencia-Salazar, G.; Gonzaléz-Maciel, A.; Reynoso-Robles, R.; Villarreal-Calderón, R.; Reed, W. Pediatric Respiratory and Systemic Effects of Chronic Air Pollution Exposure: Nose, Lung, Heart, and Brain Pathology. Toxicol. Pathol. 2007, 35, 154–162. [Google Scholar] [CrossRef]
- Young, R.P.; Hopkins, R.J.; Marsland, B. The Gut–Liver–Lung Axis. Modulation of the Innate Immune Response and Its Possible Role in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Cell Mol. Biol. 2016, 54, 161–169. [Google Scholar] [CrossRef]
- Salim, S.Y.; Kaplan, G.G.; Madsen, K.L. Air Pollution Effects on the Gut Microbiota: A Link between Exposure and Inflammatory Disease. Gut Microbes 2014, 5, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Vatanen, T.; Kostic, A.D.; d’Hennezel, E.; Siljander, H.; Franzosa, E.A.; Yassour, M.; Kolde, R.; Vlamakis, H.; Arthur, T.D.; Hämäläinen, A.-M.; et al. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell 2016, 165, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Budden, K.F.; Gellatly, S.L.; Wood, D.L.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging Pathogenic Links between Microbiota and the Gut–Lung Axis. Nat. Rev. Microbiol. 2017, 15, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Ali, S.A. Probiotics and gut microbiota: Mechanistic Insights into Gut Immune Homeostasis through TLR Pathway Regulation. Food Funct. 2022, 13, 7423–7447. [Google Scholar] [CrossRef]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef]
- Semin, I.; Ninnemann, J.; Bondareva, M.; Gimaev, I.; Kruglov, A.A. Interplay between Microbiota, Toll-like Receptors and Cytokines for the Maintenance of Epithelial Barrier Integrity. Front. Med. 2021, 8, 644333. [Google Scholar] [CrossRef]
- Thorburn, A.N.; Macia, L.; Mackay, C.R. Diet, Metabolites, and “Western-Lifestyle” Inflammatory Diseases. Immunity 2014, 40, 833–842. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Bhutia, Y.D.; Yang, S.; Ganapathy, V. Short-Chain Fatty Acid Transporters: Role in Colonic Homeostasis. Compr. Physiol. 2017, 8, 299–314. [Google Scholar] [CrossRef]
- Mazmanian, S.K.; Round, J.L.; Kasper, D.L. A Microbial Symbiosis Factor Prevents Intestinal Inflammatory Disease. Nature 2008, 453, 620–625. [Google Scholar] [CrossRef]
- Sharon, G.; Garg, N.; Debelius, J.; Knight, R.; Dorrestein, P.C.; Mazmanian, S.K. Specialized Metabolites from the Microbiome in Health and Disease. Cell Metab. 2014, 20, 719–730. [Google Scholar] [CrossRef]
- Chen, L.-W.; Chen, P.-H.; Hsu, C.-M. Commensal Microflora Contribute to Host Defense against Escherichia Coli Pneumonia through Toll-like Receptors. Shock 2011, 36, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Tsay, T.-B.; Yang, M.-C.; Chen, P.-H.; Hsu, C.-M.; Chen, L.-W. Gut Flora Enhance Bacterial Clearance in Lung through Toll-like Receptors 4. J. Biomed. Sci. 2011, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.N.; Yadav, J.S. Lung and Gut Microbiota Interactions with Air Pollution and Aging in Human Chronic Diseases. In Gut Microbiota in Aging and Chronic Diseases; Marotta, F., Ed.; Healthy Ageing and Longevity; Springer International Publishing: Cham, Switzerland, 2023; Volume 17, pp. 215–236. ISBN 978-3-031-14022-8. [Google Scholar]
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The Gut–Liver Axis and the Intersection with the Microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hall, S.; Vitetta, L. Altered Gut Microbial Metabolites Could Mediate the Effects of Risk Factors in COVID-19. Rev. Med. Virol. 2021, 31, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; De Minicis, S.; Osterreicher, C.H.; Kluwe, J.; Osawa, Y.; Brenner, D.A.; Schwabe, R.F. TLR4 Enhances TGF-Beta Signaling and Hepatic Fibrosis. Nat. Med. 2007, 13, 1324–1332. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Trauner, M. Gut-Liver Axis: Pathophysiological Concepts and Clinical Implications. Cell Metab. 2022, 34, 1700–1718. [Google Scholar] [CrossRef]
- Anand, S.; Mande, S.S. Host-Microbiome Interactions: Gut-Liver Axis and Its Connection with Other Organs. NPJ Biofilms Microbiomes 2022, 8, 89. [Google Scholar] [CrossRef]
- Hilliard, K.L.; Allen, E.; Traber, K.E.; Yamamoto, K.; Stauffer, N.M.; Wasserman, G.A.; Jones, M.R.; Mizgerd, J.P.; Quinton, L.J. The Lung-Liver Axis: A Requirement for Maximal Innate Immunity and Hepatoprotection during Pneumonia. Am. J. Respir. Cell Mol. Biol. 2015, 53, 378–390. [Google Scholar] [CrossRef]
- Herrero, R.; Sánchez, G.; Asensio, I.; López, E.; Ferruelo, A.; Vaquero, J.; Moreno, L.; de Lorenzo, A.; Bañares, R.; Lorente, J.A. Liver-Lung Interactions in Acute Respiratory Distress Syndrome. Intensive Care Med. Exp. 2020, 8, 48. [Google Scholar] [CrossRef]
- Petschow, B.; Doré, J.; Hibberd, P.; Dinan, T.; Reid, G.; Blaser, M.; Cani, P.D.; Degnan, F.H.; Foster, J.; Gibson, G. Probiotics, Prebiotics, and the Host Microbiome: The Science of Translation. Ann. N. Y. Acad. Sci. 2013, 1306, 1–17. [Google Scholar] [CrossRef]
- Chen, W.-X.; Ren, L.-H.; Shi, R.-H. Enteric Microbiota Leads to New Therapeutic Strategies for Ulcerative Colitis. World J. Gastroenterol. WJG 2014, 20, 15657. [Google Scholar] [CrossRef] [PubMed]
- Udayappan, S.D.; Hartstra, A.V.; Dallinga-Thie, G.M.; Nieuwdorp, M. Intestinal Microbiota and Faecal Transplantation as Treatment Modality for Insulin Resistance and Type 2 Diabetes Mellitus. Clin. Exp. Immunol. 2014, 177, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Dietert, R.R.; Dietert, J.M. The Microbiome and Sustainable Healthcare. Healthcare 2015, 3, 100–129. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Round, J.L. Defining Dysbiosis and Its Influence on Host Immunity and Disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef]

| Body Site | Dominant Bacterial Communities |
|---|---|
| Skin | Corynebacteria, Propionibacteria, Staphylococcus, Streptococcus, Moraxella, Dolosigranulum |
| Oral cavity | Prevotella, Veillonella, Streptococcus, Corynebacteria, Neisseria, Haemophilus, Fusobacterium, Rothia |
| Lung | Prevotella, Streptococcus, Haemophilus, Fusobacterium, Actinobacteria |
| Gut | Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria |
| Urogenital tract | Prevotella, Gardnerella, Atopobium, Lactobacillus, Escherichia, Enterococcus, Shigella, Streptococcus, Citrobacter |
| Condition | Taxa (Major Genera) |
|---|---|
| Healthy [53] | Veillonella, Fusobacterium, Prevotella, Streptococcus, Porphyromonas, Neisseria |
| Asthma [54] | Haemophilus, Streptococcus, Prevotella, Klebsiella, Moraxella |
| COPD [55,56] | Moraxella, Streptococcus, Haemophilus, Streptococcus, Pseudomonas |
| Lung cancer [57,58] | Streptococcus, Abiotrophia, Granulicatella, Veilonella, Staphylococcus, Haemophilus |
| Cystic fibrosis [59,60] | Streptococcus, Prevotella, Veillonella, Gemella, Neissera, Rothia, Actynomyces, Haemophilus |
| Idiopathic pulmonary fibrosis [61,62] | Hemophilus, Neisseria, Streptococcus, Staphylococcus, Veillonella |
| Study Population | Exposure | Sample Type | Results Summary |
|---|---|---|---|
| Healthy and COPD Volunteers [70] | PM2.5 | Sputum sample | Higher FEV1/FVC ratio to bacterial load (+), OTU (+) |
| Farmer’s Market Vendors [69] | PM2.5−200 ug/m3, PM10−300 ug/m3 | Pharyngeal swabs | Chao1 ↑, ACE ↑, correlation with microbiome: smog (+), gender (+), smoking (+), mask (−) |
| Healthy Volunteers [71] | PM2.5, PM10 | Nasal swab | Shannon (−), Chao1 (−), PD whole tree (−) |
| Asthmatic Children [73] | PM2.5 or ozone | Broncho alveolar lavage | Species richness (−), observed species (−) |
| Young Adults [74] | PM2.5 | Sputum | Cytokine ↑: IL4, IL6, IL17, TNF−a, IFN−g |
| Healthy Volunteers [65] | PM2.5, PM10, NO | Throat swab | Lung function ↓ |
| Lung Cancer Patients [72] | PM10 | Lung tissues | PD whole tree (+) |
| Children [64] | Traffic-related air pollution | Saliva and sputum | Shannon ↑, observed ASV ↑, phylogenetic diversity ↑ |
| Healthy and Lung Cancer Females [67] | Household air pollution | Sputum | Alpha diversity (−), observed species (−), unweighted UniFrac (+) |
| Healthy Subjects [66] | Household air pollution | Broncho alveolar lavage | No alpha, beta diversity change |
| Adults [75] | Indoor dust | Nasopharyngeal swabs | ASV, Shannon |
| Healthy Young Adults [68] | O3−200 ppb; 2 h | Nasal secretion | Serum CC16 ↑, FEV1 ↓, FVC ↓, glucose ↑, lactic acid ↑, D−glyceric acid ACE ↓, Simpson ↓, Shannon ↓, weighted UniFrac (#) |
| Study Population | Exposure | Sample Type | Results Summary |
|---|---|---|---|
| Children between ages 3 and 5 [90] | Air PAH level | Soil, stool, skin | PPAR (+), adipocytokine signaling pathway (+) |
| Young adults [88] | PM2.5, PM1 | Stool | Type 2 diabetes (+), Shannon (−), Chao1 (−), PD whole tree (−) |
| Adults [94] | PM2.5 | Stool | Shannon ↓ |
| Children [92] | PM10, PM2.5, smog | Gut | No Shannon and Chao1 index difference No weighted and unweighted UniFrac change |
| Adults [93] | Traffic-related air pollution, nitrogen oxides | Gut | Impaired glucose homeostasis |
| Young adults [91] | Air pollution | Gut | Shannon ↑ |
| Study Population | Exposure | Sample Type | Results Summary |
|---|---|---|---|
| C57BL/6J mice, male [96] | PM2.5 | Gut and gut content | ↑ Feces: observed OUT, Chao1, PD whole tree, unweighted UniFrac (+), Bray–Curtis similarity (+) |
| BALB/c mice, male [98] | PM2.5 | Gut | IL-6 ↑, IL-8 ↑, TNF-a ↑, OTU ↓, Chao1 ↑, Shannon↑ |
| C57BL/6 mice, male [82] | CB, O3 and CB + O3 | Fecal content | Total bacterial load ↑, SCFA: acetate ↑, propionate ↑ |
| Sprague Dawley rats, male [97] | PM2.5 | Stool | Shannon ↑, Chao1 ↑, Simpson ↓, ACE ↑, weighted UniFrac (+) |
| C57BL/6 mice, male [102] | Ultra-fine particles | Fecal content | ASV ↑, Shannon ↑ |
| Ldr KO mice [103] | Ultra-fine particles | Gut content | Chao1 ↓, Faith PD ↓, Shannon ↓, unweighted UniFrac (+), weighted UniFrac (+), ↑plasma: TNF-a, MCP-1, LPC18:1 |
| C57BL/6J mice, male [100] | Concentrated ambient particle | Feces | Glucose intolerance (+), insulin resistance (+), ACE ↓, Chao1 ↓ |
| C57BL/6 mice, female [99] | Diesel exhaust particle | Gut content | Shannon ↓, Simpson ↓, weighted UniFrac (+) Cecal SCFA ↓, triglycerides ↓ |
| C57BL/6 mice, male [79] | Diesel exhaust particle | Gut content | Chao1 ↓, ACE ↓, plasma LPS ↑, IL-13 ↑, G-CSF ↑, MIP-2 ↑, TNF-a ↑ |
| C57BL/6J mice, male [95] | Diesel exhaust particle | Feces | OUT ↓, Chao1 ↓, Shannon ↓, Goods coverage ↑, unweighted UniFrac (+) |
| BABL/c mice, female [104] | House dust | Gut content | Fast UniFrac (+), ↓ lung: IL-13, IL-4 |
| C57BL/6 mice, male [105] | Cigarette smoke | Gut content | Shannon ↓, Muc5b ↑, Muc4 ↓ |
| C57BL/6 mice [106] | Carbon nanotube + cigarette smoke | Feces | Shannon ↑, Chao1 ↑, total protein content ↑, CXCL1 ↑, TGF-beta ↑ |
| Sprague Dawley rats, male [101] | Ambient NO2 | Gut | PD whole tree ↑, unweighted UniFrac (+), cardiac mfn2 ↓, HSP70↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazumder, M.H.H.; Hussain, S. Air-Pollution-Mediated Microbial Dysbiosis in Health and Disease: Lung–Gut Axis and Beyond. J. Xenobiot. 2024, 14, 1595-1612. https://doi.org/10.3390/jox14040086
Mazumder MHH, Hussain S. Air-Pollution-Mediated Microbial Dysbiosis in Health and Disease: Lung–Gut Axis and Beyond. Journal of Xenobiotics. 2024; 14(4):1595-1612. https://doi.org/10.3390/jox14040086
Chicago/Turabian StyleMazumder, Md Habibul Hasan, and Salik Hussain. 2024. "Air-Pollution-Mediated Microbial Dysbiosis in Health and Disease: Lung–Gut Axis and Beyond" Journal of Xenobiotics 14, no. 4: 1595-1612. https://doi.org/10.3390/jox14040086
APA StyleMazumder, M. H. H., & Hussain, S. (2024). Air-Pollution-Mediated Microbial Dysbiosis in Health and Disease: Lung–Gut Axis and Beyond. Journal of Xenobiotics, 14(4), 1595-1612. https://doi.org/10.3390/jox14040086








