Environmental Stress-Induced Alterations in Embryo Developmental Morphokinetics
Abstract
1. Embryo Morphokinetics
2. Potential Factors That Affect Embryo Morphokinetics
3. Environmental Stressors
3.1. Effect of Heat Stress on the Embryo Developmental Morphokinetics
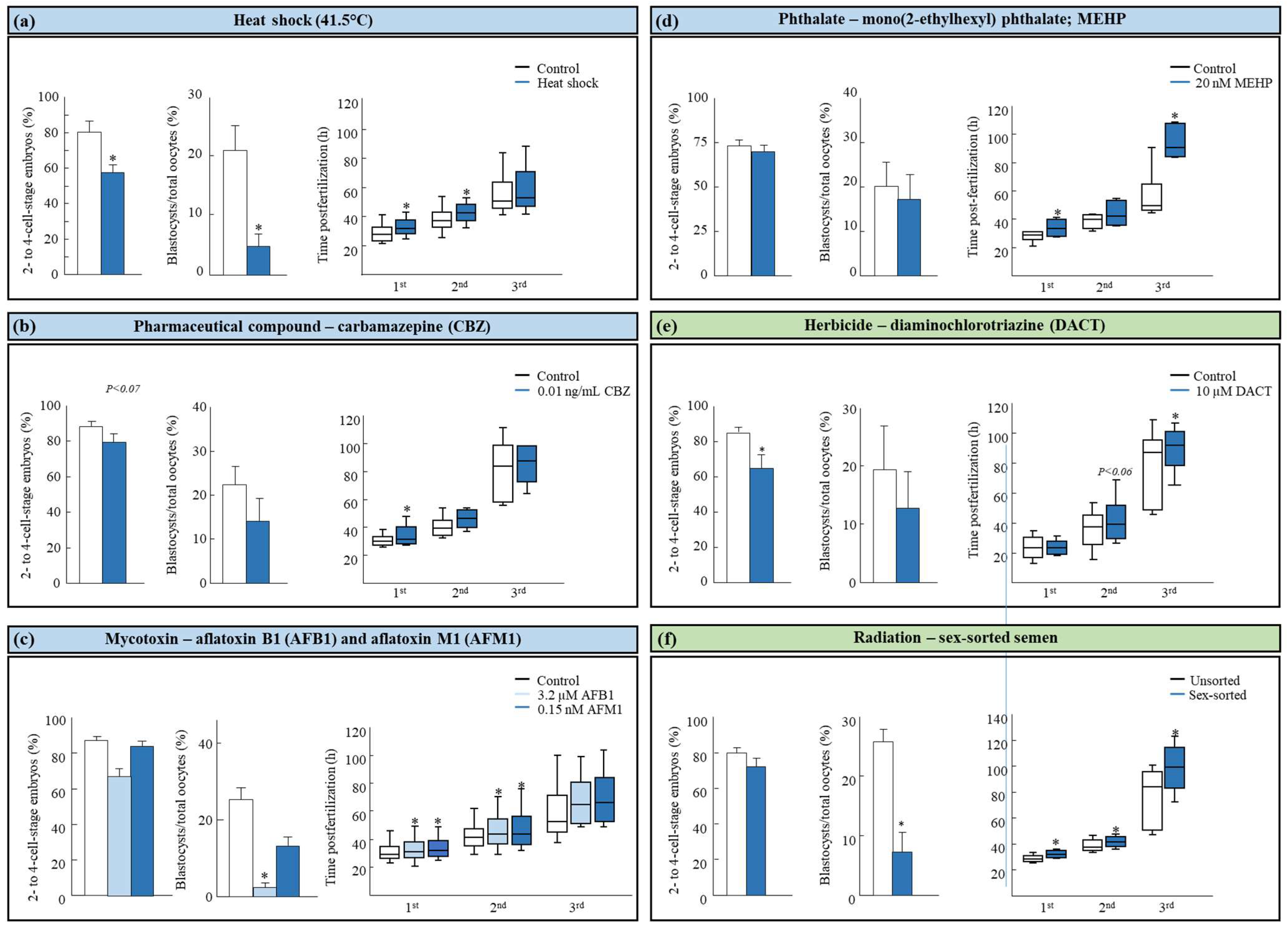
3.2. Effect of Human-Made Chemicals on the Embryo Developmental Morphokinetics
3.3. Effect of Natural Occurring Compounds on the Embryo Developmental Morphokinetics
3.4. Effect of Radiation on the Embryo Developmental Morphokinetics
The Impact of Radiation on the Spermatozoa in the Embryo Developmental Morphokinetics
4. Potential Protective Compounds to Preserve the Embryo Morphokinetics
5. Synopsis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Payne, D.; Flaherty, S.P.; Barry, M.F.; Matthews, C.D. Preliminary observations on polar body extrusion and pronuclear formation in human oocytes using time-lapse video cinematography. Hum. Reprod. 1997, 12, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Lemmen, J.G.; Agerholm, I.; Ziebe, S. Kinetic markers of human embryo quality using time-lapse recordings of IVF/ICSI-fertilized oocytes. Reprod. Biomed. Online 2008, 17, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, S.; Akai, T.; Hashiyada, Y.; Somfai, T.; Inaba, Y.; Hirayama, M.; Yamanouchi, T.; Matsuda, H.; Kobayashi, S.; Aikawa, Y.; et al. Promising system for selecting healthy in vitro-fertilized embryos in cattle. PLoS ONE 2012, 7, e36627. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Loewke, K.E.; Bossert, N.L.; Behr, B.; De Jonge, C.J.; Baer, T.M.; Reijo Pera, R.A. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat. Biotechnol. 2010, 28, 1115–1121. [Google Scholar] [CrossRef]
- Somfai, T.; Inaba, Y.; Aikawa, Y.; Ohtake, M.; Kobayashi, S.; Konishi, K.; Imai, K. Relationship between the length of cell cycles, cleavage pattern and developmental competence in bovine embryos generated by in vitro fertilization or parthenogenesis. J. Reprod. Dev. 2010, 56, 200–207. [Google Scholar] [CrossRef]
- Mizobe, Y.; Tokunaga, M.; Oya, N.; Iwakiri, R.; Yoshida, N.; Sato, Y.; Onoue, N.; Ezono, Y. Synchrony of the first division as an index of the blastocyst formation rate during embryonic development. Reprod. Med. Biol. 2017, 17, 64–70. [Google Scholar] [CrossRef]
- Meseguer, M.; Herrero, J.; Tejera, A.; Hilligsøe, K.M.; Ramsing, N.B.; Remoh, J. The use of morphokinetics as a predictor of embryo implantation. Hum. Reprod. 2011, 26, 2658–2671. [Google Scholar] [CrossRef]
- Huayhua, C.; Rodríguez, M.; Vega, J.; Briones, M.; Rodriguez-Alvarez, L.; Mellisho, E. Blastulation time measured with time-lapse system can predict in vitro viability of bovine blastocysts. PLoS ONE 2023, 18, e0289751. [Google Scholar] [CrossRef]
- Huang, T.T.; Chinn, K.; Kosasa, T.; Ahn, H.J.; Kessel, B. Morphokinetics of human blastocyst expansion in vitro. Reprod. Biomed. Online 2016, 33, 659–667. [Google Scholar] [CrossRef]
- Magata, F. Time-lapse monitoring technologies for the selection of bovine in vitro fertilized embryos with high implantation potential. Reprod. Dev. 2023, 69, 57–64. [Google Scholar] [CrossRef]
- Giménez, C.; Conversa, L.; Murria, L.; Meseguer, M. Time-lapse imaging: Morphokinetic analysis of in vitro fertilization outcomes. Fertil. Steril. 2023, 120, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Yaacobi-Artzi, S.; Kalo, D.; Roth, Z. Association between the morphokinetics of in-vitro-derived bovine embryos and the transcriptomic profile of the derived blastocysts. PLoS ONE 2022, 17, e0276642. [Google Scholar] [CrossRef] [PubMed]
- Yaacobi-Artzi, S.; Kalo, D.; Roth, Z. Morphokinetics of in vitro-derived embryos—A lesson from human and bovine studies. Dairy 2024, 5, 419–435. [Google Scholar] [CrossRef]
- Zhan, Q.; Ye, Z.; Clarke, R.; Rosenwaks, Z.; Zaninovic, N. Direct unequal cleavages: Embryo developmental competence, genetic constitution and clinical outcome. PLoS ONE 2016, 11, e0166398. [Google Scholar] [CrossRef]
- Hardarson, T.; Hanson, C.; Sjögren, A.; Lundin, K. Human embryos with unevenly sized blastomeres have lower pregnancy and implantation rates: Indications for aneuploidy and multinucleation. Hum. Reprod. 2001, 16, 313–318. [Google Scholar] [CrossRef]
- Sugimura, S.; Akai, T.; Somfai, T.; Hirayama, M.; Aikawa, Y.; Ohtake, M.; Hattori, H.; Kobayashi, S.; Hashiyada, Y.; Konishi, K.; et al. Time-Lapse Cinematography-Compatible Polystyrene-Based Microwell Culture System: A Novel Tool for Tracking the Development of Individual Bovine Embryos. Biol. Reprod. 2010, 83, 97–978. [Google Scholar] [CrossRef]
- Liu, Y.; Chapple, V.; Roberts, P.; Matson, P. Prevalence, consequence, and significance of reverse cleavage by human embryos viewed with the use of the Embryoscope time-lapse video system. Fertil. Steril. 2014, 102, 1295–1300. [Google Scholar] [CrossRef]
- Van Royen, E.V.; Mangelschots, K.; Vercruyssen, M.; Neubourg, D.D.; Valkenburg, M.; Ryckaert, G.; Gerris, J. Multinucleation in cleavage stage embryos. Hum. Reprod. 2003, 18, 1062–1069. [Google Scholar] [CrossRef]
- Gardner, D.K. Blastocyst culture: Toward single embryo transfers. Hum. Fertil. 2000, 3, 229–237. [Google Scholar] [CrossRef]
- Stringfellow, D.A.; Seide, S.M. Manual of the International Embryo Transfer Society: A Procedural Guide and General Information for the Use of Embryo Transfer Technology Emphasizing Sanitary Procedures, 3rd ed.; International Embryo Transfer Society (IETS): Savory, IL, USA, 1998. [Google Scholar]
- Fenwick, J.; Platteau, P.; Murdoch, A.P.; Herbert, M. Time from insemination to first cleavage predicts developmental competence of human preimplantation embryos in vitro. Hum. Reprod. 2002, 17, 407–412. [Google Scholar] [CrossRef]
- Milewski, R.; Kuć, P.; Kuczyńska, A.; Stankiewicz, B.; Łukaszuk, K.; Kuczyński, W. A predictive model for blastocyst formation based on morphokinetic parameters in time lapse monitoring of embryo development. J. Assist. Reprod. Genet. 2015, 32, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Rubio, I.; Kuhlmann, R.; Agerholm, I.; Kirk, J.; Herrero, J.; Escribá, M.J.; Bellver, J.; Meseguer, M. Limited implantation success of direct-cleaved human zygotes: A time-lapse study. Fertil. Steril. 2012, 98, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Holm, P.; Shukri, N.N.; Vajta, G.; Booth, P.; Bendixen, C.; Callesen, H. Developmental kinetics of the first cell cycles of bovine in vitro produced embryos in relation to their in vitro viability and sex. Theriogenology 1998, 50, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Kalo, D.; Roth, Z.; (Department of Animal Sciences, Robert H. Smith Faculty of Agriculture, Food and Environment, The Hebrew University, Rehovot, Israel). Morphokinetic of Bovine Embryos. 2023; (Unpublished work). [Google Scholar]
- Ebner, T.; Moser, M.; Sommergruber, M.; Tews, G. Selection based on morphological assessment of oocytes and embryos at different stages of preimplantation development: A review. Hum. Reprod. Update 2003, 9, 251–262. [Google Scholar] [CrossRef]
- Bellver, J.; Mifsud, A.; Grau, N.; Privitera, L.; Meseguer, M. Similar morphokinetic patterns in embryos derived from obese and normoweight infertile women: A time-lapse study. Hum. Reprod. 2013, 28, 794–800. [Google Scholar] [CrossRef]
- Fréour, T.; Dessolle, L.; Lammers, J.; Lattes, S.; Barrière, P. Comparison of embryo morphokinetics after in vitro fertilization-intracytoplasmic sperm injection in smoking and nonsmoking women. Fertil. Steril. 2013, 99, 1944–1950. [Google Scholar] [CrossRef]
- Hoek, J.; Schoenmakers, S.; van Duijn, L.; Willemsen, S.P.; van Marion, E.S.; Laven, J.S.E.; Baart, E.B.; Steegers-Theunissen, R.P.M. A higher preconceptional paternal body mass index influences fertilization rate and preimplantation embryo development. Andrology 2022, 10, 486–494. [Google Scholar] [CrossRef]
- Setti, A.S.; Braga, D.P.A.F.; Vingris, L.; Iaconelli, A., Jr.; Borges, E., Jr. Early and late paternal contribution to cell division of embryos in a time-lapse imaging incubation system. Andrologia 2021, 53, e14211. [Google Scholar] [CrossRef]
- Dal Canto, M.; Bartolacci, A.; Turchi, D.; Pignataro, D.; Lain, M.; De Ponti, E.; Brigante, C.; Mignini Renzini, M.; Buratini, J. Faster fertilization and cleavage kinetics reflect competence to achieve a live birth after intracytoplasmic sperm injection, but this association fades with maternal age. Fertil. Steril. 2021, 115, 665–672. [Google Scholar] [CrossRef]
- Liao, Q.; Huang, B.; Zhang, S.; Chen, J.; Chen, G.; Li, K.Z.; Ai, J.H. Influence of Different Quality Sperm on Early Embryo Morphokinetic Parameters and Cleavage Patterns: A Retrospective Time-lapse Study. Curr. Med. Sci. 2020, 40, 960–967. [Google Scholar] [CrossRef]
- Borges, E., Jr.; Braga, D.; Provenza, R.; Iaconelli, A., Jr.; Setti, A. Association between embryo morphokinetic development and intracytoplasmic sperm injection with epididymal sperm via time-lapse imaging. Mol. Reprod. Dev. 2024, 91, e23747. [Google Scholar] [CrossRef] [PubMed]
- Ciray, H.N.; Aksoy, T.; Goktas, C.; Ozturk, B.; Bahceci, M. Time-lapse evaluation of human embryo development in single versus sequential culture media--a sibling oocyte study. J. Assist. Reprod. Genet. 2012, 29, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.; Garrido, N.; Gadea, B.; Muñoz, M.; Pérez-Cano, I.; Meseguer, M. Oocyte insemination techniques are related to alterations of embryo developmental timing in an oocyte donation model. Reprod. Biomed. Online 2013, 27, 367–375. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kirkegaard, K.; Hindkjaer, J.J.; Ingerslev, H.J. Effect of oxygen concentration on human embryo development evaluated by time-lapse monitoring. Fertil. Steril. 2013, 99, 738–744.e4. [Google Scholar] [CrossRef]
- Lammers, J.; Reignier, A.; Splingart, C.; Catteau, A.; David, L.; Barriere, P.; Freour, T. Does sperm origin affect embryo morphokinetic parameters? J. Assist. Reprod. Genet. 2015, 32, 1325–1332. [Google Scholar] [CrossRef]
- Akhter, N.; Shahab, M. Morphokinetic analysis of human embryo development and its relationship to the female age: A retrospective time-lapse imaging study. Cell. Mol. Biol. (Noisy-le-grand). 2017, 63, 84–92. [Google Scholar] [CrossRef]
- Faramarzi, A.; Khalili, M.A.; Mangoli, E. Correlations between embryo morphokinetic development and maternal age: Results from an intracytoplasmic sperm injection program. Clin. Exp. Reprod. Med. 2019, 46, 119–124. [Google Scholar] [CrossRef]
- Almagor, M.; Or, Y.; Fieldust, S.; Shoham, Z. Irregular cleavage of early preimplantation human embryos: Characteristics of patients and pregnancy outcomes. J. Assist. Reprod. Genet. 2015, 32, 1811–1815. [Google Scholar] [CrossRef]
- Ezoe, K.; Miki, T.; Akaike, H.; Shimazaki, K.; Takahashi, T.; Tanimura, Y.; Amagai, A.; Sawado, A.; Mogi, M.; Kaneko, S.; et al. Maternal age affects pronuclear and chromatin dynamics, morula compaction and cell polarity, and blastulation of human embryos. Hum. Reprod. 2023, 38, 387–399. [Google Scholar] [CrossRef]
- Apter, S.; Ebner, T.; Freour, T.; Guns, Y.; Kovacic, B.; Le Clef, N.; Marques, M.; Meseguer, M.; Montjean, D.; Sfontouris, I.; et al. Good practice recommendations for the use of time-lapse technology. ESHRE Working group on Time-lapse technology. Hum. Reprod. Open 2020, 2020, hoaa008. [Google Scholar] [CrossRef]
- Higdon, H.L.; Blackhurst, D.W.; Boone, W.R. Incubator management in an assisted reproductive technology laboratory. Fertil. Steril. 2008, 89, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Konstantogianni, O.; Panou, T.; Zikopoulos, A.; Skentou, C.; Stavros, S.; Asimakopoulos, B. Culture of Human Embryos at High and Low Oxygen Levels. J. Clin. Med. 2024, 13, 2222. [Google Scholar] [CrossRef] [PubMed]
- Dumoulin, J.C.; Meijers, C.J.; Bras, M.; Coonen, E.; Geraedts, J.P.; Evers, J.L. Effect of oxygen concentration on human in-vitro fertilization and embryo culture. Hum. Reprod. 1999, 14, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Jelinkova, L.; Brucker, C.; Reeka, N.; Gagsteiger, F. Effect of oxygen concentration on early cleavage of human embryos in vitro. Int. Congr. Ser. 2004, 1271, 147–150. [Google Scholar] [CrossRef]
- Mestres, E.; García-Jiménez, M.; Casals, A.; Cohen, J.; Acacio, M.; Villamar, A.; Matia-Algué, Q.; Calderón, G.; Costa-Borges, N. Factors of the human embryo culture system that may affect media evaporation and osmolality. Hum. Reprod. 2021, 36, 605–613. [Google Scholar] [CrossRef]
- Bossi, R.L.; Pinto, B.C.V.; Sampaio, M.A.C.; Geber, S. How to optimize culture media osmolality during Assisted Reproductive Technologies treatments. JBRA Assist. Reprod. 2023, 27, 35–40. [Google Scholar] [CrossRef]
- Valera, M.A.; Albert, C.; Marcos, J.; Larreategui, Z.; Bori, L.; Meseguer, M. A propensity score-based, comparative study assessing humid and dry time-lapse incubation, with single-step medium, on embryo development and clinical outcomes. Hum. Reprod. 2022, 37, 1980–1993. [Google Scholar] [CrossRef]
- Roth, Z.; Kalo, D.; Komsky-Elbaz, A. Effect of environmental contamination on female and male gametes—A lesson from bovine. Anim. Reprod. 2020, 17, e20200041. [Google Scholar] [CrossRef]
- Komsky-Elbaz, A.; Kalo, D.; Roth, Z. New evidence for deleterious effects of environmental contaminants on the male gamete. Anim Reprod. Sci. 2022, 246, 106886. [Google Scholar] [CrossRef]
- Roth, Z. Heat stress, the follicle, and its enclosed oocyte: Mechanisms and potential strategies to improve fertility in dairy cows. Reprod. Domest. Anim. 2008, 43 (Suppl. 2), 238–244. [Google Scholar] [CrossRef]
- García-Ispierto, I.; López-Gatius, F.; Santolaria, P.; Yániz, J.L.; Nogareda, C.; López-Béjar, M.; De Rensis, F. Relationship between heat stress during the peri-implantation period and early fetal loss in dairy cattle. Theriogenology 2006, 65, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.; Randel, R.D.; Broussard, J.R.; Lim, J.M.; Blair, R.M.; Roussel, J.D.; Godke, R.; Hansel, W. High environmental temperature and humidity decrease oocyte quality in Bos taurus but not in Bos taurus cows. Theriogenology 1998, 49, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Al-Katanani, Y.M.; Rivera, R.M.; Hansen, P.J. Seasonal variation in development of in vitro produced bovine embryos. Vet. Rec. 2002, 150, 486–487. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.L.; Hansen, P.J. Differential responses of bovine oocytes and preimplantation embryos to heat shock. Mol. Reprod. Develop. 1997, 46, 138–145. [Google Scholar] [CrossRef]
- Roth, Z.; Hansen, P.J. Involvement of apoptosis in disruption of developmental competence of bovine oocytes by heat shock during maturation. Biol. Reprod. 2004, 71, 1898–1906. [Google Scholar] [CrossRef]
- Ju, J.C. Cellular responses of oocytes and embryos under thermal stress: Hints to molecular signaling. Anim. Reprod. 2005, 2, 79–90. [Google Scholar]
- Hansen, P.J. Exploitation of genetic and physiological determinants of embryonic resistance to elevated temperature to improve embryonic survival in dairy cattle during heat stress. Theriogenology 2007, 68 (Suppl. 1), S242–S249. [Google Scholar] [CrossRef]
- Yaacobi-Artzi, S.; Kalo, D.; Roth, Z. Seasonal variation in the morphokinetics of in-vitro-derived bovine embryos is associated with the blastocyst developmental competence and gene expression. Front. Reprod. Health 2022, 4, 1030949. [Google Scholar] [CrossRef]
- Gendelman, M.; Aroyo, A.; Yavin, S.; Roth, Z. Seasonal effects on gene expression, cleavage timing, and developmental competence of bovine preimplantation embryos. Reproduction 2010, 140, 73–82. [Google Scholar] [CrossRef]
- Yaacobi-Artzi, S.; Shimoni, C.; Kalo, D.; Hansen, P.J.; Roth, Z. Melatonin slightly alleviates the effect of heat shock on bovine oocytes and resulting blastocysts. Theriogenology 2020, 158, 477–489. [Google Scholar] [CrossRef]
- Salumets, A.; Hydén-Granskog, C.; MaÈkinen, S.; Suikkari, A.M.; Tiitinen, A.; Tuuri, T. Early cleavage predicts the viability of human embryos in elective single embryo transfer procedures. Hum. Reprod. 2003, 18, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Walters, E.A.; Brown, J.L.; Krisher, R.; Voelkel, S.; Swain, J.E. Impact of a controlled culture temperature gradient on mouse embryo development and morphokinetics. Reprod. Biomed. Online 2020, 40, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, D.F.; Makri, D.; Maalouf, M.N.; Adamova, P.; de Moraes, G.F.A.; Pinheiro, M.O.; Bernardineli, D.L.; Massaia, I.F.D.S.; Maalouf, W.E.; Lo Turco, E.G. The effects of temperature variation treatments on embryonic development: A mouse study. Sci. Rep. 2022, 12, 2489. [Google Scholar] [CrossRef] [PubMed]
- Fryc, K.; Nowak, A.; Kij, B.; Kochan, J.; Bartlewski, P.M.; Murawski, M. Timing of cleavage divisions determined with time-lapse imaging is linked to blastocyst formation rates and quality of in vitro-produced ovine embryos. Theriogenology 2021, 159, 147–152. [Google Scholar] [CrossRef]
- Angel-Velez, D.; De Coster, T.; Azari-Dolatabad, N.; Fernández-Montoro, A.; Benedetti, C.; Pavani, K.; Van Soom, A.; Bogado Pascottini, O.; Smits, K. Embryo morphokinetics derived from fresh and vitrified bovine oocytes predict blastocyst development and nuclear abnormalities. Sci. Rep. 2023, 13, 4765. [Google Scholar] [CrossRef]
- Yang, T.; Yuan, X.; Xue, Q.; Sun, L.; Xu, T.; Chen, Y.; Shi, D.; Li, X. Comparison of symmetrical and asymmetrical cleavage 2-cell embryos of porcine by Smart-seq2. Theriogenology 2023, 210, 221–226. [Google Scholar] [CrossRef]
- Hayden, C.; Wells, C.; Wiik, A.; Killingsworth, R. Machine learning identifies differences in morphokinetics of in vivo-derived bovine embryos between hot and cool seasons. In Proceedings of the 50th Annual Conference of the International Embryo Technology Society (IETS), Denver, CO, USA, 8–12 January 2024; Volume 36, pp. 270–271. [Google Scholar]
- Kalo, D.; Komsky-Elbaz, A.; Roth, Z. Effect of Carbamazepine At Environmentally Relevant Concentrations on Oocyte Developmental Competence. In Proceedings of the Society for the Study of Reproduction (SSR), Virtual Meeting, 18–20 of July 2020. [Google Scholar]
- Yaacobi-Artzi, S.; Kalo, D.; Roth, Z. Effect of the aflatoxins B1 and M1 on bovine oocyte developmental competence and embryo morphokinetics. Reprod. Toxicol. 2023, 120, 108437. [Google Scholar] [CrossRef]
- Kalo, D.; Roth, Z.; (Department of Animal Sciences, Robert H. Smith Faculty of Agriculture, Food and Environment, The Hebrew University, Rehovot, Israel). The Effect of MEHP on Bovine Embryo Morphokinetic. 2018; (Unpublished work). [Google Scholar]
- Komsky-Elbaz, A.; Kalo, D.; Roth, Z. Carryover effect of atrazine and its metabolite—From treated bovine spermatozoa to the embryo’s transcriptome. Biol. Reprod. 2021, 104, 1162–1180. [Google Scholar] [CrossRef]
- Kalo, D.; Manovich, S.; Yaacobi-Artzi, S.; Roth, Z. Using a Time-lapse system to examine the association between morphokinetic patterns and bovine embryo gender. In Proceeding of the 57th annual meeting of the Society for the Study of Reproduction (SSR), Dublin, Ireland, 15–19 July 2024. [Google Scholar]
- ATSDR, U.S. Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Di(2-Ethylhexyl)phthalate (DEHP). 2002. Available online: http://www.atsdr.cdc.gov/toxprofiles/tp9.html (accessed on 13 August 2024).
- Wang, Y.; Qian, H. Phthalates and Their Impacts on Human Health. Healthcare 2021, 9, 603. [Google Scholar] [CrossRef]
- Koch, H.M.; Calafat, A.M. Human body burdens of chemicals used in plastic manufacture. Philos. Trans. R Soc. Lond B Biol. Sci. 2009, 364, 2063–2078. [Google Scholar] [CrossRef]
- Pure Strategies. Sources of Phthalates in Dairy Farm Equipment. Prepared for Environmental Health Strategy Center and Coalition for Safer Food Processing & Packaging, with Screening and Testing by Ecology Center. 2018. Available online: https://www.kleanupkraft.org/Phthalates-Farm-Equipment.pdf (accessed on 13 August 2024).
- Koch, H.M.; Bolt, H.M.; Preuss, R.; Angerer, J. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch. Toxicol. 2005, 79, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Liu, C.; Qin, D.Y.; Yuan, X.Q.; Yao, Q.Y.; Li, N.J.; Huang, Y.; Rao, W.T.; Li, Y.Y.; Deng, Y.L.; et al. Associations between phthalate metabolite concentrations in follicular fluid and reproductive outcomes among women undergoing in vitro fertilization/intracytoplasmic sperm injection treatment. Environ. Health Perspect. 2023, 131, 127019. [Google Scholar] [CrossRef] [PubMed]
- Bellavia, A.; Zou, R.; Björvang, R.D.; Roos, K.; Sjunnesson, Y.; Hallberg, I.; Holte, J.; Pikki, A.; Lenters, V.; Portengen, L.; et al. Association between chemical mixtures and female fertility in women undergoing assisted reproduction in Sweden and Estonia. Environ. Res. 2023, 216 Pt 1, 114447. [Google Scholar] [CrossRef]
- Kalo, D.; Hadas, R.; Furman, O.; Ben-Ari, J.; Maor, Y.; Patterson, D.G.; Tomey, C.; Roth, Z. Carryover Effects of Acute DEHP Exposure on Ovarian Function and Oocyte Developmental Competence in Lactating Cows. PLoS ONE 2015, 10, e0130896. [Google Scholar] [CrossRef] [PubMed]
- Kalo, D.; Vitorino Carvalho, A.; Archilla, C.; Duranthon, V.; Moroldo, M.; Levin, Y.; Kupervaser, M.; Smith, Y.; Roth, Z. Mono(2-ethylhexyl) phthalate (MEHP) induces transcriptomic alterations in oocytes and their derived blastocysts. Toxicology 2019, 421, 59–73. [Google Scholar] [CrossRef]
- Marzano, G.; Mastrorocco, A.; Zianni, R.; Mangiacotti, M.; Chiaravalle, A.E.; Lacalandra, G.M.; Minervini, F.; Cardinalim, A.; Macciocca, M.; Vicenti, R.; et al. Altered morphokinetics in equine embryos from oocytes exposed to DEHP during IVM. Mol. Reprod. Dev. 2019, 86, 1388–1404. [Google Scholar] [CrossRef]
- Tian, W.; Liao, H.; Li, N.; Yao, W.; Li, Y.; Guo, N.; Yuan, X.; Du, Y.; Teng, X.; Li, Y.; et al. Monomethyl phthalate causes early embryo development delay, apoptosis, and energy metabolism disruptions through inducing redox imbalance. Reprod. Sci. 2024, 31, 139–149. [Google Scholar] [CrossRef]
- Chu, D.P.; Tian, S.; Sun, D.G.; Hao, C.J.; Xia, H.F.; Ma, X. Corrigendum to: Exposure to mono-n-butyl phthalate disrupts the development of preimplantation embryos. Reprod. Fertil. Develop. 2013, 26, 491. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, M.; Aitken, R.J. Exposure of spermatozoa to dibutyl phthalate induces abnormal embryonic development in a marine invertebrate Galeolaria caespitosa (Polychaeta: Serpulidae). Aquat. Toxicol. 2017, 191, 189–200. [Google Scholar] [CrossRef]
- Xu, X.; Yang, M. Effects of Environmental EDCs on Oocyte Quality, Embryo Development, and the Outcome in Human IVF Process. In Environment and Female Reproductive Health. Advances in Experimental Medicine and Biology; Zhang, H., Yan, J., Eds.; Springer: Singapore, 2021; Volume 1300. [Google Scholar] [CrossRef]
- Matuszczak, E.; Komarowska, M.D.; Debek, W.; Hermanowicz, A. The Impact of Bisphenol A on Fertility, Reproductive System, and Development: A Review of the Literature. Int. J. Endocrinol. 2019, 10, 4068717. [Google Scholar] [CrossRef]
- Sabry, R.; Saleh, A.C.; Stalker, L.; LaMarre, J.; Favetta, L.A. Effects of bisphenol A and bisphenol S on microRNA expression during bovine (Bos taurus) oocyte maturation and early embryo development. Reprod. Toxicol. 2021, 99, 96–108. [Google Scholar] [CrossRef]
- Choi, B.I.; Harvey, A.J.; Green, M.P. Bisphenol A affects early bovine embryo development and metabolism that is negated by an oestrogen receptor inhibitor. Sci. Rep. 2016, 6, 29318. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Semiz, O.; Cinar, O. Bisphenol-A induces cell cycle delay and alters centrosome and spindle microtubular organization in oocytes during meiosis. Mol. Hum. Reprod. 2005, 11, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Ko, D.H.; Lee, W.; Kim, K.R.; Chun, S.; Song, J.; Min, W.K. Body fluid concentrations of bisphenol A and their association with in vitro fertilization outcomes. Hum. Fertil. 2021, 24, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Governini, L.; Orvieto, R.; Guerranti, C.; Gambera, L.; De Leo, V.; Piomboni, P. The impact of environmental exposure to perfluorinated compounds on oocyte fertilization capacity. J. Assist. Reprod. Genet. 2011, 28, 415–418. [Google Scholar] [CrossRef]
- McCoy, J.A.; Bangma, J.T.; Reiner, J.L.; Bowden, J.A.; Schnorr, J.; Slowey, M.; O'Leary, T.; Guillette, L.J., Jr.; Parrott, B.B. Associations between perfluorinated alkyl acids in blood and ovarian follicular fluid and ovarian function in women undergoing assisted reproductive treatment. Sci. Total Environ. 2017, 605–606, 9–17. [Google Scholar] [CrossRef]
- Kim, Y.R.; White, N.; Bräunig, J.; Vijayasarathy, S.; Mueller, J.F.; Knox, C.L.; Harden, F.A.; Pacella, R.; Toms, L.L. Per- and poly-fluoroalkyl substances (PFASs) in follicular fluid from women experiencing infertility in Australia. Environ. Res. 2020, 190, 109963. [Google Scholar] [CrossRef]
- Lefebvre, T.; Fréour, T.; Duval, G.; Ploteau, S.; Marchand, P.; Le Bizec, B.; Antignac, J.P.; Cano-Sancho, G. Associations between internal concentrations of fluorinated and organochlorinated chemicals in women and in vitro fertilization outcomes: A multi-pollutant study. Environ. Pollut. 2022, 313, 120087. [Google Scholar] [CrossRef]
- Hallberg, I.; Kjellgren, J.; Persson, S.; Örn, S.; Sjunnesson, Y. Perfluorononanoic acid (PFNA) alters lipid accumulation in bovine blastocysts after oocyte exposure during in vitro maturation. Reprod. Toxicol. 2019, 84, 1–8. [Google Scholar] [CrossRef]
- González-Alvarez, M.E.; Antwi-Boasiako, C.; Keating, A.F. Effects of Per- and Polyfluoroalkylated Substances on Female Reproduction. Toxics 2024, 12, 455. [Google Scholar] [CrossRef]
- Abhilash, P.C.; Singh, N. Pesticide use and application: An Indian scenario. J. Hazard. Mater. 2009, 165, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Swan, S.H.; Kruse, R.L.; Liu, F.; Barr, D.B.; Drobnis, E.Z.; Redmon, J.B.; Wang, C.; Brazil, C.; Overstreet, J.W. Study for Future Families Research Group. Semen quality in relation to biomarkers of pesticide exposure. Environ. Health Perspect. 2003, 111, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Bretveld, R.W.; Thomas, C.M.; Scheepers, P.T.; Zielhuis, G.A.; Roeleveld, N. Pesticide exposure: The hormonal function of the female reproductive system disrupted? Reprod. Biol. Endocrinol. 2006, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- McGraw, M.S.; Daigneault, B.W. Environment to embryo: Intersections of contaminant exposure and preimplantation embryo development in agricultural animals. Biol. Reprod. 2022, 107, 869–880. [Google Scholar] [CrossRef]
- Silveyra, G.R.; Canosa, I.S.; Zanitti, M.; Rodríguez, E.M.; Medesani, D.A. Interference of an atrazine commercial formulation with the endocrine control of ovarian growth exerted by the eyestalks. Environ. Sci. Pollut. Res. 2020, 27, 965–973. [Google Scholar] [CrossRef]
- Barr, D.B.; Panuwet, P.; Nguyen, J.V.; Udunka, S.; Needham, L.L. Assessing exposure to atrazine and its metabolites using biomonitoring. Environ. Health Perspect. 2007, 115, 1474–1478. [Google Scholar] [CrossRef]
- Gely-Pernot, A.; Hao, C.; Becker, E.; Stuparevic, I.; Kervarrec, C.; Chalmel, F.; Primig, M.; Jégou, B.; Smagulova, F. The epigenetic processes of meiosis in male mice are broadly affected by the widely used herbicide atrazine. BMC Genom. 2015, 16, 885. [Google Scholar] [CrossRef]
- Rey, F.; González, M.; Zayas, M.A.; Stoker, C.; Durando, M.; Luque, E.H.; Muñoz-de-Toro, M. Prenatal exposure to pesticides disrupts testicular histoarchitecture and alters testosterone levels in male Caiman latirostris. Gen. Comp. Endocrinol. 2009, 162, 286–292. [Google Scholar] [CrossRef]
- Dill, G.M.; Sammons, R.D.; Feng, P.C.C.; Kohn, F.; Kretzner, K.; Mehrsheikh, A.; Bleeke, M.; Honegger, J.L.; Farmer, D.; Wright, D.; et al. Glyphosate: Discovery, Development, Applications, and Properties; Nandula, V.K., Ed.; Glyphosate Resistance in Crops and Weeds: Hoboken, NJ, USA, 2010. [Google Scholar]
- Dovolou, E.; Nanas, I.; Giannoulis, T.; Fytsilli, A.; Ntemka, A.; Anifandis, G.; Tsakmakidis, I.; Amiridis, G.S. The effects of a glyphosate-based herbicide on the bovine gametes during an in vitro embryo production model. Environ. Pollut. 2024, 350, 123967. [Google Scholar] [CrossRef]
- Spinaci, M.; Nerozzi, C.; Tamanini, C.L.; Bucci, D.; Galeati, G. Glyphosate and its formulation Roundup impair pig oocyte maturation. Sci. Rep. 2020, 10, 12007. [Google Scholar] [CrossRef] [PubMed]
- Fabian, D.; Bystriansky, J.; Burkuš, J.; Rehák, P.; Legáth, J.; Koppel, J. The effect of herbicide BASTA 15 on the development of mouse preimplantation embryos in vivo and in vitro. Toxicol. Vitr. 2011, 25, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, G.; Ng, W.J.; Tan, S.K. A review on removing pharmaceutical contaminants from wastewater by constructed wetlands: Design, performance and mechanism. Sci. Total Environ. 2014, 15, 908–932. [Google Scholar] [CrossRef] [PubMed]
- Grossberger, A.; Hadar, Y.; Borch, T.; Chefetz, B. Biodegradability of pharmaceutical compounds in agricultural soils irrigated with treated wastewater. Environ. Pollut. 2014, 185, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Mordechay, E.B.; Mordehay, V.; Tarchitzky, J.; Chefetz, B. Pharmaceuticals in edible crops irrigated with reclaimed wastewater: Evidence from a large survey in Israel. J. Hazar. Mater. 2021, 416, 126184. [Google Scholar] [CrossRef]
- Zhang, Y.; Geissen, S.U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef]
- Xie, Z.; Lu, G.; Liu, J.; Yan, Z.; Ma, B.; Zhang, Z.; Chen, W. Occurrence, bioaccumulation, and trophic magnification of pharmaceutically active compounds in Taihu Lake, China. Chemosphere 2015, 138, 140–147. [Google Scholar] [CrossRef]
- Andretta, R.R.; Okada, F.K.; Paccola, C.C.; Stumpp, T.; de Oliva, S.U.; Miraglia, S.M. Carbamazepine-exposure during gestation and lactation affects pubertal onset and spermatic parameters in male pubertal offspring. Reprod. Toxicol. 2014, 44, 52–62. [Google Scholar] [CrossRef]
- Nunes, M.; de Sousa, F.D.C.; Andretta, R.R.; Miraglia, S.M.; de Oliva, S.U. Carbmazepine causes changes in maternal reproductiv performance and fetal growth retarfation in rats. bioRxiv 2020, 303487. [Google Scholar]
- Afshar, M.; Moallem, S.A.; Houshang Mohammadpour, A.; Shiravi, A.; Majid Jalalian, S.; Jafar Golalipour, M. Teratogenic effects of carbamazepine on embryonic eye development in pregnant mice. Cutan. Ocul. Toxicol. 2010, 29, 10–15. [Google Scholar] [CrossRef]
- Douek-Maba, O.; Kalev-Altman, R.; Mordehay, V.; Hayby-Averbuch, H.; Shlezinger, N.; Chefetz, B.; Sela-Donenfeld, D. Maternal exposure to environmental levels of carbamazepine induces mild growth retradation in mouse embryos. bioRxiv 2023, 523650. [Google Scholar]
- Monapathi, M.E.; Oguegbulu, J.C.; Adogo, L.; Klink, M.; Okoli, B.; Mtunzi, F.; Modise, J.S. Pharmaceutical Pollution: Azole Antifungal Drugs and Resistance of Opportunistic Pathogenic Yeasts in Wastewater and Environmental Water. Appl. Environ. Soil. Sci. 2021, 2021, 9985398. [Google Scholar] [CrossRef]
- Yun, Y.; Holt, J.E.; Lane, S.I.; McLaughlin, E.A.; Merriman, J.A.; Jones, K.T. Reduced ability to recover from spindle disruption and loss of kinetochore spindle assembly checkpoint proteins in oocytes from aged mice. Cell Cycle 2014, 13, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Betzendahl, I.; Pacchierotti, F.; Ranaldi, R.; Smitz, J.; Cortvrindt, R.; Eichenlaub-Ritter, U. Aneuploidy in mouse metaphase II oocytes exposed in vivo and in vitro in preantral follicle culture to nocodazole. Mutagenesis 2005, 20, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Kratka, C.R.; Pea, J.; Lee, H.C.; Kratka, C.E.; Xu, J.; Marin, D.; Treff, N.R.; Duncan, F.E. The severity of meiotic aneuploidy is associated with altered morphokinetic variables of mouse oocyte maturation. Hum. Reprod. Open 2024, 2024, hoae023. [Google Scholar] [CrossRef]
- Ayala, A.; Parrado, J.; Bougria, M.; Machado, A. Effect of oxidative stress, produced by cumene hydroperoxide, on the various steps of protein synthesis. Modifications of elongation factor-2. J. Biol. Chem. 1996, 271, 23105–23110. [Google Scholar] [CrossRef]
- Hughes, P.M.; Morbeck, D.E.; Hudson, S.B.; Fredrickson, J.R.; Walker, D.L.; Coddington, C.C. Peroxides in mineral oil used for in vitro fertilization: Defining limits of standard quality control assays. J. Assist. Reprod. Genet. 2010, 27, 87–92. [Google Scholar] [CrossRef]
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Saari, G.N.; Kostal, J.; Williams, E.S.; Mills, M.; Gallagher, E.P.; Kavanagh, T.J.; Simcox, N.; et al. Toward the design of less hazardous chemicals: Exploring comparative oxidative stress in two common animal models. Chem. Res. Toxicol. 2017, 30, 893–904. [Google Scholar] [CrossRef]
- Adigun, N.S.; Oladiji, A.T.; Ajiboye, T.O. Antioxidant and anti-hyperlipidemic activity of hydroethanolic seed extract of Aframomum melegueta K. Schum in Triton X-100 induced hyperlipidemic rats. S. Afr. J. Bot. 2016, 105, 324–332. [Google Scholar] [CrossRef]
- Tijjani, H.; Adegunloye, A.P.; Uba, H.; Joel, E.B.; Olatunde, A. Antioxidant activities of aqueous and ethyl acetate fractions of Daucus carota L. seed against triton X-100 induced oxidative stress in mice. Sci. Afr. 2020, 8, e00429. [Google Scholar] [CrossRef]
- Wolff, H.S.; Fredrickson, J.R.; Walker, D.L.; Morbeck, D.E. Advances in quality control: Mouse embryo morphokinetics are sensitive markers of in vitro stress. Hum. Reprod. 2013, 28, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Maeda, T.; Fukunaga, E.; Shiba, R.; Okano, S.; Kinutani, M.; Horiuchi, T. Selection of high-quality and viable blastocysts based on timing of morula compaction and blastocyst formation. Reprod. Med. Biol. 2019, 19, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.; Meriano, J.; Bassil, R.; Barzilay, E.; Zilberberg, E.; Casper, R.F. Developmental potential of slow-developing embryos: Day-5 morulae compared with day-5 cavitating morulae. Fertil. Steril. 2019, 111, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Mara, L.; Parham, A.; Dattena, M. Mineral Oil for in vitro Embryo Production: What We Should Know? Arch. Razi. Inst. 2022, 77, 1325–1330. [Google Scholar] [PubMed]
- Otsuki, J.; Nagai, Y.; Chiba, K. Peroxidation of mineral oil used in droplet culture is detrimental to fertilization and embryo development. Fertil. Steril. 2007, 88, 741–743. [Google Scholar] [CrossRef]
- Ainsworth, A.J.; Fredrickson, J.R.; Morbeck, D.E. Improved detection of mineral oil toxicity using an extended mouse embryo assay. J. Assist. Reprod. Genet. 2017, 34, 391–397. [Google Scholar] [CrossRef]
- Martinez, C.A.; Nohalez, A.; Ceron, J.J.; Rubio, C.P.; Roca, J.; Cuello, C.; Rodriguez-Martinez, H.; Martinez, E.A.; Gil, M.A. Peroxidized mineral oil increases the oxidant status of culture media and inhibits in vitro porcine embryo development. Theriogenology 2017, 103, 17–23. [Google Scholar] [CrossRef]
- Deluao, J.C.; Winstanley, Y.; Robker, R.L.; Pacella-Ince, L.; Gonzalez, M.B.; McPherson, N.O. Oxidative stress and reproductive function: Reactive oxygen species in the mammalian pre-implantation embryo. Reproduction 2022, 164, F95–F108. [Google Scholar] [CrossRef]
- Atoui, A.; Cordevant, C.; Chesnot, T.; Gassilloud, B. SARS-CoV-2 in the environment: Contamination routes, detection methods, persistence and removal in wastewater treatment plants. Sci. Total Environ. 2023, 881, 163453. [Google Scholar] [CrossRef]
- Dai, Y.; Huang, K.; Zhang, B.; Zhu, L.; Xu, W. Aflatoxin B1-induced epigenetic alterations: An overview. Food Chem. Toxicol. 2017, 109 Pt 1, 683–689. [Google Scholar] [CrossRef]
- Shen, H.M.; Shi, C.Y.; Lee, H.P.; Ong, C.N. Aflatoxin B1-induced lipid peroxidation in rat liver. Toxicol. Appl. Pharmacol. 1994, 127, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Galvano, F.; Galofaro, V.; Galvano, G. Occurrence and stability of aflatoxin M1 in milk and milk products: A worldwide review. J. Food Prot. 1996, 10, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.T.; Guo, J.; Niu, Y.J.; Cui, X.S. The toxic effect of aflatoxin B1 on early porcine embryonic development. Theriogenology 2018, 118, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Applebaum, R.S.; Brackett, R.E.; Wiseman, D.W.; Marth, E.H. Aflatoxin: Toxicity to dairy cattle and occurrence in milk and milk products-a review. J. Food Prot. 1982, 45, 752–777. [Google Scholar] [CrossRef] [PubMed]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological properties and their involvement in cancer development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nut. 2004, 80, 1106–1122. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Watson, S.; Routledge, M.N. Aflatoxin exposure and associated human health effects, a review of epidemiological studies. Food Saf. 2016, 4, 14–27. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Liu, J.Q.; Zou, P.; Jia, L.; Su, Y.T.; Sun, Y.R.; Sun, S.C. Comparison of the toxic effects of different mycotoxins on porcine and mouse oocyte meiosis. PeerJ 2018, 6, e5111. [Google Scholar] [CrossRef]
- Hajarizadeh, A.; Eidi, A.; Arefian, E.; Tvrda, E.; Mohammadi-Sangcheshmeh, A. Aflatoxin B1 impairs in vitro early developmental competence of ovine oocytes. Theriogenology 2022, 183, 53–60. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Zhao, Q.H.; Duan, H.W.; Zou, Y.J.; Sun, S.C.; Hu, L.L. Aflatoxin B1 exposure disrupts organelle distribution in mouse oocytes. PeerJ 2022, 10, e13497. [Google Scholar] [CrossRef]
- Ibeh, I.N.; Saxena, D.K.; Uraih, N. Toxicity of aflatoxin: Effects on spermatozoa, oocytes, and in vitro fertilization. J. Environ. Pathol. Toxicol. Oncol. 2000, 19, 357–361. [Google Scholar] [PubMed]
- Jiang, Y.; Hansen, P.J.; Xiao, Y.; Amaral, T.F.; Vyas, D.; Adesogan, A.T. Aflatoxin compromises development of the preimplantation bovine embryo through mechanisms independent of reactive oxygen production. J. Dairy Sci. 2019, 102, 10506–10513. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aquila, M.E.; Asif, S.; Temerario, L.; Mastrorocco, A.; Marzano, G.; Martino, N.A.; Lacalandra, G.M.; Roelen, B.A.; Carluccio, A.; Robbe, D.; et al. Ochratoxin A affects oocyte maturation and subsequent embryo developmental dynamics in the juvenile sheep model. Mycotoxin. Res. 2021, 37, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, C.; Alsafi, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 422. [Google Scholar] [CrossRef]
- Colaco, S.; Chhabria, K.; Singh, D.; Bhide, A.; Singh, N.; Singh, A.; Husein, A.; Mishra, A.; Sharma, R.; Ashary, N.; et al. Expression map of entry receptors and infectivity factors for pan-coronaviruses in preimplantation and implantation stage human embryos. J. Assist. Reprod. Genet. 2021, 38, 1709–1720. [Google Scholar] [CrossRef]
- Montano, M.; Victor, A.R.; Griffin, D.K.; Duong, T.; Bolduc, N.; Farmer, A.; Garg, V.; Hadjantonakis, A.K.; Coates, A.; Barnes, F.L.; et al. SARS-CoV-2 can infect human embryos. Sci. Rep. 2022, 12, 15451. [Google Scholar] [CrossRef]
- Ma, L.; Yao, Y.; Zhang, Z.; Huang, Y.; Zhang, L.; Qiao, P.; Kang, J.; Ren, C.; Xie, W.; Liang, R.; et al. SARS-CoV-2 infection negatively impacts on the quality of embryos by delaying early embryonic development. Am. J. Reprod. Immunol. 2024, 91, e13831. [Google Scholar] [CrossRef]
- Braga, D.P.A.F.; Setti, A.S.; Iaconelli, A., Jr.; Borges, E., Jr. Previous infection with SARS-CoV-2 impacts embryo morphokinetics but not clinical outcomes in a time-lapse imaging system. Mol. Reprod. Dev. 2023, 90, 53–58. [Google Scholar] [CrossRef]
- Cao, M.; Han, Y.; Feng, T.; Lu, P.; Wang, Y.; Sun, Q.; Zhao, Z.; Pan, W. Impact of COVID-19 convalescence on pregnancy outcomes in patients undergoing IVF/ICSI during fresh ART cycles: A retrospective cohort study. Front. Endocrinol. 2024, 14, 1298995. [Google Scholar] [CrossRef]
- Li, X.F.; Zhang, Y.J.; Yao, Y.L.; Chen, M.X.; Wang, L.L.; Wang, M.D.; Hu, X.Y.; Tang, X.J.; Zhong, Z.H.; Fu, L.J.; et al. The association of post-embryo transfer SARS-CoV-2 infection with early pregnancy outcomes in in vitro fertilization: A prospective cohort study. Am. J. Obstet. Gynecol. 2024, 230, 436.e1–436.e12. [Google Scholar] [CrossRef] [PubMed]
- Aminpour, M.; Hameroff, S.; Tuszynski, J.A. How COVID-19 Hijacks the Cytoskeleton: Therapeutic Implications. Life 2022, 12, 814. [Google Scholar] [CrossRef] [PubMed]
- Zullo, F.; Fiano, V.; Gillio-Tos, A.; Leoncini, S.; Nesi, G.; Macrì, L.; Preti, M.; Rolfo, A.; Benedetto, C.; Revelli, A.; et al. Human papillomavirus infection in women undergoing in-vitro fertilization: Effects on embryo development kinetics and live birth rate. Reprod. Biol. Endocrinol. 2023, 21, 39. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.L.; Teissier, S.; Gunaratne, J.; Quek, L.S.; Bellanger, S. Stranglehold on the spindle assembly checkpoint: The human papillomavirus E2 protein provokes BUBR1-dependent aneuploidy. Cell Cycle 2015, 14, 1459–1470. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vazirov, R.A.; Makutina, V.A.; Krivonogova, A.S.; Isaeva, A.G.; Romanova, A.S. The influence of low-intensity electromagnetic radiation on the development of bovine embryos in vitro. BIO Web Conf. 2024, 108, 23004. [Google Scholar] [CrossRef]
- Koohestanidehaghi, Y.; Khalili, M.A.; Fesahat, F.; Seify, M.; Mangoli, E.; Kalantar, S.M.; Annarita Nottola, S.; Macchiarelli, G.; Grazia Palmerini, M. Detrimental effects of radiofrequency electromagnetic waves emitted by mobile phones on morphokinetics, oxidative stress, and apoptosis in mouse preimplantation embryos. Environ. Pollut. 2023, 336, 122411. [Google Scholar] [CrossRef]
- Seify, M.; Khalili, M.A.; Anbari, F.; Koohestanidehaghi, Y. Detrimental effects of electromagnetic radiation emitted from cell phone on embryo morphokinetics and blastocyst viability in mice. Zygote 2024, 32, 149–153. [Google Scholar] [CrossRef]
- Magata, F.; Urakawa, M.; Matsuda, F.; Oono, Y. Developmental kinetics and viability of bovine embryos produced in vitro with sex-sorted semen. Theriogenology 2021, 161, 243–251. [Google Scholar] [CrossRef]
- Bermejo-Alvarez, P.; Lonergan, P.; Rath, D.; Gutiérrez-Adan, A.; Rizos, D. Developmental kinetics and gene expression in male and female bovine embryos produced in vitro with sex-sorted spermatozoa. Reprod. Fertil. Dev. 2010, 22, 426–436. [Google Scholar] [CrossRef]
- Steele, H.; Makri, D.; Maalouf, W.E.; Reese, S.; Kölle, S. Bovine Sperm Sexing Alters Sperm Morphokinetics and Subsequent Early Embryonic Development. Sci. Rep. 2020, 10, 6255. [Google Scholar] [CrossRef]
- Hada, M.; Georgakilas, A.G. Formation of clustered DNA damage after high-LET irradiation: A review. J. Radiat. Res. 2008, 49, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Mavragani, I.V.; Nikitaki, Z.; Kalospyros, S.A.; Georgakilas, A.G. Ionizing Radiation and Complex DNA Damage: From Prediction to Detection Challenges and Biological Significance. Cancers 2019, 11, 1789. [Google Scholar] [CrossRef] [PubMed]
- Szumiel, I. Ionizing radiation-induced oxidative stress, epigenetic changes and genomic instability: The pivotal role of mitochondria. Int. J. Radiat. Biol. 2015, 91, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, D.M.; Brent, R.L. Effects of X Irradiation during Preimplantation Stages of Gestation on Cell Viability and Embryo Survival in the Mouse. Radiat. Res. 1978, 75, 202–216. [Google Scholar] [CrossRef]
- Honjo, Y.; Ichinohe, T. Stage-Specific Effects of Ionizing Radiation during Early Development. Int. J. Mol. Sci. 2020, 21, 3975. [Google Scholar] [CrossRef]
- Jacquet, P. Developmental defects and genomic instability after x-irradiation of wild-type and genetically modified mouse pre-implantation and early post-implantation embryos. J. Radiol. Prot. 2012, 32, R13–R36. [Google Scholar] [CrossRef]
- Matsumoto, H.; Fukuda, A.; Mizuno, S.; Hashimoto, S.; Morimoto, Y. Effect of X-ray exposure during hysterosalpingography on capabilities of female germ cells. J. Assist. Reprod. Genet. 2021, 38, 3233–3242. [Google Scholar] [CrossRef]
- Altun, G.; Deniz, Ö.G.; Yurt, K.K.; Davis, D.; Kaplan, S. Effects of mobile phone exposure on metabolomics in the male and female reproductive systems. Environ. Res. 2018, 167, 700–707. [Google Scholar] [CrossRef]
- Jaffar, F.H.F.; Osman, K.; Ismail, N.H.; Chin, K.Y.; Ibrahim, S.F. Adverse effects of wi-fi radiation on male reproductive system: A systematic review. Tohoku J. Exp. Med. 2019, 248, 169–179. [Google Scholar] [CrossRef]
- Suzuki, S.; Okutsu, M.; Suganuma, R.; Komiya, H.; Nakatani-Enomoto, S.; Kobayashi, S.; Ugawa, Y.; Tateno, H.; Fujimori, K. Influence of radiofrequency-electromagnetic waves from 3rd-generation cellular phones on fertilization and embryo development in mice. Bioelectromagnetics 2017, 38, 466–473. [Google Scholar] [CrossRef]
- Safian, F.; Khalili, M.A.; Khoradmehr, A.; Anbari, F.; Soltani, S.; Halvaei, I. Survival Assessment of Mouse Preimplantation Embryos After Exposure to Cell Phone Radiation. J. Reprod. Infertil. 2016, 17, 138–143. [Google Scholar] [PubMed]
- Fatehi, D.; Anjomshoa, M.; Mohammadi, M.; Seify, M.; Rostamzadeh, A. Biological effects of cell-phone radiofrequency waves exposure on fertilization in mice; an in vivo and in vitro study. Middle East Fertil. Soc. J. 2018, 23, 148–153. [Google Scholar] [CrossRef]
- Santini, S.J.; Cordone, V.; Falone, S.; Mijit, M.; Tatone, C.; Amicarelli, F.; Di Emidio, G. Role of mitochondria in the oxidative stress induced by electromagnetic fields: Focus on reproductive systems. Oxid. Med. Cell. Longev. 2018, 2018, 5076271. [Google Scholar] [CrossRef] [PubMed]
- Kesari, K.K.; Agarwal, A.; Henkel, R. Radiations and male fertility. Reprod. Biol. Endocrinol. 2018, 16, 118. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.F.N.; Gadella, B.M. Detection of damage in mammalian sperm cells. Theriogenology 2006, 65, 958–978. [Google Scholar] [CrossRef]
- Agarwal, A.; Desai, N.R.; Makker, K.; Varghese, A.; Mouradi, R.; Sabanegh, E.; Sharma, R. Effects of radiofrequency electromagnetic waves (RF-EMW) from cellular phones on human ejaculated semen: An in vitro pilot study. Fertil. Steril. 2009, 92, 1318–1325. [Google Scholar] [CrossRef]
- Bordignon, V.; Smith, L.C. Ultraviolet-Irradiated Spermatozoa Activate Oocytes but Arrest Preimplantation Development After Fertilization and Nuclear Transplantation in Cattle. Biol. Reprod. 1999, 61, 1513–1520. [Google Scholar] [CrossRef]
- Ghavi Hossein-Zadeh, N.; Nejati-Javaremi, A.; Miraei-Ashtiani, S.R.; Kohram, H. Bioeconomic evaluation of the use of sexed semen at different conception rates and herd sizes in Holstein populations. Anim. Reprod. Sci. 2010, 121, 17–23. [Google Scholar] [CrossRef]
- Vishwanath, R.; Moreno, J.F. Review: Semen sexing—Current state of the art with emphasis on bovine species. Animal 2018, 12, s85–s96. [Google Scholar] [CrossRef]
- Johnson, L.A. Sexing mammalian sperm for production of offspring: The state of the art. Anim. Reprod. Sci. 2000, 60, 93–107. [Google Scholar] [CrossRef]
- Espinosa-Cervantes, R.; Córdova-Izquierdo, A. Sexing sperm of domestic animals. Trop. Anim. Health Prod. 2012, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gaur, P.; Saini, G.; Saharan, P.; Bisla, A.; Yadav, V. Sex Sorted Semen—Methods, Constraints and Future Perspective. Vet. Res. Int. 2020, 8, 368–375. [Google Scholar]
- Balao da Silva, C.M.; Ortega-Ferrusola, C.; Morrell, J.M.; Rodriguez Martínez, H.; Peña, F.J. Flow Cytometric Chromosomal Sex Sorting of Stallion Spermatozoa Induces Oxidative Stress on Mitochondria and Genomic DNA. Reprod. Domest. Anim. 2016, 51, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Balao da Silva, C.M.; Ortega Ferrusola, C.; Morillo Rodriguez, A.; Gallardo Bolaños, J.M.; Plaza Dávila, M.; Morrell, J.M.; Rodriguez Martínez, H.; Tapia, J.A.; Aparicio, I.M.; Pena, F.J. Sex sorting increases the permeability of the membrane of stallion spermatozoa. Anim. Reprod. Sci. 2013, 138, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Mari, G.; Rizzato, G.; Merlo, B.; Iacono, E.; Bucci, D.; Seren, E.; Tamanini, C.; Galeati, G.; Spinaci, M. Quality and fertilizing ability in vivo of sex-sorted stallion spermatozoa. Reprod. Domest. Anim. 2010, 45, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Dennery, P.A. Effects of oxidative stress on embryonic development. Birth Defects Res. C Embryo Today 2007, 81, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Alegre, L.; Del Gallego, R.; Arrones, S.; Hernández, P.; Muñoz, M.; Meseguer, M. Novel noninvasive embryo selection algorithm combining time-lapse morphokinetics and oxidative status of the spent embryo culture medium. Fertil. Steril. 2019, 111, 918–927.e3. [Google Scholar] [CrossRef]
- Guerin, P.; Mouatassim, S.E.; Menezo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef]
- Hardeland, R.; Pandi-Perumal, S.R. Melatonin, a potent agent in antioxidative defense: Actions as a natural food constituent, gastrointestinal factor, drug and prodrug. Nutr. Metab. 2005, 2, 22. [Google Scholar] [CrossRef]
- Tamura, H.; Jozaki, M.; Tanabe, M.; Shirafuta, Y.; Mihara, Y.; Shinagawa, M.; Tamura, I.; Maekawa, R.; Sato, S.; Taketani, T.; et al. Importance of melatonin in assisted reproductive technology and ovarian aging. Int. J. Mol. Sci. 2020, 21, 1135. [Google Scholar] [CrossRef]
- Cebrian-Serrano, A.; Salvador, I.; Raga, E.; Dinnyes, A.; Silvestre, M.A. Beneficial effect of melatonin on blastocyst in vitro production from heat-stressed bovine oocytes. Reprod. Domest. Anim. 2013, 48, 738–746. [Google Scholar] [CrossRef] [PubMed]
- El-Raey, M.; Geshi, M.; Somfai, T.; Kaneda, M.; Hirako, M.; Abdel-Ghaffar, A.E.; Sosa, G.A.; El-Roos, M.E.; Nagai, T. Evidence of melatonin synthesis in the cumulus oocyte complexes and its role in enhancing oocyte maturation in vitro in cattle. Mol. Reprod. Dev. 2011, 78, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Takada, L.; Junior, A.M.; Mingoti, G.Z.; Balieiro, J.C.; Cipolla-Neto, J.; Coelho, L.A. Effect of melatonin on DNA damage of bovine cumulus cells during in vitro maturation (IVM) and on in vitro embryo development. Res. Vet. Sci. 2012, 92, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Jammoul, M.; Lawand, N. Melatonin: A Potential Shield against Electromagnetic Waves. Curr. Neuropharmacol. 2022, 20, 648–660. [Google Scholar]
- Shokri, M.; Shamsaei, M.E.; Malekshah, A.K.; Amiri, F.T. The protective effect of melatonin on radiofrequency electromagnetic fields of mobile phone-induced testicular damage in an experimental mouse model. Andrologia 2020, 52, e13834. [Google Scholar] [CrossRef]
- Wang, F.; Tian, X.; Zhou, Y.; Tan, D.; Zhu, S.; Dai, Y.; Liu, G. Melatonin improves the quality of in vitro produced (IVP) bovine embryos: Implications for blastocyst development, cryotolerance, and modifications of relevant gene expression. PLoS ONE 2014, 9, e93641. [Google Scholar] [CrossRef]
- Makarevich, A.V.; Sirotkin, A.V. The involvement of the GH/IGF-I axis in the regulation of secretory activity by bovine oviduct epithelial cells. Anim. Reprod. Sci. 1997, 48, 197–207. [Google Scholar] [CrossRef]
- Robinson, R.S.; Mann, G.E.; Gadd, T.S.; Lamming, G.E.; Wathes, D.C. The expression of the IGF system in the bovine uterus throughout the oestrous cycle and early pregnancy. J. Endocrinol. 2000, 165, 231–243. [Google Scholar] [CrossRef]
- Lonergan, P.; Gutiérrez-Adán, A.; Pintado, B.; Fair, T.; Ward, F.; Fuente, J.D.; Boland, M. Relationship between time of first cleavage and the expression of IGF-I growth factor, its receptor, and two housekeeping genes in bovine two-cell embryos and blastocysts produced in vitro. Mol. Reprod. Dev. 2000, 57, 146–152. [Google Scholar] [CrossRef]
- Jousan, F.D.; Hansen, P.J. Insulin-like growth factor-I as a survival factor for the bovine preimplantation embryo exposed to heat shock. Biol. Reprod. 2004, 71, 1665–1670. [Google Scholar] [CrossRef]
- Kurzawa, R.; Głabowski, W.; Baczkowski, T.; Brelik, P. Evaluation of mouse preimplantation embryos exposed to oxidative stress cultured with insulin-like growth factor I and II, epidermal growth factor, insulin, transferrin and selenium. Reprod. Biol. 2002, 2, 143–162. [Google Scholar]
- Michaelov, A.; Roth, Z.; (Department of Animal Sciences, Robert H. Smith Faculty of Agriculture, Food and Environment, The Hebrew University, Rehovot, Israel). The Effect of IGF-I on Bovine Embryo Morphokinetic. 2023; (Unpublished work). [Google Scholar]
- Sinbad, O.O.; Folorunsho, A.A.; Olabisi, O.L.; Ayoola, O.A.; Temitope, E.J. Vitamins as Antioxidants. J. Food Sci. Nutr. Res. 2019, 2, 214–235. [Google Scholar]
- Skowrońska, P.; Kunicki, M.; Pastuszek, E.; Konieczna, L.; Bączek, T.; Łukaszuk, K. Follicular fat-soluble vitamins as markers of oocyte competency. Syst. Biol. Reprod. Med. 2020, 66, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Gad, A.; Abu Hamed, S.; Khalifa, M.; Amin, A.; El-Sayed, A.; Swiefy, S.A.; El-Assal, S. Retinoic acid improves maturation rate and upregulates the expression of antioxidant-related genes in in vitro matured buffalo (Bubalus bubalis) oocytes. Int. J. Vet. Sci. Med. 2018, 6, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Sovernigo, T.C.; Adona, P.R.; Monzani, P.S.; Guemra, S.; Barros, F.; Lopes, F.G.; Leal, C. Effects of supplementation of medium with different antioxidants during in vitro maturation of bovine oocytes on subsequent embryo production. Reprod. Domest. Anim. 2017, 52, 561–569. [Google Scholar] [CrossRef]
- Gode, F.; Akarsu, S.; Dikmen, Z.G.; Tamer, B.; Isik, A.Z. The Effect Follicular Fluid Vitamin A, E, D and B6 on Embryo Morphokinetics and Pregnancy Rates in Patients Receiving Assisted Reproduction. Gynecol. Obstet. Reprod. Med. 2019, 25, 89–95. [Google Scholar] [CrossRef]
- Sun, W.S.; Jang, H.; Park, M.R.; Oh, K.B.; Lee, H.; Hwang, S.; Xu, L.J.; Hwang, I.S.; Lee, J.W. N-acetyl-L-cysteine Improves the developmental competence of bovine oocytes and embryos cultured in vitro by attenuating oxidative damage and apoptosis. Antioxidants 2021, 10, 860. [Google Scholar] [CrossRef]
- El-Sokary, M.M.M.; El-Naby, A.A.H.; Hameed, A.R.A.E.; Mahmoud, K.G.M.; Scholkamy, T.H. Impact of L-carnitine supplementation on the in vitro developmental competence and cryotolerance of buffalo embryos. Vet. World 2021, 14, 3164–3169. [Google Scholar] [CrossRef]
- Truong, T.T.; Soh, Y.M.; Gardner, D.K. Antioxidants improve mouse preimplantation embryo development and viability. Hum. Reprod. 2016, 31, 1445–1454. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, S.Y.; Kim, E.Y.; Kim, K.H.; Koong, M.K.; Lee, K.A. The Antioxidant Auraptene Improves Aged Oocyte Quality and Embryo Development in Mice. Antioxidants 2022, 12, 87. [Google Scholar] [CrossRef]
- Canosa, S.; Paschero, C.; Carosso, A.; Leoncini, S.; Mercaldo, N.; Gennarelli, G.; Benedetto, C.; Revelli, A. Effect of a Combination of Myo-Inositol, Alpha-Lipoic Acid, and Folic Acid on Oocyte Morphology and Embryo Morphokinetics in non-PCOS Overweight/Obese Patients Undergoing IVF: A Pilot, Prospective, Randomized Study. J. Clin. Med. 2020, 9, 2949. [Google Scholar] [CrossRef] [PubMed]
- Kermack, A.J.; Lowen, P.; Wellstead, S.J.; Fisk, H.L.; Montag, M.; Cheong, Y.; Osmond, C.; Houghton, F.D.; Calder, P.C.; Macklon, N.S. Effect of a 6-week “Mediterranean” dietary intervention on in vitro human embryo development: The Preconception Dietary Supplements in Assisted Reproduction double-blinded randomized controlled trial. Fertil. Steril. 2020, 113, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Meital, L.T.; Windsor, M.T.; Perissiou, M.; Schulze, K.; Magee, R.; Kuballa, A.; Golledge, J.; Bailey, T.G.; Askew, C.D.; Russell, F.D. Omega-3 fatty acids decrease oxidative stress and inflammation in macrophages from patients with small abdominal aortic aneurysm. Sci. Rep. 2019, 9, 12978. [Google Scholar] [CrossRef] [PubMed]
- Kalo, D.; Reches, D.; Netta, N.; Komsky-Elbaz, A.; Zeron, Y.; Moallem, U.; Roth, Z. Carryover effects of feeding bulls with an omega-3-enriched-diet—From spermatozoa to developed embryos. PLoS ONE 2022, 17, e0265650. [Google Scholar] [CrossRef]


| Stressor | Subtype Stressor | Species | Cell Type | The Effect | Reference |
|---|---|---|---|---|---|
| Phthalates | DEHP | Equine | Oocyte | Delay in the extrusion of the second polar body; reduced duration of the second cell cycle; increased abnormal divisions | Marzano et al. [84] |
| MMP | Mice | 2-cell stage embryo | Delay in the divisions into the 4- and 8-cell stages; delay in the morula and blastocyst formation | Tian et al. [85] | |
| MBP | Mice Galeolaria caespitose | 2-cell stage embryo Oocyte Spermatozoa | No effect on abnormal morphology Increased abnormal morphology | Chu et al. [86]; Lu et al. [87] | |
| DBP | |||||
| Herbicides | Bovine | Spermatozoa | Delay in the divisions into 4- and 8-cells; increase in abnormal division pattern | Komsky-Elbaz et al. [73] | |
| Pharmaceutical compound | CBZ | Bovine | Oocyte | Delay in the division into the 2- 4-, 6-, and 7-cell stages; increased abnormal divisions | Kalo et al. [70] |
| Nocodazole | Mice | Oocyte | Delay in the time of the first polar body extrusion | Zhu et al. [125] | |
| Oxidant agent | Cumene hydroperoxide | Mice | Oocyte | Delay in the divisions into the 4-, 5-, 6-, 7-, and 8-cell stages; delay in morula, blastocoel cavity formations, and blastocyst expansion | Wolff et al. [131] |
| Triton-X 100 | Mice | Oocyte | Delay in the divisions into the 2-, 3-, 4-, 5-, 6-, 7-, and 8-cell stages, morula formation, blastocoel cavity formation, and blastocyst expansion | Wolff et al. [131] | |
| Mineral oil (peroxidated) | Mice | 1-cell embryo | Delay in the division into the 2-, 3-, 4-, 5-, 6-, 7-, and 8-cell stages; delay in morula and blastocyst formation | Wolff et al. [131]; Ainsworth et al. [136] |
| Stressor | Subtype Stressor | Species | Cell Type | The Effect | Reference |
|---|---|---|---|---|---|
| Mycotoxins | AFB1, AFM1 | Bovine | Oocyte | Delay in the first, second, and third divisions, affecting the ratio between synchronous vs. asynchronous cleavages | Yaacobi-Artzi et al. [71] |
| Orchatoxin A | Sheep | Oocyte | Delay in the divisions into the 5- and 8-cell stages; embryonic arrest at the 2–4-cell and 8–16-cell stages | Dell’Aquila et al. [153] | |
| Virus | SARS-CoV-2 | Human | Positively infected women | Delay in the pronuclei appearance and in pronuclei fading; delay in the division into the 2-, 3-, 4-, and 5-cell stages; delay in blastocyst formation; prolonged transition time from the 2- to 3-cell, 3- to 4-cell, 3- to 5-cell stages, and from the 6-cell stage to blastulation | Ma et al. [158]; Braga et al. [159] |
| Papillomavirus | Human | Positively infected women | A shorter pronuclei appearance and fading; faster division into the 2-cell stage; delay in blastocyst formation | Zullo et al. [163] | |
| Radiation | X-ray | Bovine | Oocyte | Delay in the divisions from the 2- to 3-cell stages and from the 3- to 4-cell stages | Vazirov et al. [165] |
| Electromagnetic | Mice | Zygote | Delay in the division into the 2-cell to the 12-cell stage; delay in blastocyst formation and hatching; increased abnormal divisions | Koohestanidehaghi et al. [166]; Seify et al. [167] | |
| UV-laser light (used in sex-sorting method) | Bovine | Spermatozoa | Delay in all embryonic divisions; increased abnormal divisions | Kalo et al. [74]; Magata et al. [168] | |
| Delay in the first division | Bermejo-Alvarez et al. [169] | ||||
| Arrest at the zygote and the 4-cell stages; no difference in embryo kinetics | Steele et al. [170] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalo, D.; Yaacobi-Artzi, S.; Manovich, S.; Michaelov, A.; Komsky-Elbaz, A.; Roth, Z. Environmental Stress-Induced Alterations in Embryo Developmental Morphokinetics. J. Xenobiot. 2024, 14, 1613-1637. https://doi.org/10.3390/jox14040087
Kalo D, Yaacobi-Artzi S, Manovich S, Michaelov A, Komsky-Elbaz A, Roth Z. Environmental Stress-Induced Alterations in Embryo Developmental Morphokinetics. Journal of Xenobiotics. 2024; 14(4):1613-1637. https://doi.org/10.3390/jox14040087
Chicago/Turabian StyleKalo, Dorit, Shira Yaacobi-Artzi, Shir Manovich, Ariel Michaelov, Alisa Komsky-Elbaz, and Zvi Roth. 2024. "Environmental Stress-Induced Alterations in Embryo Developmental Morphokinetics" Journal of Xenobiotics 14, no. 4: 1613-1637. https://doi.org/10.3390/jox14040087
APA StyleKalo, D., Yaacobi-Artzi, S., Manovich, S., Michaelov, A., Komsky-Elbaz, A., & Roth, Z. (2024). Environmental Stress-Induced Alterations in Embryo Developmental Morphokinetics. Journal of Xenobiotics, 14(4), 1613-1637. https://doi.org/10.3390/jox14040087





