Time of Application of Desiccant Herbicides Affects Photosynthetic Pigments, Physiological Indicators, and the Quality of Cowpea Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of the Study Area and Field Conduction
2.2. Experiment I
2.2.1. Experimental Design and Treatments
2.2.2. Herbicide Application
2.3. Experiment II
2.3.1. Experimental Design and Treatments
2.3.2. Herbicide Application
2.4. Harvesting and Preparation of Seeds
2.5. Variables Analyzed
2.5.1. Experiment I
Germination Test
First Germination Count
Average Germination Speed
Germination Speed Index
Length and Dry Mass of Root and Shoot
Electrical Conductivity
Accelerated Aging Test
Preparation of the Plant Extract
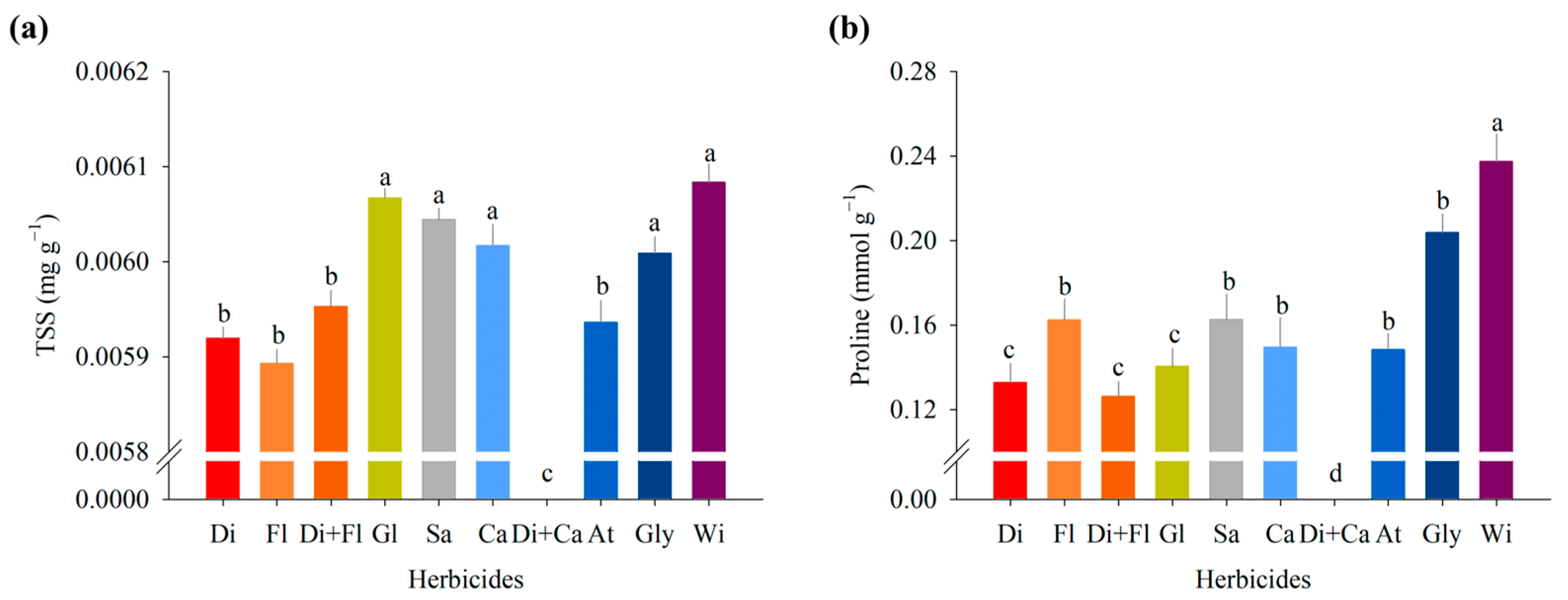
Total Soluble Sugars
Proline
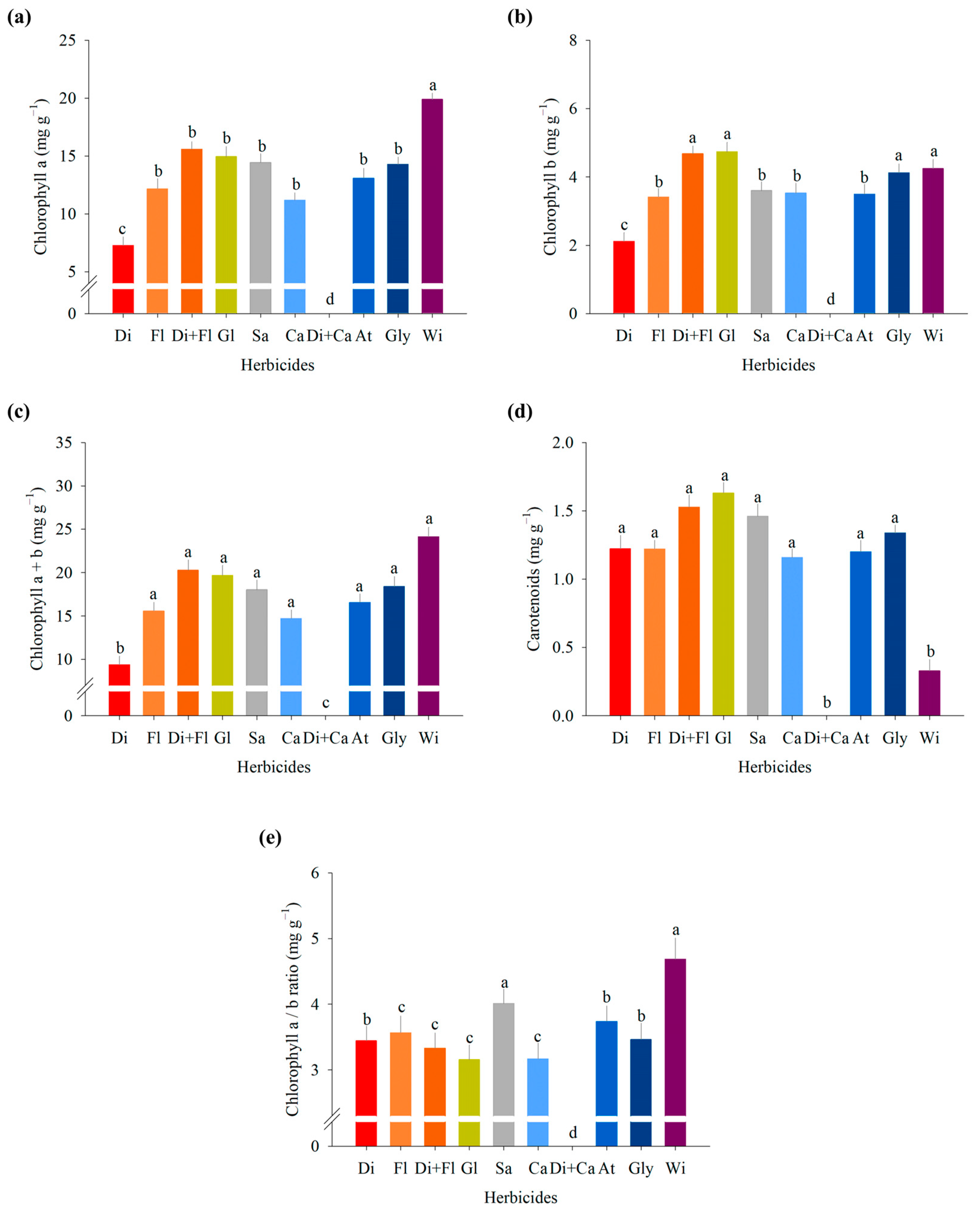
Chlorophyll and Carotenoid Content
2.5.2. Experiment II
2.6. Data Analysis
3. Results and Discussion
3.1. Experiment I
3.2. Experiment II
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jayathilake, C.; Visvanathan, R.; Deen, A.; Bangamuwage, R.; Jayawardana, B.C.; Nammi, S.; Liyanage, R. Cowpea: An overview on its nutritional facts and health benefits. J. Sci. Food Agric. 2018, 98, 4793–4806. [Google Scholar] [CrossRef] [PubMed]
- Assis, M.O.; Araujo, E.F.; Freitas, F.C.L.; Silva, L.J.; Araujo, R.F. Pre-harvest desiccation in productivity and physiological quality of cowpea seeds. Planta Daninha 2019, 37, e019177741. [Google Scholar] [CrossRef]
- Mekonnen, T.W.; Gerrano, A.S.; Mbuma, N.W.; Labuschagne, M.T. Breeding of vegetable cowpea for nutrition and climate resilience in Sub-Saharan Africa: Progress, opportunities, and challenges. Plants 2022, 11, 1583. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.d.O.; Silva, I.C.; Freitas, T.K.T.; Rodrigues, L.L.; de SabÓIa, R.C.B.; Rocha, M.d.M. Composição química e valor energético total de grãos imaturos de linhagens e cultivares de feijão caupi. Ed. Científica Digit. 2021, 2, 373–382. [Google Scholar]
- Lindemann, I.d.S.; Lang, G.H.; Hoffmann, J.F.; Rombaldi, C.V.; de Oliveira, M.; Elias, M.C.; Vanier, N.L. Foliar desiccators glyphosate, carfentrazone, and paraquat affect the technological and chemical properties of cowpea grains. J. Agric. Food Chem. 2017, 65, 6771–6778. [Google Scholar] [CrossRef]
- Freire Filho, F.R.; Ribeiro, V.Q.; Rodrigues, J.; Vieira, P. A cultura: Aspectos socioeconômicos. In Feijão-Caupi: Do Plantio à Colheita; Universidade Federal de Viçosa: Viçosa, Brazil, 2017; pp. 9–34. [Google Scholar]
- Omomowo, O.I.; Babalola, O.O. Constraints and prospects of improving cowpea productivity to ensure food, nutritional security and environmental sustainability. Front. Plant Sci. 2021, 12, 751731. [Google Scholar] [CrossRef]
- Castoldi, C.T.; Radunz, L.L.; Galon, L.; Aspiazú, I.; Forte, C.T.; Scariot, M.A.; Souza, D.O. Physiological quality of carioca bean seeds submitted to the application of desiccant herbicides in two periods. Planta Daninha 2019, 37, e019215688. [Google Scholar] [CrossRef]
- Krenchinski, F.H.; Cesco, V.J.S.; Rodrigues, D.M.; Pereira, V.G.C.; Albrecht, A.J.P.; Albrecht, L.P. Yield and physiological quality of wheat seeds after desiccation with different herbicides. J. Seed Sci. 2017, 39, 254–261. [Google Scholar] [CrossRef]
- Rigo, G.A.; Schuch, L.O.B.; Vargas, R.L.d.; Barros, W.S.; Szareski, V.J.; Carvalho, I.R.; Pedo, T. Micronutrient content and physiological quality of soybean seeds. J. Agric. Sci. 2018, 10, 223–230. [Google Scholar] [CrossRef][Green Version]
- Vargas, R.L.d.; Schuch, L.O.B.; Barros, W.S.; Rigo, G.A.; Szareski, V.J.; Carvalho, I.R.; Pimentel, J.R.; Troyjack, C.; Jaques, L.; Souza, V.Q.d. Macronutrients and micronutrients variability in soybean seeds. J. Agric. Sci. 2018, 10, 209. [Google Scholar] [CrossRef]
- Seidler, E.P.; Velho, J.P.; Christofari, L.F.; Almeida, P.S.G.; Andreatta, T. Dessecação em pré-colheita do trigo: Nova preocupação para a qualidade do cereal no consumo humano. Sci. Agrar. Parana. 2019, 18, 200–208. [Google Scholar] [CrossRef]
- Rosado, C.B.; Pereira, G.A.M.; Capobiango, N.P.; Moreira, R.P.L.; Freitas, F.C.L.; Teixeira, M.F.F.; Silva, A.A.d. Physiological quality of bean seeds after application of desiccant herbicides. Ciência Rural. 2019, 49, e20180228. [Google Scholar] [CrossRef]
- Bezerra, A.R.G.; Sediyama, T.; Cruz, C.D.; dos Santos Silva, F.C.; da Silva, A.F.; Rosa, D.P.; dos Santos Dias, L.A. Productivity and quality of soybean seeds of determinate and indeterminate growth types desiccated in pre-harvest. Aust. J. Crop Sci. 2016, 10, 693–700. [Google Scholar] [CrossRef]
- Zhang, M.; Van Veldhuizen, R. Varieties and pre harvesting treatment for growing polish canola (Brassica rapa L.) in Interior Alaska. Univers. J. Agric. Res. 2016, 4, 211–216. [Google Scholar] [CrossRef]
- Goffnett, A.M.; Sprague, C.L.; Mendoza, F.; Cichy, K.A. Preharvest herbicide treatments affect black bean desiccation, yield, and canned bean color. Crop Sci. 2016, 56, 1962–1969. [Google Scholar] [CrossRef]
- Raisse, E.R.; Assis, M.D.O.; Araujo, E.F.; Freitas, F.C.L.D.E.; Araujo, R.F. Chemical desiccants for anticipation of harvest and physiological quality of cowpea seeds. Rev. Caatinga 2020, 33, 878–887. [Google Scholar] [CrossRef]
- Rubenich, R.; Schaedler, C.E.; Zandoná, R.R.; de Melo Scalcon, R.; Chiapinotto, D.M. Efeito da reduà § ã o de luz na seletividade a herbicidas e rendimento de grã os do trigo. Rev. Bras. Herbic. 2017, 16, 296–306. [Google Scholar]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Maciel, C.D.d.G.; Iuchemin, C.E.L.; de Souza, M.V.; da Silva, A.A.P.; Karpinski, R.A.K.; Helvig, E.O.; Karpinski, P.K.K.; Baixo, B.T.; Matias, J.P. Control efficiency of black bindweed by glyphosate and glyphosate+ 2, 4-D on different application times. Rev. Bras. Herbic. 2016, 15, 380–387. [Google Scholar]
- Johnston, C.R.; Eure, P.M.; Grey, T.L.; Culpepper, A.S.; Vencill, W.K. Time of application influences translocation of auxinic herbicides in Palmer amaranth (Amaranthus palmeri). Weed Sci. 2018, 66, 4–14. [Google Scholar] [CrossRef]
- Vidal, R.A.; Merotto Jr, A.; Schaedler, C.E.; Pinto Lamego, F.; Portugal, J.; Menendes, J.; Kozlowski, L.A.; Muzell Trezzi, M.; De Prado, R. Mecanismos de ação dos herbicidas. Asp. Biol. Manejo Plantas Daninhas 2014, 10, 235–256. [Google Scholar]
- Ganie, Z.A.; Jugulam, M.; Jhala, A.J. Temperature influences efficacy, absorption, and translocation of 2, 4-D or glyphosate in glyphosate-resistant and glyphosate-susceptible common ragweed (Ambrosia artemisiifolia) and giant ragweed (Ambrosia trifida). Weed Sci. 2017, 65, 588–602. [Google Scholar] [CrossRef]
- de Paula, D.F.; Mendes, K.F.; da Silva Brochado, M.G.; Laube, A.F.S.; Rave, L.A.B. Técnicas para evitar a deriva e volatilização de herbicidas. In Desenvolvimento Sustentável, Interdisciplinaridade e Ciências Ambientais 2; Atena: Ponta Grossa, Brazil, 2021; pp. 89–116. [Google Scholar]
- Adegas, F.S.; Gazziero, D.L.P. Tecnologia de aplicação de agrotóxicos. In Tecnologias de Produção de Soja; Seixas, C.D.S., Neumaier, N., Balbinot Junior, A.A., Krzyzanowski, F.C., Leite, R.M.V.B., Eds.; Embrapa Soja: Londrina, Brazil, 2020; pp. 281–292. [Google Scholar]
- Coêlho, E.d.S.; Everthon da Silva Ribeiro, J.; Oliveira, P.H.d.A.; Lopes, W.d.A.R.; Oliveira, A.K.S.d.; Souza, M.d.F.; Lins, H.A.; Benedito, C.P.; Silveira, L.M.d.; Barros Júnior, A.P.; et al. Chemical Desiccation in the Preharvest of Cowpea: A Study of How the Time of Application Interferes in the Enzymatic and Physiological Aspects of Seedlings from Desiccated Plants. ACS Omega 2024, 9, 34893–34904. [Google Scholar] [CrossRef] [PubMed]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.d.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, H.G.; Jacomine, P.K.T.; Dos Anjos, L.H.C.; De Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; De Almeida, J.A.; de Araujo Filho, J.C.; de Oliveira, J.B.d.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos; Embrapa: Brasília, Brazil, 2018; Volume 2018, p. 356. [Google Scholar]
- Vale, J.C.d.; Bertini, C.; Borém, A. Feijão-Caupi: Do Plantio à Colheita; UFV: Viçosa, Brazil, 2017. [Google Scholar]
- Ministério da Agricultura. Brasil Regras Para Análise de Sementes, 1st ed.; Ministério da Agricultura, Pecuária e Abastecimento: Brasília, Brazil, 2009; ISBN 978-85-99851-70-8.
- Labouriau, L.G.; Valadares, M.B. On the germination of seeds of Calotropis procera (Ait.) Ait. f. Annals Braz. Acad. Sci. 1976, 48, 263–284. [Google Scholar]
- Maguire, J.D. Speed of germination—Aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Dutra, A.S.; Teófilo, E.M. Envelhecimento acelerado para avaliar o vigor de sementes de feijão caupi. Rev. Bras. Sementes 2007, 29, 193–197. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.A.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Witham, F.H.; Blaydes, D.F.; Dewlin, R.M. Experiments in Plant Physiology; Von Nostrand Reinhold Company: New York, NY, USA, 1971; pp. 55–56. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- R Core Team. In R: R Development Core Team R: A Language and Environment for Statistical Computing 2023; R Foundation for Statistical Computing: Vienna, Austria, 2023.
- Barbosa, A.P.; Zucareli, C.; Freiria, G.H.; Gomes, G.R.; Bazzo, J.H.B.; Takahashi, L.S.A. Subdoses de glifosato no processo germinativo e desenvolvimento de plântulas de milho. Rev. Bras. Milho E Sorgo 2017, 16, 240–250. [Google Scholar] [CrossRef][Green Version]
- Fipke, G.M.; Deak, E.A.; Stecca, J.D.L.; Bernardy, D.; Berger, M.; Tai, L.A.; Martin, T.N. Morphology and enzymatic activity of seedlings from wheat desiccated in pre-harvest. Acta Scientiarum. Agron. 2020, 43, e44974. [Google Scholar] [CrossRef]
- Ferreira, A.G.; Borghetti., F. Germinação: Do Básico ao Aplicado; Artmed: Porto Alegre, Brazil, 2004; p. 323. [Google Scholar]
- Krzyzanowski, F.C.; França-Neto, J.B.; Henning, A.A. A alta qualidade da semente de soja: Fator importante para a produção da cultura. Circ. Técnica 2018, 136, 1. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/177391/1/CT136-online.pdf (accessed on 18 September 2024).
- França Neto, J.B.; Krzyzanowski, F.C. O Vigor e o Desempenho das Sementes; ABRASEM—Associação Brasileira de Sementes e Mudas: Anuário, Brasília, 2018; pp. 26–30. [Google Scholar]
- Zuffo, A.; Aguilera, J.G.; Carvalho, E.R.; Teodoro, P.E. Harvest times with chemical desiccation and the effects on the enzymatic expression and physiological quality of soybean seeds. Rev. Caatinga 2020, 33, 361–370. [Google Scholar] [CrossRef]
- Marcos-Filho, J. Fisiologia de Sementes de Plantas Cultivadas, 2nd ed.; Abrates: Londrina, Brazil, 2015; p. 660. [Google Scholar]
- Marcandalli, L.H.; Lazarini, E.; Malaspina, I.C. Épocas de aplicação de dessecantes na cultura da soja: Qualidade fisiológica de sementes. Rev. Bras. Sementes 2011, 33, 241–250. [Google Scholar] [CrossRef]
- Bellé, C.; Kulczynski, S.M.; Basso, C.J.; Edu Kaspary, T.; Lamego, F.P.; Pinto, M.A.B. Yield and quality of wheat seeds as a function of desiccation stages and herbicides. J. Seed Sci. 2014, 36, 63–70. [Google Scholar] [CrossRef]
- Silva, J.N.; Costa, E.M.; Pereira, L.S.; Gonçalves, E.C.Z.; Zuchi, J.; Jakelaitis, A. Cowpea yield and quality after application of desiccating herbicides. J. Seed Sci. 2020, 42, e202042019. [Google Scholar] [CrossRef]
- Vanzolini, S.; Araki, C.A.d.S.; Silva, A.C.T.M.d.; Nakagawa, J. Teste de comprimento de plântula na avaliação da qualidade fisiológica de sementes de soja. Rev. Bras. Sementes 2007, 29, 90–96. [Google Scholar] [CrossRef]
- Daltro, E.M.F.; Albuquerque, M.C.d.F.; França Neto, J.d.B.; Guimarães, S.C.; Gazziero, D.L.P.; Henning, A.A. Aplicação de dessecantes em pré-colheita: Efeito na qualidade fisiológica de sementes de soja. Rev. Bras. Sementes 2010, 32, 111–122. [Google Scholar] [CrossRef]
- Mahapatra, K.; De, S.; Banerjee, S.; Roy, S. Pesticide mediated oxidative stress induces genotoxicity and disrupts chromatin structure in fenugreek (Trigonella foenum-graecum L.) seedlings. J. Hazard. Mater. 2019, 369, 362–374. [Google Scholar] [CrossRef]
- Takano, H.K.; Dayan, F.E. Glufosinate-ammonium: A review of the current state of knowledge. Pest Manag. Sci. 2020, 76, 3911–3925. [Google Scholar] [CrossRef] [PubMed]
- Durigon, M.R.; Camera, A.S.; Cechin, J.; Vargas, L.; Chavarria, G. Does spraying of atrazine on triazine-resistant canola hybrid impair photosynthetic processes? Planta Daninha 2019, 37, e019190367. [Google Scholar] [CrossRef]
- Botelho, F.J.E.; Oliveira, J.A.; Von Pinho, É.V.d.R.; Carvalho, E.R.; Figueiredo, Í.B.D.; Andrade, V. Qualidade de sementes de soja obtidas de diferentes cultivares submetidas à dessecação com diferentes herbicidas e épocas de aplicação. Rev. Agro@ Mbiente-Line 2016, 10, 137–144. [Google Scholar] [CrossRef][Green Version]
- Zanatta, E.; Szareski, V.J.; Carvalho, I.R.; Pimentel, J.R.; Troyjack, C.; Dellagostin, S.M.; Demari, G.H.; Lautenchleger, F.; Souza, V.Q.; Martinazzo, E.G. Pre-harvest Desiccation: Productivity and physical and physiological inferences on soybean seeds during storage. J. Agric. Sci. 2018, 10, 354–362. [Google Scholar] [CrossRef][Green Version]
- Pagliarini, M.K.; Rebouças, M.C.; de Almeida Monaco-Mello, K.; dos Santos Zomerfeld, P.; Pontim, B.C.A.; Gordin, C.R.B.; Minella, E. Pre-harvest glyphosate desiccation on barley seed quality. Res. Soc. Dev. 2021, 10, e24310716469. [Google Scholar] [CrossRef]
- Radwan, D.E.M.; Mohamed, A.K.; Fayez, K.A.; Abdelrahman, A.M. Oxidative stress caused by Basagran® herbicide is altered by salicylic acid treatments in peanut plants. Heliyon 2019, 5, e01791. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Bohn, K.K.; McKeithen, J.; Singh, A. Effects of conversion harvests on light regimes in a southern pine ecosystem in transition from intensively managed plantations to uneven-aged stands. For. Ecol. Manag. 2019, 432, 140–149. [Google Scholar] [CrossRef]
- Sandmann, G. Bleaching herbicides: Action mechanism in carotenoid biosynthesis, structural requirements and engineering of resistance. In Herbicide Classes in Development: Mode of Action, Targets, Genetic Engineering, Chemistry; Springer: Berlin/Heidelberg, Germany, 2002; pp. 43–57. [Google Scholar]
- Rosas-Saavedra, C.; Stange, C. Biosynthesis of carotenoids in plants: Enzymes and color. In Carotenoids in Nature: Biosynthesis, Regulation and Function; Springer International Publishing: Cham, Switzerland, 2016; pp. 35–69. [Google Scholar]
- Agostinetto, D.; Perboni, L.T.; Langaro, A.C.; Gomes, J.; Fraga, D.S.; Franco, J.J. Changes in photosynthesis and oxidative stress in wheat plants submmited to herbicides application. Planta Daninha 2016, 34, 01–09. [Google Scholar] [CrossRef]
- Salem, R.E.M.E.; El-Sobki, A.E.A. Physiological and biochemical parameters as an index for herbicides damage in wheat plants. Egypt. Acad. J. Biol. Sci. F. Toxicol. Pest Control 2021, 13, 25–35. [Google Scholar] [CrossRef]
- Sharma, A.; Yuan, H.; Kumar, V.; Ramakrishnan, M.; Kohli, S.K.; Kaur, R.; Thukral, A.K.; Bhardwaj, R.; Zheng, B. Castasterone attenuates insecticide induced phytotoxicity in mustard. Ecotoxicol. Environ. Saf. 2019, 179, 50–61. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Singh, R.; Thukral, A.K.; Bhardwaj, R. Effect of seed pre-soaking with 24-epibrassinolide on growth and photosynthetic parameters of Brassica juncea L. in imidacloprid soil. Ecotoxicol. Environ. Saf. 2016, 133, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Kaya, A.; Doganlar, Z.B. Exogenous jasmonic acid induces stress tolerance in tobacco (Nicotiana tabacum) exposed to imazapic. Ecotoxicol. Environ. Saf. 2016, 124, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Galon, L.; Weirich, S.N.; Franceschetti, M.B.; Aspiazu, I.; da Silva, A.F.; Forte, C.T.; Rossetto, E.R.d.O.; Brandler, D.; Perin, G.F.; Ulkovski, C. Selectivity of saflufenacil applied alone or mixed to glyphosate in maize. J. Agric. Stud. 2020, 8, 775–787. [Google Scholar] [CrossRef]
- Amaral, C.L.; Santos, J.I.; Portugal, C.R.S.; Braga, A.F.; Alves, P. Crescimento de Plântulas de Vernonia ferruginea Submetidas a Estresse Térmico. Planta Daninha 2020, 38, e020178700. [Google Scholar] [CrossRef]
- Barrozo, L.M.; Costa, T.d.J.F.; Gomes, D.S.; dos Santos, J.C.; de Moura Lima, J.F.; da Costa Zanatta, T.S.; Diniz, A.A.; de Oliveira Filho, A.S.B. Estresse térmico e tratamento químico no potencial fisiológico de sementes de Zea mays L. Rev. Ibero-Am. Ciências Ambient. 2020, 11, 126–136. [Google Scholar] [CrossRef]
- Silva, P.V.d.; Mendonça Bezera, M.; Medeiros, E.S.d.; Carvalho Dambrós, T.; Mauad, M.; Monquero, P.A.; Alves Nunes, F.; Schedenffeldt, B.F. Pre-harvest desiccation strategies of soybean culture: A scenario without paraquat. J. Environ. Sci. Health Part B 2022, 57, 710–719. [Google Scholar] [CrossRef]
- Bazzo, J.H.B.; Riede, C.R.; Arruda, K.M.A.; Zucareli, C.; Fonseca, I.C.d.B. Topdressing nitrogen fertilization associated with trinexapac-ethyl on industrial quality of oat grains. Rev. Ceres 2021, 68, 47–54. [Google Scholar] [CrossRef]
- Henning, F.A.; Mertz, L.M.; Jacob Junior, E.A.; Machado, R.D.; Fiss, G.; Zimmer, P.D. Composição química e mobilização de reservas em sementes de soja de alto e baixo vigor. Bragantia 2010, 69, 727–734. [Google Scholar] [CrossRef]
- Chao, S.; Mitchell, J.; Fukai, S. Factors determining genotypic variation in the speed of rice germination. Agronomy 2021, 11, 1614. [Google Scholar] [CrossRef]
- Santos, F.L.; Bertacine, F.; Souza, J.S.; Simões, I.; Bossolani, J.W.; Sá, M.E. A influência de dessecante na qualidade fisiológica de sementes de soja. Rev. Bras. Eng. Biossistemas 2018, 12, 68–76. [Google Scholar]
- Karim, M.M.; Rahman, M.L.; Ferdush, J.; Tareq, M.Z.; Mia, M.M.; Sultan, M.T.; Himel, R.M. Yield, quality and cost of jute (Corchorus species) seed production as influenced by herbicide application time. Int. J. Adv. Geosci. 2020, 8, 153–159. [Google Scholar] [CrossRef]
- Toledo, M.Z.; Cavariani, C.; França-Neto, J.d.B. Qualidade fisiológica de sementes de soja colhidas em duas épocas após dessecação com glyphosate. Rev. Bras. Sementes 2012, 34, 134–142. [Google Scholar] [CrossRef]
- Barbaś, P.; Sawicka, B.; Marczak, B.K.; Pszczółkowski, P. Effect of mechanical and herbicide treatments on weed densities and biomass in two potato cultivars. Agriculture 2020, 10, 455. [Google Scholar] [CrossRef]
- Gonzales, J.L.S.; Paula, R.C.d.; Valeri, S.V. Teste de condutividade elétrica em sementes de Albizia hassleri (Chodat) Burkart. Fabaceae-Mimosoideae. Rev. Árvore 2009, 33, 625–634. [Google Scholar] [CrossRef]
| pH in H2O | EC | P | K+ | Na+ | Ca2+ | Mg2+ | |
|---|---|---|---|---|---|---|---|
| dS m−1 | mg dm−3 | cmolc dm−3 | |||||
| 20 cm | 7.56 | 0.08 | 156.77 | 156.00 | 15.20 | 3.50 | 0.86 |
| 40 cm | 7.45 | 0.05 | 106.23 | 145.87 | 15.20 | 3.00 | 0.43 |
| Active Ingredient | Commercial Product | Commercial Product Dose | Applied Dose of Active Ingredient (a.i.)/Acid Equivalent (a.e.) |
|---|---|---|---|
| Diquat | Reglone | 2.0 L ha−1 | 400.00 g a.i. ha−1 |
| Flumioxazin | Sumyzin | 50 mL ha−1 | 25.00 g a.i. ha−1 * |
| Diquat + flumioxazin | Reglone + Sumyzin500 | 2.0 L ha−1 + 50 mL ha−1 | 400.00 g a.i. ha−1 + 25.00 g a.i. ha−1 * |
| Glufosinate | Fascinate BR | 2.0 L ha−1 | 400.00 g a.i. ha−1 |
| Saflufenacil | Heat | 140 g ha−1 | 98.00 g a.i. ha−1 |
| Carfentrazone-ethyl | Aurora | 125 mL ha−1 | 50.00 g a.i. ha−1 * |
| Diquat + carfentrazone | Reglone + Aurora | 2.0 L ha−1 + 125 mL ha−1 | 400.00 g a.i. ha−1 + 50.00 g a.i. ha−1 * |
| Atrazine | Herbitrin | 5.0 L ha−1 | 2.50 g a.i. ha−1 * |
| Glyphosate | Roundup original DI | 4.0 L ha−1 | 1.480 g a.e. ha−1 |
| Herbicides | Normal Seedlings (%) | Abnormal Seedlings (%) | Dead Seeds (%) |
|---|---|---|---|
| Diquat | 49 b | 49 c | 2 a |
| Flumioxazin | 35 c | 64 b | 0 a |
| Diquat + flumioxazin | 55 b | 44 c | 1 a |
| Glufosinate | 59 b | 40 c | 1 a |
| Saflufenacil | 51 b | 48 c | 0 a |
| Carfentrazone | 49 b | 48 c | 2 a |
| Diquat + carfentrazone | 0 d | 100 a | 0 a |
| Atrazine | 43 c | 55 b | 1 a |
| Glyphosate | 56 b | 43 c | 0 a |
| Witness (control) | 82 a | 17 d | 0 a |
| CV (%) | 7.80 | 7.91 | 6.50 |
| Herbicides | FGC | AGS | GSI |
|---|---|---|---|
| Diquat | 45 b ± 4.96 | 0.3211 a ± 0.028 | 7.962 b ± 1.23 |
| Flumioxazin | 33 c ± 5.78 | 0.3218 a ± 0.035 | 5.787 c ± 0.87 |
| Diquat + flumioxazin | 52 b ± 4.38 | 0.3288 a ± 0.026 | 8.383 b ± 1.04 |
| Glufosinate | 55 b ± 4.66 | 0.3259 a ± 0.028 | 9.662 b ± 1.28 |
| Saflufenacil | 36 c ± 5.71 | 0.2876 b ± 0.023 | 7.712 b ± 1.23 |
| Carfentrazone | 47 b ± 5.12 | 0.3258 a ± 0.023 | 8.120 b ± 1.21 |
| Diquat + carfentrazone | 0 d ± 0.00 | 0.0000 c ± 0.000 | 0.000 d ± 0.00 |
| Atrazine | 40 c ± 5.05 | 0.3206 a ± 0.027 | 7.066 c ± 1.14 |
| Glyphosate | 52 b ± 5.12 | 0.3231 a ± 0.025 | 9.129 b ± 1.21 |
| Witness (control) | 76 a ± 6.15 | 0.3236 a ± 0.030 | 13.466 a ± 1.81 |
| Herbicides | RL (cm Seedling−1) | SL (cm Seedling−1) | RDM (g Seedling−1) | SDM (g Seedling−1) |
|---|---|---|---|---|
| Diquat | 8.81 b ± 1.12 | 6.65 c ± 0.85 | 0.30 a ± 0.04 | 2.53 a ± 0.25 |
| Flumioxazin | 9.32 b ± 1.08 | 6.48 c ± 0.78 | 0.20 b ± 0.06 | 1.90 b ± 0.27 |
| Diquat + flumioxazin | 9.22 b ± 1.15 | 6.65 c ± 0.82 | 0.22 b ± 0.05 | 2.28 a ± 0.27 |
| Glufosinate | 9.07 b ± 1.10 | 5.28 c ± 0.90 | 0.24 b ± 0.05 | 2.47 a ± 0.26 |
| Saflufenacil | 8.73 b ± 1.05 | 7.38 b ± 0.80 | 0.23 b ± 0.05 | 1.98 b ± 0.28 |
| Carfentrazone | 9.72 b ± 1.20 | 7.36 b ± 0.75 | 0.24 b ± 0.06 | 1.91 b ± 0.29 |
| Diquat + carfentrazone | 0.00 c ± 0.00 | 0.00 d ± 0.00 | 0.00 d ± 0.00 | 0.00 d ± 0.00 |
| Atrazine | 9.89 b ± 1.18 | 7.31 b ± 0.85 | 0.19 c ± 0.05 | 1.59 c ± 0.27 |
| Glyphosate | 10.20 b ± 1.22 | 7.21 b ± 0.80 | 0.29 a ± 0.06 | 2.33 a ± 0.28 |
| Witness | 11.31 a ± 1.25 | 8.21 a ± 0.90 | 0.32 a ± 0.05 | 2.82 a ± 0.30 |
| Herbicides | EC (μS cm−1g−1) | AA |
|---|---|---|
| Diquat | 22.61 a ± 1.50 | 0 c ± 0.00 |
| Flumioxazin | 24.43 a ± 1.75 | 98 a ± 2.50 |
| Diquat + flumioxazin | 22.85 a ± 1.60 | 92 b ± 3.00 |
| Glufosinate | 23.51 a ± 1.80 | 99 a ± 2.00 |
| Saflufenacil | 21.91 a ± 1.55 | 96 a ± 2.75 |
| Carfentrazone | 23.81 a ± 1.70 | 0 c ± 0.00 |
| Diquat + carfentrazone | 19.71 b ± 1.45 | 94 b ± 3.25 |
| Atrazine | 24.35 a ± 1.65 | 96 a ± 2.50 |
| Glyphosate | 19.96 b ± 1.50 | 93 b ± 3.00 |
| Witness | 16.89 c ± 1.85 | 100 a ± 1.75 |
| Variables | Herbicides | Times (h) | ||
|---|---|---|---|---|
| 6 a.m. | 12 p.m. | 6 p.m. | ||
| Normal seedlings (%) | Diquat | 47.7 bA | 38.8 aB | 46.0 aA |
| Diquat + carfentrazone | 56.5 aA | 39.0 aC | 46.5 aB | |
| Diquat + flumioxazin | 47.0 bA | 35.5 aB | 44.5 aA | |
| Witness | 67.0 α | |||
| CV (%) | 7.95 | |||
| Abnormal seedlings (%) | Diquat | 45.2 bA | 47.5 bA | 50.2 aA |
| Diquat + carfentrazone | 41.0 bC | 57.0 aA | 50.5 aB | |
| Diquat + flumioxazin | 51.0 aA | 55.7 aA | 52.5 aA | |
| Witness | 31.5 α | |||
| CV (%) | 7.93 | |||
| Dead seeds (%) | Diquat | 7.0 aB | 14.0 aA | 3.7 aB α |
| Diquat + carfentrazone | 2.5 bA α | 4.0 cA α | 3.0 aA α | |
| Diquat + flumioxazin | 2.0 bB α | 8.7 bA | 3.0 aB α | |
| Witness | 1.5 α | |||
| CV (%) | 9.01 | |||
| Variables | Herbicides | Times (h) | ||
|---|---|---|---|---|
| 6 a.m. | 12 p.m. | 6 p.m. | ||
| FGC (%) | Diquat | 14.5 aA | 7.5 bC | 11.5 bB |
| Diquat + carfentrazone | 14.5 aA | 10.5 aB | 10.5 bB | |
| Diquat + flumioxazin | 14.5 aA | 7.5 bB | 13.0 aA | |
| Witness | 25.0 α | |||
| AGS (days) | Diquat | 0.5356 aA α | 0.4703 aB α | 0.4484 aB α |
| Diquat + carfentrazone | 0.5721 aA α | 0.4666 aB α | 0.4934 aB α | |
| Diquat + flumioxazin | 0.5089 aA α | 0.4057 aB | 0.4945 aA α | |
| Witness | 0.5234 α | |||
| GSI | Diquat | 12.46 aA | 13.37 aA | 13.62 aA |
| Diquat + carfentrazone | 14.35 aA | 10.91 aB | 13.45 aA | |
| Diquat + flumioxazin | 14.24 aA | 10.83 aB | 11.87 aA | |
| Witness | 21.37 α | |||
| Variables | Herbicides | Times (h) | ||
|---|---|---|---|---|
| 6 a.m. | 12 p.m. | 6 p.m. | ||
| RL (cm seedling−1) | Diquat | 9.345 aA α | 9.624 aA α | 10.606 aA α |
| Diquat + carfentrazone | 10.853 aA α | 7.229 aB | 8.530 bA | |
| Diquat + flumioxazin | 8.212 bA | 8.762 aA | 6.103 bB | |
| Witness | 11.781 α | |||
| SL (cm seedling−1) | Diquat | 7.142 aA | 6.325 aA | 6.365 aA |
| Diquat + carfentrazone | 7.415 aA α | 6.361 aB | 6.105 aB | |
| Diquat + flumioxazin | 6.197 bA | 5.661 aA | 6.153 aA | |
| Witness | 8.108 α | |||
| RDM (g seedling−1) | Diquat | 0.0132 aA | 0.0121 aA | 0.0138 aA |
| Diquat + carfentrazone | 0.0127 aA | 0.0125 aA | 0.0144 aA α | |
| Diquat + flumioxazin | 0.0129 aA | 0.0114 aA | 0.0105 bB | |
| Witness | 0.0175 α | |||
| SDM (g seedling−1) | Diquat | 0.1460 aA α | 0.1468 aA α | 0.1406 aA α |
| Diquat + carfentrazone | 0.1496 aA α | 0.1249 bB | 0.1371 bA α | |
| Diquat + flumioxazin | 0.1438 aA α | 0.1418 aA α | 0.1254 bB | |
| Witness | 0.1459 α | |||
| Variables | Herbicides | Times (h) | ||
|---|---|---|---|---|
| 6 a.m. | 12 p.m. | 6 p.m. | ||
| RL (cm seedling−1) | Diquat | 9.345 aA α | 9.624 aA α | 10.606 aA α |
| Diquat + carfentrazone | 10.853 aA α | 7.229 aB | 8.530 aB | |
| Diquat + flumioxazin | 8.212 aA | 8.762 aA | 6.103 bB | |
| Witness | 11.781 α | |||
| SL (cm seedling−1) | Diquat | 7.142 aA | 6.325 aA | 6.365 aA |
| Diquat + carfentrazone | 7.415 aA α | 6.361 aB | 6.105 aB | |
| Diquat + flumioxazin | 6.197 bA | 5.661 aA | 6.153 aA | |
| Witness | 8.108 α | |||
| RDM (g seedling−1) | Diquat | 0.0132 aA | 0.0121 aA | 0.0138 aA |
| Diquat + carfentrazone | 0.0127 aA | 0.0125 aA | 0.0144 aA α | |
| Diquat + flumioxazin | 0.0129 aA | 0.0114 aB | 0.0105 bB | |
| Witness | 0.0175 α | |||
| SDM (g seedling−1) | Diquat | 0.1460 aA α | 0.1468 aA α | 0.1406 aA α |
| Diquat + carfentrazone | 0.1496 aA α | 0.1249 bB | 0.1371 bA α | |
| Diquat + flumioxazin | 0.1438 aA α | 0.1418 aA α | 0.1254 bB | |
| Witness | 0.1459 α | |||
| Variables | Herbicides | Times (h) | ||
|---|---|---|---|---|
| 6 a.m. | 12 p.m. | 6 p.m. | ||
| AA (%) | Diquat | 40.5 aA | 29.5 bB | 38.7 aA |
| Diquat + carfentrazone | 39.5 aA | 33.7 aB | 33.7 bB | |
| Diquat + flumioxazin | 40.0 aA | 27.7 bC | 35.2 bB | |
| Witness | 45.75 α | |||
| EC (μS cm−1g−1) | Diquat | 72.46 aB | 83.61 bA | 75.18 aB |
| Diquat + carfentrazone | 72.56 aB | 92.97 bA | 78.90 aB | |
| Diquat + flumioxazin | 75.46 aA | 76.90 aA | 78.58 aA | |
| Witness | 66.66 α | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coêlho, E.d.S.; Ribeiro, J.E.d.S.; Lopes, W.d.A.R.; Oliveira, A.K.S.d.; Oliveira, P.H.d.A.; Santos, G.L.d.; Barbosa, E.d.S.; Silva, V.N.S.e.; Lins, H.A.; Benedito, C.P.; et al. Time of Application of Desiccant Herbicides Affects Photosynthetic Pigments, Physiological Indicators, and the Quality of Cowpea Seeds. J. Xenobiot. 2024, 14, 1312-1331. https://doi.org/10.3390/jox14030074
Coêlho EdS, Ribeiro JEdS, Lopes WdAR, Oliveira AKSd, Oliveira PHdA, Santos GLd, Barbosa EdS, Silva VNSe, Lins HA, Benedito CP, et al. Time of Application of Desiccant Herbicides Affects Photosynthetic Pigments, Physiological Indicators, and the Quality of Cowpea Seeds. Journal of Xenobiotics. 2024; 14(3):1312-1331. https://doi.org/10.3390/jox14030074
Chicago/Turabian StyleCoêlho, Ester dos Santos, João Everthon da Silva Ribeiro, Welder de Araújo Rangel Lopes, Anna Kézia Soares de Oliveira, Pablo Henrique de Almeida Oliveira, Gisele Lopes dos Santos, Ewerton da Silva Barbosa, Valécia Nogueira Santos e Silva, Hamurábi Anizio Lins, Clarisse Pereira Benedito, and et al. 2024. "Time of Application of Desiccant Herbicides Affects Photosynthetic Pigments, Physiological Indicators, and the Quality of Cowpea Seeds" Journal of Xenobiotics 14, no. 3: 1312-1331. https://doi.org/10.3390/jox14030074
APA StyleCoêlho, E. d. S., Ribeiro, J. E. d. S., Lopes, W. d. A. R., Oliveira, A. K. S. d., Oliveira, P. H. d. A., Santos, G. L. d., Barbosa, E. d. S., Silva, V. N. S. e., Lins, H. A., Benedito, C. P., Silveira, L. M. d., Araujo Filho, A. C. d., Silva, D. V., & Barros Júnior, A. P. (2024). Time of Application of Desiccant Herbicides Affects Photosynthetic Pigments, Physiological Indicators, and the Quality of Cowpea Seeds. Journal of Xenobiotics, 14(3), 1312-1331. https://doi.org/10.3390/jox14030074







