Vanadium Toxicity Is Altered by Global Warming Conditions in Sea Urchin Embryos: Metal Bioaccumulation, Cell Stress Response and Apoptosis
Abstract
1. Introduction
2. Materials and Methods
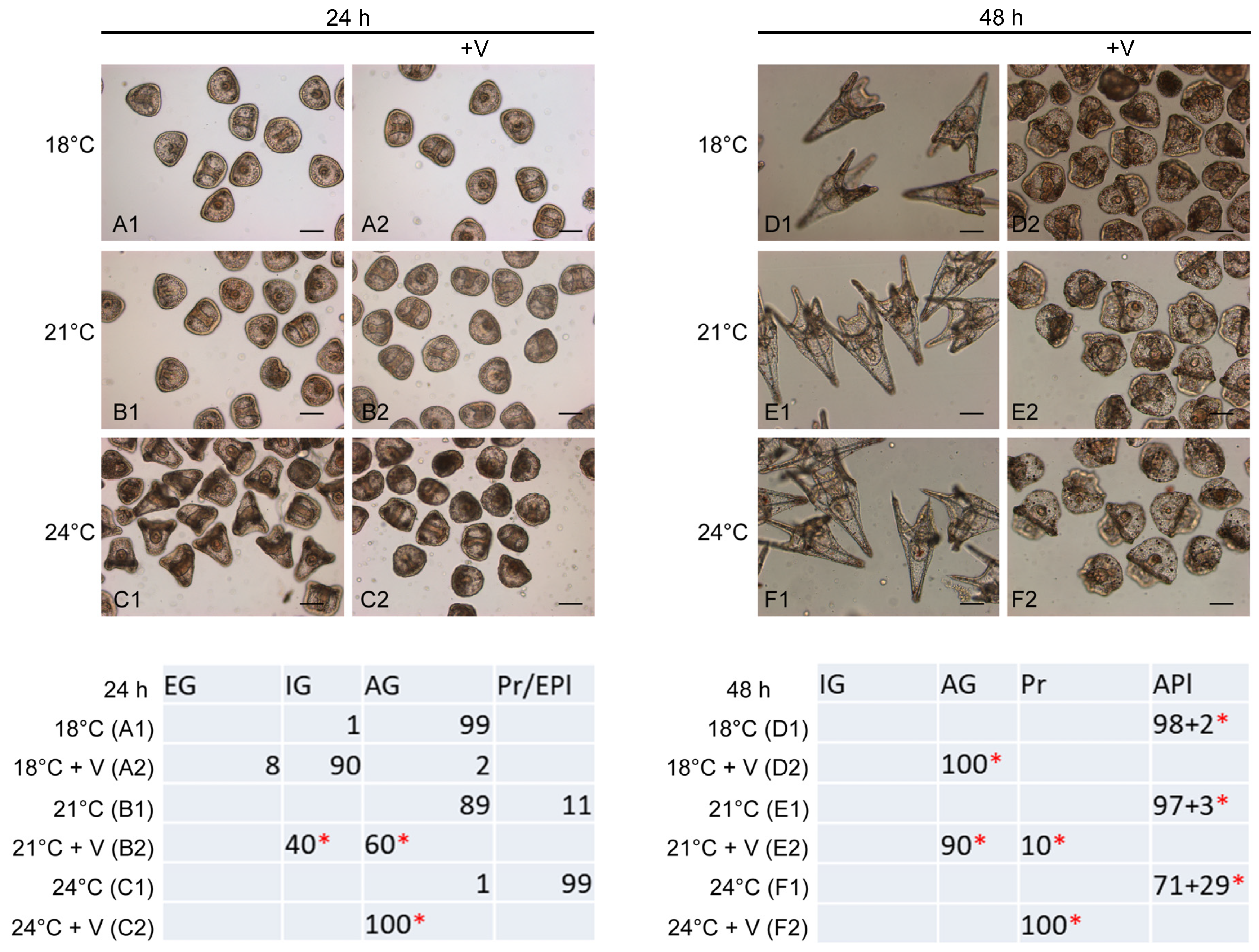
2.1. Embryos Cultures, Treatments, and Morphological Analyses
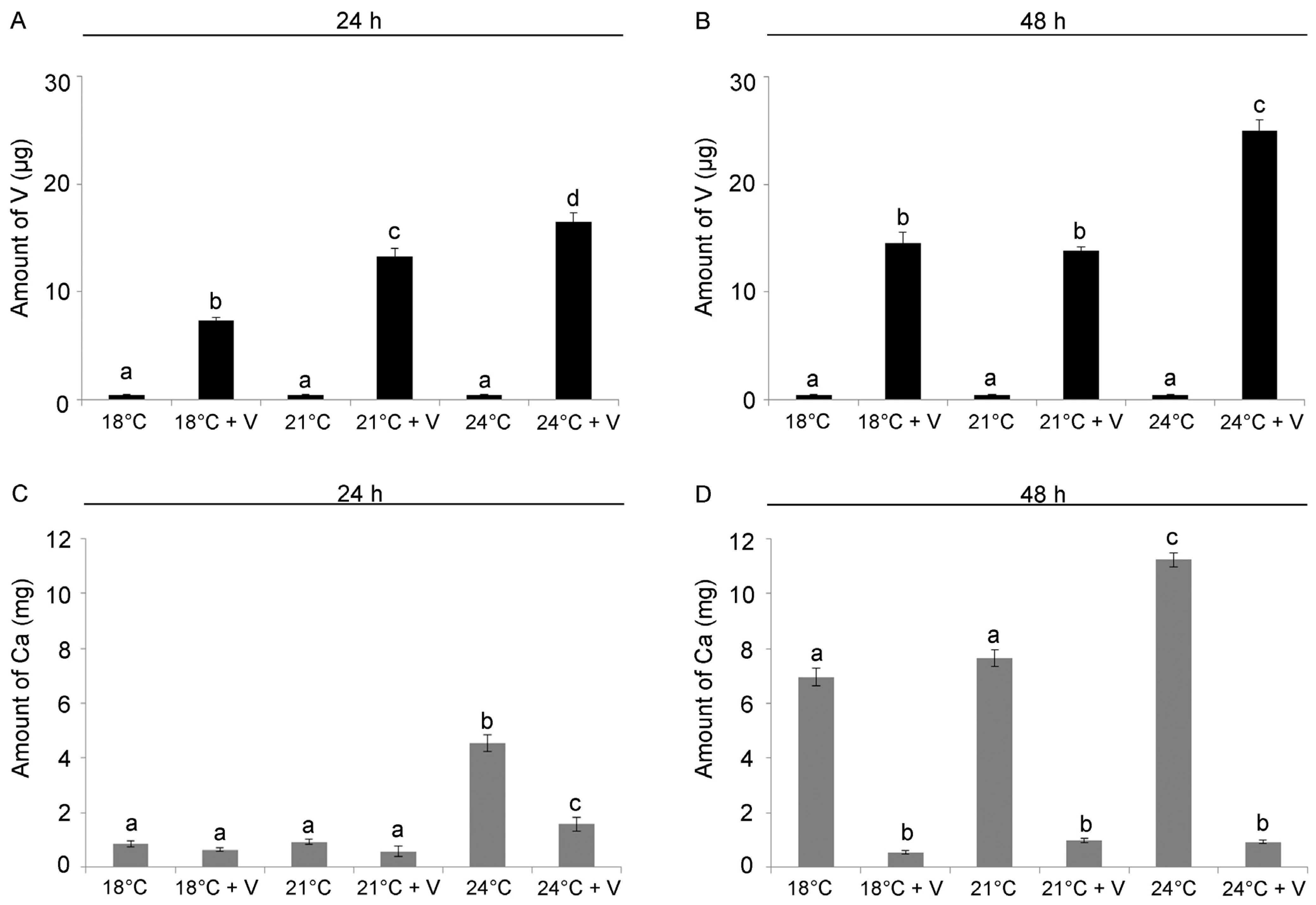
2.2. V and Ca Inductively Coupled Plasma Mass Spectrometry Analyses
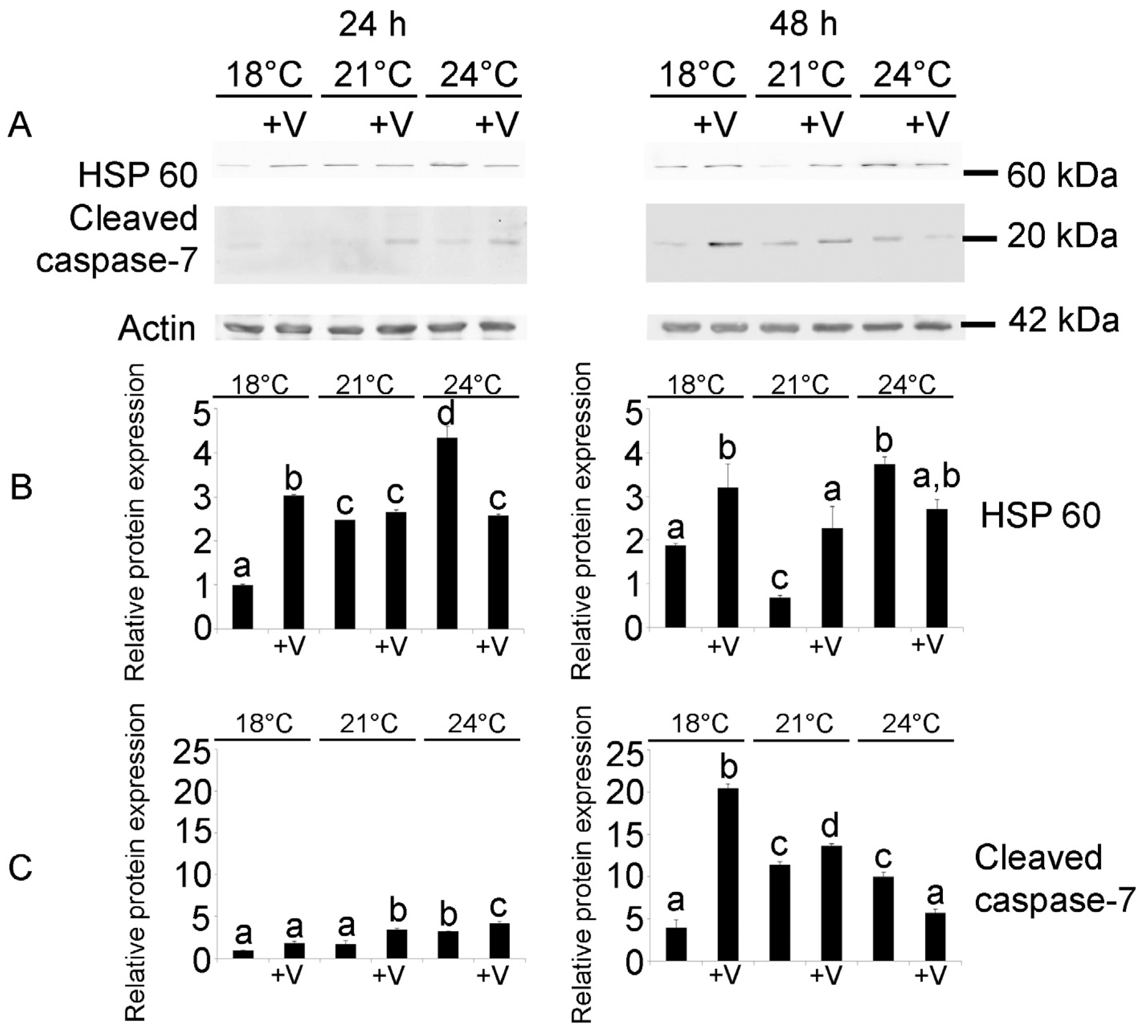
2.3. Immunoblot Detection
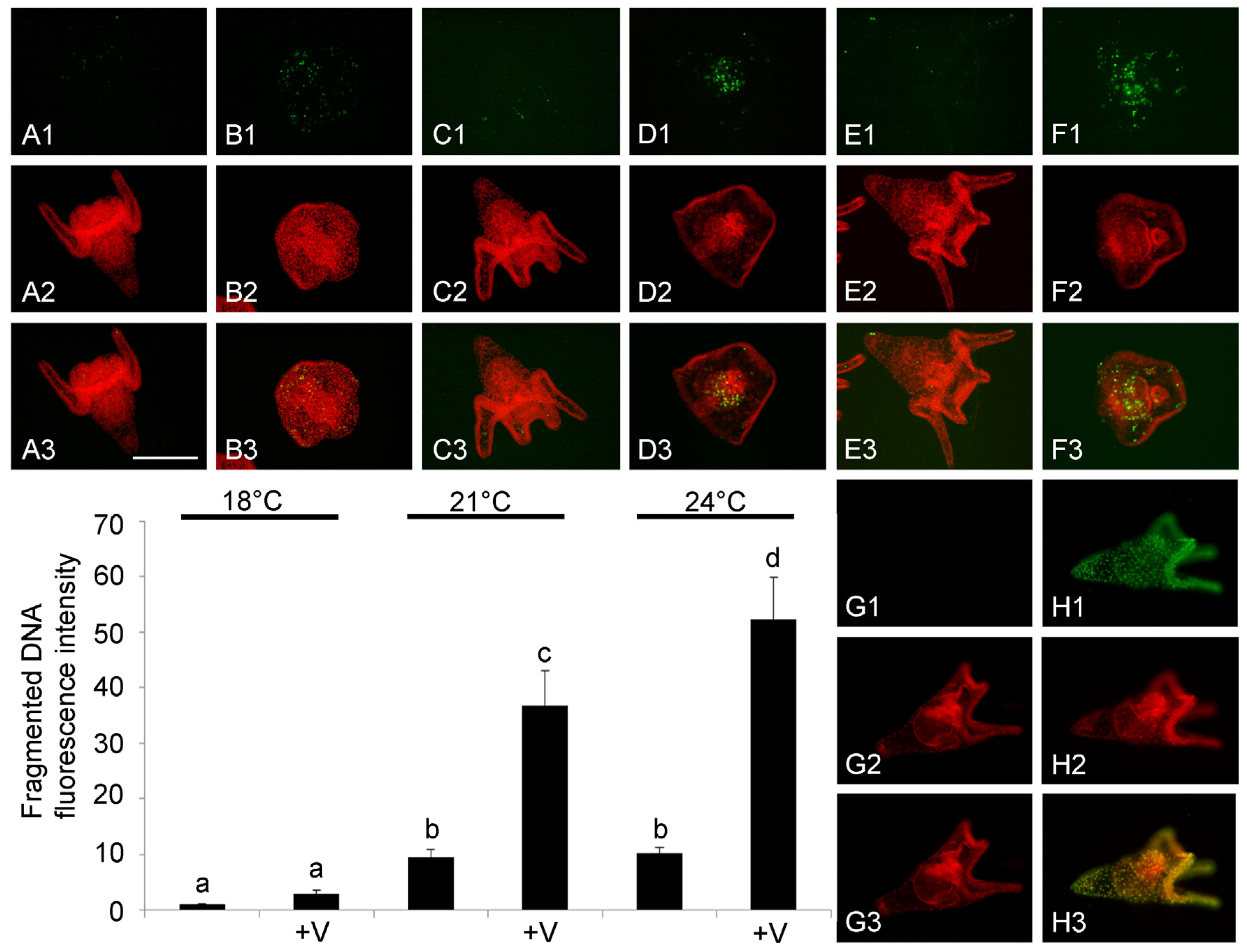
2.4. Terminal Deoxynucleotidyl Transferase (TdT) dUTP Nick-End Labeling (TUNEL) Assay
2.5. Statistical Analysis
3. Results
3.1. Temperature Increase Alters the Effects of V on Embryo Development
3.2. V and Ca Content Are Modulated by Temperature Increase
3.3. Embryonic Cytoprotection and Apoptosis
3.4. DNA Fragmentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reichelt-Brushett, A. Marine Pollution—Monitoring, Management and Mitigation; Springer Textbooks in Earth Sciences, Geography and Environment; Springer: Berlin, Germany, 2023. [Google Scholar] [CrossRef]
- Ivanov, A.S.; Parker, B.F.; Zhang, Z.; Aguila, B.; Sun, Q.; Ma, S.; Jansone-Popova, S.; Arnold, J.; Mayes, R.T.; Dai, S.; et al. Siderophore-inspired chelator hijacks uranium from aqueous medium. Nat. Commun. 2019, 10, 819. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, M.; Rizwan, M.S.; Xiong, S.; Li, H.; Ashraf, M.; Shahzad, S.M.; Shahzad, M.; Rizwan, M.; Tu, S. Vanadium, recent advancements and research prospects: A review. Environ. Int. 2015, 80, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Vanitec. 2011–2020 Vanadium Statistics. 2021. Available online: http://www.vanitec.org/vanadium/production-consumption (accessed on 17 June 2021).
- Tulcan, R.X.S.; Ouyang, W.; Lin, C.; He, M.; Wang, B. Vanadium pollution and health risks in marine ecosystems: Anthropogenic sources over natural contributions. Water Res. 2021, 207, 117838. [Google Scholar] [CrossRef]
- Naeem, A.; Westerhoff, P.; Mustafa, S. Vanadium removal by metal (hydr)oxide adsorbents. Water Res. 2007, 41, 1596–1602. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 35–115. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- Cerrano, C.; Bavestrello, G.; Bianchi, C.N.; Cattaneoavietti, R.; Bava, S.; Morganti, C.; Morri, C.; Picco, P.; Sara, G.; Schiaparelli, S.; et al. Catastrophic massâ-mortality episode of gorgonians and other organisms in the Ligurian Sea (North-Western Mediterranean), summer 1999. Ecol. Lett. 2000, 3, 284–293. [Google Scholar] [CrossRef]
- Garrabou, J.; Gómez-Gras, D.; Medrano, A.; Cerrano, C.; Ponti, M.; Schlegel, R.; Bensoussan, N.; Turicchia, E.; Sini, M.; Gerovasileiou, V.; et al. Marine heatwaves drive recurrent mass mortalities in the Mediterranean Sea. Glob. Chang. Biol. 2022, 28, 5708–5725. [Google Scholar] [CrossRef]
- Holbrook, N.J.; Scannell, H.A.; Sen Gupta, A.; Benthuysen, J.A.; Feng, M.; Oliver, E.C.J.; Alexander, L.V.; Burrows, M.T.; Donat, M.G.; Hobday, A.J.; et al. A global assessment of marine heatwaves and their drivers. Nat. Commun. 2019, 10, 2624. [Google Scholar] [CrossRef]
- Lionello, P.; Scarascia, L. The relation between climate change in the Mediterranean region and global warming. Reg. Environ. Chang. 2018, 18, 1481–1493. [Google Scholar] [CrossRef]
- Bertucci, J.I.; Veloso-Cerredelo, C.; Bellas, J. Global climate change increases the impact of pollutant mixtures in the model species Paracentrotus lividus. Sci. Total Environ. 2023, 893, 164837. [Google Scholar] [CrossRef]
- Martino, C.; Byrne, M.; Roccheri, M.C.; Chiarelli, R. Interactive effects of increased temperature and gadolinium pollution in Paracentrotus lividus sea urchin embryos: A climate change perspective. Aquat. Toxicol. 2021, 232, 105750. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, I.M.; Lannig, M.G. Interactive effects of metal pollution and temperature on metabolism in aquatic ectotherms: Implications of global climate change. Clim. Res. 2008, 37, 181–201. [Google Scholar] [CrossRef]
- Mubiana, V.K.; Blust, R. Effects of temperature on scope for growth and accumulation of Cd, Co, Cu and Pb by the marine bivalve Mytilus edulis. Mar. Environ. Res. 2007, 63, 219–235. [Google Scholar] [CrossRef]
- Guinot, D.; Ureña, R.; Pastor, A.; Varó, I.; del Ramo, J.; Torreblanca, A. Long-term effect of temperature on bioaccumulation of dietary metals and metallothionein induction in Sparus aurata. Chemosphere 2012, 87, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Coppola, F.; Almeida, Â.; Henriques, B.; Soares, A.M.V.M.; Figueira, E.; Pereira, E.; Freitas, R. Biochemical responses and accumulation patterns of Mytilus galloprovincialis exposed to thermal stress and Arsenic contamination. Ecotoxicol. Environ. Saf. 2018, 147, 954–962. [Google Scholar] [CrossRef]
- Byrne, M. Impact of ocean warming and ocean acidification on marine invertebrate life history stages: Vulnerabilities and potential for persistence in a changing ocean. Oceanogr. Mar. Biol. Ann. Rev. 2011, 49, 1–42. [Google Scholar]
- Matranga, V.; Pinsino, A.; Bonaventura, R.; Costa, C.; Karakostis, K.; Martino, C.; Russo, R.; Zito, F. Cellular and molecular bases of biomineralization in sea urchin embryos. Cah. Biol. Mar. 2013, 54, 467–478. [Google Scholar]
- Chiarelli, R.; Martino, C.; Roccheri, M.C. Cadmium stress effects indicating marine pollution in different species of sea urchin employed as environmental bioindicators. Cell Stress Chaperones 2019, 24, 675–687. [Google Scholar] [CrossRef]
- Oliveri, P.; Tu, Q.; Davidson, E.H. Global regulatory logic for specification of an embryonic cell lineage. Proc. Natl. Acad. Sci. USA 2008, 105, 5955–5962. [Google Scholar] [CrossRef]
- Byrne, M.; Lamare, M.; Winter, D.; Dworjanyn, S.A.; Uthicke, S. The stunting effect of a high CO2 ocean on calcification and development in sea urchin larvae, a synthesis from the tropics to the poles. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120439. [Google Scholar] [CrossRef]
- Martino, R.; Chiarelli, M.C.; Roccheri, V.; Matranga, M.; Byrne, M. Effects of magnesium deprivation on development and biomineralization in the sea urchin Arbacia lixula. Invertebr. Reprod. Dev. 2019, 63, 165–176. [Google Scholar] [CrossRef]
- Chiarelli, R.; Agnello, M.; Roccheri, M.C. Sea urchin embryos as a model system for studying autophagy induced by cadmium stress. Autophagy 2011, 7, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Pinsino, A.; Roccheri, M.C.; Costa, C.; Matranga, V. Manganese interferes with calcium, perturbs ERK signaling, and produces embryos with no skeleton. Toxicol. Sci. 2011, 123, 217–230. [Google Scholar] [CrossRef]
- Martino, C.; Costa, C.; Roccheri, M.C.; Koop, D.; Scudiero, R.; Byrne, M. Gadolinium perturbs expression of skeletogenic genes, calcium uptake and larval development in phylogenetically distant sea urchin species. Aquat. Toxicol. 2018, 194, 57–66. [Google Scholar] [CrossRef]
- Chiarelli, R.; Martino, C.; Roccheri, M.C.; Cancemi, P. Toxic effects induced by vanadium on sea urchin embryos. Chemosphere 2021, 274, 129843. [Google Scholar] [CrossRef]
- Chiarelli, R.; Scudiero, R.; Memoli, V.; Roccheri, M.C.; Martino, C. Toxicity of Vanadium during Development of Sea Urchin Embryos: Bioaccumulation, Calcium Depletion, ERK Modulation and Cell-Selective Apoptosis. Int. J. Mol. Sci. 2022, 23, 6239. [Google Scholar] [CrossRef] [PubMed]
- Martino, C.; Chiarelli, R.; Bosco, L.; Roccheri, M.C. Induction of skeletal abnormalities and autophagy in Paracentrotus lividus sea urchin embryos exposed to gadolinium. Mar. Environ. Res. 2017, 130, 12–20. [Google Scholar] [CrossRef]
- Roccheri, M.C.; Agnello, M.; Bonaventura, R.; Matranga, V. Cadmium induces the expression of specific stress proteins in sea urchin embryos. Biochem. Biophys. Res. Commun. 2004, 321, 80–87. [Google Scholar] [CrossRef]
- Rana, D.; Kumar, A. Is there a Role for Sodium Orthovanadate in the Treatment of Diabetes? Curr. Diabetes Rev. 2019, 15, 284–287. [Google Scholar] [CrossRef]
- Savoca, D.; Pace, A.; Arizza, V.; Arculeo, M.; Melfi, R. Controlled uptake of PFOA in adult specimens of Paracentrotus lividus and evaluation of gene expression in their gonads and embryos. Environ. Sci. Pollut. Res. Int. 2023, 30, 26094–26106. [Google Scholar] [CrossRef]
- Ulbricht, R.J.; Pritchard, A.W. Effect of temperature on the metabolic rate of sea urchins. Biol. Bull. 1972, 142, 178–185. [Google Scholar] [CrossRef]
- Mohammed, E.H.; Wang, G.; Jiang, J. The effects of nickel on the reproductive ability of three different marine copepods. Ecotoxicology 2010, 19, 911–916. [Google Scholar] [CrossRef]
- Agnello, M.; Filosto, S.; Scudiero, R.; Rinaldi, A.M.; Roccheri, M.C. Cadmium induces an apoptotic response in sea urchin embryos. Cell Stress Chaperones 2007, 12, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Strathmann, R.R.; Fenaux, L.; Strathmann, M.F. Heterochronic developmental plasticity in larval sea urchins and its implications for evolution of nonfeeding larvae. Evolution 1992, 46, 972–986. [Google Scholar] [CrossRef]
- Somero, G.N. The cellular stress response and temperature: Function, regulation, and evolution. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2020, 333, 379–397. [Google Scholar] [CrossRef] [PubMed]
- Dokladny, K.; Myers, O.B.; Moseley, P.L. Heat shock response and autophagy—Cooperation and control. Autophagy 2015, 11, 200–213. [Google Scholar] [CrossRef]
- Roccheri, M.C.; Barbata, G.; Cardinale, F.; Tipa, C.; Bosco, L.; Oliva, O.A.; Cascino, D.; Giudice, G. Apoptosis in sea urchin embryos. Biochem. Biophys. Res. Commun. 1997, 240, 359–366. [Google Scholar] [CrossRef]
- Chiarelli, R.; Martino, C.; Agnello, M.; Bosco, L.; Roccheri, M.C. Autophagy as a defense strategy against stress: Focus on Paracentrotus lividus sea urchin embryos exposed to cadmium. Cell Stress Chaperones 2016, 21, 19–27. [Google Scholar] [CrossRef]
- Martino, C.; Badalamenti, R.; Frinchi, M.; Chiarelli, R.; Palumbo Piccionello, A.; Urone, G.; Mauro, M.; Arizza, V.; Luparello, C.; Di Liberto, V.; et al. The stunting effect of an oxylipins-containing macroalgae extract on sea urchin reproduction and neuroblastoma cells viability. Chemosphere 2024, 359, 142278. [Google Scholar] [CrossRef]
- Chiarelli, R.; Agnello, M.; Bosco, L.; Roccheri, M.C. Sea urchin embryos exposed to cadmium as an experimental model for studying the relationship between autophagy and apoptosis. Mar. Environ. Res. 2014, 93, 47–55. [Google Scholar] [CrossRef]
- Pirotta, E.; Thomas, L.; Costa, D.P.; Hall, A.J.; Harris, C.M.; Harwood, J.; Kraus, S.D.; Miller, P.J.O.; Moore, M.J.; Photopoulou, T.; et al. Understanding the combined effects of multiple stressors: A new perspective on a longstanding challenge. Sci. Total Environ. 2022, 821, 153322. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martino, C.; Geraci, F.; Scudiero, R.; Barone, G.; Naselli, F.; Chiarelli, R. Vanadium Toxicity Is Altered by Global Warming Conditions in Sea Urchin Embryos: Metal Bioaccumulation, Cell Stress Response and Apoptosis. J. Xenobiot. 2024, 14, 1130-1142. https://doi.org/10.3390/jox14030064
Martino C, Geraci F, Scudiero R, Barone G, Naselli F, Chiarelli R. Vanadium Toxicity Is Altered by Global Warming Conditions in Sea Urchin Embryos: Metal Bioaccumulation, Cell Stress Response and Apoptosis. Journal of Xenobiotics. 2024; 14(3):1130-1142. https://doi.org/10.3390/jox14030064
Chicago/Turabian StyleMartino, Chiara, Fabiana Geraci, Rosaria Scudiero, Giampaolo Barone, Flores Naselli, and Roberto Chiarelli. 2024. "Vanadium Toxicity Is Altered by Global Warming Conditions in Sea Urchin Embryos: Metal Bioaccumulation, Cell Stress Response and Apoptosis" Journal of Xenobiotics 14, no. 3: 1130-1142. https://doi.org/10.3390/jox14030064
APA StyleMartino, C., Geraci, F., Scudiero, R., Barone, G., Naselli, F., & Chiarelli, R. (2024). Vanadium Toxicity Is Altered by Global Warming Conditions in Sea Urchin Embryos: Metal Bioaccumulation, Cell Stress Response and Apoptosis. Journal of Xenobiotics, 14(3), 1130-1142. https://doi.org/10.3390/jox14030064







