Berberine Attenuates Acetamiprid Exposure-Induced Mitochondrial Dysfunction and Apoptosis in Rats via Regulating the Antioxidant Defense System
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals and Their Care
2.3. Experimental Design
2.4. Preparation of Mitochondria
2.5. Complex I Activity Assay
2.6. Complex II Activity Assay
2.7. Complex IV Activity Assay
2.8. Semi-Quantitative PCR Analysis
2.9. Western Blotting
2.9.1. Sample Preparation for Western Blotting
2.9.2. Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.10. Electron Microscopy
2.11. Statistical Analysis
3. Results
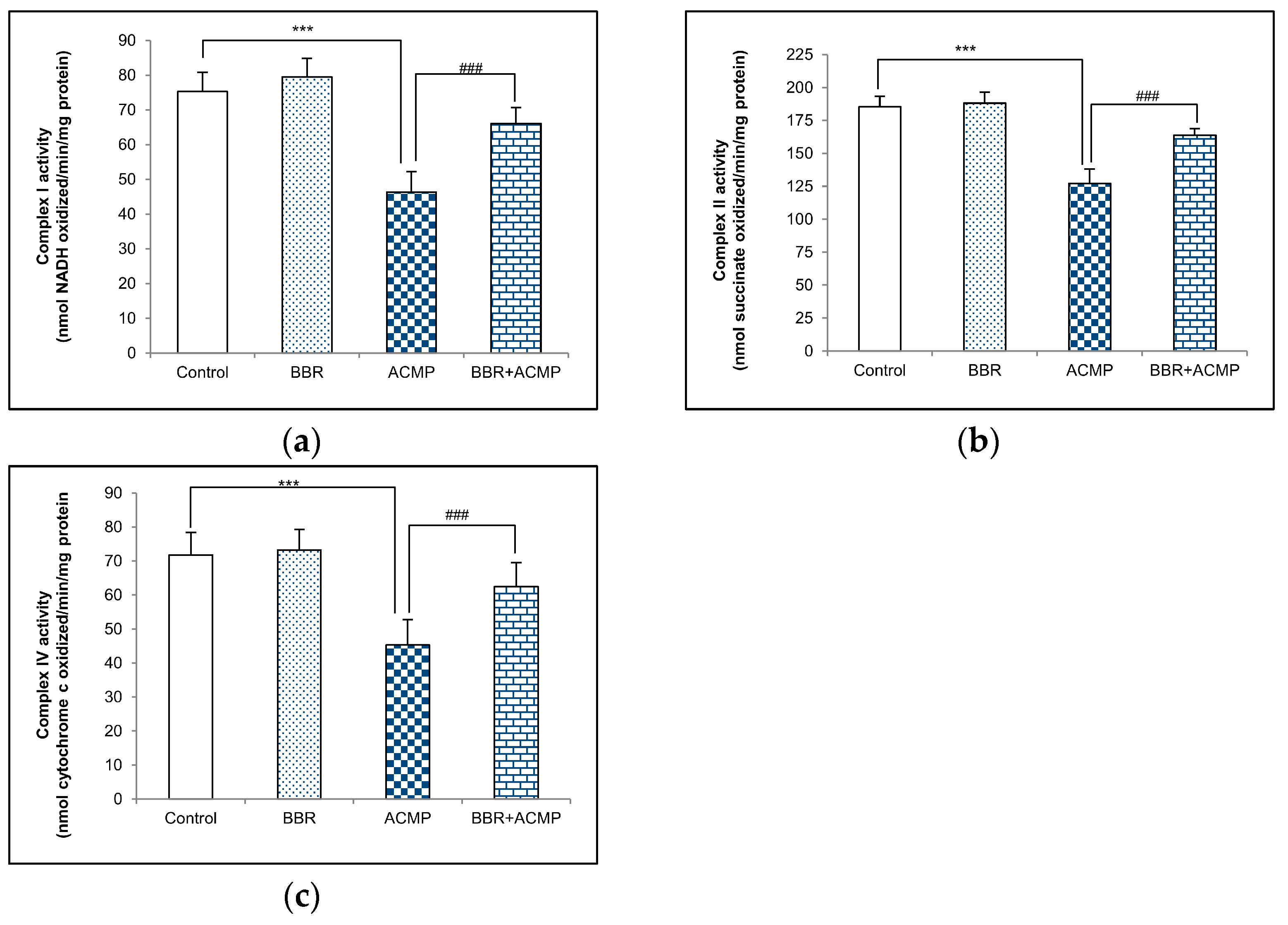
3.1. Complex I Activity Assay
3.2. Complex II Activity Assay
3.3. Complex IV Activity Assay
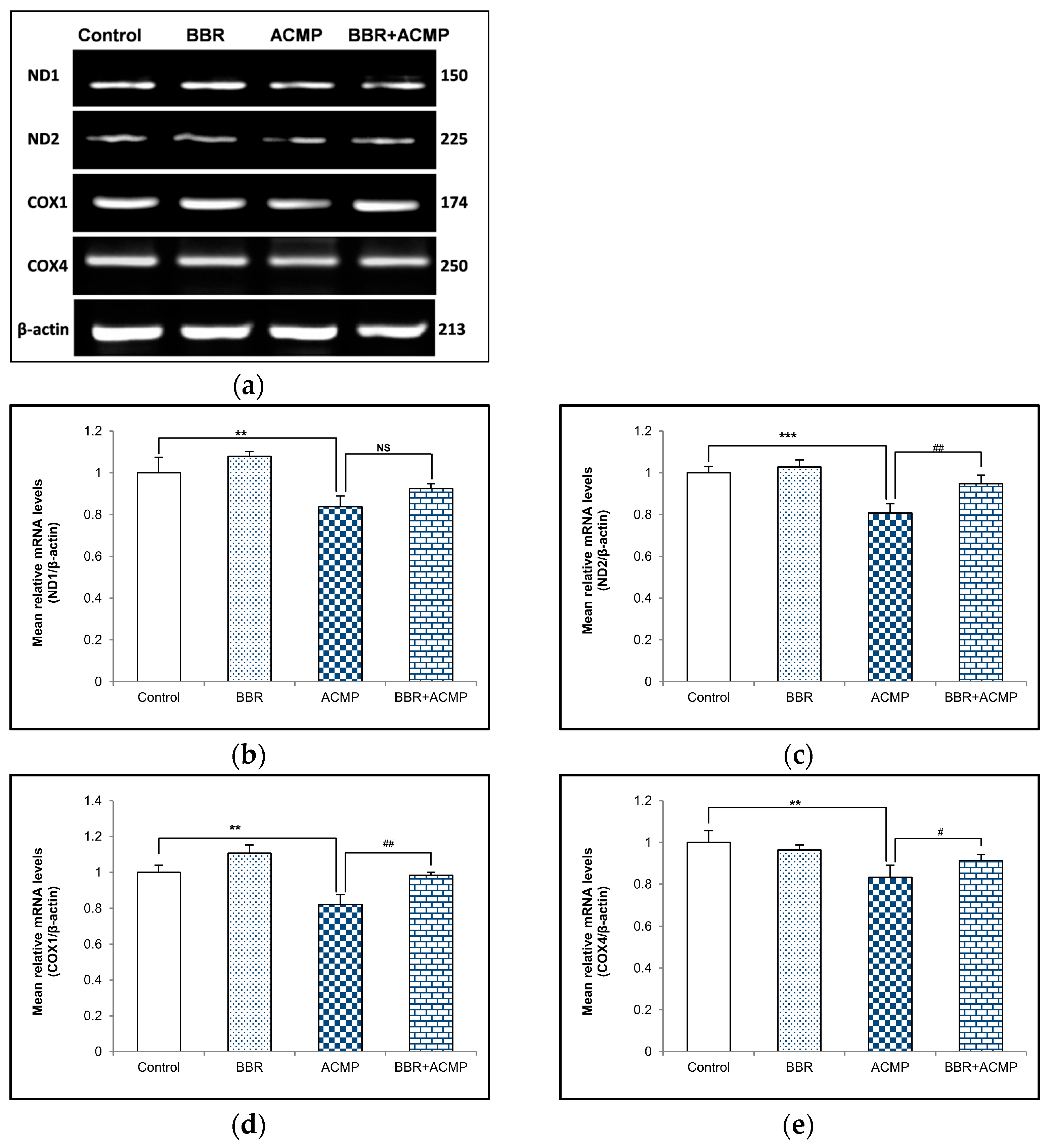
3.4. mRNA Expression of Mitochondrial Subunits
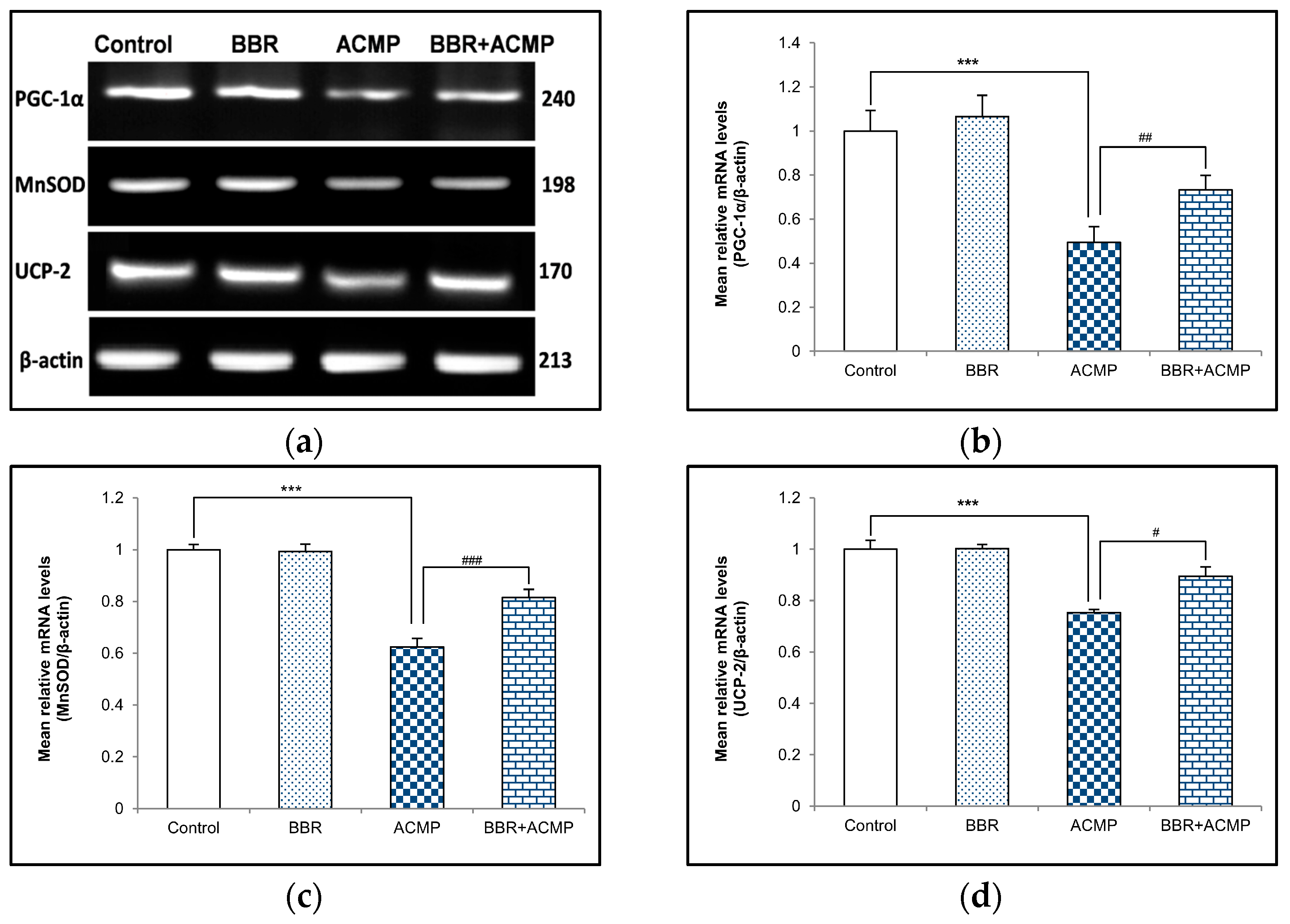
3.5. mRNA Expression of PGC-1α, MnSOD, and UCP-2
3.6. Western Blotting Analysis
3.7. Electron Microscopic Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zoumenou, B.G.Y.M.; Aïna, M.P.; Imorou Toko, I.; Igout, A.; Douny, C.; Brose, F.; Schiffers, B.; Gouda, I.; Chabi Sika, K.; Kestemont, P.; et al. Occurrence of Acetamiprid Residues in Water Reservoirs in the Cotton Basin of Northern Benin. Bull. Environ. Contam. Toxicol. 2019, 102, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Bonmatin, J.-M.; Mitchell, E.A.; Glauser, G.; Lumawig-Heitzman, E.; Claveria, F.; van Lexmond, M.B.; Taira, K.; Sánchez-Bayo, F. Residues of Neonicotinoids in Soil, Water and People’s Hair: A Case Study from Three Agricultural Regions of the Philippines. Sci. Total Environ. 2021, 757, 143822. [Google Scholar] [CrossRef]
- Craddock, H.A.; Huang, D.; Turner, P.C.; Quirós-Alcalá, L.; Payne-Sturges, D.C. Trends in Neonicotinoid Pesticide Residues in Food and Water in the United States 1999–2015. Environ. Health 2019, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, G.; Kuribayashi, R.; Ikenaka, Y.; Ichise, T.; Nakayama, S.M.M.; Ishizuka, M.; Taira, K.; Fujioka, K.; Sairenchi, T.; Kobashi, G.; et al. LC-ESI/MS/MS Analysis of Neonicotinoids in Urine of Very Low Birth Weight Infants at Birth. PLoS ONE 2019, 14, e0219208. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, J.; Aoi, A.; Ueda, Y.; Oya, N.; Sugiura, Y.; Ito, Y.; Ebara, T.; Kamijima, M. Biomonitoring Method for Neonicotinoid Insecticides in Urine of Non-Toilet-Trained Children Using LC-MS/MS. Food Addit. Contam. Part A 2020, 37, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Kabata, R.; Nanayakkara, S.; Senevirathna, S.; Harada, K.H.; Chandrajith, R.; Hitomi, T.; Abeysekera, T.; Takasuga, T.; Koizumi, A. Neonicotinoid concentrations in urine from chronic kidney disease patients in the North Central Region of Sri Lanka. J. Occup. Health 2016, 58, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Yanagawa, Y.; Nishikawa, K.; Matsumoto, N.; Sakamoto, T. Two Cases of Acute Poisoning with Acetamiprid in Humans. Clin. Toxicol. 2010, 48, 851–853. [Google Scholar] [CrossRef]
- Yeter, O.; Aydın, A. Determination of Acetamiprid and IM-1-2 in PostMortem Human Blood, Liver, Stomach Contents by HPLC-DAD. J. Forensic Sci. 2014, 59, 287–292. [Google Scholar] [CrossRef]
- Chakroun, S.; Ezzi, L.; Grissa, I.; Kerkeni, E.; Neffati, F.; Bhouri, R.; Sallem, A.; Najjar, M.F.; Hassine, M.; Mehdi, M.; et al. Hematological, Biochemical, and Toxicopathic Effects of Subchronic Acetamiprid Toxicity in Wistar Rats. Environ. Sci. Pollut. Res. 2016, 23, 25191–25199. [Google Scholar] [CrossRef]
- Arıcan, E.Y.; Gökçeoğlu Kayalı, D.; Ulus Karaca, B.; Boran, T.; Öztürk, N.; Okyar, A.; Ercan, F.; Özhan, G. Reproductive Effects of Subchronic Exposure to Acetamiprid in Male Rats. Sci. Rep. 2020, 10, 8985. [Google Scholar] [CrossRef]
- Phogat, A.; Singh, J.; Kumar, V.; Malik, V. Berberine Mitigates Acetamiprid-Induced Hepatotoxicity and Inflammation via Regulating Endogenous Antioxidants and NF-κB/TNF-α Signaling in Rats. Environ. Sci. Pollut. Res. 2023, 30, 87412–87423. [Google Scholar] [CrossRef] [PubMed]
- Phogat, A.; Singh, J.; Malik, V.; Kumar, V. Neuroprotective Potential of Berberine against Acetamiprid Induced Toxicity in Rats: Implication of Oxidative Stress, Mitochondrial Alterations, and Structural Changes in Brain Regions. J. Biochem. Mol. Toxicol. 2023, 37, e23434. [Google Scholar] [CrossRef]
- Singh, J.; Phogat, A.; Kumar, V.; Malik, V. N-acetylcysteine ameliorates monocrotophos exposure-induced mitochondrial dysfunctions in rat liver. Toxicol. Mech. Methods 2022, 32, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Prakash, C.; Soni, M.; Kumar, V. Biochemical and molecular alterations following arsenic-induced oxidative stress and mitochondrial dysfunction in rat brain. Biol. Trace Elem. Res. 2015, 167, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial Dysfunction and Oxidative Stress in Neurodegenerative Diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Mehndiratta, M.M.; Aggarwal, P.; Singal, R.K.; Munjal, Y.P. Mitochondrial Cytopathies; WHO IMSEAR: New Delhi, India, 2000.
- Murphy, M.P. How Mitochondria Produce Reactive Oxygen Species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Rodriguez-Rocha, H.; Garcia-Garcia, A.; Pickett, C.; Li, S.; Jones, J.; Chen, H.; Webb, B.; Choi, J.; Zhou, Y.; Zimmerman, M.C.; et al. Compartmentalized Oxidative Stress in Dopaminergic Cell Death Induced by Pesticides and Complex I Inhibitors: Distinct Roles of Superoxide Anion and Superoxide Dismutases. Free Radic. Biol. Med. 2013, 61, 370–383. [Google Scholar] [CrossRef]
- Mattiasson, G.; Shamloo, M.; Gido, G.; Mathi, K.; Tomasevic, G.; Yi, S.; Warden, C.H.; Castilho, R.F.; Melcher, T.; Gonzalez-Zulueta, M. Uncoupling Protein-2 Prevents Neuronal Death and Diminishes Brain Dysfunction after Stroke and Brain Trauma. Nat. Med. 2003, 9, 1062–1068. [Google Scholar] [CrossRef]
- Deierborg, O.T.; Wieloch, T.; Diano, S.; Warden, C.H.; Horvath, T.L.; Mattiasson, G. Overexpression of UCP2 Protects Thalamic Neurons Following Global Ischemia in the Mouse. J. Cereb. Blood Flow Metab. 2008, 28, 1186–1195. [Google Scholar] [CrossRef]
- Indo, H.P.; Yen, H.-C.; Nakanishi, I.; Matsumoto, K.; Tamura, M.; Nagano, Y.; Matsui, H.; Gusev, O.; Cornette, R.; Okuda, T. A Mitochondrial Superoxide Theory for Oxidative Stress Diseases and Aging. J. Clin. Biochem. Nutr. 2015, 56, 1–7. [Google Scholar] [CrossRef]
- Holley, A.K.; Dhar, S.K.; Clair, D.K.S. Manganese Superoxide Dismutase versus P53: The Mitochondrial Center. Ann. N. Y. Acad. Sci. 2010, 1201, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Zhang, J.; Hou, X.; Zhang, S.; Tan, J.; Chen, Y.; Yang, W.; Zeng, J.; Han, Y.; Liu, X.; et al. Acetamiprid Inhibits Testosterone Synthesis by Affecting the Mitochondrial Function and Cytoplasmic Adenosine Triphosphate Production in Rat Leydig Cells. Biol. Reprod. 2017, 96, 936. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, S.; Kebieche, M.; Rouabhi, R.; Touahria, C.; Lahouel, A.; Lakroun, Z.; Henine, S.; Soulimani, R. Alteration of Membrane Integrity and Respiratory Function of Brain Mitochondria in the Rats Chronically Exposed to a Low Dose of Acetamiprid. Environ. Sci. Pollut. Res. Int. 2017, 24, 22258–22264. [Google Scholar] [CrossRef] [PubMed]
- Valle, I.; Alvarez-Barrientos, A.; Arza, E.; Lamas, S.; Monsalve, M. PGC-1α Regulates the Mitochondrial Antioxidant Defense System in Vascular Endothelial Cells. Cardiovasc. Res. 2005, 66, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Hock, M.B.; Kralli, A. Transcriptional Control of Mitochondrial Biogenesis and Function. Annu. Rev. Physiol. 2009, 71, 177–203. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative Stress: The Mitochondria-Dependent and Mitochondria-Independent Pathways of Apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, S.; Chafaa, S.; Lakroun, Z.; Rouabhi, R.; Touahria, C.; Kebieche, M.; Soulimani, R. Neuronal Apoptosis and Imbalance of Neurotransmitters Induced by Acetamiprid in Rats. Toxicol. Environ. Health Sci. 2019, 11, 305–311. [Google Scholar] [CrossRef]
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Mitochondrial Oxidative Stress: Implications for Cell Death. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 143–183. [Google Scholar] [CrossRef]
- Ott, M.; Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria, Oxidative Stress and Cell Death. Apoptosis 2007, 12, 913–922. [Google Scholar] [CrossRef]
- Habtemariam, S. Berberine and Inflammatory Bowel Disease: A Concise Review. Pharmacol. Res. 2016, 113, 592–599. [Google Scholar] [CrossRef]
- Coelho, A.R.; Martins, T.R.; Couto, R.; Deus, C.; Pereira, C.V.; Simões, R.F.; Rizvanov, A.A.; Silva, F.; Cunha-Oliveira, T.; Oliveira, P.J. Berberine-Induced Cardioprotection and Sirt3 Modulation in Doxorubicin-Treated H9c2 Cardiomyoblasts. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2904–2923. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Su, P.; Lv, C.; Guo, L.; Cao, G.; Qin, C.; Zhang, W. Berberine Alleviates Amyloid β-Induced Mitochondrial Dysfunction and Synaptic Loss. Oxidative Med. Cell. Longev. 2019, 2019, 7593608. [Google Scholar] [CrossRef]
- Gomes, A.P.; Duarte, F.V.; Nunes, P.; Hubbard, B.P.; Teodoro, J.S.; Varela, A.T.; Jones, J.G.; Sinclair, D.A.; Palmeira, C.M.; Rolo, A.P. Berberine Protects against High Fat Diet-Induced Dysfunction in Muscle Mitochondria by Inducing SIRT1-Dependent Mitochondrial Biogenesis. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, J.; Gao, Y.; Yu, X.; Xu, G.; Huang, Y. Uncoupling Protein-2 Mediates the Protective Action of Berberine against Oxidative Stress in Rat Insulinoma INS-1 E Cells and in Diabetic Mouse Islets. Br. J. Pharmacol. 2014, 171, 3246–3254. [Google Scholar] [CrossRef]
- Brooks, S.P.; Lampi, B.J.; Bihun, C.G. The influence of euthanasia methods on rat liver metabolism. Contemp. Top. Lab. Anim. Sci. 1999, 38, 19–24. [Google Scholar] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Kaur, P.; Radotra, B.; Minz, R.; Gill, K. Impaired Mitochondrial Energy Metabolism and Neuronal Apoptotic Cell Death after Chronic Dichlorvos (OP) Exposure in Rat Brain. NeuroToxicology 2007, 28, 1208–1219. [Google Scholar] [CrossRef]
- Sandhir, R.; Sood, A.; Mehrotra, A.; Kamboj, S. N-Acetylcysteine Reverses Mitochondrial Dysfunctions and Behavioral Abnormalities in 3-Nitropropionic Acid-Induced Huntington’s Disease. Neuro-Degener. Dis. 2012, 9, 145–157. [Google Scholar] [CrossRef]
- Singh, D.P.; Chopra, K. Verapamil Augments the Neuroprotectant Action of Berberine in Rat Model of Transient Global Cerebral Ischemia. Eur. J. Pharmacol. 2013, 720, 98–106. [Google Scholar] [CrossRef]
- Li, J.; Jiang, R.; Cong, X.; Zhao, Y. UCP2 Gene Polymorphisms in Obesity and Diabetes, and the Role of UCP2 in Cancer. FEBS Lett. 2019, 593, 2525–2534. [Google Scholar] [CrossRef]
- Mattiasson, G.; Sullivan, P.G. The Emerging Functions of UCP2 in Health, Disease, and Therapeutics. Antioxid. Redox Signal. 2006, 8, 1–38. [Google Scholar] [CrossRef]
- Ježek, P.; Holendová, B.; Garlid, K.D.; Jabůrek, M. Mitochondrial Uncoupling Proteins: Subtle Regulators of Cellular Redox Signaling. Antioxid. Redox Signal. 2018, 29, 667–714. [Google Scholar] [CrossRef] [PubMed]
- Shaker, F.H.; El-Derany, M.O.; Wahdan, S.A.; El-Demerdash, E.; El-Mesallamy, H.O. Berberine Ameliorates Doxorubicin-Induced Cognitive Impairment (Chemobrain) in Rats. Life Sci. 2021, 269, 119078. [Google Scholar] [CrossRef]
- Abdel, M.A.E. The Neuroprotective Effect of Berberine in Mercury-Induced Neurotoxicity in Rats. Metab. Brain Dis. 2015, 30, 935–942. [Google Scholar] [CrossRef]
- Xuan, W.; Wang, H.; Zhou, P.; Ye, T.; Gao, H.; Ye, S.; Wang, J.; Chen, M.; Song, H.; Wang, Y. Berberine Ameliorates Rats Model of Combined Alzheimer’s Disease and Type 2 Diabetes Mellitus via the Suppression of Endoplasmic Reticulum Stress. 3 Biotech 2020, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.G.; Ahmed, K.A. Neuroprotective Potential of Berberine Against Doxorubicin-Induced Toxicity in Rat’s Brain. Neurochem. Res. 2021, 46, 3247–3263. [Google Scholar] [CrossRef]
- Singh, S.; Pathak, N.; Fatima, E.; Negi, A.S. Plant Isoquinoline Alkaloids: Advances in the Chemistry and Biology of Berberine. Eur. J. Med. Chem. 2021, 226, 113839. [Google Scholar] [CrossRef] [PubMed]



| Primer | Accession | Primer Sequence(5′-3′) | Size (bp) |
|---|---|---|---|
| ND1 | EU104724.1 | F-TGGCCTTCCTCACCCTAGTA | 150 |
| R-AGGTGGTTAGAGGGCGTATG | |||
| ND2 | EU104725.1 | F-TCATCAGTCTTTGTTGGCGC | 225 |
| R-TCATGCGAGTGAGAGTGTGT | |||
| COX1 | KF011917.1 | F-CACATGAGCAAAAGCCCACT | 174 |
| R-ACGGCCGTAAGTGAGATGAA | |||
| COX4 | NM_017202.1 | F-GACTACCCCTTGCCTGATGT | 250 |
| R-ACACGTAGCTCTTCTCCCAG | |||
| PGC-1α | NM_031347.1 | F-AGCCTCTTTGCCCAGATCTT | 240 |
| R-GCAATCCGTCTTCATCCACC | |||
| UCP-2 | NM_019354.3 | F-AGACCATTGCACGAGAGGAA | 170 |
| R-AGAAGTGAAGTGGCAAGGGA | |||
| MnSOD | NM_017051.2 | F-ACAGGCCTTATTCCACTGCT | 198 |
| R-CTACAAAACACCCACCACGG | |||
| β-actin | V01217.1 | F-TTGCCCTAGACTTCGAGCAA | 213 |
| R-AGACTTACAGTGTGGCCTCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phogat, A.; Singh, J.; Sheoran, R.; Hasanpuri, A.; Chaudhary, A.; Bhardwaj, S.; Antil, S.; Kumar, V.; Prakash, C.; Malik, V. Berberine Attenuates Acetamiprid Exposure-Induced Mitochondrial Dysfunction and Apoptosis in Rats via Regulating the Antioxidant Defense System. J. Xenobiot. 2024, 14, 1079-1092. https://doi.org/10.3390/jox14030061
Phogat A, Singh J, Sheoran R, Hasanpuri A, Chaudhary A, Bhardwaj S, Antil S, Kumar V, Prakash C, Malik V. Berberine Attenuates Acetamiprid Exposure-Induced Mitochondrial Dysfunction and Apoptosis in Rats via Regulating the Antioxidant Defense System. Journal of Xenobiotics. 2024; 14(3):1079-1092. https://doi.org/10.3390/jox14030061
Chicago/Turabian StylePhogat, Annu, Jagjeet Singh, Reena Sheoran, Arun Hasanpuri, Aakash Chaudhary, Shakti Bhardwaj, Sandeep Antil, Vijay Kumar, Chandra Prakash, and Vinay Malik. 2024. "Berberine Attenuates Acetamiprid Exposure-Induced Mitochondrial Dysfunction and Apoptosis in Rats via Regulating the Antioxidant Defense System" Journal of Xenobiotics 14, no. 3: 1079-1092. https://doi.org/10.3390/jox14030061
APA StylePhogat, A., Singh, J., Sheoran, R., Hasanpuri, A., Chaudhary, A., Bhardwaj, S., Antil, S., Kumar, V., Prakash, C., & Malik, V. (2024). Berberine Attenuates Acetamiprid Exposure-Induced Mitochondrial Dysfunction and Apoptosis in Rats via Regulating the Antioxidant Defense System. Journal of Xenobiotics, 14(3), 1079-1092. https://doi.org/10.3390/jox14030061






