Effect of Plant Growth-Promoting Bacteria on Antioxidant Status, Acetolactate Synthase Activity, and Growth of Common Wheat and Canola Exposed to Metsulfuron-Methyl
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Microorganisms
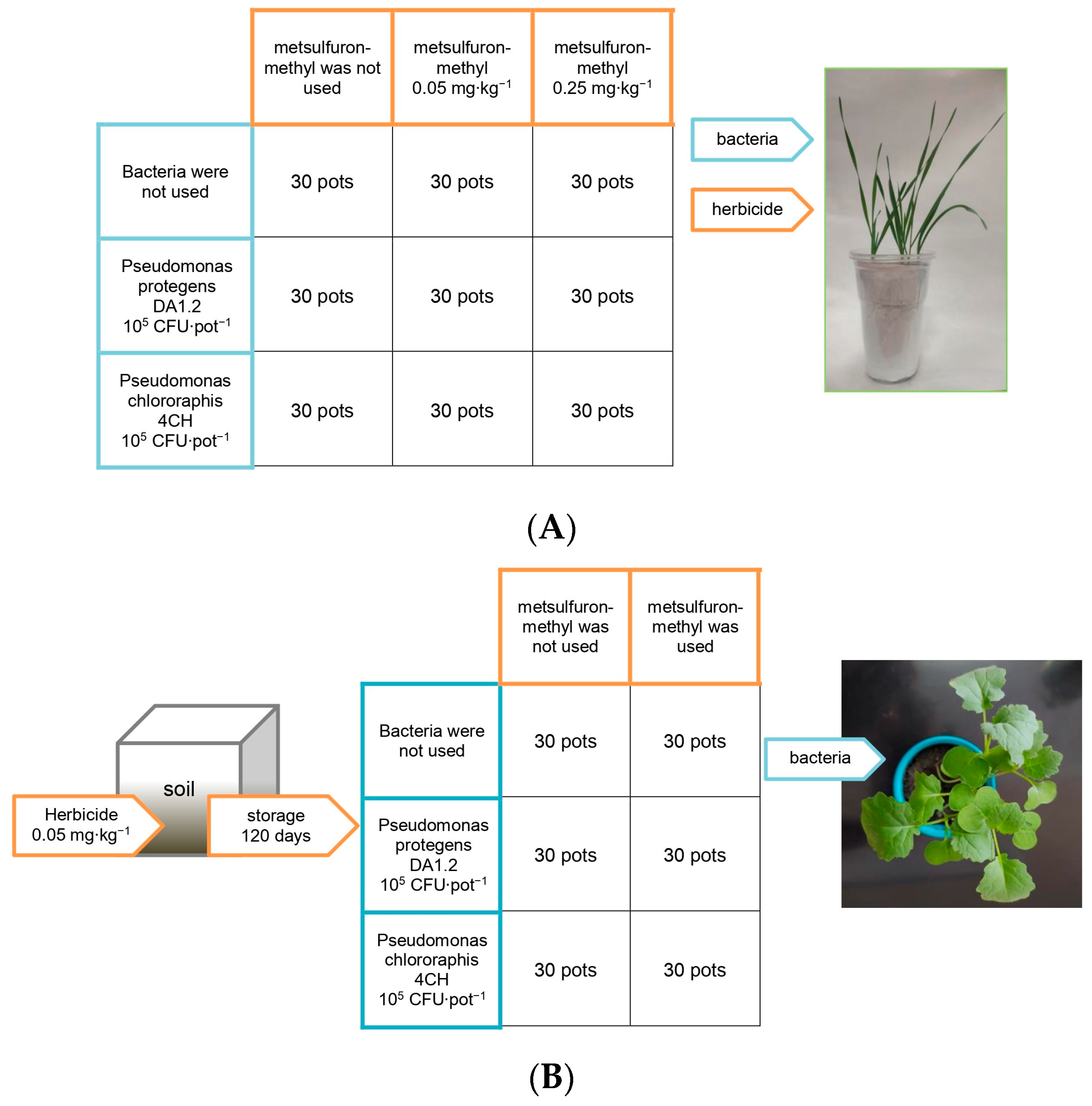
2.2. Experimental Details
2.3. Non-Enzymatic Antioxidants
2.4. Malondialdehyde Assay
2.5. Antioxidant Enzyme Assays in Crude Leaf Extract
2.6. In Vivo Activity of ALS
2.7. Statistical Analysis
3. Results
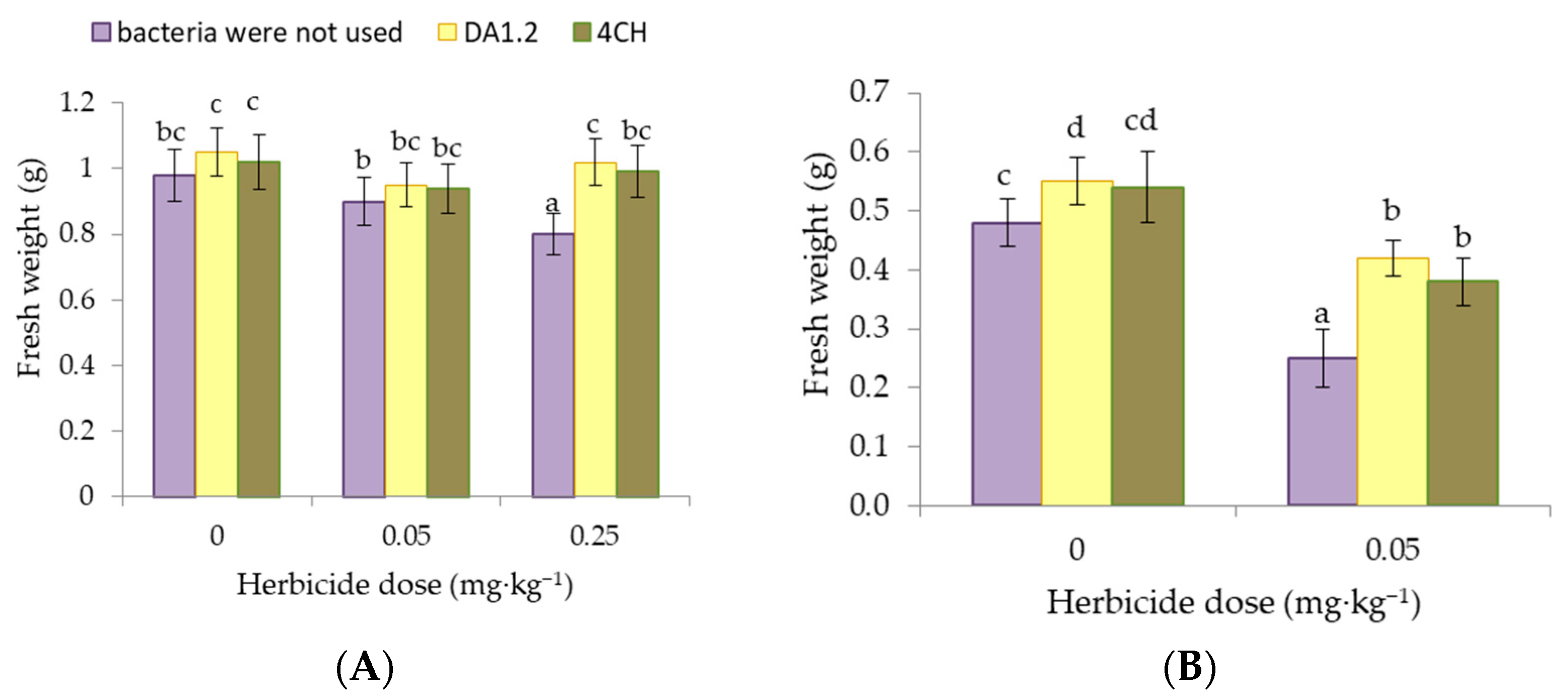
3.1. Plant Weight
3.2. Lipid Peroxidation
3.3. Non-Enzymatic Antioxidants
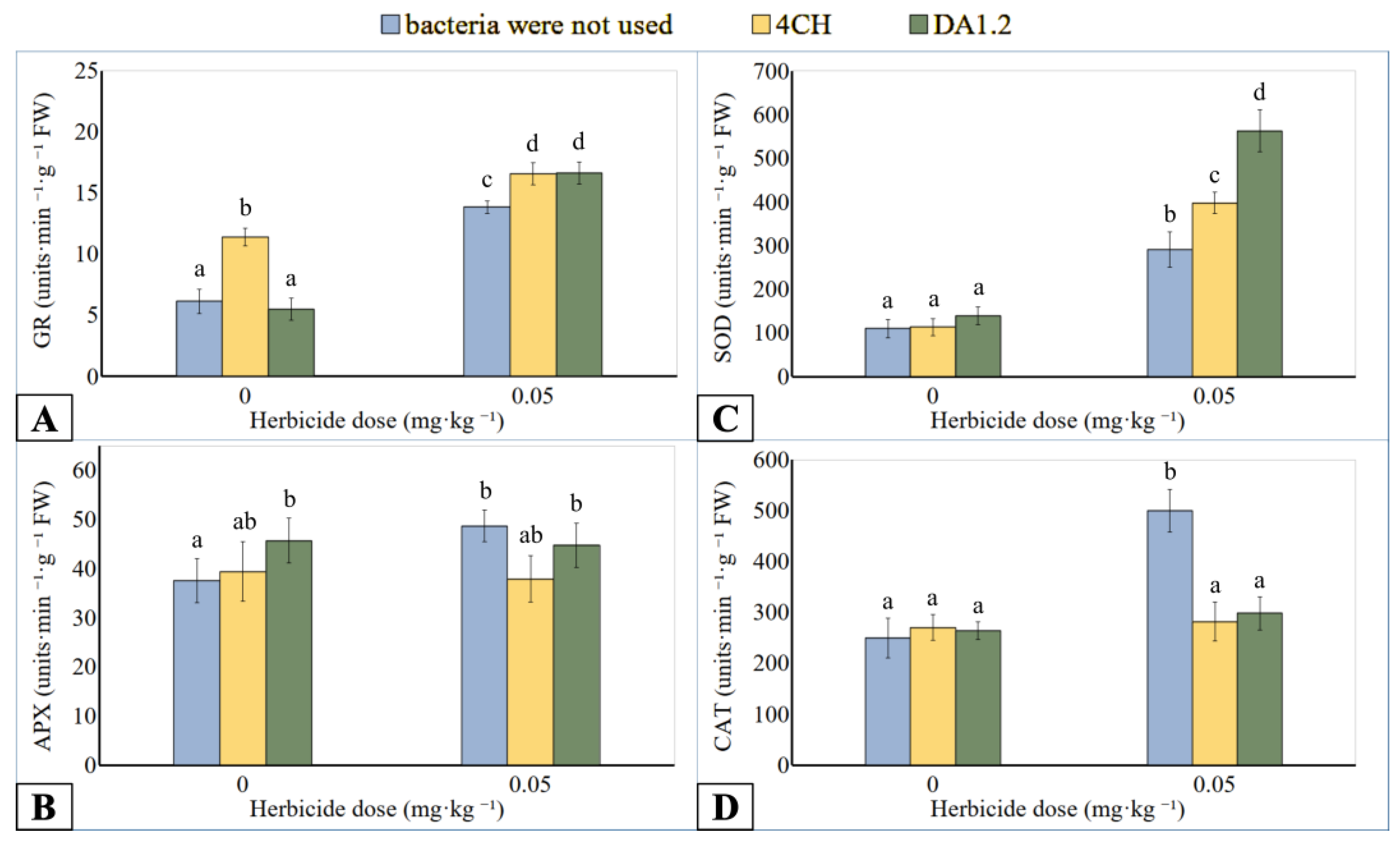
3.4. Antioxidant Enzymes
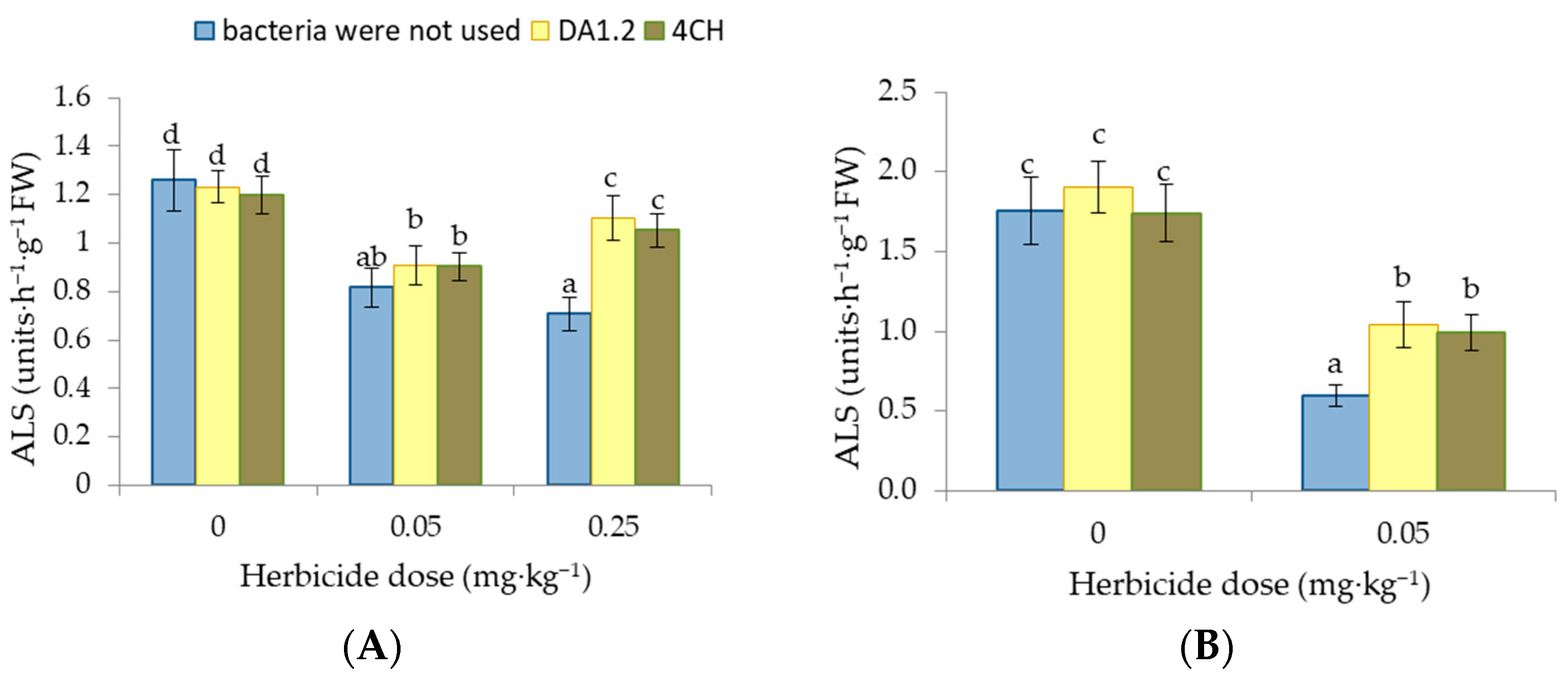
3.5. ALS Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarmah, A.K.; Sabadie, J. Hydrolysis of sulfonylurea herbicides in soils and aqueous solutions: A review. J. Agric. Food Chem. 2002, 50, 6253–6265. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Yates, S.Y.; Zhang, J.; Gan, J.; Ma, J.; Wu, J.; Xuan, R. Mineralization of metsulfuron-methyl in Chinese paddy soils. Chemosphere 2010, 78, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-J.; Xu, J.-X.; Muhammad, A.; Ma, G.-R. Effect of bound residues of metsulfuron-methyl in soil on rice growth. Chemosphere 2005, 58, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Kaur, T.; Brar, L.S. Residual effect of wheat applied sulfonylurea herbicides on succeeding crops as affected by soil pH. Indian J. Weed Sci. 2014, 46, 241–243. [Google Scholar]
- Mehdizadeh, M.; Alebrahim, M.T.; Roushani, M.; Streibig, J.G. Evaluation of four different crops’ sensitivity to sulfosulfuron and tribenuron methyl soil residues. Acta Agric. Scand. Sect. B Soil Plant Sci. 2016, 66, 706–713. [Google Scholar] [CrossRef]
- Ruiu, L. Plant-Growth-Promoting Bacteria (PGPB) against Insects and Other Agricultural Pests. Agronomy 2020, 10, 861. [Google Scholar] [CrossRef]
- Stegelmeier, A.A.; Rose, D.M.; Joris, B.R.; Glick, B.R. The Use of PGPB to Promote Plant Hydroponic Growth. Plants 2022, 11, 2783. [Google Scholar] [CrossRef] [PubMed]
- Poria, V.; Dębiec-Andrzejewska, K.; Fiodor, A.; Lyzohub, M.; Ajijah, N.; Singh, S.; Pranaw, K. Plant Growth-Promoting Bacteria (PGPB) integrated phytotechnology: A sustainable approach for remediation of marginal lands. Front. Plant Sci. 2022, 13, 99986. [Google Scholar] [CrossRef]
- Ajijah, N.; Fiodor, A.; Pandey, A.K.; Rana, A.; Pranaw, K. Plant Growth-Promoting Bacteria (PGPB) with Biofilm-Forming Ability: A Multifaceted Agent for Sustainable griculture. Diversity 2023, 15, 112. [Google Scholar] [CrossRef]
- Boro, M.; Sannyasi, S.; Chettri, D.; Verma, A.K. Microorganisms in biological control strategies to manage microbial plant pathogens: A review. Arch. Microbiol. 2022, 204, 666. [Google Scholar] [CrossRef]
- Ali, S.A.; Kornaros, M.; Manni, A.; Al-Tohamy, R.; El-Raheem, A.; El-Shanshoury, R.; Matter, I.M.; Elsamahy, T.; Sobhy, M.; Sun, J. Advances in microorganisms-based biofertilizers: Major mechanisms and applications. In Biofertilizers; Rakshit, A., Meena, V.S., Parihar, M., Singh, H.B., Singh, A.K., Eds.; Woodhead Publishing: Cambridge, UK, 2021; Volume 1, pp. 371–385. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; Santoyo, G.; Glick, B.R. Recent Advances in the Bacterial Phytohormone Modulation of Plant Growth. Plants 2023, 12, 606. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Mosqueda, M.d.C.; Glick, B.R.; Santoyo, G. ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Mishra, J.; Arora, N.K. Plant growth promoting bacteria for combating salinity stress in plants—Recent developments and prospects: A review. Microbiol. Res. 2021, 252, 126861. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Yuan, M.M.; Li, J. Editorial: Microbe assisted plant resistance to abiotic stresses. Front. Plant Sci. 2023, 14, 1277682. [Google Scholar] [CrossRef] [PubMed]
- Ahemad, M.; Khan, M.S. Ameliorative effects of Mesorhizobium sp. MRC4 on chickpea yield and yield components under different doses of herbicide stress. Pest. Biochem. Physiol. 2010, 98, 183–190. [Google Scholar] [CrossRef]
- Motamedi, M.; Zahedi, M.; Karimmojeni, H.; Baldwin, T.C.; Motamedi, H. Rhizosphere-associated bacteria as biofertilizers in herbicide-treated alfalfa (Medicago sativa). J. Soil Sci. Plant Nutr. 2023, 23, 2585–2598. [Google Scholar] [CrossRef]
- Bourahla, M.; Djebbar, R.; Kaci, Y.; Abrous-Belbachir, O. Alleviation of bleaching herbicide toxicity by PGPR strain isolated from wheat rhizosphere. Analele Univ. Oradea Fasc. Biol. 2018, 25, 74–83. [Google Scholar]
- Bakaeva, M.; Chetverikov, S.; Timergalin, M.; Feoktistova, A.; Rameev, T.; Chetverikova, D.; Kenjieva, A.; Starikov, S.; Sharipov, D.; Hkudaygulov, G. PGP-Bacterium Pseudomonas protegens improves bread wheat growth and mitigates herbicide and drought stress. Plants 2022, 11, 3289. [Google Scholar] [CrossRef]
- Jiang, Z.; Jiang, D.; Zhou, Q.; Zheng, Z.; Cao, B.; Meng, Q.; Qu, J.; Wang, Y.; Zhang, Y. Enhancing the atrazine tolerance of Pennisetum americanum (L.) K. Schum by inoculating with indole-3-acetic acid producing strain Pseudomonas chlororaphis PAS18. Ecotoxicol. Environ. Saf. 2020, 202, 110854. [Google Scholar] [CrossRef]
- Chennappa, G.; Sreenivasa, M.Y.; Nagaraja, H. Azotobacter salinestris: A novel pesticide-degrading and prominent biocontrol PGPR bacteria. In Microorganisms for Green Revolution. Microorganisms for Sustainability; Panpatte, D., Jhala, Y., Shelat, H., Vyas, R., Eds.; Springer: Singapore, 2018; Volume 7, pp. 23–43. [Google Scholar] [CrossRef]
- Traxler, C.; Gaines, T.A.; Küpper, A.; Luemmen, P.; Dayan, F.E. The nexus between reactive oxygen species and the mechanism of action of herbicides. J. Biol. Chem. 2023, 299, 105267. [Google Scholar] [CrossRef]
- Jain, S.; Rai, P.; Singh, J.; Singh, V.P.; Prasad, R.; Rana, S.; Deshmukh, R.; Tripathi, D.K.; Sharma, S. Exogenous addition of silicon alleviates metsulfuron methyl induced stress in wheat seedlings. Plant Physiol. Biochem. 2021, 167, 705–712. [Google Scholar] [CrossRef]
- Liu, X.-Q.; Yu, C.-Y.; Dong, J.-G.; Hu, S.-W.; Xu, A.-X. Acetolactate synthase-inhibiting gametocide amidosulfuron causes chloroplast destruction, tissue autophagy, and elevation of ethylene release in rapeseed. Front. Plant Sci. 2017, 8, 1625. [Google Scholar] [CrossRef] [PubMed]
- Chetverikov, S.P.; Chetverikova, D.V.; Bakaeva, M.D.; Kenjieva, A.A.; Starikov, S.N.; Sultangazin, Z.R. A promising herbicide resistant bacterial strain of Pseudomonas protegens for stimulation of the growth of agricultural cereal grains. Appl. Biochem. Microbiol. 2021, 57, 110–116. [Google Scholar] [CrossRef]
- Chetverikov, S.P.; Chetverikova, D.V.; Kenjieva, A.A.; Bakaeva, M.D. New herbicide-tolerant strains of microorganisms for the protection of crops. Agrochem. Ecol. Probl. 2020, 4, 35–39. (In Russian) [Google Scholar] [CrossRef]
- Dewhirst, R.A.; Clarkson, G.J.J.; Rothwell, S.D.; Fry, S.C. Novel insights into ascorbate retention and degradation during the washing and post-harvest storage of spinach and other salad leaves. Food Chem. 2017, 233, 237–246. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–211. [Google Scholar] [CrossRef]
- Hodges, D.M.; Delong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid). Anal. Biochem. 1988, 175, 408–413. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Elavarthi, S.; Martin, B. Spectrophotometric Assays for antioxidant enzymes in plants. In Plant Stress Tolerance. Methods in Molecular Biology; Sunkar, R., Ed.; Humana Press: New York City, NY, USA, 2010; Volume 639, pp. 273–280. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Simpson, D.; Stoller, E.; Wax, L. An in vivo Acetolactate Synthase Assay. Weed Technol. 1995, 9, 17–22. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, W.; Zhang, Y.; Liu, K.K. Action mechanisms of acetolactate synthase-inhibiting herbicides. Pest. Biochem. Physiol. 2007, 89, 89–96. [Google Scholar] [CrossRef]
- Eceiza, M.V.; Barco-Antoñanzas, M.; Gil-Monreal, M.; Huybrechts, M.; Zabalza, A.; Cuypers, A.; Royuela, M. Role of oxidative stress in the physiology of sensitive and resistant Amaranthus palmeri populations treated with herbicides inhibiting acetolactate synthase. Front. Plant Sci. 2023, 13, 1040456. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.I.H.; Alkhudairi, U.A.; Alhusayni, S.A.S. Alleviation of herbicide toxicity in Solanum lycopersicum L.—An antioxidant stimulation approach. Plants 2022, 11, 2261. [Google Scholar] [CrossRef] [PubMed]
- Hkudaygulov, G.; Chetverikova, D.; Bakaeva, M.; Kenjieva, A.; Chetverikov, S. Plant growth promoting Rhizobacteria strain role in protecting crops sensitive to sulfonylurea herbicides from stress. J. Crop Protect. 2022, 11, 515–524. [Google Scholar]
- Iwaniuk, P.; Konecki, R.; Kaczynski, P.; Rysbekova, A.; Lozowicka, B. Influence of seven levels of chemical/biostimulator protection on amino acid profile and yield traits in wheat. Crop J. 2022, 10, 1198–1206. [Google Scholar] [CrossRef]
- Lei, Q.; Zhong, J.; Chen, S.F.; Wu, S.; Huang, Y.; Guo, P.; Mishra, S.; Bhatt, K.; Chen, S. Microbial degradation as a powerful weapon in the removal of sulfonylurea herbicides. Environ. Res. 2023, 235, 116570. [Google Scholar] [CrossRef]
- Palma-Bautista, C.; Vázquez-García, J.G.; de Portugal, J.; Bastida, F.; Alcántara-de la Cruz, R.; Osuna-Ruiz, M.D.; Torra, J.; De Prado, R. Enhanced detoxification via Cyt-P450 governs cross-tolerance to ALS-inhibiting herbicides in weed species of Centaurea. Environ. Pollut. 2023, 322, 121140. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Hasanuzzaman, M.; Gill, R.; Trived, D.K.; Ahmad, I.; Pereira, E.; Tuteja, N. Glutathione reductase and glutathione: A boon in disguise for plant abiotic stress defense operations. Plant Physiol. Biochem. 2013, 70, 204–212. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Panwar, M.; Tewari, R.; Nayyar, H. Native halo-tolerant plant growth promoting rhizobacteria Enterococcus and Pantoea sp. improve seed yield of Mungbean (Vigna radiata L.) under soil salinity by reducing sodium uptake and stress injury. Physiol. Mol. Biol. Plants 2016, 22, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, P.; Zhang, Y.; Jin, C.; Guan, C. A novel PGPR strain Kocuria rhizophila Y1 enhances salt stress tolerance in maize by regulating phytohormone levels, nutrient acquisition, redox potential, ion homeostasis, photosynthetic capacity and stress-responsive genes expression. Environ. Exp. Bot. 2020, 174, 104023. [Google Scholar] [CrossRef]
- Cummins, I.; Dixon, D.P.; Freitag-Pohl, S.; Skipsey, M.; Edwards, R. Multiple roles for plant glutathione transferases in xenobiotic detoxification. Drug Metabol. Rev. 2011, 43, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Gaafar, R.M.; Osman, M.E.-A.H.; Abo-Shady, A.M.; Almohisen, I.A.A.; Badawy, G.A.; El-Nagar, M.M.F.; Ismail, G.A. Role of Antioxidant Enzymes and Glutathione S-transferase in bromoxynil herbicide stress tolerance in wheat plants. Plants 2022, 11, 2679. [Google Scholar] [CrossRef] [PubMed]
- Amador, V.C.; da Silva, E.F.; Nadvorny, D.; Maia, R.T. Possible metsulfuron herbicide detoxification by a Oryza sativa L. glutathione S-transferase enzyme. Braz. Arch. Biol. Technol. 2020, 63, e20180571. [Google Scholar] [CrossRef]
- Benidire, L.; Madline, A.; Pereira, S.I.A.; Castro, P.M.L.; Boularbah, A. Synergistic effect of organo-mineral amendments and plant growth-promoting rhizobacteria (PGPR) on the establishment of vegetation cover and amelioration of mine tailings. Chemosphere 2021, 262, 127803. [Google Scholar] [CrossRef]
- Han, H.S.; Lee, K.D. Plant growth promoting rhizobacteria effect on antioxidant status, photosynthesis, mineral uptake and growth of lettuce under soil salinity. Res. J. Agric. Biol. Sci. 2005, 1, 210–215. [Google Scholar]
- Barrett, M. Reduction of Imazaquin Injury to Corn (Zea mays) and Sorghum (Sorghum bicolor) with antidotes. Weed Sci. 1989, 37, 34–41. [Google Scholar] [CrossRef]
- Hatzios, K.K.; Burgos, N. Metabolism-based herbicide resistance: Regulationby safeners. Weed Sci. 2004, 52, 454–467. [Google Scholar] [CrossRef]
- Lu, Y.C.; Zhang, S.; Yang, H. Acceleration of the herbicide isoproturon degradation in wheat by glycosyltransferases and salicylic acid. J. Hazard. Mater. 2015, 283, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Farago, S.; Brunold, C.; Kreuz, K. Herbicide safeners and glutathione metabolism. Phisiol. Plant. 1994, 91, 537–542. [Google Scholar] [CrossRef]
- Wang, X.; Wu, L.; Xie, J.; Li, T.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Herbicide isoproturon aggravates the damage of low temperature stress and exogenous ascorbic acid alleviates the combined stress in wheat seedlings. Plant Growth Regul. 2018, 84, 293–301. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, X.; Li, F.; Shi, Z. Effects of nicosulfuron on growth, oxidative damage, and the ascorbate-glutathione pathway in paired nearly isogenic lines of waxy maize (Zea mays L.). Pest. Biochem. Physiol. 2018, 145, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Kandoliya, U.K.; Vakharia, D.N. Ascorbic acid and ascorbate peroxidase based defence system induced by Pseudomonas fluorescens against wilt pathogen in chickpea. Int. J. Plant Prot. 2015, 8, 86–92. [Google Scholar] [CrossRef]
- Islam, F.; Yasmeen, T.; Ali, Q.; Ali, S.; Arif, M.S.; Hussain, S.; Rizvi, H. Influence of Pseudomonas aeruginosa as PGPR on oxidative stress tolerance in wheat under Zn stress. Ecotoxicol. Environ. Safe. 2014, 104, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Neshat, M.; Abbasi, A.; Hosseinzadeh, A.; Sarikhani, M.R.; Dadashi Chavan, D.; Rasoulnia, A. Plant growth promoting bacteria (PGPR) induce antioxidant tolerance against salinity stress through biochemical and physiological mechanisms. Physiol. Mol. Biol. Plants 2022, 28, 347–361. [Google Scholar] [CrossRef]
- Rossi, M.; Borromeo, I.; Capo, C.; Glick, B.R.; Del Gallo, M.; Pietrini, F.; Forni, C. PGPB Improve Photosynthetic Activity and Tolerance to Oxidative Stress in Brassica napus Grown on Salinized Soils. Appl. Sci. 2021, 11, 11442. [Google Scholar] [CrossRef]
- Raihan, M.R.H.; Rahman, M.; Mahmud, N.U.; Adak, M.K.; Islam, T.; Fujita, M.; Hasanuzzaman, M. Application of Rhizobacteria, Paraburkholderia fungorum and Delftia sp. Confer Cadmium Tolerance in Rapeseed (Brassica campestris) through Modulating Antioxidant Defense and Glyoxalase Systems. Plants 2022, 11, 2738. [Google Scholar] [CrossRef]
- Sood, G.; Kaushal, R.; Sharma, M. Significance of inoculation with Bacillus subtilis to alleviate drought stress in wheat (Triticum aestivum L.). Vegetos 2020, 33, 782–792. [Google Scholar] [CrossRef]
- Yaseen, R.; Aziz, O.; Saleem, M.H.; Riaz, M.; Zafar-ul-Hye, M.; Rehman, M.; Ali, S.; Rizwan, M.; Nasser Alyemeni, M.; El-Serehy, H.A.; et al. Ameliorating the Drought Stress for Wheat Growth through Application of ACC-Deaminase Containing Rhizobacteria along with Biogas Slurry. Sustainability 2020, 12, 6022. [Google Scholar] [CrossRef]
- Ilyas, N.; Mazhar, R.; Yasmin, H.; Khan, W.; Iqbal, S.; Enshasy, H.E.; Dailin, D.J. Rhizobacteria Isolated from Saline Soil Induce Systemic Tolerance in Wheat (Triticum aestivum L.) against Salinity Stress. Agronomy 2020, 10, 989. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Łuniewski, S.; Kaczyński, P.; Łozowicka, B. The Influence of Humic Acids and Nitrophenols on Metabolic Compounds and Pesticide Behavior in Wheat under Biotic Stress. Agronomy 2023, 13, 1378. [Google Scholar] [CrossRef]
- Nivetha, N.; Lavanya, A.K.; Vikram, K.V.; Asha, A.D.; Sruthi, K.S.; Bandeppa, S.; Annapurna, K.; Paul, S. PGPR-Mediated Regulation of Antioxidants: Prospects for Abiotic Stress Management in Plants. In Antioxidants in Plant-Microbe Interaction; Singh, H.B., Vaishnav, A., Sayyed, R., Eds.; Springer: Singapore, 2021; pp. 471–497. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Fini, A.; Gori, A.; Guidi, L.; Landi, M.; Sebastiani, F.; Tattini, M. Are Flavonoids Effective Antioxidants in Plants? Twenty Years of Our Investigation. Antioxidant 2020, 9, 1098. [Google Scholar] [CrossRef] [PubMed]
- Chamam, A.; Sanguin, H.; Bellvert, F.; Meiffren, G.; Comte, G.; Wisniewski-Dye, F.; Bertrand, C.; Prigent-Combaret, C. Plant secondary metabolite profiling evidences strain-dependent effect in the Azospirillum-Oryza sativa association. Phytochemistry 2013, 87, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Nasab, R.S.; Yali, M.P.; Bozorg-Amirkalaee, M. Effects of humic acid and plant growth-promoting rhizobacteria (PGPR) on induced resistance of canola to Brevicoryne brassicae L. Bull. Entomol. Res. 2019, 109, 479–489. [Google Scholar] [CrossRef]
- Cummins, I.; Bryant, D.N.; Edwards, R. Safener responsiveness and multiple herbicide resistance in the weed black-grass (Alopecurus myosuroides). Plant Biotech. J. 2009, 7, 807–820. [Google Scholar] [CrossRef]
- Li, Z.-J.; Wang, H.-Z.; Xu, J.-M.; Wu, J.-J.; Ma, G.-R. Response of rice varieties to bound residues of metsulfuron-methyl in a paddy soil. Pedosphere 2007, 17, 487–492. [Google Scholar] [CrossRef]


| Properties | Bacterial Strains | |
|---|---|---|
| P. protegens DA1.2 | P. chlororaphis 4CH | |
| Indole-3-acetic acid production, mg/L | 0.870 | 0.837 |
| P solubilization | capable | capable |
| Nitrogenase activity, nmol C2H4 h−1 mL−1 | 21.3 | 30.5 |
| Phytopathogenic fungi suppressed by bacteria | Alternaria alternata (Fr.) Keissl., A. solani Sorauer, Bipolaris sorokiniana (Sacc.) Shoemaker, Botrytis cinerea Persoon, Fusarium culmorum (W.G. Smith) Sacc., F. gibbosum Appel et Wollenw, F. graminearum Schwabe, Rhizoctonia solani J.G. Kuehn. | A. alternata (Fr.) Keissl., A. solani Sorauer, B. sorokiniana (Sacc.) Shoemaker, Bot. cinerea Persoon, F. culmorum (W.G. Smith) Sacc., R. solani J.G. Kuehn. |
| Bacterium | Herbicide Dose, mg∙kg−1 | Ascorbic Acid, µg∙g−1 FW | GSH, µg∙g−1 FW | Flavonoids, Units |
|---|---|---|---|---|
| Without bacterium | 0 | 532 ± 39 cde * | 30.39 ± 2.04 b | 0.226 ± 0.019 a |
| 0.05 | 667 ± 45 f | 20.95 ± 1.78 a | 0.237 ± 0.013 a | |
| 0.25 | 256 ± 37 b | 24.55 ± 1.95 a | 0.284 ± 0.008 c | |
| Pseudomonas protegens DA1.2 | 0 | 480 ± 34 c | 37.25 ± 2.28 d | 0.246 ± 0.007 ab |
| 0.05 | 538 ± 28 de | 35.69 ± 1.74 cd | 0.259 ± 0.009 b | |
| 0.25 | 126 ± 21 a | 41.13 ± 1.90 de | 0.301 ± 0.014 d | |
| P. chlororaphis 4CH | 0 | 580 ± 30 e | 35.62 ± 1.67 cd | 0.232 ± 0.008 a |
| 0.05 | 460 ± 45 c | 33.61 ± 1.41 bc | 0.265 ± 0.011 b | |
| 0.25 | 599 ± 29 ef | 42.39 ± 2.33 e | 0.324 ± 0.015 d |
| Bacterium | Initial Dose of Herbicide, mg∙kg−1 | Ascorbic Acid, µg∙g−1 FW | GSH, µg∙g−1 FW | Flavonoids, Units |
|---|---|---|---|---|
| Without bacterium | 0 | 401 ± 32 b * | 27.63 ± 1.57 a | 0.185 ± 0.021 a |
| 0.05 | 520 ± 41 c | 34.54 ± 1.89 b | 0.264 ± 0.007 c | |
| Pseudomonas protegens DA1.2 | 0 | 434 ± 36 b | 30.67 ± 2.11 ab | 0.229 ± 0.015 b |
| 0.05 | 262 ± 39 a | 106.43 ± 4.25 d | 0.323 ± 0.015 d | |
| P. chlororaphis 4CH | 0 | 453 ± 43 bc | 34.82 ± 1.60 b | 0.203 ± 0.023 ab |
| 0.05 | 478 ± 35 c | 51.28 ± 5.05 c | 0.309 ± 0.009 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakaeva, M.; Chetverikov, S.; Starikov, S.; Kendjieva, A.; Khudaygulov, G.; Chetverikova, D. Effect of Plant Growth-Promoting Bacteria on Antioxidant Status, Acetolactate Synthase Activity, and Growth of Common Wheat and Canola Exposed to Metsulfuron-Methyl. J. Xenobiot. 2024, 14, 79-95. https://doi.org/10.3390/jox14010005
Bakaeva M, Chetverikov S, Starikov S, Kendjieva A, Khudaygulov G, Chetverikova D. Effect of Plant Growth-Promoting Bacteria on Antioxidant Status, Acetolactate Synthase Activity, and Growth of Common Wheat and Canola Exposed to Metsulfuron-Methyl. Journal of Xenobiotics. 2024; 14(1):79-95. https://doi.org/10.3390/jox14010005
Chicago/Turabian StyleBakaeva, Margarita, Sergey Chetverikov, Sergey Starikov, Aliya Kendjieva, Gaisar Khudaygulov, and Darya Chetverikova. 2024. "Effect of Plant Growth-Promoting Bacteria on Antioxidant Status, Acetolactate Synthase Activity, and Growth of Common Wheat and Canola Exposed to Metsulfuron-Methyl" Journal of Xenobiotics 14, no. 1: 79-95. https://doi.org/10.3390/jox14010005
APA StyleBakaeva, M., Chetverikov, S., Starikov, S., Kendjieva, A., Khudaygulov, G., & Chetverikova, D. (2024). Effect of Plant Growth-Promoting Bacteria on Antioxidant Status, Acetolactate Synthase Activity, and Growth of Common Wheat and Canola Exposed to Metsulfuron-Methyl. Journal of Xenobiotics, 14(1), 79-95. https://doi.org/10.3390/jox14010005








