Relevance of Carcinogen-Induced Preclinical Cancer Models
Abstract
1. Introduction
2. Examples of Chemical Carcinogens in Mouse Cancer Models
2.1. AOM
2.2. Cerulein
2.3. DEN
2.4. DMBA
2.5. DSS
2.6. Urethane
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Cancer Society. Known and Probable Human Carcinogens. 2022. Available online: https://www.cancer.org/cancer/cancer-causes/general-info/known-and-probable-human-carcinogens.html (accessed on 5 November 2023).
- Occupational Safety and Health Administration. Chemical Hazards and Toxic Substances. 2019. Available online: https://www.osha.gov/chemical-hazards-toxic-substances (accessed on 5 November 2023).
- National Cancer Institute. Environmental Carcinogens and Cancer Risk. 2022. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/substances/environmental-carcinogens (accessed on 5 November 2023).
- Belpoggi, F.; Soffritti, M.; Tibaldi, E.; Falcioni, L.; Bua, L. Results of long-term experimental studies on the carcinogenicity of formaldehyde and acetaldehyde in rats. Ann. N. Y. Acad. Sci. 2006, 1076, 190–202. [Google Scholar]
- Boffetta, P.; Jourenkova, N.; Gustavsson, P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control 1997, 8, 444–472. [Google Scholar] [CrossRef] [PubMed]
- Cogliano, V.J.; Baan, R.; Straif, K.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; et al. Preventable exposures associated with human cancers. J. Natl. Cancer Inst. 2011, 103, 1827–1839. [Google Scholar] [CrossRef] [PubMed]
- DeMarini, D.M. Genotoxicity of tobacco smoke and tobacco smoke condensate: A review. Mutat. Res. Rev. Mutat. Res. 2004, 567, 447–474. [Google Scholar] [CrossRef] [PubMed]
- Feron, V.J.; Kruysse, A.; Til, H.P.; Immel, H.R.; Cassee, F.R. Aldehydes: Occurrence, carcinogenic potential, mechanism of action and risk assessment. Mutat. Res. Rev. Genet. Toxicol. 1991, 259, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Feron, V.J.; Kruysse, A.; Woutersen, R.A.; Immel, H.R.; Til, H.P. Toxicology of chemical mixtures: International programme on chemical safety. Environ. Health Perspect. 1988, 78, 165–171. [Google Scholar]
- Grosse, Y.; Loomis, D.; Guyton, K.Z.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K.; et al. Carcinogenicity of fluoro-edenite, silicon carbide fibres and whiskers, and carbon nanotubes. Lancet Oncol. 2018, 19, 504–505. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Chemical Agents and Related Occupations. A Review of Human Carcinogens; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization: Geneve, Switzerland, 2012; Volume 100F, pp. 9–562. [Google Scholar]
- International Agency for Research on Cancer. List of Classifications by Cancer Sites with Sufficient or Limited Evidence in Humans; IARC: Lyon, France, 2019; Volumes 1–125. [Google Scholar]
- International Agency for Research on Cancer. Agents Classified by the IARC Monographs; IARC: Lyon, France, 2022; Volumes 1–131. [Google Scholar]
- Hakura, A.; Koyama, N.; Seki, Y.; Sonoda, J.; Asakura, S. o-Aminoazotoluene, 7,12-dimethylbenz[a]anthracene, and N-ethyl-N-nitrosourea, which are mutagenic but not carcinogenic in the colon, rapidly induce colonic tumors in mice with dextran sulfate sodium-induced colitis. Genes Environ. 2022, 44, 11. [Google Scholar] [CrossRef]
- Bareham, B.; Georgakopoulos, N.; Matas-Céspedes, A.; Curran, M.; Saeb-Parsy, K. Modeling human tumor-immune environments in vivo for the preclinical assessment of immunotherapies. Cancer immunol, immunother. 2021, 70, 2737–2750. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M.; Hocevar, B.A. Oxidative stress and oxidative damage in carcinogenesis. Toxicol. Pathol. 2009, 38, 96–109. [Google Scholar] [CrossRef]
- National Toxicology Program. Report on Carcinogens; United States Department of Health and Human Services: Washington, DC, USA, 2016. [Google Scholar]
- Ivanisevic, T.; Steklov, M.; Lechat, B.; Cawthorne, C.; Gsell, W.; Velde, G.V.; Deroose, C.; Van Laere, K.; Himmelreich, U.; Sewduth, R.N.; et al. Targeted STAT1 therapy for LZTR1-driven peripheral nerve sheath tumor. Cancer Commun. 2023, 43, 1386–1390. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. NTP Technical Report on the Toxicology and Carcinogenesis Studies of Cerulein (CAS No. 17650–98–5) in F344/N Rats and B6C3F1/N Mice (Pancreatitis, Chronic); United States Department of Health and Human Services: Washington, DC, USA, 2019. [Google Scholar]
- National Toxicology Program. NTP Technical Report on the Toxicology and Carcinogenesis Studies of Diethylnitrosamine (CAS No. 55–18–5) in F344/N Rats and B6C3F1/N Mice (Gavage Studies); United States Department of Health and Human Services: Washington, DC, USA, 2021. [Google Scholar]
- National Toxicology Program. NTP Technical Report on the Toxicology and Carcinogenesis Studies of 7,12-dimethylbenz[a]anthracene (CAS No. 57–97–6) in F344/N Rats and B6C3F1/N Mice (Dermal Studies); United States Department of Health and Human Services: Washington, DC, USA, 2022. [Google Scholar]
- National Toxicology Program. NTP Technical Report on the Toxicology and Carcinogenesis Studies of Dextran Sulfate Sodium (CAS No. 9011–18–1) in F344/N Rats and B6C3F1/N Mice (Gavage Studies); United States Department of Health and Human Services: Washington, DC, USA, 2022. [Google Scholar]
- National Toxicology Program. NTP Technical Report on the Toxicology and Carcinogenesis Studies of Urethane (CAS No. 51–79–6) in B6C3F1/N Mice (Gavage Studies); United States Department of Health and Human Services: Washington, DC, USA, 2022. [Google Scholar]
- Chen, J.; Huang, X.-F. The signal pathways in azoxymethane-induced colon cancer and preventive implications. Cancer Biol. Ther. 2009, 8, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Tung, N.H.; Shoyama, Y.; Sugie, S.; Mori, H.; Tanaka, T. Chemopreventive effect of curcumin and (-)-epigallocatechin gallate on azoxymethane-induced aberrant crypt foci and colorectal tumors in rats. Cancer Lett. 2003, 196, 129–138. [Google Scholar]
- Mori, H.; Kawabata, K.; Matsunaga, K.; Sugie, S. Chemoprevention of azoxymethane-induced rat colon carcinogenesis by dietary capsaicin and rotenone. Int. J. Cancer 2004, 110, 436–441. [Google Scholar]
- Snider, A.J.; Bialkowska, A.B.; Ghaleb, A.M.; Yang, V.W.; Obeid, L.M.; Hannun, Y.A. Murine Model for Colitis-Associated Cancer of the Colon. Mouse Models Drug Discov. Methods Protoc. 2016, 1438, 245–254. [Google Scholar]
- Uragami, T.; Ando, Y.; Aoi, M.; Fukui, T.; Matsumoto, Y.; Horitani, S.; Tomiyama, T.; Okazaki, K.; Tsuneyama, K.; Tanaka, H.; et al. Establishment of a Novel Colitis-Associated Cancer Mouse Model Showing Flat Invasive Neoplasia. Dig. Dis. Sci. 2023, 68, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, Q.; Wang, C.; Wang, G. Differential susceptibility of mouse strains on pancreatic injury and regeneration in cerulein-induced pancreatitis. Int. J. Clin. Exp. Pathol. 2017, 1, 9934–9944. [Google Scholar]
- Gitto, S.B.; Nakkina, S.P.; Beardsley, J.M.; Parikh, J.G.; Altomare, D.A. Induction of pancreatitis in mice with susceptibility to pancreatic cancer. Methods Cell Biol. 2022, 168, 139–159. [Google Scholar]
- Minaga, K.; Watanabe, T.; Kamata, K.; Kudo, M.; Strober, W. A Mouse Model of Acute and Chronic Pancreatitis. Curr. Protoc. 2022, 2, e422. [Google Scholar] [CrossRef]
- Ahmadi, A.; Nikkhoo, B.; Mokarizadeh, A.; Rahmani, M.R.; Fakhari, S.; Mohammadi, M.; Jalili, A. An optimised mouse model of chronic pancreatitis with a combination of ethanol and cerulein. Cent. Eur. J. Immunol. 2016, 41, 54–63. [Google Scholar] [CrossRef]
- Schulien, I.; Hasselblatt, P. Diethylnitrosamine-induced liver tumorigenesis in mice. Methods Cell Biol. 2021, 163, 137–152. [Google Scholar] [PubMed]
- Nie, Y.; Huang, B.; Hu, A.L.; Xu, Y.Y.; Zou, Y.; Liu, Y.; Liu, J. Antitumor effects of cadmium against diethylnitrosamine-induced liver tumors in mice. Oncol. Lett. 2022, 23, 33. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.K.; Li, C.; Zhang, R.Y.; Wei, D.; Shang, Y.K.; Yong, Y.L.; Kong, L.M.; Zheng, N.S.; Liu, K.; Lu, M.; et al. EYA2 suppresses the progression of hepatocellular carcinoma via SOCS3-mediated blockade of JAK/STAT signaling. Mol. Cancer 2021, 20, 79. [Google Scholar] [CrossRef] [PubMed]
- Yim, S.Y.; Shim, J.J.; Shin, J.H.; Jeong, Y.S.; Kang, S.H.; Kim, S.B.; Eun, Y.G.; Lee, D.J.; Conner, E.A.; Factor, V.M.; et al. Integrated Genomic Comparison of Mouse Models Reveals Their Clinical Resemblance to Human Liver Cancer. Mol. Cancer Res. 2018, 16, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.; Pyao, Y.; Jung, Y.; Lee, J.I.; Lee, W.K. A Modified Protocol of Diethylnitrosamine Administration in Mice to Model Hepatocellular Carcinoma. Int. J. Mol. Sci. 2020, 21, 5461. [Google Scholar] [CrossRef] [PubMed]
- Budán, F.; Varjas, T.; Nowrasteh, G.; Prantner, I.; Varga, Z.; Ember, A.; Cseh, J.; Gombos, K.; Pázsit, E.; Gobel, G.; et al. Early modification of c-myc, Ha-ras and p53 expressions by chemical carcinogens (DMBA, MNU). In Vivo 2009, 23, 591–598. [Google Scholar] [PubMed]
- Buqué, A.; Perez-Lanzón, M.; Petroni, G.; Humeau, J.; Bloy, N.; Yamazaki, T.; Sato, A.; Kroemer, G.; Galluzzi, L. MPA/DMBA-driven mammary carcinomas. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 163, pp. 1–19. [Google Scholar]
- Stewart, M.K.; Bechberger, J.F.; Welch, I.; Naus, C.C.; Laird, D.W. Cx26 knockout predisposes the mammary gland to primary mammary tumors in a DMBA-induced mouse model of breast cancer. Oncotarget 2015, 6, 37185–37199. [Google Scholar] [CrossRef] [PubMed]
- Slaga, T.J. SENCAR mouse skin tumorigenesis model versus other strains and stocks of mice. Environ. Health Perspect. 1986, 68, 27–32. [Google Scholar] [CrossRef]
- Plante, I. Dimethylbenz(a)anthracene-induced mammary tumorigenesis in mice. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 163, pp. 21–44. [Google Scholar]
- Schwarz, M.; Münzel, P.A.; Braeuning, A. Non-melanoma skin cancer in mouse and man. Arch. Toxicol. 2013, 87, 783–798. [Google Scholar] [CrossRef]
- Schepelmann, M.; Kupper, N.; Gushchina, V.; Mesteri, I.; Manhardt, T.; Moritsch, S.; Müller, C.; Piatek, K.; Salzmann, M.; Vlasaty, A.; et al. AOM/DSS Induced Colitis-Associated Colorectal Cancer in 14-Month-Old Female Balb/C and C57/Bl6 Mice-A Pilot Study. Int. J. Mol. Sci. 2022, 23, 5278. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Gao, H.; Hou, X.; Chen, Y.; Xu, K. Pretreatment with IPA ameliorates colitis in mice: Colon transcriptome and fecal 16S amplicon profiling. Front. Immunol. 2022, 13, 1014881. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Schwertassek, U.; Seydel, A.; Weber, K.; Falk, W.; Hauschildt, S.; Lehmann, J. A refined and translationally relevant model of chronic DSS colitis in BALB/c mice. Lab. Anim. 2018, 52, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lin, H.; Cao, M.; Lin, T.; Lin, A.; Xu, W.; Wang, H.; He, J.; Li, Y.; Tang, H.; et al. Shifts and importance of viable bacteria in treatment of DSS-induced ulcerative colitis mice with FMT. Front. Cell. Infect. Microbiol. 2023, 13, 1124256. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Xu, Y.; Han, X.; Liu, X.; Xie, R.; Cheng, Z.; Fu, X. Transcriptomic and Proteomic Study on the High-Fat Diet Combined with AOM/DSS-Induced Adenomatous Polyps in Mice. Front. Oncol. 2021, 11, 736225. [Google Scholar] [CrossRef]
- Ghanayem, B.I. Inhibition of urethane-induced carcinogenicity in Cyp2e1−/− in comparison to Cyp2e1+/+ mice. Toxicol. Sci. 2007, 95, 331–3391. [Google Scholar] [CrossRef][Green Version]
- Malkinson, A.M. Primary lung tumors in mice as an aid for understanding, preventing, and treating human adenocarcinoma of the lung. Lung Cancer 2001, 32, 265–2792. [Google Scholar] [CrossRef]
- Hynds, R.E.; Frese, K.K.; Pearce, D.R.; Grönroos, E.; Dive, C.; Swanton, C. Progress towards non-small-cell lung cancer models that represent clinical evolutionary trajectories. Open Biol. 2021, 11, 200247. [Google Scholar] [CrossRef]
- Regala, R.P.; Weems, C.; Jamieson, L.; Copland, J.A.; Thompson, E.A.; Fields, A.P. Atypical protein kinase Ciota plays a critical role in human lung cancer cell growth and tumorigenicity. J. Biol. Chem. 2011, 286, 30367–303774. [Google Scholar]
- Radwan, E.; Ali, M.; Faied, S.M.A.; Omar, H.M.; Mohamed, W.S.; Abd-Elghaffar, S.K.; Sayed, A.A. Novel therapeutic regimens for urethane-induced early lung cancer in rats: Combined cisplatin nanoparticles with vitamin-D3. IUBMB Life 2021, 73, 362–374. [Google Scholar] [CrossRef]
- Teixeira, D.; Almeida, J.S.; Visniauskas, B.; Gomes, G.N.; Hirata, A.E.; Bueno, V. Myeloid-derived suppressor cells and associated events in urethane-induced lung cancer. Clinics 2013, 68, 858–864. [Google Scholar] [CrossRef]
- Paranjpe, M.G.; Rudmann, D.; Sargeant, A.; Morse, M.; Yonpiam, R.; Bonnette, K.; Albretsen, J.; Papagiannis, C. Proposal to Eliminate Urethane-Treated Positive Control Dose Groups in 26-Week Tg.rasH2 Carcinogenicity Studies. Int. J. Toxicol. 2021, 40, 207–210. [Google Scholar] [CrossRef]
- Shah, S.A.; Paranjpe, M.G.; Atkins, P.I.; Zahalka, E.A. Reduction in the number of animals and the evaluation period for the positive control group in Tg.rasH2 short-term carcinogenicity studies. Int. J. Toxicol. 2012, 31, 423–429. [Google Scholar] [CrossRef]
- Morton, D.; Alden, C.L.; Roth, A.J.; Usui, T. The Tg rasH2 mouse in cancer hazard identification. Toxicol. Pathol. 2002, 30, 139–146. [Google Scholar] [CrossRef]
- Heeschen, C.; Jang, J.J.; Weis, M.; Pathak, A.; Kaji, S.; Hu, R.S.; Tsao, P.S.; Johnson, F.L.; Cooke, J.P. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat. Med. 2001, 7, 833–839. [Google Scholar] [CrossRef]
- Robinson, B.W.S.; Redwood, A.J.; Creaney, J. How Our Continuing Studies of the Pre-clinical Inbred Mouse Models of Mesothelioma Have Influenced the Development of New Therapies. Front. Pharmacol. 2022, 13, 858557. [Google Scholar] [CrossRef]
- Wang, J.P.; Qi, L.; Moore, M.R.; Ng, J.C. A review of animal models for the study of arsenic carcinogenesis. Toxicol. Lett. 2002, 133, 17–31. [Google Scholar] [CrossRef]
- Portier, C.J. A comprehensive analysis of the animal carcinogenicity data for glyphosate from chronic exposure rodent carcinogenicity studies. Env. Health 2020, 19, 18. [Google Scholar] [CrossRef]
- Schetter, A.J.; Heegaard, N.H.; Harris, C.C. Inflammation and cancer: Interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis 2010, 31, 37–49. [Google Scholar] [CrossRef]
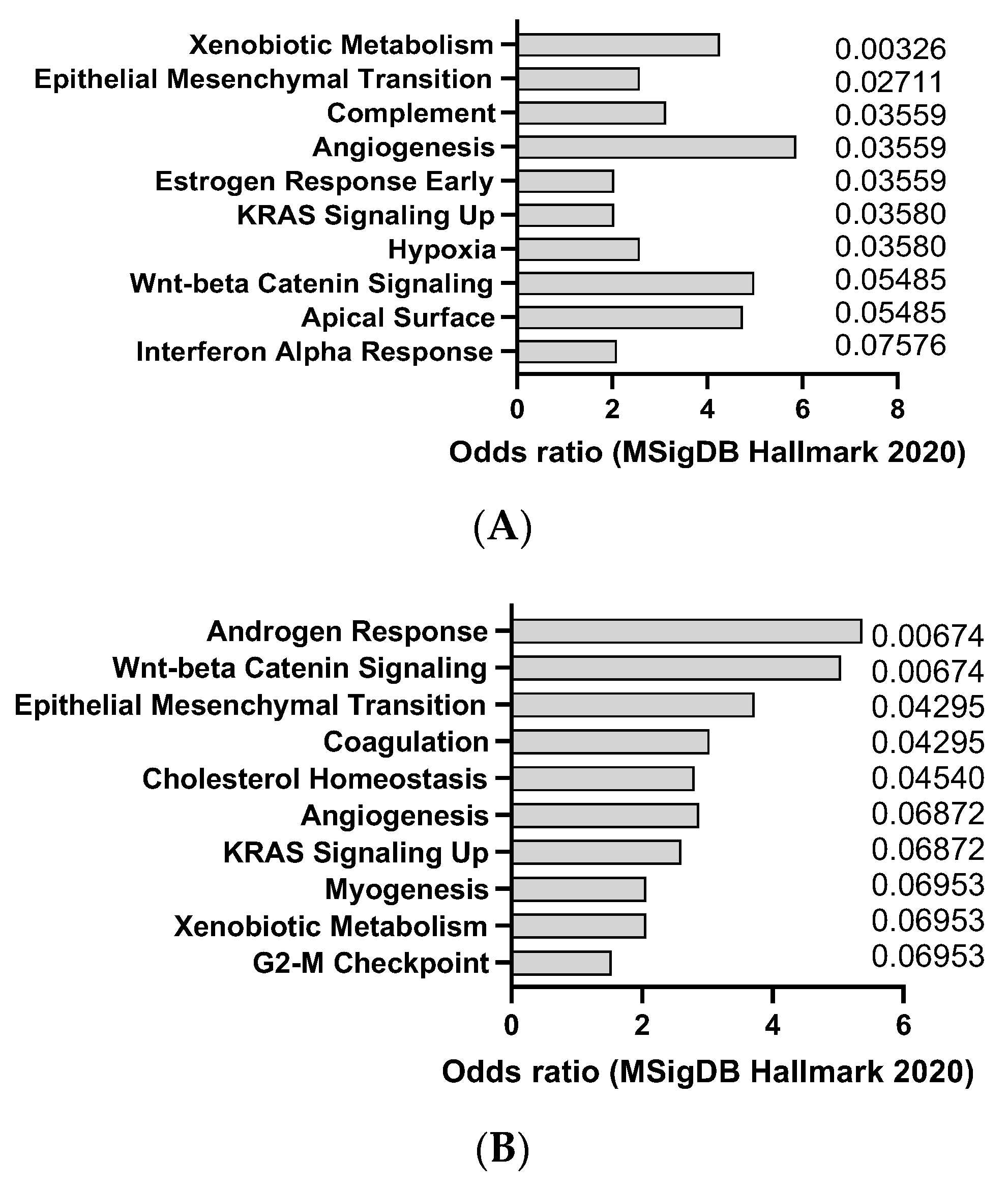

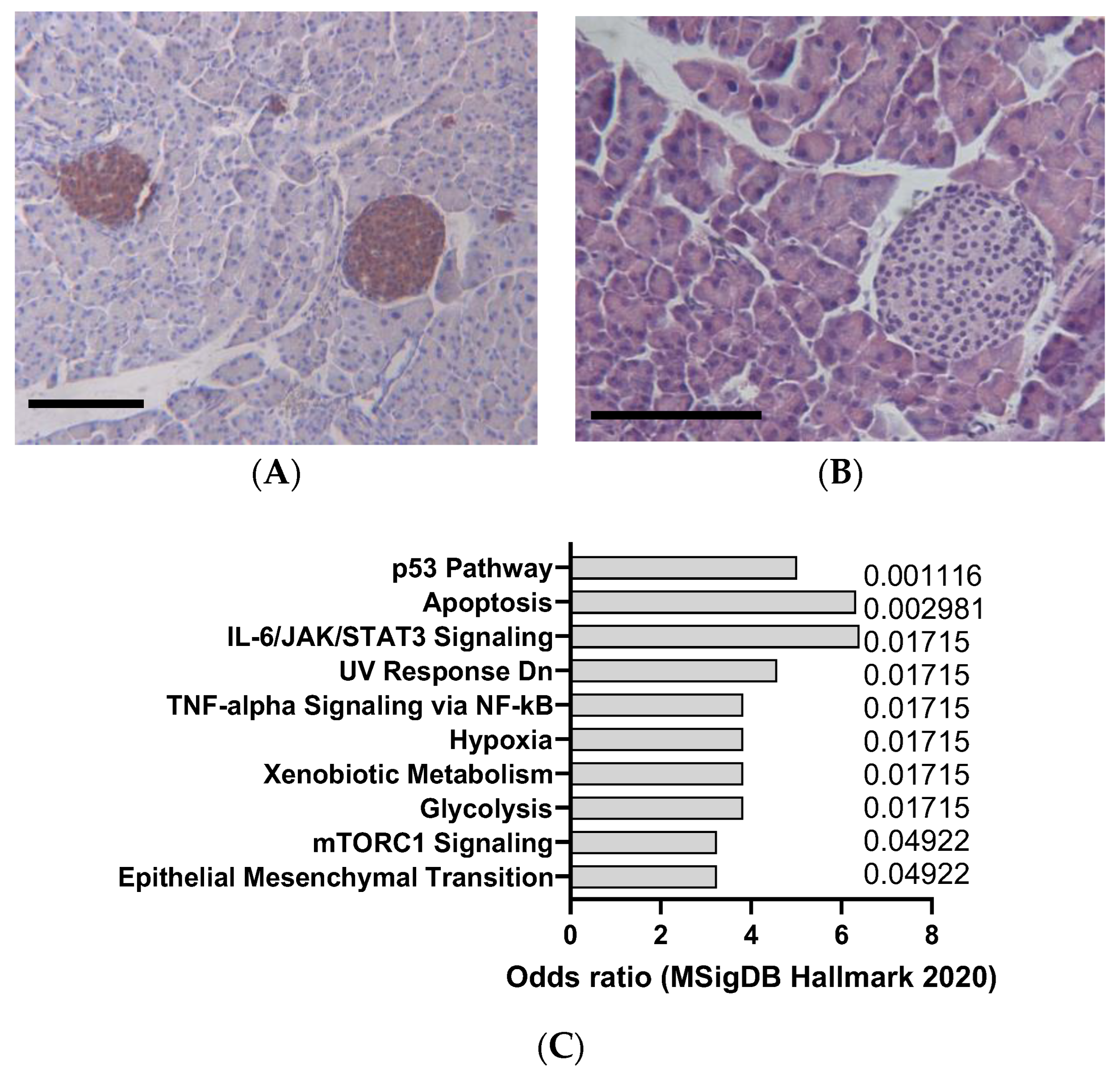

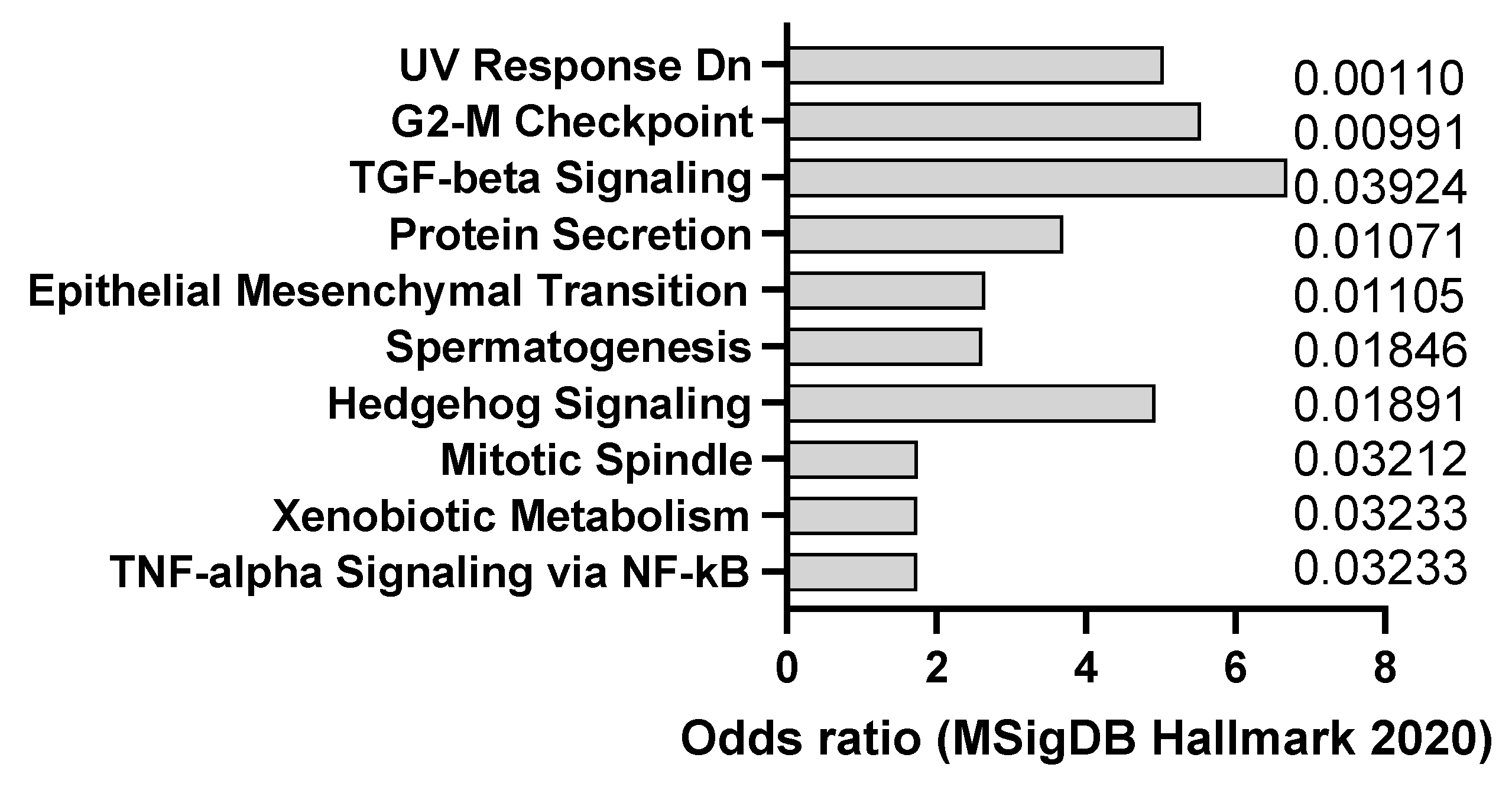
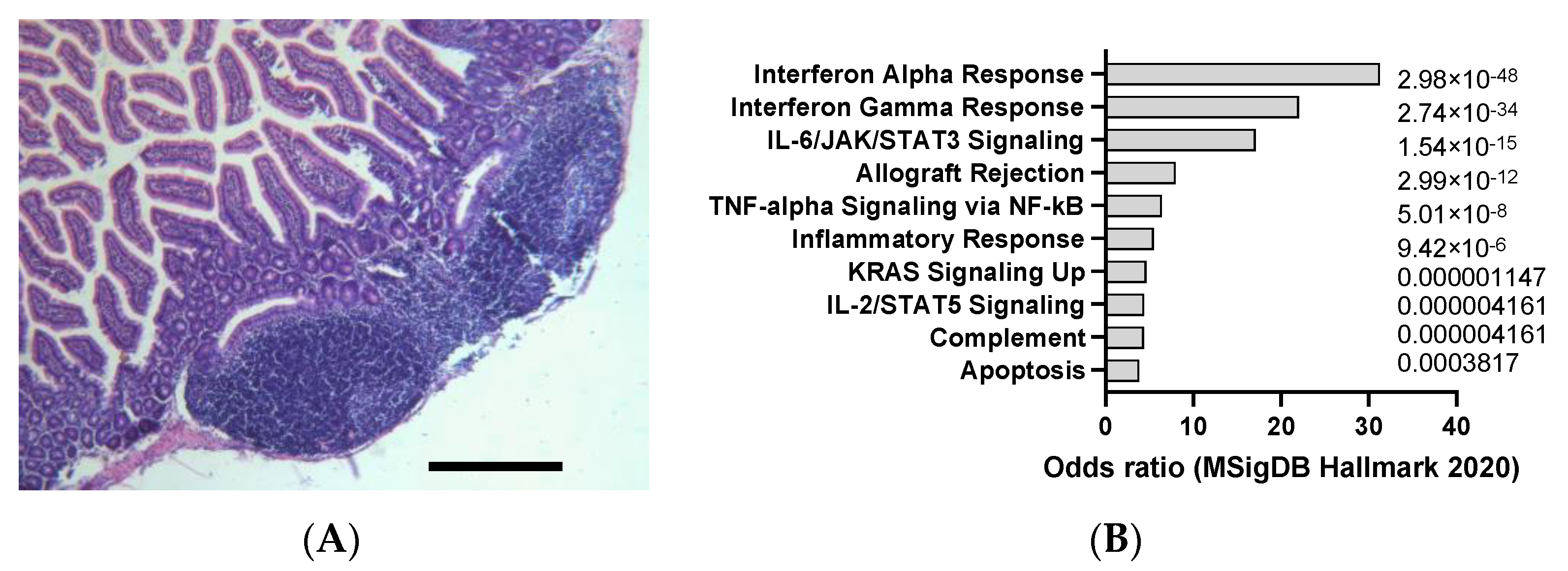
| Agent | Mechanism of Action | Organ Affected | Clinical Relevance |
|---|---|---|---|
| Azoxymethane (AOM) | Nitroso compound that requires metabolic activation to generate a DNA-reactive mutagen that induces colon cancer in mice and rats | Colon | A widely used carcinogen to study chemically induced colorectal carcinogenesis and is an agent for studying fulminant hepatic failure |
| Cerulein (also called ceruletide or caerulein) [19] | Ten amino acid oligopeptide that induces the secretion of pancreatic enzymes, causing inflammation and fibrosis | Pancreas | A potent carcinogen. It is used in paralytic ileus and as diagnostic aid in pancreatic malfunction. It is present in the skin of the Australian green tree frog |
| DEN (diethylnitrosamine) [20] | Nitrosamine that forms nitrosylated DNA adducts and activates oncogenes | Liver, colon, stomach, pancreas | A potent hepatocarcinogen and carcinogen for the digestive tract in rodents that is found in tobacco smoke |
| DMBA [21] | Polycyclic aromatic hydrocarbon (PAH) that can induce breast cancer by causing DNA damage | Breast, skin | A potent carcinogen for mice and humans, used to specifically quantify phlorotannins |
| DSS (dextran sulfate sodium) [22] | Sulfated polysaccharide that induces colitis-associated colorectal cancer by activating nuclear factor-kappa B and transforming growth factor-beta signaling pathways | Colon | A common environmental pollutant and dietary component that can cause inflammation and cancer in humans |
| Urethane [23] | Aromatic hydrocarbon that induces DNA damage and mutations in various tissues | Liver, lung, kidney, bladder, brain | A widely used solvent and industrial chemical that can cause cancer in humans |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sewduth, R.N.; Georgelou, K. Relevance of Carcinogen-Induced Preclinical Cancer Models. J. Xenobiot. 2024, 14, 96-109. https://doi.org/10.3390/jox14010006
Sewduth RN, Georgelou K. Relevance of Carcinogen-Induced Preclinical Cancer Models. Journal of Xenobiotics. 2024; 14(1):96-109. https://doi.org/10.3390/jox14010006
Chicago/Turabian StyleSewduth, Raj N., and Konstantina Georgelou. 2024. "Relevance of Carcinogen-Induced Preclinical Cancer Models" Journal of Xenobiotics 14, no. 1: 96-109. https://doi.org/10.3390/jox14010006
APA StyleSewduth, R. N., & Georgelou, K. (2024). Relevance of Carcinogen-Induced Preclinical Cancer Models. Journal of Xenobiotics, 14(1), 96-109. https://doi.org/10.3390/jox14010006






