Modulation of the Mas-Related G Protein-Coupled Receptor X2 (MRGPRX2) by Xenobiotic Compounds and Its Relevance to Human Diseases
Abstract
1. Introduction
2. Pathophysiological Basis
2.1. Mast Cell Characteristics
2.2. Structure and Regulation of MRGPRX2 Function
2.3. Role of MRGPRX2 in MC-Driven Skin Diseases
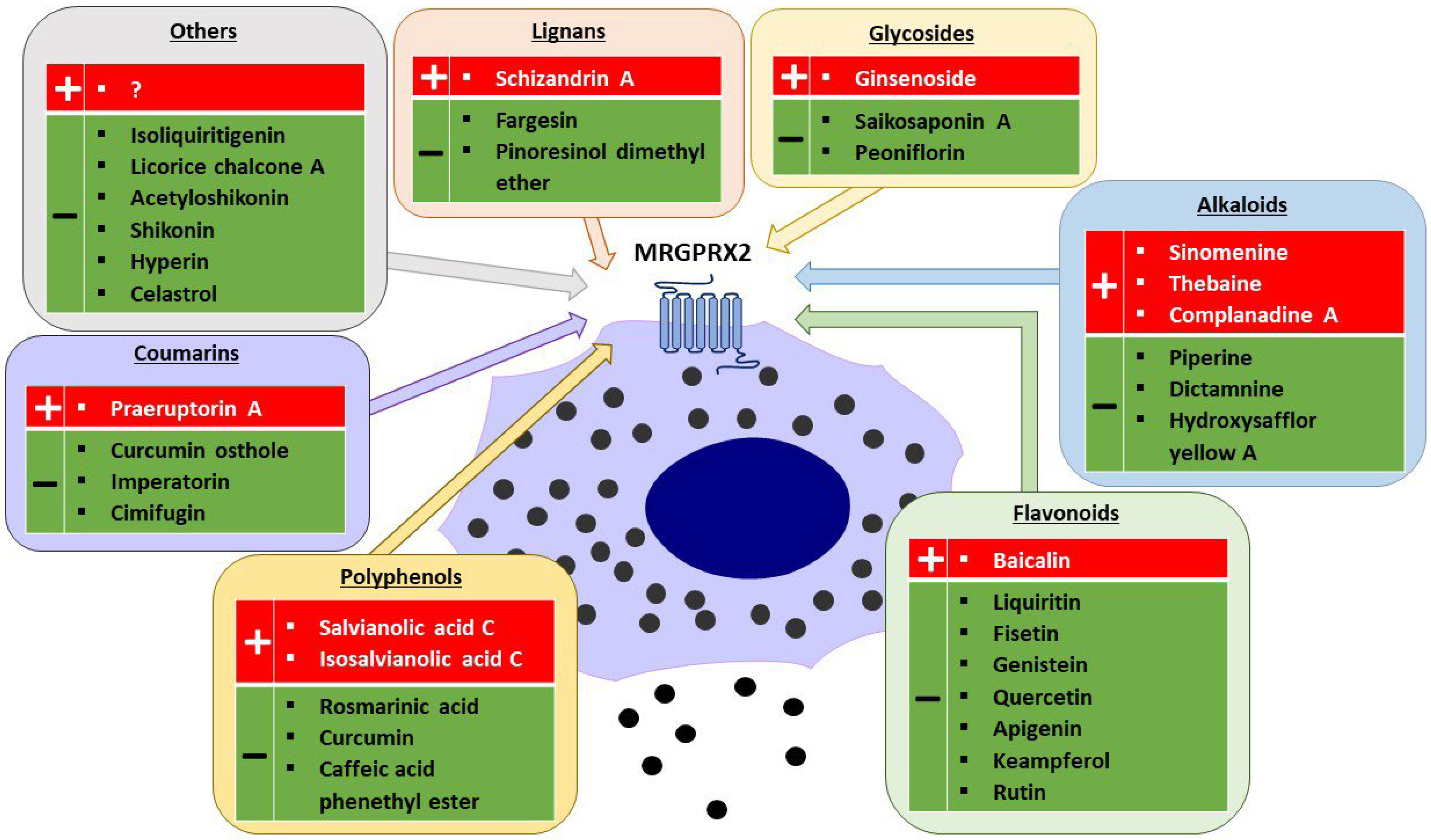
3. Traditional Chinese Medicines and Plant-Derived Compounds
3.1. TCM Compounds in Evidence-Based Medicine and Their Potential for Use in Humans
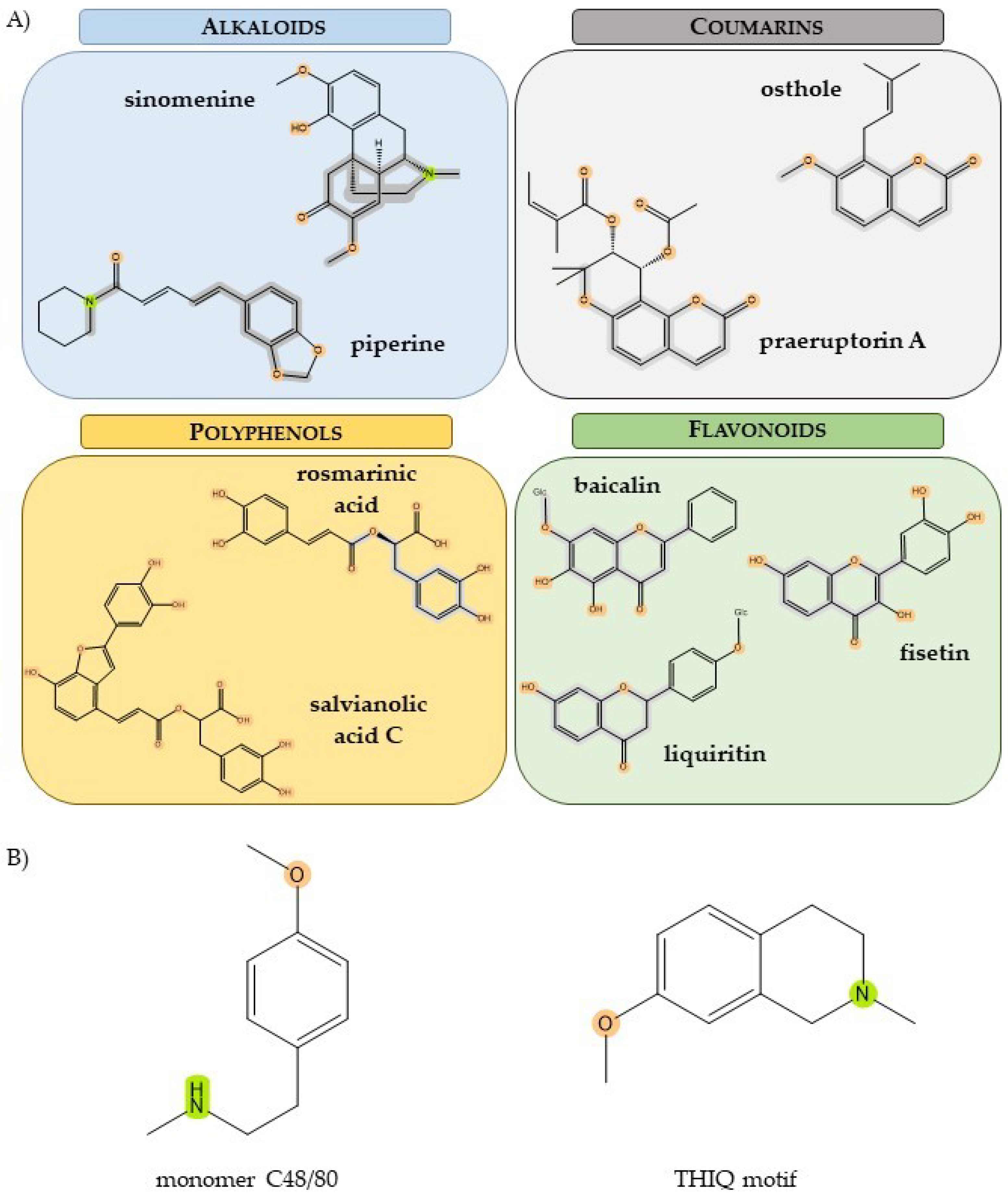
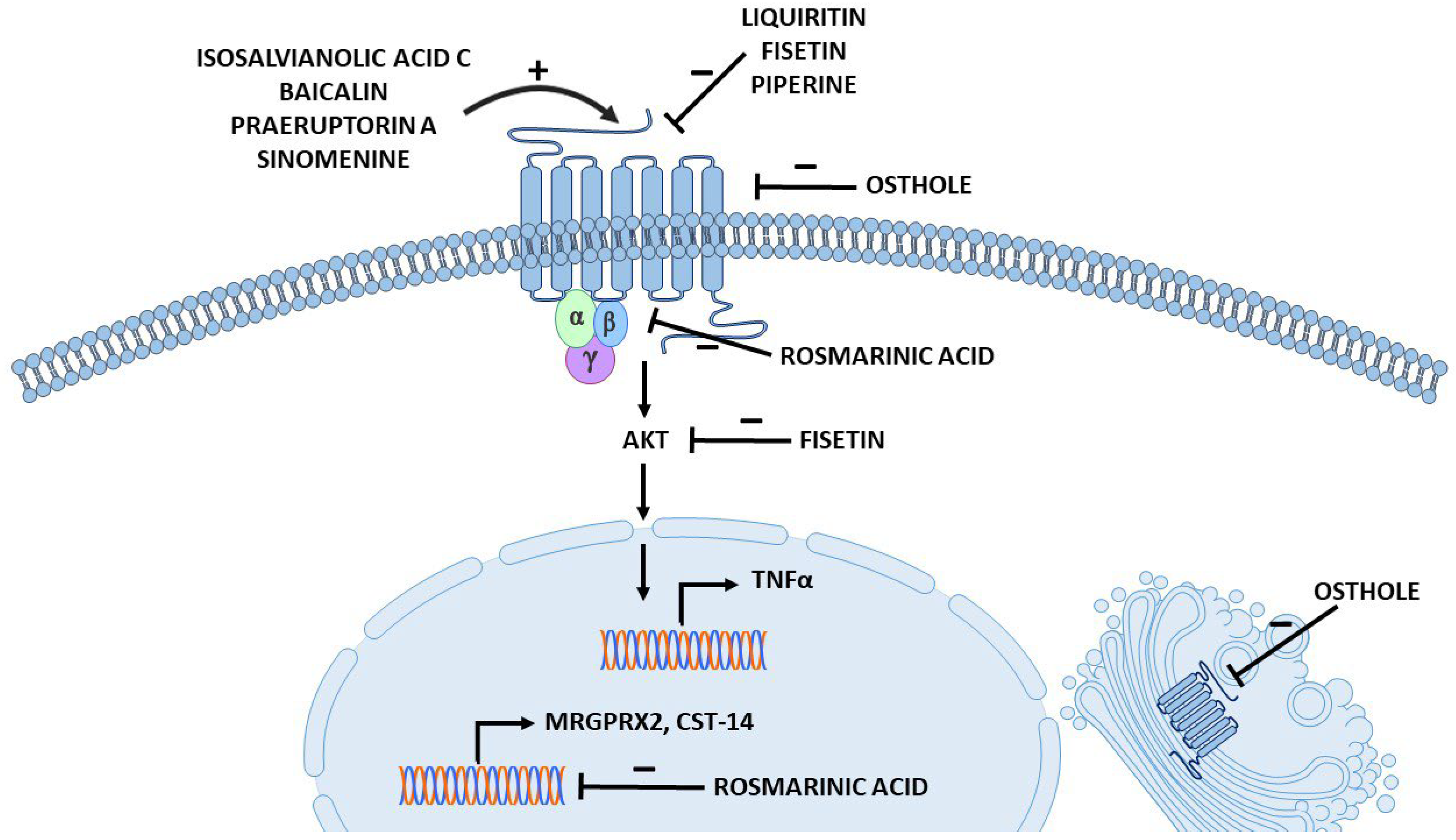
3.2. Polyphenols
3.2.1. Salvanolic Acid C and Isosalvanolic Acid C
3.2.2. Rosmarinic Acid
3.3. Flavonoids
3.3.1. Baicalin
3.3.2. Liquiritin from Licorice Extract
3.3.3. Fisetin
3.4. Coumarins
3.4.1. Praeruptorin A
3.4.2. Osthole
3.5. Alkaloids
3.5.1. Sinomenine
3.5.2. Piperine
| Compound | Experimental Model or Methods | Primary Outcome Measure | Key Conclusions about Compound Activity | References | MRGPRX2 Inhibition and/or Activation | EC50 and/or IC50 for MRGPRX2 (Experimental Model and Assay) | Cmax in Plasma |
|---|---|---|---|---|---|---|---|
| Salvianolic acid | Molecular docking, molecular dynamics | Inhibition of PI3K and mTOR | A candidate for in vitro experiments in breast cancer studies | [89] | Activation * [37] | EC50 = 15.70 ± 4.62 μM (MPMC, β-hexosaminidase release assay) [37] | 171.48 ± 9.42 ng/mL 1 (0.00024 μM) [158] |
| Rosmarinic acid | Mouse and rat models | Behavioral tests | Antinociceptive and anti-inflammatory activity | [130] | Inhibition [72] /no effect [35,40] 2 | IC50 = 1.8 mM (MRGPRX2-HEK293 cells, retention time on CMC column) [40] IC50 cannot be calculated (MRGPRX2-HEK293 cells, intracellular Ca2+ mobilization assay) [35] | |
| Carrageenan-induced pleurisy and paw edema tests in rats | Behavioral tests | Potential for anti-inflammatory and antinociceptive activity | [129] | ||||
| PC12 cells | Amyloid β-induced cellular reactive oxygen species generation | A candidate for neuroprotective treatment of Alzheimer’s disease | [159] | 162.20 ± 40.20 nmol/L (0.162 mM) [160] | |||
| Mouse model of cardiac fibrosis | Morphological examination, echocardiography | Promising as a therapeutic agent against cardiac fibrosis | [161] | ||||
| Baicalin | Mouse model of anxiety/ depression | Depression-like behaviors | Improvement of anxiety/ depression-like behaviors | [162] | Activation * [33,133] | NA | - |
| Rat model of peridontitis | Toll-like receptor expression | Potential for treatment of periodontitis | [163] | ||||
| Mouse model | Tumor growth | Potential for treatment of lung cancer | [87] | ||||
| Liquiritin | Rat model | Cell viability, inflammatory cytokine expression | Beneficial impact on pressure ulcers | [164] | Inhibition [41] | NA | - |
| Rat model | Behavioral tests | Potential for treatment of bone cancer pain | [165] | ||||
| PC12 cells | Expression of proteins involved in signalling pathway | Neuroprotective activity | [166] | ||||
| Diabetic mouse model | α-glucosidase inhibition | Potential for treating diabetes | [167] | ||||
| H9C2 cells | Cell viability level | Cardioprotective effect | [168] | ||||
| Fisetin | Male C57bl/6 J mice | Histopathological and serological injury markers | Protection against septic acute kidney injury | [142] | Inhibition [42] | NA | - |
| Prostate and lung adenocarcinoma cells | Inhibition of the PI3K/AKT and the mTOR pathways | Potential as adjuvant with chemotherapeutic drugs | [143] | ||||
| Osthole | Pulmonary inflammation induced in mice | Inflammatory parameters in BAL fluid | Potential for inhibition of inflammation in chronic obstructive pulmonary disease | [169] | Inhibition [34]/activation [38] 3 | NA | - |
| Mouse model | Itch–scratch response | Antipruritic activity | [170] | ||||
| Mouse monocyte-macrophage cells | Inflammatory mediators’ level | Potential for treatment of ulcerative colitis | [92] | ||||
| Model of middle cerebral artery occlusion in rats | Determination of the infarct area | Potential for neuroprotective therapy in ischemic stroke | [93] | ||||
| Bleomycin induced pulmonary fibrosis in rats | Expression of inflammatory mediators | Beneficial effects in tested model | [171] | ||||
| Cervical cancer cell lines | Cancer cell viability, proliferation, and migration and invasion | Potential as adjuvant treatment for cervical cancer | [172] | ||||
| Human gastric cancer cells | Cell proliferation and apoptosis | Potential for inhibition of gastric cancer cells proliferation | [88] | ||||
| Osteosarcoma cell lines | Cell viability | Potential for osteosarcoma treatment | [173] | ||||
| Tumor-bearing mice | Survival days | Potential for developing antitumor drugs | [174] | ||||
| Diabetic mice | PPAR activation | Potential for treatment of diabetes | [175] | ||||
| Skeletal muscle cells | Expression of AMP-activated protein kinase and glucose transporter 4 | Potential for treatment of diabetes | [176] | ||||
| Praeruptorin A | Mouse macrophages | Expression of NF-κB-related proteins | Potential as a drug for viral infection | [177] | Activation [38] | NA | - |
| Human hepatocellular carcinoma | Migration and invasion of tested cells | Potential as a therapeutic agent in human hepatocellular carcinoma | [178] | ||||
| Sinomenine | Rat neuron–glial cultures | Expression of TNF-α, prostaglandin E2, and reactive oxygen species | Potential for treatment of inflammation-mediated neuro-degenerative diseases | [179] | Activation [32,39,43,153,155] | EC50 = 2.16 µM (LAD2 cells, intracellular Ca2+ mobilization assay) [32] EC50 = 1.84 µM (MRGPRX2-HEK293 cells, intracellular Ca2+ mobilization assay) [32] EC50 = 2.77 ± 0.44 µM (MRGPRX2-HEK293 cells, intracellular Ca2+ mobilization assay) [153] EC50 = 2318 ± 314 µM (MrgprB2-HEK293 cells, intracellular Ca2+ mobilization assay) [153] | |
| Rats and mice models | Behavioral tests | Analgesic effect in rodent models | [180] | 123 ± 22 ng/mL (0.00037 µM) [181] | |||
| Human bladder cancer cell line | P-glycoprotein expression | A candidate for treatment of bladder cancer | [182] | ||||
| Mouse model of middle cerebral artery occlusion | Brain edema, neuronal apoptosis, neurological deficiency | A candidate for stroke therapy | [183] | ||||
| Microglial cells | Amyloid β-induced levels of reactive oxygen species and nitric oxide | Potential for treatment of Alzheimer’s diseases | [184] | ||||
| Piperine | Cervical cancer and non-tumoral cell lines | Cell proliferation, viability, and migration | Potential as complementary treatment in cervical cancer | [185] | Inhibition [36,38] | NA | - |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dispenza, M.C.; Metcalfe, D.D.; Olivera, A. Research Advances in Mast Cell Biology and Their Translation into Novel Therapies for Anaphylaxis. J. Allergy Clin. Immunol. Pract. 2023, 11, 2032–2042. [Google Scholar] [CrossRef]
- Jutel, M.; Agache, I.; Zemelka-Wiacek, M.; Akdis, M.; Chivato, T.; del Giacco, S.; Gajdanowicz, P.; Gracia, I.E.; Klimek, L.; Lauerma, A.; et al. Nomenclature of allergic diseases and hypersensitivity reactions: Adapted to modern needs: An EAACI position paper. Allergy 2023, 78, 2851–2874. [Google Scholar] [CrossRef] [PubMed]
- Tatemoto, K.; Nozaki, Y.; Tsuda, R.; Konno, S.; Tomura, K.; Furuno, M.; Ogasawara, H.; Edamura, K.; Takagi, H.; Iwamura, H.; et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem. Biophys. Res. Commun. 2006, 349, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- McNeil, B.D.; Pundir, P.; Meeker, S.; Han, L.; Undem, B.J.; Kulka, M.; Dong, X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015, 519, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Porebski, G.; Kwiecien, K.; Pawica, M.; Kwitniewski, M. Mas-Related G Protein-Coupled Receptor-X2 (MRGPRX2) in Drug Hypersensitivity Reactions. Front. Immunol. 2018, 9, 3027. [Google Scholar] [CrossRef] [PubMed]
- St John, A.L.; Abraham, S.N. Innate immunity and its regulation by mast cells. J. Immunol. 2013, 190, 4458–4463. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, H.; Noguchi, M. Therapeutic Potential of MRGPRX2 Inhibitors on Mast Cells. Cells 2021, 10, 2906. [Google Scholar] [CrossRef]
- Roy, S.; Chompunud Na Ayudhya, C.; Thapaliya, M.; Deepak, V.; Ali, H. Multifaceted MRGPRX2: New insight into the role of mast cells in health and disease. J. Allergy Clin. Immunol. 2021, 148, 293–308. [Google Scholar] [CrossRef]
- Kumar, M.; Duraisamy, K.; Chow, B.K. Unlocking the Non-IgE-Mediated Pseudo-Allergic Reaction Puzzle with Mas-Related G-Protein Coupled Receptor Member X2 (MRGPRX2). Cells 2021, 10, 1033. [Google Scholar] [CrossRef]
- Baldo, B.A. MRGPRX2, drug pseudoallergies, inflammatory diseases, mechanisms and distinguishing MRGPRX2- and IgE/FcεRI-mediated events. Br. J. Clin. Pharmacol. 2023, 89, 3232–3246. [Google Scholar] [CrossRef]
- Quan, P.L.; Sabaté-Brescó, M.; Guo, Y.; Martín, M.; Gastaminza, G. The Multifaceted Mas-Related G Protein-Coupled Receptor Member X2 in Allergic Diseases and Beyond. Int. J. Mol. Sci. 2021, 22, 4421. [Google Scholar] [CrossRef]
- West, P.W.; Bulfone-Paus, S. Mast cell tissue heterogeneity and specificity of immune cell recruitment. Front. Immunol. 2022, 13, 932090. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Wöhrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2020, 58, 342–365. [Google Scholar] [CrossRef]
- Wernersson, S.; Pejler, G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar] [CrossRef]
- Gordon, J.R.; Galli, S.J. Release of both preformed and newly synthesized tumor necrosis factor alpha (TNF-alpha)/cachectin by mouse mast cells stimulated via the Fc epsilon RI. A mechanism for the sustained action of mast cell-derived TNF-alpha during IgE-dependent biological responses. J. Exp. Med. 1991, 174, 103–107. [Google Scholar] [CrossRef]
- Molderings, G.J.; Afrin, L.B. A survey of the currently known mast cell mediators with potential relevance for therapy of mast cell-induced symptoms. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 2881–2891. [Google Scholar] [CrossRef]
- Boyce, J.A. Mast cells and eicosanoid mediators: A system of reciprocal paracrine and autocrine regulation. Immunol. Rev. 2007, 217, 168–185. [Google Scholar] [CrossRef]
- Vliagoftis, H.; Befus, A.D. Rapidly changing perspectives about mast cells at mucosal surfaces. Immunol. Rev. 2005, 206, 190–203. [Google Scholar] [CrossRef]
- Wasiuk, A.; de Vries, V.C.; Hartmann, K.; Roers, A.; Noelle, R.J. Mast cells as regulators of adaptive immunity to tumours. Clin. Exp. Immunol. 2009, 155, 140–146. [Google Scholar] [CrossRef]
- Bischoff, S.C. Role of mast cells in allergic and non-allergic immune responses: Comparison of human and murine data. Nat. Rev. Immunol. 2007, 7, 93–104. [Google Scholar] [CrossRef]
- Oskeritzian, C.A.; Zhao, W.; Min, H.K.; Xia, H.Z.; Pozez, A.; Kiev, J.; Schwartz, L.B. Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J. Allergy Clin. Immunol. 2005, 115, 1162–1168. [Google Scholar] [CrossRef]
- Krishnaswamy, G.; Ajitawi, O.; Chi, D.S. The human mast cell: An overview. Methods Mol. Biol. 2006, 315, 13–34. [Google Scholar] [CrossRef]
- Tauber, M.; Basso, L.; Martin, J.; Bostan, L.; Pinto, M.M.; Thierry, G.R.; Houmadi, R.; Serhan, N.; Loste, A.; Blériot, C.; et al. Landscape of mast cell populations across organs in mice and humans. J. Exp. Med. 2023, 220, e20230570, Erratum in J. Exp. Med. 2024, 221, e2023057001172024c. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, D.; Kashiwakura, J.; Kita, H.; Kikukawa, Y.; Fujitani, Y.; Sasaki-Sakamoto, T.; Kuroda, K.; Nunomura, S.; Hayama, K.; Terui, T.; et al. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J. Allergy Clin. Immunol. 2014, 134, 622–633.e9. [Google Scholar] [CrossRef]
- Pyatilova, P.; Ashry, T.; Luo, Y.; He, J.; Bonnekoh, H.; Jiao, Q.; Moñino-Romero, S.; Hu, M.; Scheffel, J.; Frischbutter, S.; et al. The Number of MRGPRX2-Expressing Cells Is Increased in Skin Lesions of Patients with Indolent Systemic Mastocytosis, but Is Not Linked to Symptom Severity. Front. Immunol. 2022, 13, 930945. [Google Scholar] [CrossRef]
- Manorak, W.; Idahosa, C.; Gupta, K.; Roy, S.; Panettieri, R., Jr.; Ali, H. Upregulation of Mas-related G Protein coupled receptor X2 in asthmatic lung mast cells and its activation by the novel neuropeptide hemokinin-1. Respir. Res. 2018, 19, 1. [Google Scholar] [CrossRef]
- Ray, P.; Torck, A.; Quigley, L.; Wangzhou, A.; Neiman, M.; Rao, C.; Lam, T.; Kim, J.Y.; Kim, T.H.; Zhang, M.Q.; et al. Comparative transcriptome profiling of the human and mouse dorsal root ganglia: An RNA-seq-based resource for pain and sensory neuroscience research. Pain 2018, 159, 1325–1345. [Google Scholar] [CrossRef]
- Kühn, H.; Kolkhir, P.; Babina, M.; Düll, M.; Frischbutter, S.; Fok, J.S.; Jiao, Q.; Metz, M.; Scheffel, J.; Wolf, K.; et al. Mas-related G protein-coupled receptor X2 and its activators in dermatologic allergies. J. Allergy Clin. Immunol. 2021, 147, 456–469. [Google Scholar] [CrossRef]
- Arifuzzaman, M.; Mobley, Y.R.; Choi, H.W.; Bist, P.; Salinas, C.A.; Brown, Z.D.; Chen, S.L.; Staats, H.F.; Abraham, S.N. MRGPR-mediated activation of local mast cells clears cutaneous bacterial infection and protects against reinfection. Sci. Adv. 2019, 5, eaav0216. [Google Scholar] [CrossRef]
- Seldeslachts, A.; Peigneur, S.; Mebs, D.; Tytgat, J. Unraveling the venom chemistry with evidence for histamine as key regulator in the envenomation by caterpillar Automeris zaruma. Front. Immunol. 2022, 13, 972442. [Google Scholar] [CrossRef]
- Wang, L.; Hu, G.Z.; Lu, Y.; Jiang, S.J.; Qi, J.; Su, H. Anti-pseudo-allergic components in licorice extract inhibit mast cell degranulation and calcium influx. Chin. J. Nat. Med. 2022, 20, 421–431. [Google Scholar] [CrossRef]
- Lei, P.; Liu, Y.; Ding, Y.; Su, X.; Liang, J.; Chen, H.; Ma, W. Thebaine induces anaphylactic reactions via the MRGPRX2 receptor pathway on mast cells. Cell. Immunol. 2022, 375, 104514. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Che, D.; Zeng, Y.; Wu, Y.; Qin, Q.; Wang, N. Baicalin induces Mrgprb2-dependent pseudo-allergy in mice. Immunol. Lett. 2020, 226, 55–61. [Google Scholar] [CrossRef]
- Callahan, B.N.; Kammala, A.K.; Syed, M.; Yang, C.; Occhiuto, C.J.; Nellutla, R.; Chumanevich, A.P.; Oskeritzian, C.A.; Das, R.; Subramanian, H. Osthole, a Natural Plant Derivative Inhibits MRGPRX2 Induced Mast Cell Responses. Front. Immunol. 2020, 11, 703. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, Y.; Wang, J.; Zhang, Y.; Hou, Y.; Qin, Q.; Ma, W.; Wang, N. Discovery and analysis the anti-pseudo-allergic components from Perilla frutescens leaves by overexpressed MRGPRX2 cell membrane chromatography coupled with HPLC-ESI-IT-TOF system. J. Pharm. Pharmacol. 2020, 72, 852–862. [Google Scholar] [CrossRef]
- Qiao, C.; Hu, S.; Che, D.; Wang, J.; Gao, J.; Ma, R.; Jiang, W.; Zhang, T.; Liu, R. The anti-anaphylactoid effects of Piperine through regulating MAS-related G protein-coupled receptor X2 activation. Phytother. Res. 2020, 34, 1409–1420. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, C.; Hou, Y.; Sun, W.; Che, D.; Yang, L.; Zhang, T.; Sun, M.; He, H.; He, L. Simultaneous identification of three pseudoallergic components in Danshen injection by using high-expression Mas-related G protein coupled receptor X2 cell membrane chromatography coupled online to HPLC-ESI-MS/MS. J. Sep. Sci. 2018, 41, 2488–2497. [Google Scholar] [CrossRef]
- Han, S.; Lv, Y.; Kong, L.; Sun, Y.; Fu, J.; Li, L.; He, L. Simultaneous identification of the anaphylactoid components from traditional Chinese medicine injections using rat basophilic leukemia 2H3 and laboratory of allergic disease 2 dual-mixed/cell membrane chromatography model. Electrophoresis 2018, 39, 1181–1189. [Google Scholar] [CrossRef]
- Lansu, K.; Karpiak, J.; Liu, J.; Huang, X.P.; McCorvy, J.D.; Kroeze, W.K.; Che, T.; Nagase, H.; Carroll, F.I.; Jin, J.; et al. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat. Chem. Biol. 2017, 13, 529–536. [Google Scholar] [CrossRef]
- Adhikari, N.; Shim, W.S. Caffeic acid phenethyl ester inhibits pseudo-allergic reactions via inhibition of MRGPRX2/MrgprB2-dependent mast cell degranulation. Arch. Pharm. Res. 2022, 45, 644–657. [Google Scholar] [CrossRef]
- Wang, L.; Huang, C.; Li, Z.; Hu, G.; Qi, J.; Fan, Z. Liquiritin inhibits MRGPRX2-mediated pseudo-allergy through the PI3K/AKT and PLCγ signaling pathways. Heliyon 2023, 9, e13290. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Dang, B.; Hu, S.; Zhao, C.; Wang, Y.; Yuan, Y.; Liu, R. Fisetin alleviates chronic urticaria by inhibiting mast cell activation via MRGPRX2. J. Pharm. Pharmacol. 2023, 75, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Fu, J.; Gao, C.; Wang, H.; Wang, S.; Liang, P.; Han, S.; Lv, Y.; He, L. MrgX2-SNAP-tag/cell membrane chromatography model coupled with liquid chromatography-mass spectrometry for anti-pseudo-allergic compound screening in Arnebiae Radix. Anal. Bioanal. Chem. 2022, 414, 5741–5753. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hu, S.; Ding, Y.; Wang, J.; Wang, Y.; Gao, J.; He, L. Dictamnine is an effective anti-anaphylactoid compound acting via the MrgX2 receptor located on mast cells. Phytother. Res. 2021, 35, 3181–3193. [Google Scholar] [CrossRef]
- Sun, W.; Wang, S.; Liang, P.; Zhou, H.; Zhang, L.; Jia, Q.; Fu, J.; Lv, Y.; Han, S. Pseudo-allergic compounds screened from Shengmai injection by using high-expression Mas-related G protein-coupled receptor X2 cell membrane chromatography online coupled with liquid chromatography and mass spectrometry. J. Sep. Sci. 2021, 44, 1421–1429. [Google Scholar] [CrossRef]
- Wang, N.; Wang, J.; Zhang, Y.; Zeng, Y.; Hu, S.; Bai, H.; Hou, Y.; Wang, C.; He, H.; He, L. Imperatorin ameliorates mast cell-mediated allergic airway inflammation by inhibiting MRGPRX2 and CamKII/ERK signaling pathway. Biochem. Pharmacol. 2021, 184, 114401. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Jia, Q.; Sun, W.; Fu, J.; Lv, Y.; Han, S. Cell membrane chromatography coupled online with LC-MS to screen anti-anaphylactoid components from Magnolia biondii Pamp. targeting on Mas-related G protein-coupled receptor X2. J. Sep. Sci. 2020, 43, 2571–2578. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Wang, J.; Liu, R.; Zhang, G.; Dong, K.; Zhang, T. Paeoniflorin inhibits MRGPRX2-mediated pseudo-allergic reaction via calcium signaling pathway. Phytother. Res. 2020, 34, 401–408. [Google Scholar] [CrossRef]
- Jia, Q.; Sun, W.; Zhang, L.; Fu, J.; Lv, Y.; Lin, Y.; Han, S. Screening the anti-allergic components in Saposhnikoviae Radix using high-expression Mas-related G protein-coupled receptor X2 cell membrane chromatography online coupled with liquid chromatography and mass spectrometry. J. Sep. Sci. 2019, 42, 2351–2359. [Google Scholar] [CrossRef]
- Ding, Y.; Che, D.; Li, C.; Cao, J.; Wang, J.; Ma, P.; Zhao, T.; An, H.; Zhang, T. Quercetin inhibits Mrgprx2-induced pseudo-allergic reaction via PLCγ-IP3R related Ca2+ fluctuations. Int. Immunopharmacol. 2019, 66, 185–197. [Google Scholar] [CrossRef]
- Lin, Y.; Lv, Y.; Fu, J.; Jia, Q.; Han, S. A high expression Mas-related G protein coupled receptor X2 cell membrane chromatography coupled with liquid chromatography and mass spectrometry method for screening potential anaphylactoid components in kudiezi injection. J. Pharm. Biomed. Anal. 2018, 159, 483–489. [Google Scholar] [CrossRef]
- Hou, Y.; Che, D.; Ma, P.; Zhao, T.; Zeng, Y.; Wang, N. Anti-pseudo-allergy effect of isoliquiritigenin is MRGPRX2-dependent. Immunol. Lett. 2018, 198, 52–59. [Google Scholar] [CrossRef]
- Wang, N.; Che, D.; Zhang, T.; Liu, R.; Cao, J.; Wang, J.; Zhao, T.; Ma, P.; Dong, X.; He, L. Saikosaponin A inhibits compound 48/80-induced pseudo-allergy via the Mrgprx2 pathway in vitro and in vivo. Biochem. Pharmacol. 2018, 148, 147–154. [Google Scholar] [CrossRef]
- Johnson, T.; Siegel, D. Complanadine A, a selective agonist for the Mas-related G protein-coupled receptor X2. Bioorg. Med. Chem. Lett. 2014, 24, 3512–3515. [Google Scholar] [CrossRef]
- Yao, C.; Ye, W.; Chen, M. Inhibition of Mast Cell Degranulation in Atopic Dermatitis by Celastrol through Suppressing MRGPRX2. Dis. Markers 2023, 2023, 9049256. [Google Scholar] [CrossRef]
- Ye, F.; Jiang, Y.; Zhang, J.; Zong, Y.; Yu, M.; Chen, C.; Zhu, C.; Yang, Y.; Jia, K.; Chen, G.; et al. Water Extract of Senecio scandens Buch.Ham Ameliorates Pruritus by Inhibiting MrgprB2 Receptor. J. Inflamm. Res. 2022, 15, 5989–5998. [Google Scholar] [CrossRef]
- Jiang, Y.; Zong, Y.; Du, Y.; Zhang, M.; Ye, F.; Zhang, J.; Yang, Y.; Zhu, C.; Tang, Z. Curcumin inhibits the pruritus in mice through mast cell MrgprB2 receptor. Inflamm. Res. 2023, 72, 933–945. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; Hu, S.; Ding, Y.; Jia, Q.; Zhu, J.; An, H. Kaempferol ameliorates secretagogue-induced pseudo-allergic reactions via inhibiting intracellular calcium fluctuation. J. Pharm. Pharmacol. 2020, 72, 1221–1231. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, T.; Che, D.; Cao, J.; Wang, J.; Lv, Y.; Ma, P.; Ding, Y.; Wang, N.; Wang, X.; et al. The anti-anaphylactoid effects of hydroxysafflor yellow A on the suppression of mast cell Ca2+ influx and degranulation. Phytomedicine 2018, 48, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Roth, B.L. The structure, function, and pharmacology of MRGPRs. Trends Pharmacol. Sci. 2023, 44, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Subramanian, H.; Klos, A.; Ali, H. Phosphorylation of C3a receptor at multiple sites mediates desensitization, β-arrestin-2 recruitment and inhibition of NF-κB activity in mast cells. PLoS ONE 2012, 7, e46369. [Google Scholar] [CrossRef]
- Cahill, T.J., 3rd; Thomsen, A.R.; Tarrasch, J.T.; Plouffe, B.; Nguyen, A.H.; Yang, F.; Huang, L.Y.; Kahsai, A.W.; Bassoni, D.L.; Gavino, B.J.; et al. Distinct conformations of GPCR-β-arrestin complexes mediate desensitization, signaling, and endocytosis. Proc. Natl. Acad. Sci. USA 2017, 114, 2562–2567. [Google Scholar] [CrossRef]
- Mi, Y.N.; Ping, N.N.; Cao, Y.X. Ligands and Signaling of Mas-Related G Protein-Coupled Receptor-X2 in Mast Cell Activation. Rev. Physiol. Biochem. Pharmacol. 2021, 179, 139–188. [Google Scholar] [CrossRef]
- Chompunud Na Ayudhya, C.; Roy, S.; Alkanfari, I.; Ganguly, A.; Ali, H. Identification of Gain and Loss of Function Missense Variants in MRGPRX2’s Transmembrane and Intracellular Domains for Mast Cell Activation by Substance P. Int. J. Mol. Sci. 2019, 20, 5247. [Google Scholar] [CrossRef]
- Venkatakrishnan, A.J.; Deupi, X.; Lebon, G.; Heydenreich, F.M.; Flock, T.; Miljus, T.; Balaji, S.; Bouvier, M.; Veprintsev, D.B.; Tate, C.G.; et al. Diverse activation pathways in class A GPCRs converge near the G-protein-coupling region. Nature 2016, 536, 484–487. [Google Scholar] [CrossRef]
- Chompunud Na Ayudhya, C.; Amponnawarat, A.; Ali, H. Substance P Serves as a Balanced Agonist for MRGPRX2 and a Single Tyrosine Residue Is Required for β-Arrestin Recruitment and Receptor Internalization. Int. J. Mol. Sci. 2021, 22, 5318. [Google Scholar] [CrossRef]
- Murakami, T.; Suzuki, K.; Niyonsaba, F.; Tada, H.; Reich, J.; Tamura, H.; Nagaoka, I. MrgX2-mediated internalization of LL-37 and degranulation of human LAD2 mast cells. Mol. Med. Rep. 2018, 18, 4951–4959. [Google Scholar] [CrossRef]
- Song, J.; Xian, D.; Yang, L.; Xiong, X.; Lai, R.; Zhong, J. Pruritus: Progress toward Pathogenesis and Treatment. Biomed. Res. Int. 2018, 2018, 9625936. [Google Scholar] [CrossRef]
- Cao, C.; Kang, H.J.; Singh, I.; Chen, H.; Zhang, C.; Ye, W.; Hayes, B.W.; Liu, J.; Gumpper, R.H.; Bender, B.J.; et al. Structure, function and pharmacology of human itch GPCRs. Nature 2021, 600, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Meixiong, J.; Anderson, M.; Limjunyawong, N.; Sabbagh, M.F.; Hu, E.; Mack, M.R.; Oetjen, L.K.; Wang, F.; Kim, B.S.; Dong, X. Activation of Mast-Cell-Expressed Mas-Related G-Protein-Coupled Receptors Drives Non-histaminergic Itch. Immunity 2019, 50, 1163–1171.e5. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; Pyatilova, P.; Ashry, T.; Jiao, Q.; Abad-Perez, A.T.; Altrichter, S.; Vera Ayala, C.E.; Church, M.K.; He, J.; Lohse, K.; et al. Mast cells, cortistatin, and its receptor, MRGPRX2, are linked to the pathogenesis of chronic prurigo. J. Allergy Clin. Immunol. 2022, 149, 1998–2009.e5. [Google Scholar] [CrossRef]
- Ding, Y.; Ma, T.; Zhang, Y.; Zhao, C.; Wang, C.; Wang, Z. Rosmarinic acid ameliorates skin inflammation and pruritus in allergic contact dermatitis by inhibiting mast cell-mediated MRGPRX2/PLCγ1 signaling pathway. Int. Immunopharmacol. 2023, 117, 110003. [Google Scholar] [CrossRef]
- Kabashima, K.; Irie, H. Interleukin-31 as a Clinical Target for Pruritus Treatment. Front. Med. 2021, 8, 638325. [Google Scholar] [CrossRef]
- Zheng, T.; Oh, M.H.; Oh, S.Y.; Schroeder, J.T.; Glick, A.B.; Zhu, Z. Transgenic expression of interleukin-13 in the skin induces a pruritic dermatitis and skin remodeling. J. Investig. Dermatol. 2009, 129, 742–751. [Google Scholar] [CrossRef]
- Facheris, P.; Jeffery, J.; Del Duca, E.; Guttman-Yassky, E. The translational revolution in atopic dermatitis: The paradigm shift from pathogenesis to treatment. Cell. Mol. Immunol. 2023, 20, 448–474. [Google Scholar] [CrossRef]
- Nassau, S.; Fonacier, L. Allergic Contact Dermatitis. Med. Clin. N. Am. 2020, 104, 61–76. [Google Scholar] [CrossRef]
- Zuberbier, T.; Abdul Latiff, A.H.; Abuzakouk, M.; Aquilina, S.; Asero, R.; Baker, D.; Ballmer-Weber, B.; Bangert, C.; Ben-Shoshan, M.; Bernstein, J.A.; et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy 2022, 77, 734–766. [Google Scholar] [CrossRef]
- Greiwe, J.; Bernstein, J.A. Therapy of antihistamine-resistant chronic spontaneous urticaria. Expert. Rev. Clin. Immunol. 2017, 13, 311–318. [Google Scholar] [CrossRef]
- Li, L.; Liu, R.; Peng, C.; Chen, X.; Li, J. Pharmacogenomics for the efficacy and side effects of antihistamines. Exp. Dermatol. 2022, 31, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Karalliedde, L.D.; Kappagoda, C.T. The challenge of traditional Chinese medicines for allopathic practitioners. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1967–H1969. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.H.L.; Ng, T. Traditional Chinese Medicine (TCM) and Allergic Diseases. Curr. Allergy Asthma Rep. 2020, 20, 67. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Chen, G.; Xian, Y.; Zhang, H.; Wu, Y.; Yang, Y.; Wu, J.; Wang, C.; He, S.; et al. Traditional Chinese medicine compound (Tongxinluo) and clinical outcomes of patients with acute myocardial infarction: The CTS-AMI randomized clinical trial. JAMA 2023, 330, 1534–1545. [Google Scholar] [CrossRef]
- Sackett, D.L.; Rosenberg, W.M.C.; Gray, J.A.M.; Haynes, R.B.; Richardson, W.S. Evidence based medicine: What it is and what it isn’t. Br. Med. J. 1996, 312, 71–72. [Google Scholar] [CrossRef]
- Fung, F.Y.; Linn, Y.C. Developing traditional chinese medicine in the era of evidence-based medicine: Current evidences and challenges. Evid.-Based Complement. Alternat. Med. 2015, 2015, 425037. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.W.T.; Poon, S.L.; Huang, M.N.; Lim, J.Q.; Boot, A.; Yu, W.; Suzuki, Y.; Thangaraju, S.; Ng, C.C.Y.; Tan, P.; et al. Aristolochic acids and their derivatives are widely implicated in liver cancers in Taiwan and throughout Asia. Sci. Transl. Med. 2017, 9, eaan6446. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Chen, R.; Hu, D.; Li, W.; Li, X.; Chen, X.; Huang, B. The quality of reports of randomized clinical trials on traditional Chinese medicine treatments: A systematic review of articles indexed in the China National Knowledge Infrastructure database from 2005 to 2012. BMC Complement. Altern. Med. 2014, 14, 362. [Google Scholar] [CrossRef]
- Cathcart, M.C.; Useckaite, Z.; Drakeford, C.; Semik, V.; Lysaght, J.; Gately, K.; O’Byrne, K.J.; Pidgeon, G.P. Anti-cancer effects of baicalein in non-small cell lung cancer in-vitro and in-vivo. BMC Cancer 2016, 16, 707. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Zhang, Y. Osthole inhibits gastric cancer cell proliferation through regulation of PI3K/AKT. PLoS ONE 2018, 13, e0193449. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.H.; Manandhar, S.; Choudhary, S.S.; Priya, K.; Gujaran, T.V.; Mehta, C.H.; Nayak, U.Y.; Pai, K.S.R. Identification of phytochemical as a dual inhibitor of PI3K and mTOR: A structure-based computational approach. Mol. Divers. 2023, 27, 2015–2036. [Google Scholar] [CrossRef]
- Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. Available online: https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm (accessed on 29 February 2024).
- European Medicined Agency. Available online: https://www.ema.europa.eu/en/medicines (accessed on 29 February 2024).
- Fan, H.; Gao, Z.; Ji, K.; Li, X.; Wu, J.; Liu, Y.; Wang, X.; Liang, H.; Liu, Y.; Li, X.; et al. The in vitro and in vivo anti-inflammatory effect of osthole, the major natural coumarin from Cnidium monnieri (L.) Cuss, via the blocking of the activation of the NF-κB and MAPK/p38 pathways. Phytomedicine 2019, 58, 152864. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Gong, Q.; Wang, L.; Shi, J. Osthole attenuates focal inflammatory reaction following permanent middle cerebral artery occlusion in rats. Biol. Pharm. Bull. 2012, 35, 1686–1690. [Google Scholar] [CrossRef]
- Wang, J.; Fu, Y.; Wei, Z.; He, X.; Shi, M.; Kou, J.; Zhou, E.; Liu, W.; Yang, Z.; Guo, C. Anti-asthmatic activity of osthole in an ovalbumin-induced asthma murine model. Respir. Physiol. Neurobiol. 2017, 239, 64–69. [Google Scholar] [CrossRef]
- Chiang, C.Y.; Lee, C.C.; Fan, C.K.; Huang, H.M.; Chiang, B.L.; Lee, Y.L. Osthole treatment ameliorates Th2-mediated allergic asthma and exerts immunomodulatory effects on dendritic cell maturation and function. Cell. Mol. Immunol. 2017, 14, 935–947. [Google Scholar] [CrossRef]
- Matsuda, H.; Tomohiro, N.; Ido, Y.; Kubo, M. Anti-allergic effects of cnidii monnieri fructus (dried fruits of Cnidium monnieri) and its major component, osthol. Biol. Pharm. Bull. 2002, 25, 809–812. [Google Scholar] [CrossRef]
- Fu, X.; Hong, C. Osthole attenuates mouse atopic dermatitis by inhibiting thymic stromal lymphopoietin production from keratinocytes. Exp. Dermatol. 2019, 28, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Hada, Y.; Uchida, H.A.; Wada, J. Fisetin Attenuates Lipopolysaccharide-Induced Inflammatory Responses in Macrophage. Biomed. Res. Int. 2021, 2021, 5570885. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.R.; Park, H.J. Antiallergic effect of fisetin on IgE-mediated mast cell activation in vitro and on passive cutaneous anaphylaxis (PCA). J. Nutr. Biochem. 2017, 48, 103–111. [Google Scholar] [CrossRef]
- Huajuan, J.; Xulong, H.; Bin, X.; Yue, W.; Yongfeng, Z.; Chaoxiang, R.; Jin, P. Chinese herbal injection for cardio-cerebrovascular disease: Overview and challenges. Front. Pharmacol. 2023, 14, 1038906. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Li, M.; Chen, F.; Chen, L.; Jiang, Z.; Zhao, L. The Efficacy of Danshen Injection as Adjunctive Therapy in Treating Angina Pectoris: A Systematic Review and Meta-Analysis. Heart Lung Circ. 2018, 27, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liu, L.; Qi, C.; Deng, B.; Cai, X. Sinomenine versus NSAIDs for the treatment of rheumatoid arthritis: A systematic review and meta-analysis. Planta Med. 2008, 74, 1423–1429. [Google Scholar] [CrossRef]
- Bajek-Bil, A.; Chmiel, M.; Włoch, A.; Stompor-Gorący, M. Baicalin-Current Trends in Detection Methods and Health-Promoting Properties. Pharmaceuticals 2023, 16, 570. [Google Scholar] [CrossRef]
- Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, M.F.; Kandasamy, G.; Vasudevan, R.; Ali, M.S.; Amir, M. Glycyrrhiza glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology. Plants 2021, 10, 2751. [Google Scholar] [CrossRef]
- Sarkhail, P.; Shafiee, A.; Sarkheil, P. Biological activities and pharmacokinetics of praeruptorins from Peucedanum species: A systematic review. Biomed Res. Int. 2013, 2013, 343808. [Google Scholar] [CrossRef]
- Meghwal, M.; Goswami, T.K. Piper nigrum and piperine: An update. Phytother. Res. 2013, 27, 1121–1130. [Google Scholar] [CrossRef]
- Yu, H.; Qiu, J.F.; Ma, L.J.; Hu, Y.J.; Li, P.; Wan, J.B. Phytochemical and phytopharmacological review of Perilla frutescens L. (Labiatae), a traditional edible-medicinal herb in China. Food Chem. Toxicol. 2017, 108 Pt B, 375–391. [Google Scholar] [CrossRef]
- Wollam, J.; Solomon, M.; Villescaz, C.; Anderson, S.; Freeman, D.; Vasquez, A.; Pisacane, C.; Vest, A.; Napora, J.; Charlot, B.; et al. MRGPRX2 small molecule antagonists potently inhibit agonist-induced skin mast cell degranulation. Allergy 2023, 78, 204. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Nationial Library of Medicine. MEDLINE. Available online: https://clinicaltrials.gov (accessed on 28 February 2024).
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef] [PubMed]
- Vaisman, N.; Niv, E. Daily consumption of red grape cell powder in a dietary dose improves cardiovascular parameters: A double blind, placebo-controlled, randomized study. Int. J. Food Sci. Nutr. 2015, 66, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Matsumae, T.; Kataoka, T.; Yazaki, Y.; Yamaguchi, H. Effect of acacia polyphenol on glucose homeostasis in subjects with impaired glucose tolerance: A randomized multicenter feeding trial. Exp. Ther. Med. 2013, 5, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, M.; Chaiyakunapruk, N.; Jacob, S.A.; Palanisamy, U.D. Prebiotic potential of polyphenols, its effect on gut microbiota and anthropometric/clinical markers: A systematic review of randomised controlled trials. Trends Food Sci. Technol. 2020, 99, 634–649. [Google Scholar] [CrossRef]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Wang, P.; Said, J.W.; Huang, M.; Grogan, T.; Elashoff, D.; Carpenter, C.L.; Heber, D.; Aronson, W.J. Randomized clinical trial of brewed green and black tea in men with prostate cancer prior to prostatectomy. Prostate 2015, 75, 550–559. [Google Scholar] [CrossRef] [PubMed]
- González Arbeláez, L.F.; Ciocci Pardo, A.; Fantinelli, J.C.; Schinella, G.R.; Mosca, S.M.; Ríos, J.L. Cardioprotection and natural polyphenols: An update of clinical and experimental studies. Food Funct. 2018, 9, 6129–6145. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Valderas-Martinez, P.; Casas, R.; Arranz, S.; Guillén, M.; Lamuela-Raventós, R.M.; Llorach, R.; Andres-Lacueva, C.; et al. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: A randomized clinical trial. Clin. Nutr. 2013, 32, 200–206. [Google Scholar] [CrossRef]
- Cao, H.; Ou, J.; Chen, L.; Zhang, Y.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J. Dietary polyphenols and type 2 diabetes: Human Study and Clinical Trial. Crit. Rev. Food Sci. Nutr. 2019, 59, 3371–3379. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, J.; Hou, Y.; Fu, J.; Wei, D.; Jia, Q.; Lv, Y.; Wang, C.; Han, S.; He, L. Isosalvianolic acid C-induced pseudo-allergic reactions via the mast cell specific receptor MRGPRX2. Int. Immunopharmacol. 2019, 71, 22–31. [Google Scholar] [CrossRef]
- Zhu, C.; Cao, H.; Zhou, X.; Dong, C.; Luo, J.; Zhang, C.; Liu, J.; Ling, Y. Meta-analysis of the clinical value of Danshen injection and Huangqi injection in liver cirrhosis. Evid.-Based Complement. Alternat. Med. 2013, 2013, 842824. [Google Scholar] [CrossRef]
- Guo, S.; Wu, J.; Ni, M.; Jia, S.; Zhang, J.; Zhou, W.; Liu, X.; Wang, M.; Zhang, X. Comparative Efficacy of Danshen Class Injections for Treating Acute Coronary Syndrome: A Multidimensional Bayesian Network Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2020, 11, 1260. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, Q.; Marshall, G.; Cui, X.; Cheng, L.; Li, Y.; Shang, H.; Zhang, B.; Li, Y. Adverse drug reactions and adverse events of 33 varieties of traditional Chinese medicine injections on National Essential medicines List (2004 edition) of China: An overview on published literatures. J. Evid.-Based Med. 2010, 3, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Wang, D.W.; Meng, L.; Wang, Y.Q. Analysis of anaphylactic shock caused by 17 types of traditional Chinese medicine injections used to treat cardiovascular and cerebrovascular diseases. Biomed. Res. Int. 2015, 2015, 420607. [Google Scholar] [CrossRef][Green Version]
- Xu, J.; Zeng, S.; Chen, X.; Qu, H. Isolation and identification of degradation products of salvianolic acid A by NMR and LC-MS. Fitoterapia 2011, 82, 260–266. [Google Scholar] [CrossRef]
- Mahalakshmi, B.; Huang, C.Y.; Lee, S.D.; Maurya, N.; Kiefer, R.; Kumar, B.V. Review of Danshen: From its metabolism to possible mechanisms of its biological activities. J. Funct. Foods 2021, 85, 104613. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, H.-J.; Wang, X.-W. One case of Salvia miltiorrhiza injection intravenous drip too fast induced allergic reaction. Nurs. Pract. Res. 2008, 21, 46. [Google Scholar]
- Jiang, H.-Q.; Chen, S.-Y. One case of high concentration Salvia miltiorrhiza injection induced hypovolemic shock. Strait Pharm. J. 2008, 12, 102. [Google Scholar]
- Takaki, I.; Bersani-Amado, L.E.; Vendruscolo, A.; Sartoretto, S.M.; Diniz, S.P.; Bersani-Amado, C.A.; Cuman, R.K. Anti-inflammatory and antinociceptive effects of Rosmarinus officinalis L. essential oil in experimental animal models. J. Med. Food 2008, 11, 741–746. [Google Scholar] [CrossRef] [PubMed]
- González-Trujano, M.E.; Peña, E.I.; Martínez, A.L.; Moreno, J.; Guevara-Fefer, P.; Déciga-Campos, M.; López-Muñoz, F.J. Evaluation of the antinociceptive effect of Rosmarinus officinalis L. using three different experimental models in rodents. J. Ethnopharmacol. 2007, 111, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.P.; Jin, G.J.; Xiong, Y.; Hu, P.F.; Bao, J.P.; Wu, L.D. Rosmarinic acid down-regulates NO and PGE2 expression via MAPK pathway in rat chondrocytes. J. Cell. Mol. Med. 2018, 22, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Dang, B.; Zhang, Y.; Hu, S.; Wang, Y.; Zhao, C.; Zhang, T.; Gao, Z. Paeonol attenuates Substance P-induced urticaria by inhibiting Src kinase phosphorylation in mast cells. Cell. Immunol. 2023, 388–389, 104728. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lv, Y.; Kong, L.; Che, D.; Liu, R.; Fu, J.; Cao, J.; Wang, J.; Wang, C.; He, H.; et al. Use of the relative release index for histamine in LAD2 cells to evaluate the potential anaphylactoid effects of drugs. Sci. Rep. 2017, 7, 13714. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, Y.; Zeng, Y.; Hu, S.; Bai, H.; Wang, J.; Jing, H.; Wang, N. Licochalcone A inhibits MAS-related GPR family member X2-induced pseudo-allergic reaction by suppressing nuclear migration of nuclear factor-κB. Phytother. Res. 2021, 35, 6270–6280. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, C.; Wang, P.; Ding, N.; Gao, J.; Zhang, Y.; Ma, Z.; Lei, H.; Li, Q.; Jian, L.; et al. Baicalin and rutin are major constituents in Shuanghuanglian injection involving anaphylactoid reaction. J. Tradit. Chin. Med. 2017, 37, 412–420. [Google Scholar]
- Tan, L.; Li, M.; Lin, Y. Safety Concerns of Traditional Chinese Medicine Injections Used in Chinese Children. Evid.-Based Complement. Alternat. Med. 2019, 2019, 8310368. [Google Scholar] [CrossRef]
- Gao, Y.; Hou, R.; Han, Y.; Fei, Q.; Cai, R.; Qi, Y. Shuang-Huang-Lian injection induces an immediate hypersensitivity reaction via C5a but not IgE. Sci. Rep. 2018, 8, 3572. [Google Scholar] [CrossRef]
- Cheng, M.; Zhang, J.; Yang, L.; Shen, S.; Li, P.; Yao, S.; Qu, H.; Li, J.; Yao, C.; Wei, W.; et al. Recent advances in chemical analysis of licorice (Gan-Cao). Fitoterapia 2021, 149, 104803. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Sak, K.; Tuli, H.S.; Buttar, H.S.; Bishayee, A. Fisetin: A bioactive phytochemical with potential for cancer prevention and pharmacotherapy. Life Sci. 2018, 194, 75–87. [Google Scholar] [CrossRef]
- Terahara, N. Flavonoids in foods: A review. Nat. Prod. Commun. 2015, 10, 521–528. [Google Scholar] [CrossRef]
- Ren, Q.; Guo, F.; Tao, S.; Huang, R.; Ma, L.; Fu, P. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways in septic AKI mice. Biomed. Pharmacother. 2020, 122, 109772. [Google Scholar] [CrossRef]
- Adhami, V.M.; Syed, D.N.; Khan, N.; Mukhtar, H. Dietary flavonoid fisetin: A novel dual inhibitor of PI3K/Akt and mTOR for prostate cancer management. Biochem. Pharmacol. 2012, 84, 1277–1281. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Esnault, S.; Adhami, V.M.; Noll, A.L.; Banang-Mbeumi, S.; Roy, T.; Singh, S.S.; Huang, S.; Kousoulas, K.G.; Mukhtar, H. Fisetin, a 3,7,3’,4’-Tetrahydroxyflavone Inhibits the PI3K/Akt/mTOR and MAPK Pathways and Ameliorates Psoriasis Pathology in 2D and 3D Organotypic Human Inflammatory Skin Models. Cells 2019, 8, 1089. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Cruz-Martins, N.; López-Jornet, P.; Lopez, E.P.; Harun, N.; Yeskaliyeva, B.; Beyatli, A.; Sytar, O.; Shaheen, S.; Sharopov, F.; et al. Natural Coumarins: Exploring the Pharmacological Complexity and Underlying Molecular Mechanisms. Oxid. Med. Cell. Longev. 2021, 2021, 6492346. [Google Scholar] [CrossRef]
- Torres, R.; Faini, F.; Modak, B.; Urbina, F.; Labbé, C.; Guerrero, J. Antioxidant activity of coumarins and flavonols from the resinous exudate of Haplopappus multifolius. Phytochemistry 2006, 67, 984–987. [Google Scholar] [CrossRef]
- Kirsch, G.; Abdelwahab, A.B.; Chaimbault, P. Natural and Synthetic Coumarins with Effects on Inflammation. Molecules 2016, 21, 1322. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Kadhum, A.A.; Mohamad, A.B. Antifungal activities of new coumarins. Molecules 2012, 17, 5713–5723. [Google Scholar] [CrossRef]
- Warhi, T.; Sabt, A.; Elkaeed, E.B.; Eldehna, W.M. Recent advancements of coumarin-based anticancer agents: An up-to-date review. Bioorg. Chem. 2020, 103, 104163. [Google Scholar] [CrossRef]
- Bubols, G.B.; Vianna, D.D.R.; Medina-Remon, A.; von Poser, G.; Maria Lamuela-Raventos, R.; Lucia Eifler-Lima, V.; Cristina Garcia, S. The antioxidant activity of coumarins and flavonoids. Mini Rev. Med. Chem. 2013, 13, 318–334. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L. Progress in the Chemistry of Naturally Occurring Coumarins. Prog. Chem. Org. Nat. Prod. 2017, 106, 241–304. [Google Scholar] [CrossRef]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in Chemical Structures and Biological Properties of Plant Alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef]
- Liu, R.; Che, D.; Zhao, T.; Pundir, P.; Cao, J.; Lv, Y.; Wang, J.; Ma, P.; Fu, J.; Wang, N.; et al. MRGPRX2 is essential for sinomenine hydrochloride induced anaphylactoid reactions. Biochem. Pharmacol. 2017, 146, 214–223. [Google Scholar] [CrossRef]
- Chen, D.P.; Wong, C.K.; Leung, P.C.; Fung, K.P.; Lau, C.B.; Lau, C.P.; Li, E.K.; Tam, L.S.; Lam, C.W. Anti-inflammatory activities of Chinese herbal medicine sinomenine and Liang Miao San on tumor necrosis factor-α-activated human fibroblast-like synoviocytes in rheumatoid arthritis. J. Ethnopharmacol. 2011, 137, 457–468. [Google Scholar] [CrossRef]
- Wei, D.; Hu, T.; Hou, Y.J.; Wang, X.J.; Lu, J.Y.; Ge, S.; Wang, C.; He, H.Z. MRGPRX2 is critical for clozapine induced pseudo-allergic reactions. Immunopharmacol. Immunotoxicol. 2021, 43, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, R.; Che, D.; Pundir, P.; Wang, N.; Han, S.; Cao, J.; Lv, Y.; Dong, H.; Fang, F.; et al. A Mast Cell–Specific Receptor Is Critical for Granuloma Induced by Intrathecal Morphine Infusion. J. Immunol. 2019, 203, 1701–1714. [Google Scholar] [CrossRef]
- Akuzawa, N.; Obinata, H.; Izumi, T.; Takeda, S. Morphine Is an Exogenous Ligand for MrgX2, a G Protein-Coupled Receptor for Cortistatin. J. Cell Anim. Biol. 2009, 3, 216–221. [Google Scholar]
- Song, J.; Zhang, W.; Sun, J.; Zhang, X.; Xu, X.; Zhang, L.; Feng, Z.; Du, G. Determination of salvianolic acid C in rat plasma using liquid chromatography-mass spectrometry and its application to pharmacokinetic study. Biomed. Chromatogr. 2016, 30, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Rong, H.; Liang, Y.; Niu, Y. Rosmarinic acid attenuates β-amyloid-induced oxidative stress via Akt/GSK-3β/Fyn-mediated Nrf2 activation in PC12 cells. Free Radic. Biol. Med. 2018, 120, 114–123. [Google Scholar] [CrossRef]
- Hitl, M.; Kladar, N.; Gavarić, N.; Božin, B. Rosmarinic Acid-Human Pharmacokinetics and Health Benefits. Planta Med. 2021, 87, 273–282. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Z.G.; Yuan, Y.P.; Xu, S.C.; Wei, W.Y.; Song, P.; Kong, C.Y.; Deng, W.; Tang, Q.Z. Rosmarinic acid attenuates cardiac fibrosis following long-term pressure overload via AMPKα/Smad3 signaling. Cell Death Dis. 2018, 9, 102. [Google Scholar] [CrossRef]
- Zhang, K.; Pan, X.; Wang, F.; Ma, J.; Su, G.; Dong, Y.; Yang, J.; Wu, C. Baicalin promotes hippocampal neurogenesis via SGK1- and FKBP5-mediated glucocorticoid receptor phosphorylation in a neuroendocrine mouse model of anxiety/depression. Sci. Rep. 2016, 6, 30951. [Google Scholar] [CrossRef]
- Sun, J.Y.; Li, D.L.; Dong, Y.; Zhu, C.H.; Liu, J.; Li, J.D.; Zhou, T.; Gou, J.Z.; Li, A.; Zang, W.J. Baicalin inhibits toll-like receptor 2/4 expression and downstream signaling in rat experimental periodontitis. Int. Immunopharmacol. 2016, 36, 86–93. [Google Scholar] [CrossRef]
- Yang, X.; Dang, X.; Zhang, X.; Zhao, S. Liquiritin reduces lipopolysaccharide-aroused HaCaT cell inflammation damage via regulation of microRNA-31/MyD88. Int. Immunopharmacol. 2021, 101 Pt B, 108283. [Google Scholar] [CrossRef]
- Ni, H.; Xu, M.; Xie, K.; Fei, Y.; Deng, H.; He, Q.; Wang, T.; Liu, S.; Zhu, J.; Xu, L.; et al. Liquiritin Alleviates Pain Through Inhibiting CXCL1/CXCR2 Signaling Pathway in Bone Cancer Pain Rat. Front. Pharmacol. 2020, 11, 436. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qin, X.; Tian, J.; Gao, X.; Wu, X.; Du, G.; Zhou, Y. Liquiritin protects PC12 cells from corticosterone-induced neurotoxicity via regulation of metabolic disorders, attenuation ERK1/2-NF-κB pathway, activation Nrf2-Keap1 pathway, and inhibition mitochondrial apoptosis pathway. Food Chem. Toxicol. 2020, 146, 111801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, T.; Zhang, X.J.; Zhu, Z.Y. Hypoglycemic effect of glycyrrhizic acid, a natural non-carbohydrate sweetener, on streptozotocin-induced diabetic mice. Food Funct. 2020, 11, 4160–4170. [Google Scholar] [CrossRef] [PubMed]
- Thu, V.T.; Yen, N.T.H.; Ly, N.T.H. Liquiritin from Radix Glycyrrhizae Protects Cardiac Mitochondria from Hypoxia/Reoxygenation Damage. J. Anal. Methods Chem. 2021, 2021, 1857464. [Google Scholar] [CrossRef]
- Kwak, H.G.; Lim, H.B. Inhibitory effects of Cnidium monnieri fruit extract on pulmonary inflammation in mice induced by cigarette smoke condensate and lipopolysaccharide. Chin. J. Nat. Med. 2014, 12, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Basnet, P.; Yasuda, I.; Kumagai, N.; Tohda, C.; Nojima, H.; Kuraishi, Y.; Komatsu, K. Inhibition of itch-scratch response by fruits of Cnidium monnieri in mice. Biol. Pharm. Bull. 2001, 24, 1012–1015. [Google Scholar] [CrossRef]
- Hao, Y.; Liu, Y. Osthole Alleviates Bleomycin-Induced Pulmonary Fibrosis via Modulating Angiotensin-Converting Enzyme 2/Angiotensin-(1-7) Axis and Decreasing Inflammation Responses in Rats. Biol. Pharm. Bull. 2016, 39, 457–465. [Google Scholar] [CrossRef]
- Che, Y.; Li, J.; Li, Z.; Li, J.; Wang, S.; Yan, Y.; Zou, K.; Zou, L. Osthole enhances antitumor activity and irradiation sensitivity of cervical cancer cells by suppressing ATM/NF-κB signaling. Oncol. Rep. 2018, 40, 737–747. [Google Scholar] [CrossRef]
- Wang, L.; Yang, L.; Lu, Y.; Chen, Y.; Liu, T.; Peng, Y.; Zhou, Y.; Cao, Y.; Bi, Z.; Liu, T.; et al. Osthole Induces Cell Cycle Arrest and Inhibits Migration and Invasion via PTEN/Akt Pathways in Osteosarcoma. Cell. Physiol. Biochem. 2016, 38, 2173–2182. [Google Scholar] [CrossRef]
- Chou, S.Y.; Hsu, C.S.; Wang, K.T.; Wang, M.C.; Wang, C.C. Antitumor effects of Osthol from Cnidium monnieri: An in vitro and in vivo study. Phytother. Res. 2007, 21, 226–230. [Google Scholar] [CrossRef]
- Liang, H.J.; Suk, F.M.; Wang, C.K.; Hung, L.F.; Liu, D.Z.; Chen, N.Q.; Chen, Y.C.; Chang, C.C.; Liang, Y.C. Osthole, a potential antidiabetic agent, alleviates hyperglycemia in db/db mice. Chem. Biol. Interact. 2009, 181, 309–315. [Google Scholar] [CrossRef]
- Lee, W.H.; Lin, R.J.; Lin, S.Y.; Chen, Y.C.; Lin, H.M.; Liang, Y.C. Osthole enhances glucose uptake through activation of AMP-activated protein kinase in skeletal muscle cells. J. Agric. Food Chem. 2011, 59, 12874–12881. [Google Scholar] [CrossRef]
- Hu, J.; Liu, R.; Yang, Z.; Pan, X.; Li, Y.; Gong, Y.; Guo, D. Praeruptorin A inhibits the activation of NF-κB pathway and the expressions of inflammatory factors in poly (I:C)-induced RAW264.7 cells. Chem. Biol. Drug Des. 2023, 102, 1110–1120. [Google Scholar] [CrossRef]
- Yu, C.L.; Yu, Y.L.; Yang, S.F.; Hsu, C.E.; Lin, C.L.; Hsieh, Y.H.; Chiou, H.L. Praeruptorin A reduces metastasis of human hepatocellular carcinoma cells by targeting ERK/MMP1 signaling pathway. Environ. Toxicol. 2021, 36, 540–549. [Google Scholar] [CrossRef]
- Qian, L.; Xu, Z.; Zhang, W.; Wilson, B.; Hong, J.S.; Flood, P.M. Sinomenine, a natural dextrorotatory morphinan analog, is anti-inflammatory and neuroprotective through inhibition of microglial NADPH oxidase. J. Neuroinflamm. 2007, 4, 23. [Google Scholar] [CrossRef]
- Gao, T.; Hao, J.; Wiesenfeld-Hallin, Z.; Wang, D.Q.; Xu, X.J. Analgesic effect of sinomenine in rodents after inflammation and nerve injury. Eur. J. Pharmacol. 2013, 721, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Su, M.X.; Song, M.; Sun, D.Z.; Zhao, H.; Gu, X.; Zhu, L.; Zhan, X.L.; Xu, Z.N.; Wen, A.D.; Hang, T.J. Determination of sinomenine sustained-release capsules in healthy Chinese volunteers by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 889–890, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.; Lu, X.; Wu, K.; Zeng, J.; Gao, Y.; Shi, Q.; Wang, X.; Chang, L.S.; He, D. Sinomenine reverses multidrug resistance in bladder cancer cells via P-glycoprotein-dependent and independent manners. Pharmazie 2014, 69, 48–54. [Google Scholar] [PubMed]
- Qiu, J.; Wang, M.; Zhang, J.; Cai, Q.; Lu, D.; Li, Y.; Dong, Y.; Zhao, T.; Chen, H. The neuroprotection of Sinomenine against ischemic stroke in mice by suppressing NLRP3 inflammasome via AMPK signaling. Int. Immunopharmacol. 2016, 40, 492–500. [Google Scholar] [CrossRef]
- Shukla, S.M.; Sharma, S.K. Sinomenine inhibits microglial activation by Aβ and confers neuroprotection. J. Neuroinflamm. 2011, 8, 117. [Google Scholar] [CrossRef]
- Cardoso, L.P.; de Sousa, S.O.; Gusson-Zanetoni, J.P.; de Melo Moreira Silva, L.L.; Frigieri, B.M.; Henrique, T.; Tajara, E.H.; Oliani, S.M.; Rodrigues-Lisoni, F.C. Piperine Reduces Neoplastic Progression in Cervical Cancer Cells by Downregulating the Cyclooxygenase 2 Pathway. Pharmaceuticals 2023, 16, 103. [Google Scholar] [CrossRef] [PubMed]
- McNeil, B.D. MRGPRX2 and Adverse Drug Reactions. Front. Immunol. 2021, 12, 676354. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Liao, X.; Wieland, L.S.; Hu, J.; Wang, Y.; Kim, T.H.; Liu, J.P.; Zhan, S.; Robinson, N. Cochrane systematic reviews on traditional Chinese medicine: What matters-the quantity or quality of evidence? Phytomedicine 2022, 98, 153921. [Google Scholar] [CrossRef]
- You, L.; Liang, K.; An, R.; Wang, X. The path towards FDA approval: A challenging journey for traditional Chinese medicine. Pharmacol. Res. 2022, 182, 106314. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Thai, S.; Lu, W.; Sun, S.; Tang, H.; Zhai, S.; Wang, T. Traditional Chinese medicine and drug-induced anaphylaxis: Data from the Beijing pharmacovigilance database. Int. J. Clin. Pharm. 2018, 40, 921–927. [Google Scholar] [CrossRef]
- GTEx Consortium. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef]
- Subramanian, H.; Gupta, K.; Ali, H. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J. Allergy Clin. Immunol. 2016, 138, 700–710. [Google Scholar] [CrossRef]
- Azimi, E.; Reddy, V.B.; Shade, K.C.; Anthony, R.M.; Talbot, S.; Pereira, P.J.S.; Lerner, E.A. Dual action of neurokinin-1 antagonists on Mas-related GPCRs. JCI Insight 2016, 1, e89362. [Google Scholar] [CrossRef]
- Peng, X.; Knapp, B.I.; Bidlack, J.M.; Neumeyer, J.L. Pharmacological properties of bivalent ligands containing butorphan linked to nalbuphine, naltrexone, and naloxone at mu, delta, and kappa opioid receptors. J. Med. Chem. 2007, 50, 2254–2258. [Google Scholar] [CrossRef]
- Rehrauer, K.J.; Cunningham, C.W. IUPHAR Review—Bivalent and bifunctional opioid receptor ligands as novel analgesics. Pharmacol. Res. 2023, 197, 106966. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.W.; Elballa, W.M.; Vold, S.U. Bifunctional opioid receptor ligands as novel analgesics. Neuropharmacology 2019, 151, 195–207. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziadowiec, A.; Popiolek, I.; Kwitniewski, M.; Porebski, G. Modulation of the Mas-Related G Protein-Coupled Receptor X2 (MRGPRX2) by Xenobiotic Compounds and Its Relevance to Human Diseases. J. Xenobiot. 2024, 14, 380-403. https://doi.org/10.3390/jox14010024
Dziadowiec A, Popiolek I, Kwitniewski M, Porebski G. Modulation of the Mas-Related G Protein-Coupled Receptor X2 (MRGPRX2) by Xenobiotic Compounds and Its Relevance to Human Diseases. Journal of Xenobiotics. 2024; 14(1):380-403. https://doi.org/10.3390/jox14010024
Chicago/Turabian StyleDziadowiec, Alicja, Iwona Popiolek, Mateusz Kwitniewski, and Grzegorz Porebski. 2024. "Modulation of the Mas-Related G Protein-Coupled Receptor X2 (MRGPRX2) by Xenobiotic Compounds and Its Relevance to Human Diseases" Journal of Xenobiotics 14, no. 1: 380-403. https://doi.org/10.3390/jox14010024
APA StyleDziadowiec, A., Popiolek, I., Kwitniewski, M., & Porebski, G. (2024). Modulation of the Mas-Related G Protein-Coupled Receptor X2 (MRGPRX2) by Xenobiotic Compounds and Its Relevance to Human Diseases. Journal of Xenobiotics, 14(1), 380-403. https://doi.org/10.3390/jox14010024






