Carbon Nanotubes and Graphene Materials as Xenobiotics in Living Systems: Is There a Consensus on Their Safety?
Abstract
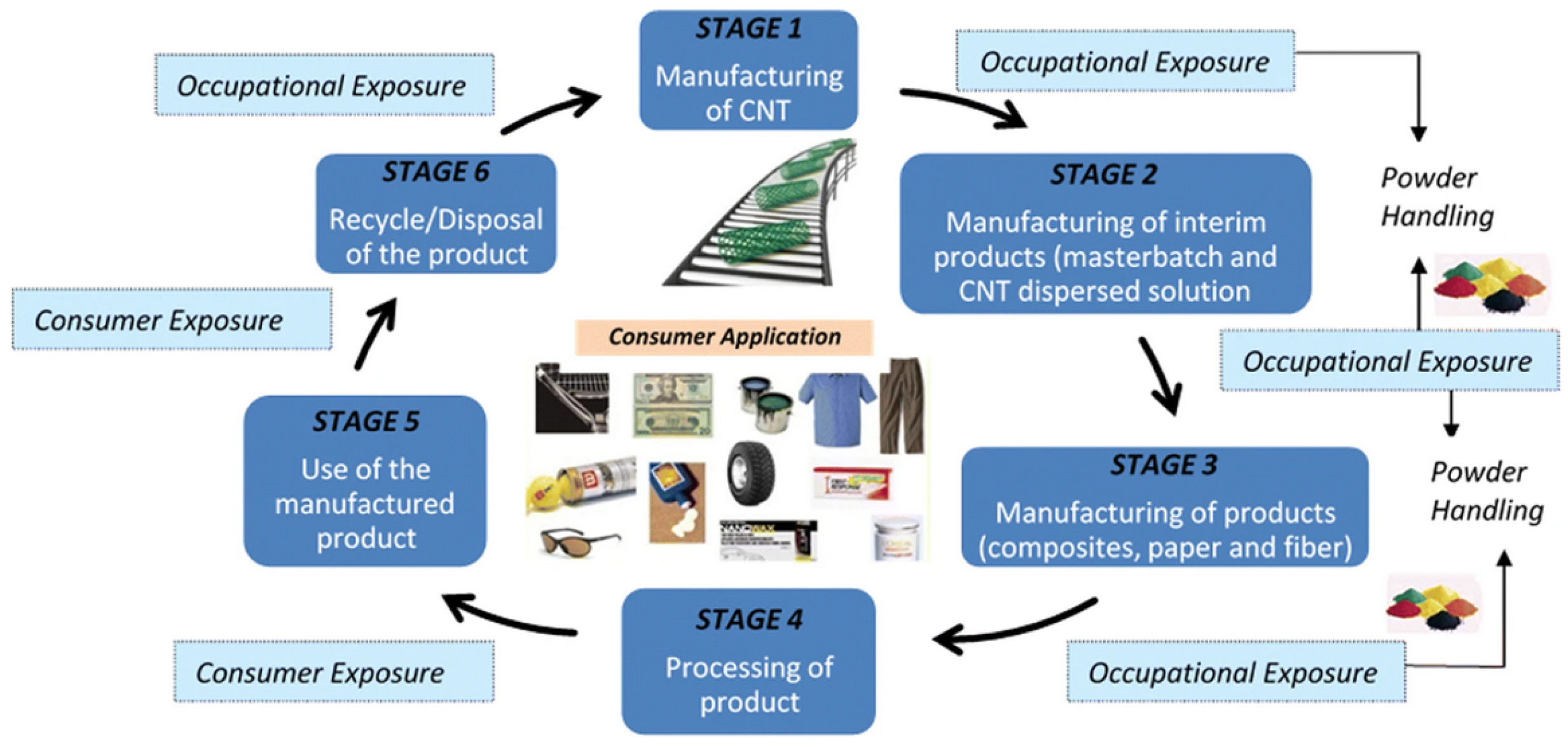
1. Introduction
2. Properties and Applications of Carbon Nanotubes and Graphene Materials
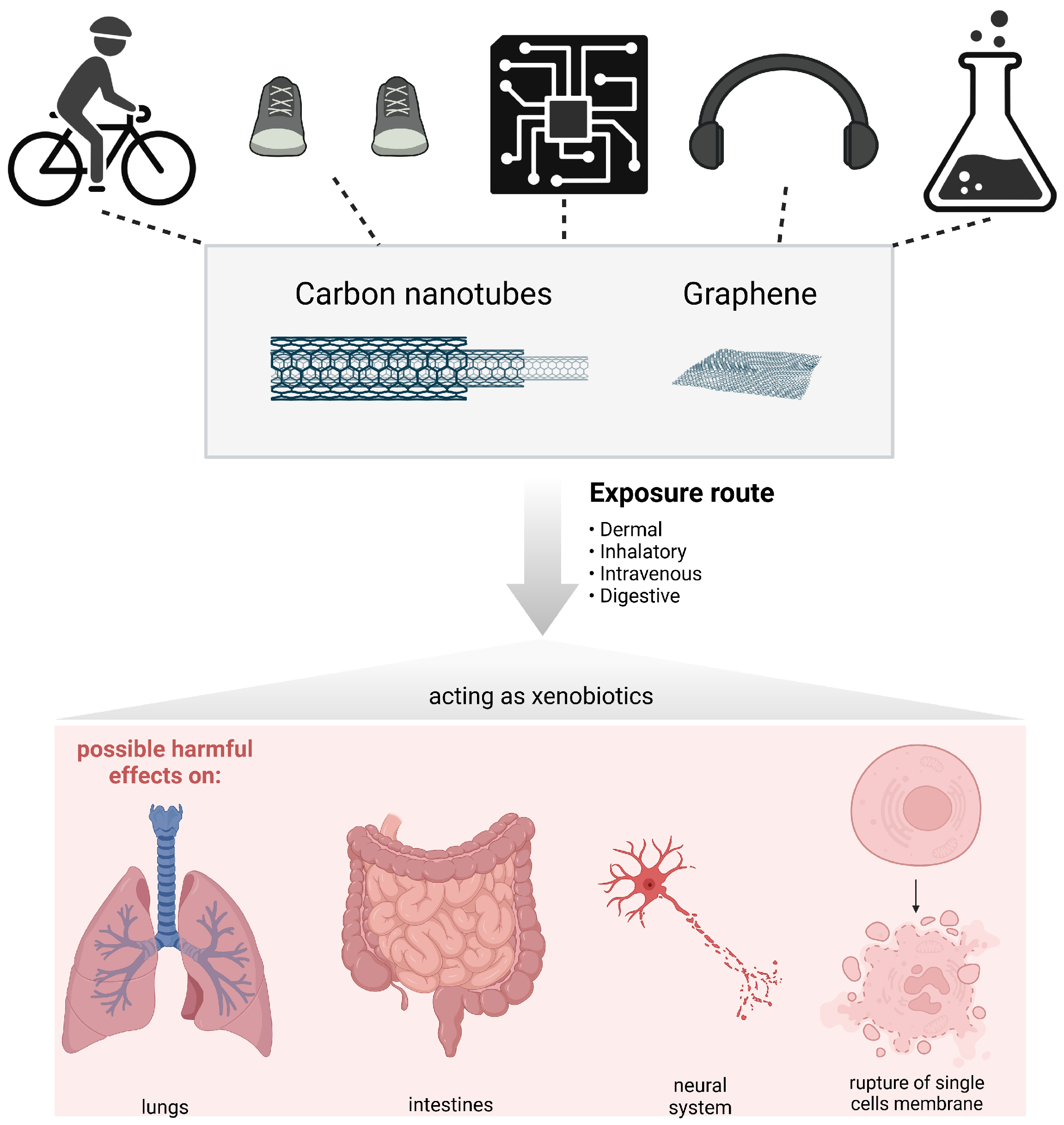
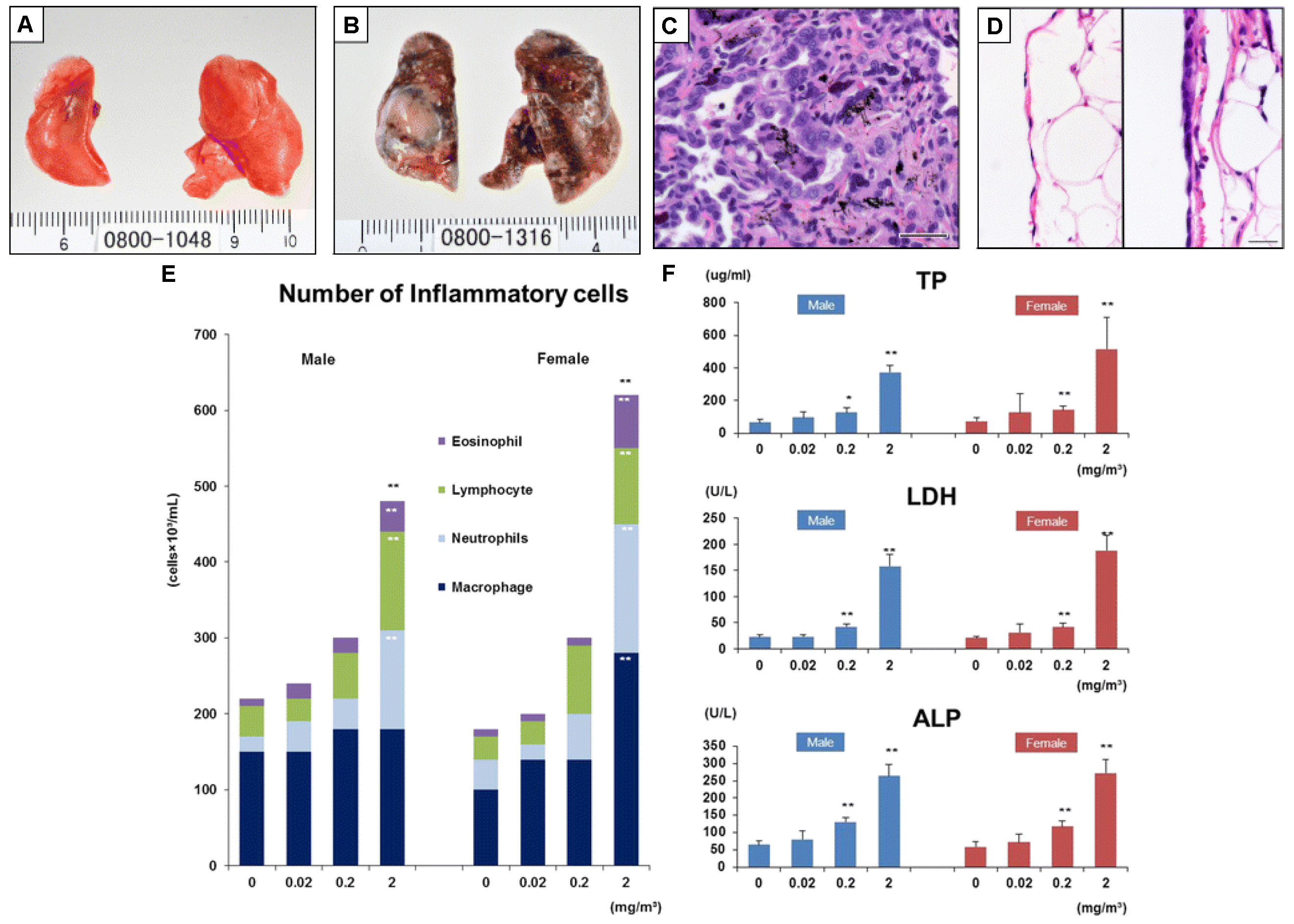
3. Our Current Understanding of the Safety of Carbon Nanotubes and Graphene Materials
4. Regulation and Guidelines
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNTs | carbon nanotubes |
| CVD | chemical vapor deposition |
| SWCNTs | single-walled carbon nanotubes |
| MWCNTs | multi-walled carbon nanotubes |
| PAHs | polycyclic aromatic hydrocarbons |
| Ch | chiral vector |
| TEM | transmission electron microscopy |
| SEM | scanning electron microscopy |
| TGA | thermogravimetric analysis |
| GO | graphene oxide |
| AFM | atomic force microscopy |
| HR-TEM | high-resolution transmission electron microscopy |
| STEM | scanning transmission electron microscopy |
| DNA | deoxyribonucleic acid |
| COSHH | Control of Substances Hazardous to Health |
| DSEAR | Dangerous Substances and Explosive Atmospheres Regulations |
| REACH | Registration, Evaluation, Authorization and Restriction of Chemicals |
| EPA | Environmental Protection Agency |
| NIOSH | National Institute for Occupational Safety and Health |
| IARC | The International Agency for Research on Cancer |
References
- Valsami-Jones, E.; Lynch, I. How safe are nanomaterials? Science 2015, 350, 388–389. [Google Scholar] [CrossRef] [PubMed]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Oyabu, T.; Myojo, T.; Morimoto, Y.; Ogami, A.; Hirohashi, M.; Yamamoto, M.; Todoroki, M.; Mizuguchi, Y.; Hashiba, M.; Lee, B.W.; et al. Biopersistence of inhaled MWCNT in rat lungs in a 4-week well-characterized exposure. Inhal. Toxicol. 2011, 23, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Chernova, T.; Murphy, F.A.; Galavotti, S.; Sun, X.M.; Powley, I.R.; Grosso, S.; Schinwald, A.; Zacarias-Cabeza, J.; Dudek, K.M.; Dinsdale, D.; et al. Long-fiber carbon nanotubes replicate asbestos-induced mesothelioma with disruption of the tumor suppressor gene Cdkn2a (Ink4a/Arf). Curr. Biol. 2017, 27, 3302–3314. [Google Scholar] [CrossRef] [PubMed]
- Podeszwa, R. Interactions of graphene sheets deduced from properties of polycyclic aromatic hydrocarbons. J. Chem. Phys. 2010, 132, 044704. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, H.; von Dem Bussche, A.; Creighton, M.; Hurt, R.H.; Kane, A.B.; Gao, H. Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites. Proc. Natl. Acad. Sci. USA 2013, 110, 12295–12300. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Rathinavel, S.; Priyadharshini, K.; Panda, D. A review on carbon nanotube: An overview of synthesis, properties, functionalization, characterization, and the application. Mater. Sci. Eng. B 2021, 268, 115095. [Google Scholar] [CrossRef]
- Boumia, L.; Zidour, M.; Benzair, A.; Tounsi, A. A Timoshenko beam model for vibration analysis of chiral single-walled carbon nanotubes. Phys. E Low-Dimens. Syst. Nanostruct. 2014, 59, 186–191. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Bandow, S.; Iijima, S. Synthesis of carbon nanohorn particles by simple pulsed arc discharge ignited between pre-heated carbon rods. Chem. Phys. Lett. 2004, 389, 181–185. [Google Scholar] [CrossRef]
- Chrzanowska, J.; Hoffman, J.; Małolepszy, A.; Mazurkiewicz, M.; Kowalewski, T.A.; Szymanski, Z.; Stobinski, L. Synthesis of carbon nanotubes by the laser ablation method: Effect of laser wavelength. Phys. Status Solidi 2015, 252, 1860–1867. [Google Scholar] [CrossRef]
- Terranova, M.L.; Sessa, V.; Rossi, M. The world of carbon nanotubes: An overview of CVD growth methodologies. Chem. Vap. Depos. 2006, 12, 315–325. [Google Scholar] [CrossRef]
- Vander Wal, R.L.; Berger, G.M.; Hall, L.J. Single-walled carbon nanotube synthesis via a multi-stage flame configuration. J. Phys. Chem. B 2002, 106, 3564–3567. [Google Scholar] [CrossRef]
- Kadam, V. Carbon Nanotubes and its Applications: A Review Asian. J. Pharm. Clin. Res. 2009, 2, 17–27. [Google Scholar]
- Hou, P.X.; Liu, C.; Cheng, H.M. Purification of carbon nanotubes. Carbon 2008, 46, 2003–2025. [Google Scholar] [CrossRef]
- Tobias, G.; Shao, L.; Salzmann, C.G.; Huh, Y.; Green, M.L. Purification and opening of carbon nanotubes using steam. J. Phys. Chem. B 2006, 110, 22318–22322. [Google Scholar] [CrossRef]
- Ballesteros, B.; Tobias, G.; Shao, L.; Pellicer, E.; Nogués, J.; Mendoza, E.; Green, M.L. Steam Purification for the Removal of Graphitic Shells Coating Catalytic Particles and the Shortening of Single-Walled Carbon Nanotubes. Small 2008, 4, 1501–1506. [Google Scholar] [CrossRef]
- Herrero-Latorre, C.; Álvarez-Méndez, J.; Barciela-García, J.; García-Martín, S.; Peña-Crecente, R. Characterization of carbon nanotubes and analytical methods for their determination in environmental and biological samples: A review. Anal. Chim. Acta 2015, 853, 77–94. [Google Scholar] [CrossRef]
- Branca, C.; Frusteri, F.; Magazu, V.; Mangione, A. Characterization of carbon nanotubes by TEM and infrared spectroscopy. J. Phys. Chem. B 2004, 108, 3469–3473. [Google Scholar] [CrossRef]
- Yu, M.; Dyer, M.J.; Skidmore, G.D.; Rohrs, H.W.; Lu, X.; Ausman, K.D.; Von Ehr, J.R.; Ruoff, R.S. Three-dimensional manipulation of carbon nanotubes under a scanning electron microscope. Nanotechnology 1999, 10, 244. [Google Scholar] [CrossRef]
- Dresselhaus, M.; Dresselhaus, G.; Charlier, J.C.; Hernandez, E. Electronic, thermal and mechanical properties of carbon nanotubes. Philos. Trans. R. Soc. London Ser. A Math. Phys. Eng. Sci. 2004, 362, 2065–2098. [Google Scholar] [CrossRef] [PubMed]
- Soldano, C.; Mahmood, A.; Dujardin, E. Production, properties and potential of graphene. Carbon 2010, 48, 2127–2150. [Google Scholar] [CrossRef]
- Mas-Balleste, R.; Gomez-Navarro, C.; Gomez-Herrero, J.; Zamora, F. 2D materials: To graphene and beyond. Nanoscale 2011, 3, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; MacDonald, A.H. Graphene: Exploring carbon flatland. Phys. Today 2007, 60, 35–41. [Google Scholar] [CrossRef]
- Ritter, K.A.; Lyding, J.W. Characterization of nanometer-sized, mechanically exfoliated graphene on the H-passivated Si (100) surface using scanning tunneling microscopy. Nanotechnology 2007, 19, 015704. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.S.; Coleman, K.S. Graphene synthesis: Relationship to applications. Nanoscale 2013, 5, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Sisto, T.J.; Zakharov, L.N.; White, B.M.; Jasti, R. Towards pi-extended cycloparaphenylenes as seeds for CNT growth: Investigating strain relieving ring-openings and rearrangements. Chem. Sci. 2016, 7, 3681–3688. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, M.S.A.; Uddin, M.N.; Islam, M.M.; Bipasha, F.A.; Hossain, S.S. Synthesis of graphene. Int. Nano Lett. 2016, 6, 65–83. [Google Scholar] [CrossRef]
- Shams, S.S.; Zhang, R.; Zhu, J. Graphene synthesis: A Review. Mater. Sci. Pol. 2015, 33, 566–578. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, I.; et al. Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Kim, J.; Cote, L.J.; Kim, F.; Huang, J. Visualizing graphene based sheets by fluorescence quenching microscopy. J. Am. Chem. Soc. 2010, 132, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Paredes, J.; Villar-Rodil, S.; Solís-Fernández, P.; Martínez-Alonso, A.; Tascon, J. Atomic force and scanning tunneling microscopy imaging of graphene nanosheets derived from graphite oxide. Langmuir 2009, 25, 5957–5968. [Google Scholar] [CrossRef] [PubMed]
- Gass, M.H.; Bangert, U.; Bleloch, A.L.; Wang, P.; Nair, R.R.; Geim, A. Free-standing graphene at atomic resolution. Nat. Nanotechnol. 2008, 3, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.; Dutta, D.; Sarkar, A.; Chattopadhyay, P. Functionalized carbon nanotubes: Synthesis, properties and applications in water purification, drug delivery, and material and biomedical sciences. Nanoscale Adv. 2021, 3, 5722–5744. [Google Scholar] [CrossRef]
- Sun, Y.P.; Fu, K.; Lin, Y.; Huang, W. Functionalized carbon nanotubes: Properties and applications. Accounts Chem. Res. 2002, 35, 1096–1104. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of graphene: Covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Xiao, Y.; Pang, Y.X.; Yan, Y.; Qian, P.; Zhao, H.; Manickam, S.; Wu, T.; Pang, C.H. Synthesis and Functionalization of Graphene Materials for Biomedical Applications: Recent Advances, Challenges, and Perspectives. Adv. Sci. 2023, 10, 2205292. [Google Scholar] [CrossRef]
- Yang, G.; Li, L.; Lee, W.B.; Ng, M.C. Structure of graphene and its disorders: A review. Sci. Technol. Adv. Mater. 2018, 19, 613–648. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.S. Carbon nanotubes? properties and applications: A review. Carbon Lett. 2013, 14, 131–144. [Google Scholar] [CrossRef]
- Popov, V.N. Carbon nanotubes: Properties and application. Mater. Sci. Eng. R Rep. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Ebbesen, T.; Lezec, H.; Hiura, H.; Bennett, J.; Ghaemi, H.; Thio, T. Electrical conductivity of individual carbon nanotubes. Nature 1996, 382, 54–56. [Google Scholar] [CrossRef]
- Ruoff, R.S.; Tersoff, J.; Lorents, D.C.; Subramoney, S.; Chan, B. Radial deformation of carbon nanotubes by van der Waals forces. Nature 1993, 364, 514–516. [Google Scholar] [CrossRef]
- Kim, P.; Shi, L.; Majumdar, A.; McEuen, P.L. Thermal transport measurements of individual multiwalled nanotubes. Phys. Rev. Lett. 2001, 87, 215502. [Google Scholar] [CrossRef]
- Bolotin, K.I.; Sikes, K.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Tian, C.; Girit, C.; Zettl, A.; Crommie, M.; Shen, Y.R. Gate-variable optical transitions in graphene. Science 2008, 320, 206–209. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Li, Q.; Carpick, R.; Kysar, J.W.; Hone, J. Elastic and frictional properties of graphene. Phys. Status Solidi 2009, 246, 2562–2567. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.H.; Yu, T.; Lu, Y.H.; Wang, Y.Y.; Feng, Y.P.; Shen, Z.X. Uniaxial strain on graphene: Raman spectroscopy study and band-gap opening. ACS Nano 2008, 2, 2301–2305. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Zhou, Q.; Chang, J.; Liu, Y.; Lin, L. Graphene and carbon nanotube (CNT) in MEMS/NEMS applications. Microelectron. Eng. 2015, 132, 192–206. [Google Scholar] [CrossRef]
- Biswas, C.; Lee, Y.H. Graphene versus carbon nanotubes in electronic devices. Adv. Funct. Mater. 2011, 21, 3806–3826. [Google Scholar] [CrossRef]
- Liang, M.; Luo, B.; Zhi, L. Application of graphene and graphene-based materials in clean energy-related devices. Int. J. Energy Res. 2009, 33, 1161–1170. [Google Scholar] [CrossRef]
- Wen, L.; Li, F.; Cheng, H.M. Carbon nanotubes and graphene for flexible electrochemical energy storage: From materials to devices. Adv. Mater. 2016, 28, 4306–4337. [Google Scholar] [CrossRef]
- Gendron, D.; Ansaldo, A.; Bubak, G.; Ceseracciu, L.; Vamvounis, G.; Ricci, D. Poly (ionic liquid)-carbon nanotubes self-supported, highly electroconductive composites and their application in electroactive devices. Compos. Sci. Technol. 2015, 117, 364–370. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Gendron, D.; Bubak, G.; Ceseracciu, L.; Ricciardella, F.; Ansaldo, A.; Ricci, D. Significant strain and force improvements of single-walled carbon nanotube actuator: A metal chalcogenides approach. Sens. Actuators B Chem. 2016, 230, 673–683. [Google Scholar] [CrossRef]
- Maheswaran, R.; Shanmugavel, B.P. A critical review of the role of carbon nanotubes in the progress of next-generation electronic applications. J. Electron. Mater. 2022, 51, 2786–2800. [Google Scholar] [CrossRef]
- Gendron, D.; Bubak, G.; Ceseracciu, L.; Ansaldo, A.; Ricci, D. The electrolyte layer composition: A key element for improving the performance of carbon nanotube actuator. Sens. Actuators B Chem. 2016, 222, 1073–1082. [Google Scholar] [CrossRef]
- Simon, J.; Flahaut, E.; Golzio, M. Overview of carbon nanotubes for biomedical applications. Materials 2019, 12, 624. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, R.; Ilyas, A.M.; Hasan, A.; Arnaout, A.; Ahmed, F.; Memic, A. Carbon nanotubes in biomedical applications: Factors, mechanisms, and remedies of toxicity: Miniperspective. J. Med. Chem. 2016, 59, 8149–8167. [Google Scholar] [CrossRef] [PubMed]
- Roldo, M.; Fatouros, D.G. Biomedical applications of carbon nanotubes. Annu. Rep. Sect. C 2013, 109, 10–35. [Google Scholar] [CrossRef]
- Raphey, V.; Henna, T.; Nivitha, K.; Mufeedha, P.; Sabu, C.; Pramod, K. Advanced biomedical applications of carbon nanotube. Mater. Sci. Eng. C 2019, 100, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Shen, H.; Wang, Y.; Chu, X.; Xie, J.; Zhou, N.; Shen, J. Biomedical application of graphene: From drug delivery, tumor therapy, to theranostics. Colloids Surf. B Biointerfaces 2020, 185, 110596. [Google Scholar] [CrossRef]
- Ghosal, K.; Sarkar, K. Biomedical applications of graphene nanomaterials and beyond. ACS Biomater. Sci. Eng. 2018, 4, 2653–2703. [Google Scholar] [CrossRef]
- Kam, N.W.S.; O’Connell, M.; Wisdom, J.A.; Dai, H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc. Natl. Acad. Sci. USA 2005, 102, 11600–11605. [Google Scholar] [CrossRef]
- Wang, W.; Su, H.; Wu, Y.; Zhou, T.; Li, T. Biosensing and biomedical applications of graphene: A review of current progress and future prospect. J. Electrochem. Soc. 2019, 166, B505. [Google Scholar] [CrossRef]
- Aschberger, K.; Johnston, H.J.; Stone, V.; Aitken, R.J.; Hankin, S.M.; Peters, S.A.; Tran, C.L.; Christensen, F.M. Review of carbon nanotubes toxicity and exposure—Appraisal of human health risk assessment based on open literature. Crit. Rev. Toxicol. 2010, 40, 759–790. [Google Scholar] [CrossRef]
- Boczkowski, J.; Lanone, S. Respiratory toxicities of nanomaterials—a focus on carbon nanotubes. Adv. Drug Deliv. Rev. 2012, 64, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Maynard, A.D.; Baron, P.A.; Foley, M.; Shvedova, A.A.; Kisin, E.R.; Castranova, V. Exposure to carbon nanotube material: Aerosol release during the handling of unrefined single-walled carbon nanotube material. J. Toxicol. Environ. Health Part A 2004, 67, 87–107. [Google Scholar] [CrossRef]
- Gangoli, V.S.; Godwin, M.A.; Reddy, G.; Bradley, R.K.; Barron, A.R. The state of HiPco single-walled carbon nanotubes in 2019. C 2019, 5, 65. [Google Scholar] [CrossRef]
- Han, J.H.; Lee, E.J.; Lee, J.H.; So, K.P.; Lee, Y.H.; Bae, G.N.; Lee, S.B.; Ji, J.H.; Cho, M.H.; Yu, I.J. Monitoring multiwalled carbon nanotube exposure in carbon nanotube research facility. Inhal. Toxicol. 2008, 20, 741–749. [Google Scholar] [CrossRef]
- Govindaraj, P.; Mirabedini, A.; Jin, X.; Antiohos, D.; Salim, N.; Aitchison, P.; Parker, J.; Fuss, F.K.; Hameed, N. Health and safety perspectives of graphene in wearables and hybrid materials. J. Mater. Sci. Technol. 2023, 155, 10–32. [Google Scholar] [CrossRef]
- Wick, P.; Louw-Gaume, A.E.; Kucki, M.; Krug, H.F.; Kostarelos, K.; Fadeel, B.; Dawson, K.A.; Salvati, A.; Vázquez, E.; Ballerini, L.; et al. Classification framework for graphene-based materials. Angew. Chem. Int. Ed. 2014, 53, 7714. [Google Scholar] [CrossRef] [PubMed]
- Pelin, M.; Sosa, S.; Prato, M.; Tubaro, A. Occupational exposure to graphene based nanomaterials: Risk assessment. Nanoscale 2018, 10, 15894–15903. [Google Scholar] [CrossRef]
- Bussy, C.; Ali-Boucetta, H.; Kostarelos, K. Safety considerations for graphene: Lessons learnt from carbon nanotubes. Accounts Chem. Res. 2013, 46, 692–701. [Google Scholar] [CrossRef]
- Rhazouani, A.; Gamrani, H.; El Achaby, M.; Aziz, K.; Gebrati, L.; Uddin, M.S.; Aziz, F. Synthesis and toxicity of graphene oxide nanoparticles: A literature review of in vitro and in vivo studies. BioMed Res. Int. 2021, 2021. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29. [Google Scholar] [CrossRef]
- Catania, F.; Marras, E.; Giorcelli, M.; Jagdale, P.; Lavagna, L.; Tagliaferro, A.; Bartoli, M. A review on recent advancements of graphene and graphene-related materials in biological applications. Appl. Sci. 2021, 11, 614. [Google Scholar] [CrossRef]
- Ghiasvand Mohammadkhani, L.; Khoshkam, M.; Kompany-Zareh, M.; Amiri, M.; Ramazani, A. Metabolomics approach to study in vivo toxicity of graphene oxide nanosheets. J. Appl. Toxicol. 2022, 42, 506–515. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Chen, Z.; Gu, Z.; Yan, L.; Zhao, F.; Zhang, A. Toxicological evaluation of graphene-family nanomaterials. J. Nanosci. Nanotechnol. 2020, 20, 1993–2006. [Google Scholar] [CrossRef] [PubMed]
- Orsu, P.; Koyyada, A. Recent progresses and challenges in graphene based nano materials for advanced therapeutical applications: A comprehensive review. Mater. Today Commun. 2020, 22, 100823. [Google Scholar] [CrossRef]
- Achawi, S.; Pourchez, J.; Feneon, B.; Forest, V. Graphene-based materials in vitro toxicity and their structure–activity relationships: A systematic literature review. Chem. Res. Toxicol. 2021, 34, 2003–2018. [Google Scholar] [CrossRef] [PubMed]
- Pathmanapan, S.; Periyathambi, P.; Anandasadagopan, S.K. Fibrin hydrogel incorporated with graphene oxide functionalized nanocomposite scaffolds for bone repair—In vitro and in vivo study. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102251. [Google Scholar] [CrossRef] [PubMed]
- Maulvi, F.A.; Soni, P.D.; Patel, P.J.; Desai, A.R.; Desai, D.T.; Shukla, M.R.; Shah, S.A.; Shah, D.O.; Willcox, M.D. Controlled bimatoprost release from graphene oxide laden contact lenses: In vitro and in vivo studies. Colloids Surfaces B Biointerfaces 2021, 208, 112096. [Google Scholar] [CrossRef]
- Burgum, M.J.; Clift, M.J.; Evans, S.J.; Hondow, N.; Miller, M.; Lopez, S.B.; Williams, A.; Tarat, A.; Jenkins, G.J.; Doak, S.H. In Vitro Primary-Indirect Genotoxicity in Bronchial Epithelial Cells Promoted by Industrially Relevant Few-Layer Graphene. Small 2021, 17, 2002551. [Google Scholar] [CrossRef]
- Esmaeili, Y.; Bidram, E.; Zarrabi, A.; Amini, A.; Cheng, C. Graphene oxide and its derivatives as promising In-vitro bio-imaging platforms. Sci. Rep. 2020, 10, 18052. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, Y.; Wu, J.; Wang, Y.; Yang, X.; Yang, R.; Wang, B.; Yang, J.; Zhang, N. Graphene oxide can induce in vitro and in vivo mutagenesis. Sci. Rep. 2013, 3, 3469. [Google Scholar] [CrossRef]
- Pang, L.; Dai, C.; Bi, L.; Guo, Z.; Fan, J. Biosafety and antibacterial ability of graphene and graphene oxide in vitro and in vivo. Nanoscale Res. Lett. 2017, 12, 1–9. [Google Scholar] [CrossRef]
- Sajid, M.I.; Jamshaid, U.; Jamshaid, T.; Zafar, N.; Fessi, H.; Elaissari, A. Carbon nanotubes from synthesis to in vivo biomedical applications. Int. J. Pharm. 2016, 501, 278–299. [Google Scholar] [CrossRef]
- Bardhan, N.M.; Ghosh, D.; Belcher, A.M. Carbon nanotubes as in vivo bacterial probes. Nat. Commun. 2014, 5, 4918. [Google Scholar] [CrossRef]
- Kato, T.; Totsuka, Y.; Ishino, K.; Matsumoto, Y.; Tada, Y.; Nakae, D.; Goto, S.; Masuda, S.; Ogo, S.; Kawanishi, M.; et al. Genotoxicity of multi-walled carbon nanotubes in both in vitro and in vivo assay systems. Nanotoxicology 2013, 7, 452–461. [Google Scholar] [CrossRef]
- Kafa, H.; Wang, J.T.W.; Rubio, N.; Venner, K.; Anderson, G.; Pach, E.; Ballesteros, B.; Preston, J.E.; Abbott, N.J.; Al-Jamal, K.T. The interaction of carbon nanotubes with an in vitro blood–brain barrier model and mouse brain in vivo. Biomaterials 2015, 53, 437–452. [Google Scholar] [CrossRef]
- Iverson, N.M.; Barone, P.W.; Shandell, M.; Trudel, L.J.; Sen, S.; Sen, F.; Ivanov, V.; Atolia, E.; Farias, E.; McNicholas, T.P.; et al. In vivo biosensing via tissue-localizable near-infrared-fluorescent single-walled carbon nanotubes. Nat. Nanotechnol. 2013, 8, 873–880. [Google Scholar] [CrossRef]
- Fujita, K.; Fukuda, M.; Endoh, S.; Maru, J.; Kato, H.; Nakamura, A.; Shinohara, N.; Uchino, K.; Honda, K. Size effects of single-walled carbon nanotubes on in vivo and in vitro pulmonary toxicity. Inhal. Toxicol. 2015, 27, 207–223. [Google Scholar] [CrossRef]
- He, H.; Pham-Huy, L.A.; Dramou, P.; Xiao, D.; Zuo, P.; Pham-Huy, C. Carbon nanotubes: Applications in pharmacy and medicine. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Fisher, C.; Rider, A.E.; Han, Z.J.; Kumar, S.; Levchenko, I.; Ostrikov, K. Applications and nanotoxicity of carbon nanotubes and graphene in biomedicine. J. Nanomater. 2012, 2012, 1–19. [Google Scholar] [CrossRef]
- Nasirzadeh, N.; Rasoulzadeh, Y.; Rezazadeh Azari, M.; Mohammadian, Y. Cellular toxicity of multi-walled carbon nanotubes on human lung cells. J. Chem. Health Risks 2020, 10, 135–144. [Google Scholar]
- Sousa, S.P.; Peixoto, T.; Santos, R.M.; Lopes, A.; Paiva, M.d.C.; Marques, A.T. Health and safety concerns related to CNT and graphene products, and related composites. J. Compos. Sci. 2020, 4, 106. [Google Scholar] [CrossRef]
- van Zandwijk, N.; Frank, A.L. Awareness: Potential toxicities of carbon nanotubes. Transl. Lung Cancer Res. 2019, 8, S471. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, B.; Bussy, C.; Merino, S.; Vázquez, E.; Flahaut, E.; Mouchet, F.; Evariste, L.; Gauthier, L.; Koivisto, A.J.; Vogel, U.; et al. Safety assessment of graphene-based materials: Focus on human health and the environment. ACS Nano 2018, 12, 10582–10620. [Google Scholar] [CrossRef] [PubMed]
- Canu, I.G.; Bateson, T.F.; Bouvard, V.; Debia, M.; Dion, C.; Savolainen, K.; Yu, I.J. Human exposure to carbon-based fibrous nanomaterials: A review. Int. J. Hyg. Environ. Health 2016, 219, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ. Sci. Technol. 2009, 43, 9216–9222. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, B.; Mokra, K.; Michałowicz, J. Benzo [a] pyrene—environmental occurrence, human exposure, and mechanisms of toxicity. Int. J. Mol. Sci. 2022, 23, 6348. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.A.; Poland, C.A.; Duffin, R.; Al-Jamal, K.T.; Ali-Boucetta, H.; Nunes, A.; Byrne, F.; Prina-Mello, A.; Volkov, Y.; Li, S.; et al. Length-dependent retention of carbon nanotubes in the pleural space of mice initiates sustained inflammation and progressive fibrosis on the parietal pleura. Am. J. Pathol. 2011, 178, 2587–2600. [Google Scholar] [CrossRef]
- Kasai, T.; Umeda, Y.; Ohnishi, M.; Mine, T.; Kondo, H.; Takeuchi, T.; Matsumoto, M.; Fukushima, S. Lung carcinogenicity of inhaled multi-walled carbon nanotube in rats. Part. Fibre Toxicol. 2015, 13, 1–19. [Google Scholar] [CrossRef]
- Liu, X.T.; Mu, X.Y.; Wu, X.L.; Meng, L.X.; Guan, W.B.; Qiang, Y.; Hua, S.; Wang, C.J.; Li, X.F. Toxicity of multi-walled carbon nanotubes, graphene oxide, and reduced graphene oxide to zebrafish embryos. Biomed. Environ. Sci. 2014, 27, 676–683. [Google Scholar]
- Omiecinski, C.J.; Vanden Heuvel, J.P.; Perdew, G.H.; Peters, J.M. Xenobiotic metabolism, disposition, and regulation by receptors: From biochemical phenomenon to predictors of major toxicities. Toxicol. Sci. 2011, 120, S49–S75. [Google Scholar] [CrossRef]
- Gu, X.; Manautou, J.E. Molecular mechanisms underlying chemical liver injury. Expert Rev. Mol. Med. 2012, 14, e4. [Google Scholar] [CrossRef]
- Curtis, D. Casarett and Doull’s Toxicology The Basic Science of Poisons, 8th ed.; McGraw-Hill: New York, NY, USA, 2013. [Google Scholar]
- Esteves, F.; Rueff, J.; Kranendonk, M. The central role of cytochrome P450 in xenobiotic metabolism—A brief review on a fascinating enzyme family. J. Xenobiotics 2021, 11, 94–114. [Google Scholar] [CrossRef]
- Ji, Z.; Zhang, D.; Li, L.; Shen, X.; Deng, X.; Dong, L.; Wu, M.; Liu, Y. The hepatotoxicity of multi-walled carbon nanotubes in mice. Nanotechnology 2009, 20, 445101. [Google Scholar] [CrossRef]
- Awasthi, K.K.; John, P.; Awasthi, A.; Awasthi, K. Multi walled carbon nano tubes induced hepatotoxicity in Swiss albino mice. Micron 2013, 44, 359–364. [Google Scholar] [CrossRef]
- Adedara, I.A.; Anao, O.O.; Forcados, G.E.; Awogbindin, I.O.; Agbowo, A.; Ola-Davies, O.E.; Patlolla, A.K.; Tchounwou, P.B.; Farombi, E.O. Low doses of multi-walled carbon nanotubes elicit hepatotoxicity in rats with markers of oxidative stress and induction of pro-inflammatory cytokines. Biochem. Biophys. Res. Commun. 2018, 503, 3167–3173. [Google Scholar] [CrossRef]
- Nirmal, N.K.; Awasthi, K.K.; John, P.J. Hepatotoxicity of graphene oxide in Wistar rats. Environ. Sci. Pollut. Res. 2021, 28, 46367–46376. [Google Scholar] [CrossRef]
- Guo, X.; Mei, N. Assessment of the toxic potential of graphene family nanomaterials. J. Food Drug Anal. 2014, 22, 105–115. [Google Scholar] [CrossRef]
- Volkov, Y.; McIntyre, J.; Prina-Mello, A. Graphene toxicity as a double-edged sword of risks and exploitable opportunities: A critical analysis of the most recent trends and developments. 2D Mater. 2017, 4, 022001. [Google Scholar] [CrossRef]
- Tabish, T.A.; Pranjol, M.Z.I.; Jabeen, F.; Abdullah, T.; Latif, A.; Khalid, A.; Ali, M.; Hayat, H.; Winyard, P.G.; Whatmore, J.L.; et al. Investigation into the toxic effects of graphene nanopores on lung cancer cells and biological tissues. Appl. Mater. Today 2018, 12, 389–401. [Google Scholar] [CrossRef]
- Martín, C.; Kostarelos, K.; Prato, M.; Bianco, A. Biocompatibility and biodegradability of 2D materials: Graphene and beyond. Chem. Commun. 2019, 55, 5540–5546. [Google Scholar] [CrossRef]
- Zhu, Y.; Ran, T.; Li, Y.; Guo, J.; Li, W. Dependence of the cytotoxicity of multi-walled carbon nanotubes on the culture medium. Nanotechnology 2006, 17, 4668. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Aitken, R.; Tran, L.; Stone, V.; Duffin, R.; Forrest, G.; Alexander, A. Carbon nanotubes: A review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol. Sci. 2006, 92, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhao, F.; Li, S.; Hu, Z.; Zhao, Y. Low-toxic and safe nanomaterials by surface-chemical design, carbon nanotubes, fullerenes, metallofullerenes, and graphenes. Nanoscale 2011, 3, 362–382. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Y.; Sun, B.; Chen, C. Understanding the toxicity of carbon nanotubes. Accounts Chem. Res. 2013, 46, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Chetyrkina, M.R.; Fedorov, F.S.; Nasibulin, A.G. In vitro toxicity of carbon nanotubes: A systematic review. RSC Adv. 2022, 12, 16235–16256. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; McNeil, S.E. Understanding the correlation between in vitro and in vivo immunotoxicity tests for nanomedicines. J. Control. Release 2013, 172, 456–466. [Google Scholar] [CrossRef]
- Andersen, M.L.; Winter, L.M. Animal models in biological and biomedical research-experimental and ethical concerns. An. Acad. Bras. Ciências 2017, 91, e20170238. [Google Scholar] [CrossRef]
- Kiani, A.K.; Pheby, D.; Henehan, G.; Brown, R.; Sieving, P.; Sykora, P.; Marks, R.; Falsini, B.; Capodicasa, N.; Miertus, S.; et al. Ethical considerations regarding animal experimentation. J. Prev. Med. Hyg. 2022, 63, E255. [Google Scholar]
- Robinson, N.B.; Krieger, K.; Khan, F.M.; Huffman, W.; Chang, M.; Naik, A.; Yongle, R.; Hameed, I.; Krieger, K.; Girardi, L.N.; et al. The current state of animal models in research: A review. Int. J. Surg. 2019, 72, 9–13. [Google Scholar] [CrossRef]
- Freires, I.A.; Morelo, D.F.C.; Soares, L.F.F.; Costa, I.S.; de Araújo, L.P.; Breseghello, I.; Abdalla, H.B.; Lazarini, J.G.; Rosalen, P.L.; Pigossi, S.C.; et al. Progress and promise of alternative animal and non-animal methods in biomedical research. Arch. Toxicol. 2023, 97, 2329–2342. [Google Scholar] [CrossRef]
- Khabib, M.N.H.; Sivasanku, Y.; Lee, H.B.; Kumar, S.; Kue, C.S. Alternative animal models in predictive toxicology. Toxicology 2022, 465, 153053. [Google Scholar] [CrossRef]
- IARC. Some Nanomaterialsand Some Fibreson the Evaluation of Carcinogenic Risks to Human; IARC: Lyon, France, 2017; Volume 111, pp. 1–325. [Google Scholar]
- WHO. WHO Guidelines on Protecting Workers from Potential Risks of Manufactured Nanomaterials; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Bergamaschi, E.; Garzaro, G.; Wilson Jones, G.; Buglisi, M.; Caniglia, M.; Godono, A.; Bosio, D.; Fenoglio, I.; Guseva Canu, I. Occupational exposure to carbon nanotubes and carbon nanofibres: More than a cobweb. Nanomaterials 2021, 11, 745. [Google Scholar] [CrossRef]
- Castranova, V.; Schulte, P.A.; Zumwalde, R.D. Occupational nanosafety considerations for carbon nanotubes and carbon nanofibers. Accounts Chem. Res. 2013, 46, 642–649. [Google Scholar] [CrossRef]
- Goverment of Canada. Engineered Nanoparticles—Health and Safety Considerations; Goverment of Canada: Ottawa, ON, Canada, 2016.
- Goverment of United Kingdom. Using Nanomaterials at Work; Goverment of United Kingdom: London, UK, 2013.
- US EPA. Control of Nanoscale Materials under the Toxic Substances Control Act; US EPA: Washington, DC, USA, 2023.
- Department of Occupational Safety and Health. Guidelines on Control and Safe Handling of Nanomaterials; Department of Occupational Safety and Health: Putrajaya, Malaysia, 2018.
- Pietroiusti, A.; Stockmann-Juvala, H.; Lucaroni, F.; Savolainen, K. Nanomaterial exposure, toxicity, and impact on human health. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1513. [Google Scholar] [CrossRef]
- Lee, J.S.; Choi, Y.C.; Shin, J.H.; Lee, J.H.; Lee, Y.; Park, S.Y.; Baek, J.E.; Park, J.D.; Ahn, K.; Yu, I.J. Health surveillance study of workers who manufacture multi-walled carbon nanotubes. Nanotoxicology 2015, 9, 802–811. [Google Scholar] [CrossRef]
- Schulte, P.A.; Leso, V.; Niang, M.; Iavicoli, I. Current state of knowledge on the health effects of engineered nanomaterials in workers: A systematic review of human studies and epidemiological investigations. Scand. J. Work. Environ. Health 2019, 45, 217. [Google Scholar] [CrossRef]
- Dahm, M.M.; Evans, D.E.; Schubauer-Berigan, M.K.; Birch, M.E.; Fernback, J.E. Occupational exposure assessment in carbon nanotube and nanofiber primary and secondary manufacturers. Ann. Occup. Hyg. 2012, 56, 542–556. [Google Scholar]
- Taghavi, S.M.; Momenpour, M.; Azarian, M.; Ahmadian, M.; Souri, F.; Taghavi, S.A.; Sadeghain, M.; Karchani, M. Effects of nanoparticles on the environment and outdoor workplaces. Electron. Physician 2013, 5, 706. [Google Scholar]
- NIOSH. Occupational Exposure to Carbon Nanotubes and Nanofibers. Curr. Intell. Bull. 2013, 65, 145. [Google Scholar]
- Wu, W.T.; Jung, W.T.; Lee, H.L. Lipid peroxidation metabolites associated with biomarkers of inflammation and oxidation stress in workers handling carbon nanotubes and metal oxide nanoparticles. Nanotoxicology 2021, 15, 577–587. [Google Scholar] [CrossRef]
- Nasirzadeh, N.; Azari, M.R.; Rasoulzadeh, Y.; Mohammadian, Y. An assessment of the cytotoxic effects of graphene nanoparticles on the epithelial cells of the human lung. Toxicol. Ind. Health 2019, 35, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, P.; Hedmer, M.; Rissler, J. Carbon nanotubes-Exposure, toxicology and protective measures in the work environment. In Kunskapsöversikt; Arbetsmiljöverket: Goteborg, Sweden, 2011. [Google Scholar]
- Canu, I.G.; Batsungnoen, K.; Maynard, A.; Hopf, N. State of knowledge on the occupational exposure to carbon nanotubes. Int. J. Hyg. Environ. Health 2020, 225, 113472. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Leo, B.F.; Murphy, F. The toxic truth about carbon nanotubes in water purification: A perspective view. Nanoscale Res. Lett. 2018, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Kobayashi, N. Evaluations of the Carcinogenicity of Carbon Nanotubes, Fluoro-Edinite, and Silicon Carbide by the International Agency for Research on Cancer (IARC). Nihon Eiseigaku Zasshi. Jpn. J. Hyg. 2016, 71, 252–259. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fukushima, S.; Kasai, T.; Umeda, Y.; Ohnishi, M.; Sasaki, T.; Matsumoto, M. Carcinogenicity of multi-walled carbon nanotubes: Challenging issue on hazard assessment. J. Occup. Health 2018, 60, 10–30. [Google Scholar] [CrossRef] [PubMed]
- Barbarino, M.; Giordano, A. Assessment of the carcinogenicity of carbon nanotubes in the respiratory system. Cancers 2021, 13, 1318. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Castro-Wallace, S.L.; Rodrigues, D.F. Acute toxicity of graphene nanoplatelets on biological wastewater treatment process. Environ. Sci. Nano 2017, 4, 160–169. [Google Scholar] [CrossRef]
- Martín-de Lucía, I.; Campos-Mañas, M.C.; Agüera, A.; Leganés, F.; Fernández-Piñas, F.; Rosal, R. Combined toxicity of graphene oxide and wastewater to the green alga Chlamydomonas reinhardtii. Environ. Sci. Nano 2018, 5, 1729–1744. [Google Scholar] [CrossRef]
- Bubak, G.; Ansaldo, A.; Gendron, D.; Brayda, L.; Ceseracciu, L.; Ricci, D. Parylene coated carbon nanotube actuators for tactile stimulation. In Proceedings of the Electroactive Polymer Actuators and Devices (EAPAD) 2015, San Diego, CA, USA, 9–12 March 2015; Volume 9430, pp. 215–222. [Google Scholar]
- Wan, Y.J.; Gong, L.X.; Tang, L.C.; Wu, L.B.; Jiang, J.X. Mechanical properties of epoxy composites filled with silane-functionalized graphene oxide. Compos. Part A Appl. Sci. Manuf. 2014, 64, 79–89. [Google Scholar] [CrossRef]
- Bubak, G.; Gendron, D.; Ceseracciu, L.; Ansaldo, A.; Ricci, D. Parylene-coated ionic liquid–carbon nanotube actuators for user-safe haptic devices. ACS Appl. Mater. Interfaces 2015, 7, 15542–15550. [Google Scholar] [CrossRef]
- McNally, T.; Pötschke, P. Polymer-Carbon Nanotube Composites: Preparation, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]


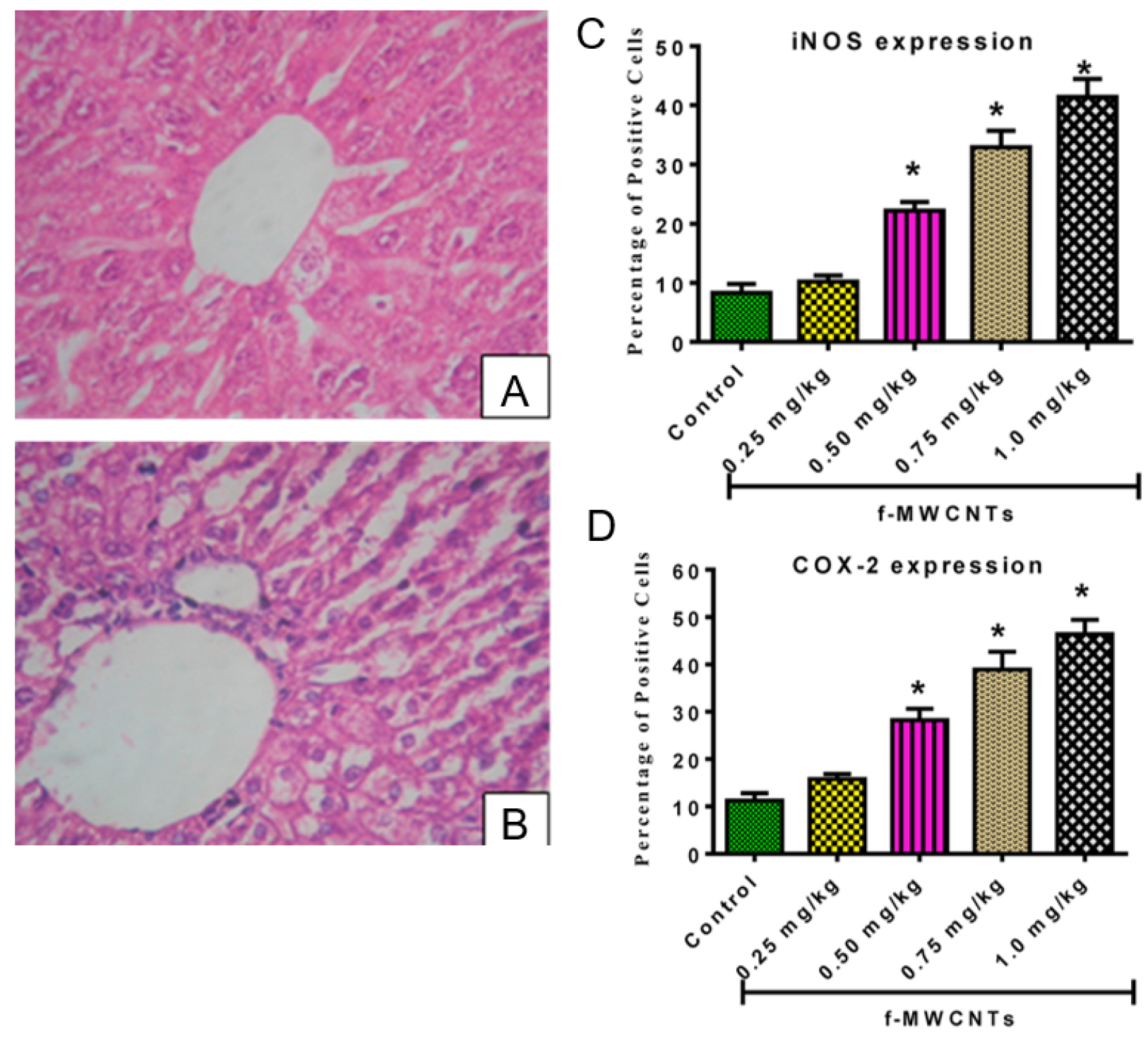
| Reference, Year, Paper Type | Summary | What Was Tested and How? | Additional Comments |
|---|---|---|---|
| [118], 2014, review | Graphene, graphene oxide, and reduced graphene oxide elicit toxic effects both in vitro and in vivo. Surface modifications can significantly reduce their toxic interactions with living systems. | Discussed toxicological effects and potential toxicity mechanisms of graphene, graphene oxide, and reduced graphene oxide in bacteria and mammalian cells. | The generation of reactive oxygen species (ROS) is the most commonly acknowledged mechanism responsible for the toxicity induced by graphene nanomaterials in living systems. The toxicity of graphene nanomaterials in biological systems can be significantly influenced by their physicochemical properties (i.e., particle size, particulate form, surface functional groups, and oxygen content/surface charges). |
| [119], 2017, review | Graphene toxicity is a double-edged sword in terms of risks and opportunities. Authors confirm that the most recent research reports establish that graphene in any of its numerous forms and derivatives must be treated as a potentially hazardous material. | Analysis of the effects of graphene on microorganisms, protozoa, plants, invertebrates and lower vertebrates as well as in vitro and in vivo models. | The significant discrepancy and frequent controversy among experimental results, even within closely related models, emphasize the need for systematic, coordinated, multi-center research. This should include thorough physicochemical characterization of the graphene materials used in each study. |
| [120], 2018, original article | Graphene nanopores are likely to have a low bioavailability in lung cancer cells and rats. Graphene nanopores caused early apoptosis in both SKMES-1 and A549 lung cancer cells. | in vitro and in vivo studies, early and late apoptosis studies in SKMES-1 and A549 cells, sub chronic toxicity in rats intraperitoneally injected with graphene material, blood biochemistry, liver and kidney enzymes functions analysis, oxidative stress biomarkers, histological examinations. | We have described graphene nanopores as they are a relatively new derivative of graphene. The authors showed that cellular toxicity increases with the dose when studying single vs. multiple doses. However, an appropriate control is with reduced graphene oxide, which is the source material for nanopores. |
| [121], 2019, review | The biodegradability of flat nanomaterials is essential in living organisms. Oxidative enzymes (i.e., peroxidases) can catalyse the degradation of graphene oxide in test tubes, in vitro and in vivo. The biodegradation of both single- and few-layer graphene was proven. | Analysis of state-of-the-art publications concerning the biocompatibility and biodegradability of graphene-related materials. | Most of the research conducted so far has been in vitro studies, but it is crucial to expand critical validation tests to whole-model organisms/animal models. |
| Reference, Year, Paper Type | Summary | What Was Tested and How? | Additional Comments |
|---|---|---|---|
| [122], 2006, original article | Multi-walled carbon nanotubes might be either toxic or non-toxic depending on the medium used to cultivate Tetrahymena pyriformis. | Experiments with various doses of multi-walled carbon nanotubes (MWNTs). Evaluated growth of Tetrahymena pyriformis, level of malondialdehyde, and superoxide dismutase activity. | Studies regarding the biological effects of the interaction of MWCNTs with certain ingredients of culture media would help us understand the mechanisms carbon nanotube toxicity to living systems. |
| [123], 2006, review | CNTs appear to possess a unique ability to stimulate mesenchymal cell growth and cause granuloma formation and fibrogenesis. In several studies, CNTs show more adverse effects than the same mass of NP carbon and quartz—used as a benchmark of particle toxicity. | Analysis of workplace safety based on the published toxicity of inhaled CNTs, mesenchymal cell growth, granuloma formation, fibrogenesis, oxidative stress, and inflammation. | CNTs should be considered in the same way as other bio-persistent fibers in workplace risk assessments. Therefore, similar similar control and assessment approaches should be taken. |
| [124], 2011, feature article | How to create low-toxic CNTs. The toxicity grade for a nanomaterial depends on more than ten factors (i.e., adsorbability, size, surface charge, or chemical modifications). | The analysis of biocompatibility, adme regulation, toxicity of carbon nanotubes, nanotoxicity mechanisms, and cellular and molecular interactions. | It is possible to alter the biological and toxicological properties of carbon nanomaterials by chemical modifications, therefore affecting the way in which these modified CNTs interact with biological systems. |
| [125], 2013, review | The underlying mechanisms of CNT toxicity include oxidative stress, inflammatory responses, malignant transformation, DNA damage and mutation, and the formation of granulomas and interstitial fibrosis. | Oxidative stress, inflammatory responses, malignant transformation, DNA damage and mutation (errors in chromosome number and disruption of mitotic spindles), formation of granulomas, and interstitial fibrosis. Reviewed data from several cell lines and animal models (rats, mice). | Researchers need to standardize their choices in terms of the cell line, animal species, and exposure conditions to ensure comparable results among different institutions and countries. |
| [126], 2022, review | CNTs located on a substrate had negligible impact, i.e., 90% of studies report good viability and cell behavior similar to the control; therefore, CNTs could be considered as a prospective conductive substrate for cell culture. CNTs are a promising platform for fundamental studies in targeted drug delivery, chemotherapy, tissue engineering, biosensing fields, etc. | Analysis of nearly 200 original publications regarding parameters such as toxicity doses, studied animal cell types, the impact of incubation time, applied toxicity tests, and viability. | Diameter, length, purification procedure, and synthesis may significantly affect CNT toxicity. The authors emphasize an urgent need for a straightforward, standardized, universal approach for the testing of the materials’ safety or toxic impact before conducting costly and time-consuming studies according to guidelines such as the OECD principles for proper laboratory practice. |
| Agent | IARC Group | Year | CAS |
|---|---|---|---|
| Carbon black | 2B | 2010 | 1333-86-4 |
| CNTs, single-walled | 3 | 2017 | 308068-56-6 |
| CNTs, multi-walled, MWCNT-7 | 2B | 2017 | 308068-56-6 |
| CNsT, multi-walled, other than MWCNT-7 | 3 | 2017 | 308068-56-6 |
| Graphene-based materials | — | — | 7782-42-5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gendron, D.; Bubak, G. Carbon Nanotubes and Graphene Materials as Xenobiotics in Living Systems: Is There a Consensus on Their Safety? J. Xenobiot. 2023, 13, 740-760. https://doi.org/10.3390/jox13040047
Gendron D, Bubak G. Carbon Nanotubes and Graphene Materials as Xenobiotics in Living Systems: Is There a Consensus on Their Safety? Journal of Xenobiotics. 2023; 13(4):740-760. https://doi.org/10.3390/jox13040047
Chicago/Turabian StyleGendron, David, and Grzegorz Bubak. 2023. "Carbon Nanotubes and Graphene Materials as Xenobiotics in Living Systems: Is There a Consensus on Their Safety?" Journal of Xenobiotics 13, no. 4: 740-760. https://doi.org/10.3390/jox13040047
APA StyleGendron, D., & Bubak, G. (2023). Carbon Nanotubes and Graphene Materials as Xenobiotics in Living Systems: Is There a Consensus on Their Safety? Journal of Xenobiotics, 13(4), 740-760. https://doi.org/10.3390/jox13040047






