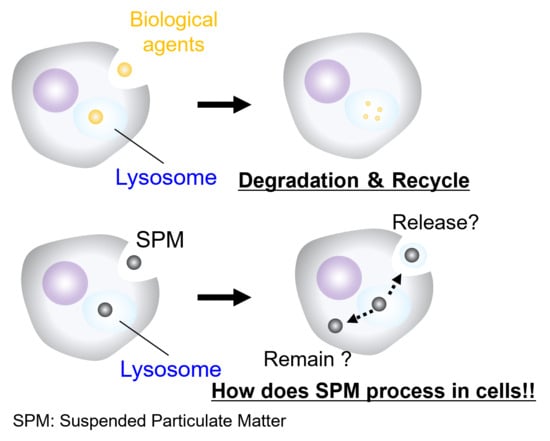
The Cellular Accumulation of Vehicle Exhaust Particulates Changes the Acidic pH Environment of Lysosomes in BEAS-2B Airway Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
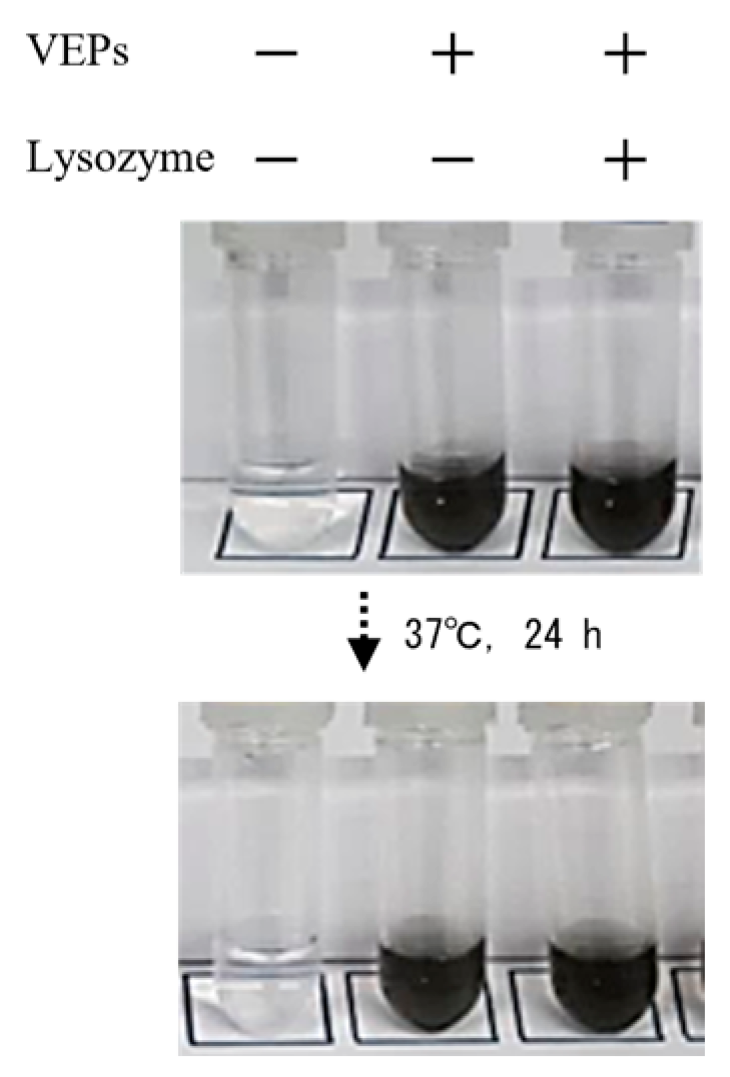
2.2. Model Materials of SPM
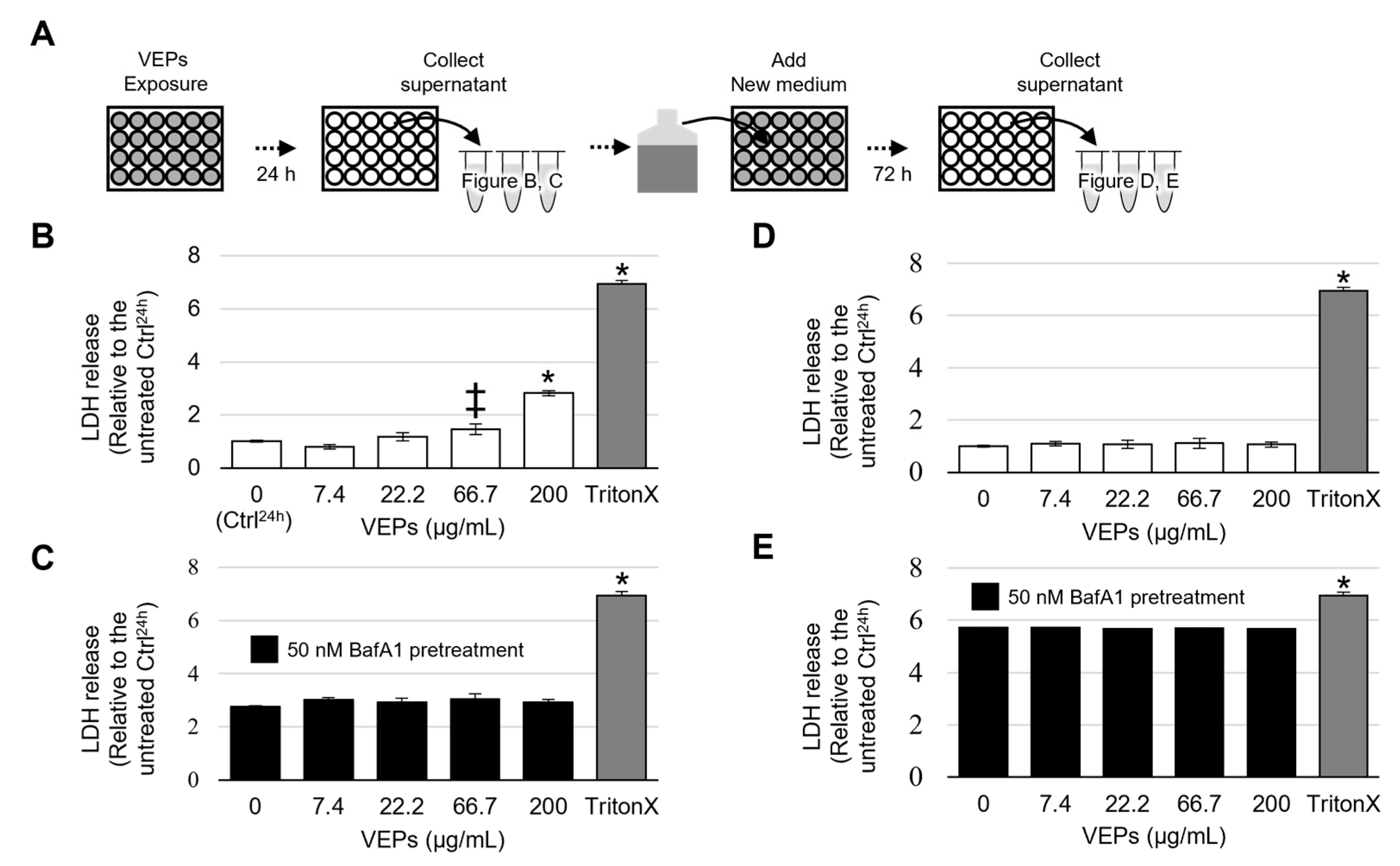
2.3. Lactate Dehydrogenase Cytotoxicity Assay
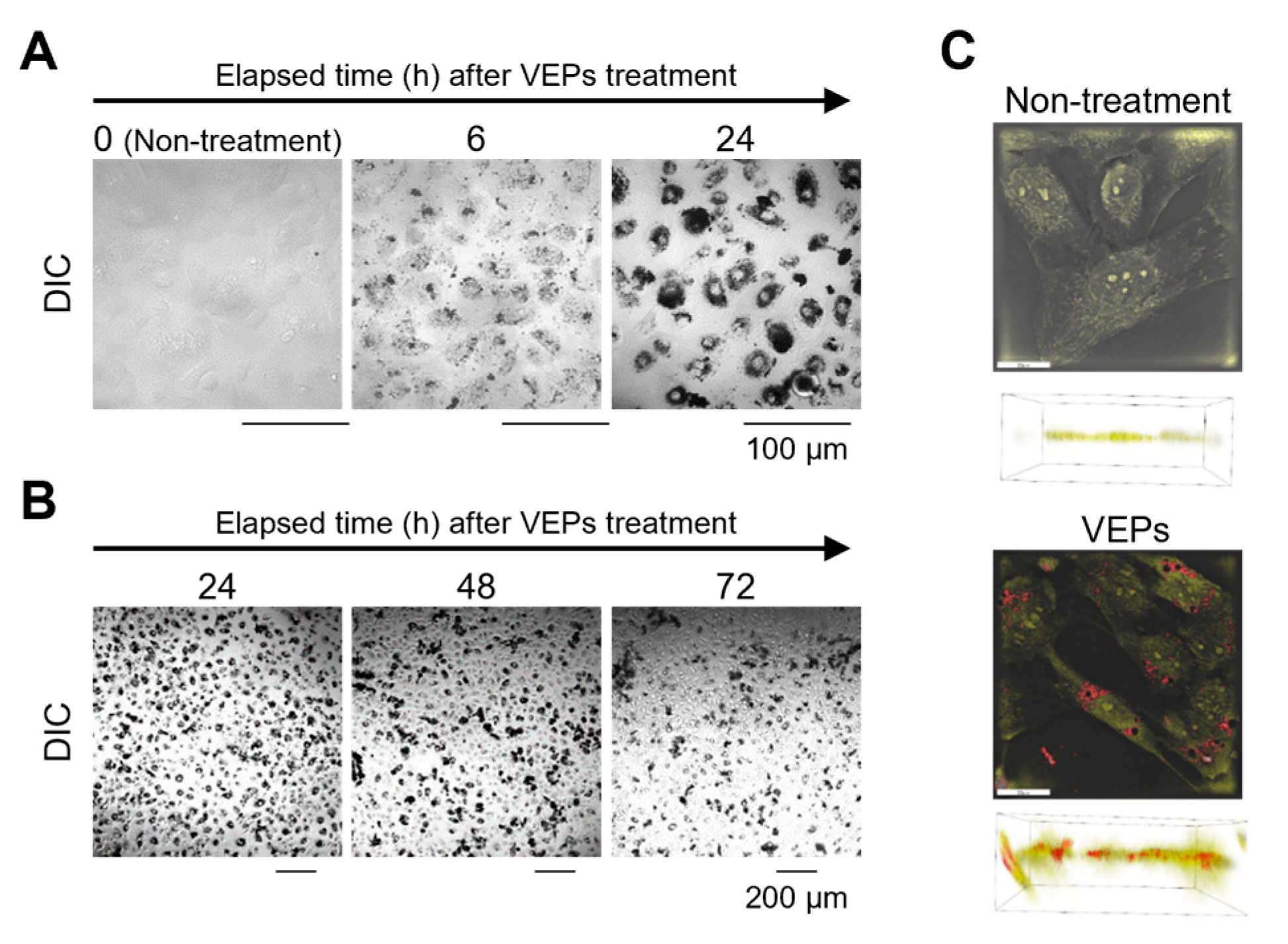
2.4. Intracellular Dynamics of VEPs
2.5. Lysosomal Acidification Stability
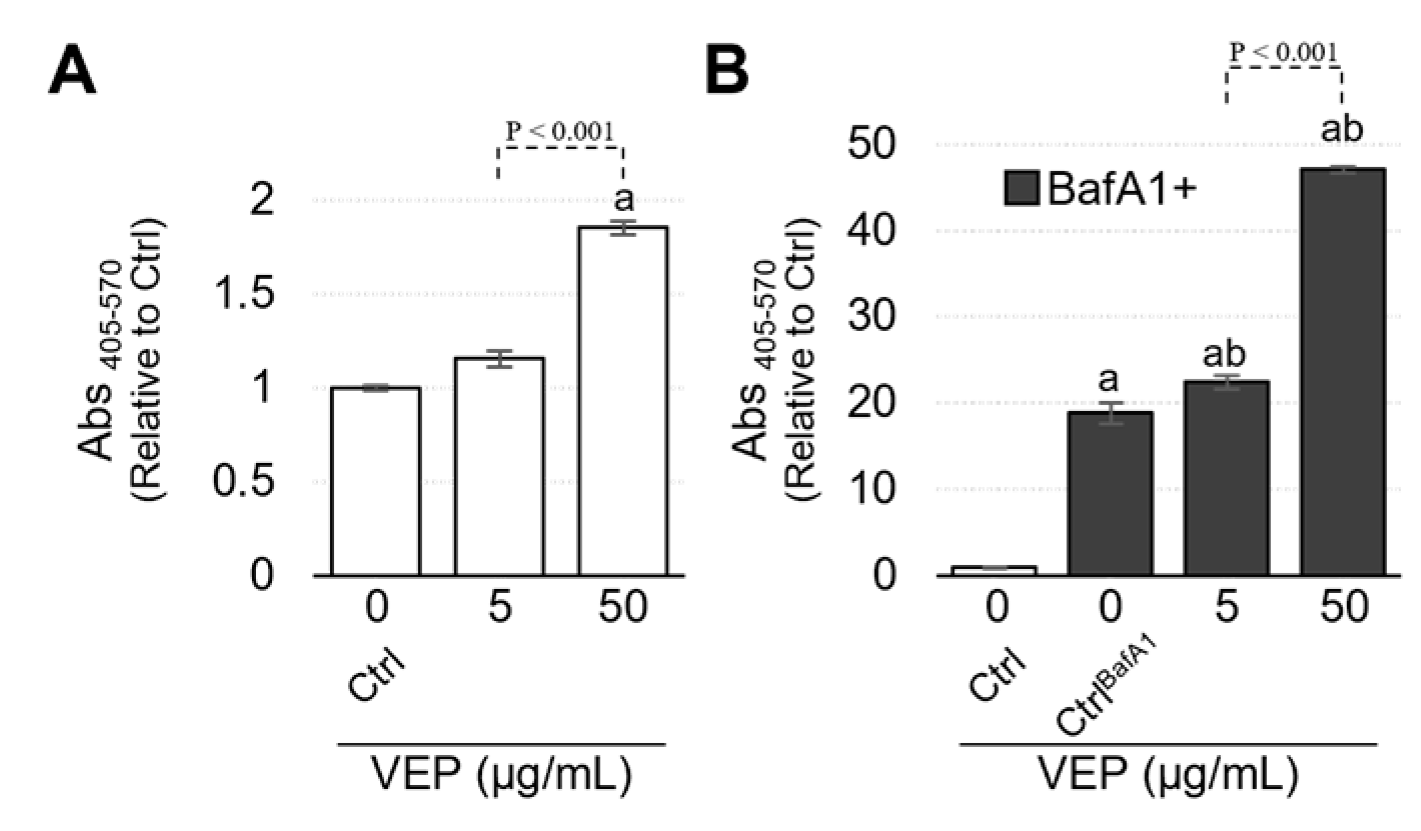
2.6. Beta Hexosaminidase (β-HEX) Activity Assay
2.7. Statistical Analysis
3. Results
3.1. Cytotoxicity of VEPs
3.2. Chronological Observation of VEPs’ Ingestion and Evacuation
3.3. Lysosomes Were Deoxidized by the Accumulation of VEPs
3.4. VEPs Promote the Release of β HEX
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, R.; Jin, X.; Li, H.; Huang, L.; Li, Z. The mechanisms of PM2.5 and its main components penetrate into HUVEC cells and effects on cell organelles. Chemosphere 2020, 241, 125127. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wang, J.; Li, J.; Jiang, N.; Zhang, R.; Yang, W.; Yao, W.; Wu, W. Oxidative stress and endocytosis are involved in upregulation of interleukin-8 expression in airway cells exposed to PM2.5. Environ. Toxicol. 2016, 31, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Hanley, S.; Cooper, K. Sorting Nexins in Protein Homeostasis. Cells 2021, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Isaka, Y.; Yoshimori, T. Autophagy and kidney inflammation. Autophagy 2017, 13, 997–1003. [Google Scholar] [CrossRef]
- Emanuel, R.; Sergin, I.; Bhattacharya, S.; Turner, J.; Epelman, S.; Settembre, C.; Diwan, A.; Ballabio, A.; Razani, B. Induction of lysosomal biogenesis in atherosclerotic macrophages can rescue lipid-induced lysosomal dysfunction and downstream sequelae. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1942–1952. [Google Scholar] [CrossRef]
- Khatib, I.; Rychter, P.; Falfushynska, H. Pesticide Pollution: Detrimental Outcomes and Possible Mechanisms of Fish Exposure to Common Organophosphates and Triazines. J. Xenobiot. 2022, 12, 236–265. [Google Scholar] [CrossRef]
- Schroder, K.; Zhou, R.; Tschopp, J. The NLRP3 inflammasome: A sensor for metabolic danger? Science 2010, 327, 296–300. [Google Scholar] [CrossRef]
- Martinon, F.; Pétrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef]
- Duan, S.; Wang, N.; Huang, L.; Zhao, Y.; Shao, H.; Jin, Y.; Zhang, R.; Li, C.; Wu, W.; Wang, J.; et al. NLRP3 inflammasome activation is associated with PM 2.5-induced cardiac functional and pathological injury in mice. Environ. Toxicol. 2019, 34, 1246–1254. [Google Scholar] [CrossRef]
- Niu, L.; Li, L.; Xing, C.; Luo, B.; Hu, C.; Song, M.; Niu, J.; Ruan, Y.; Sun, X.; Lei, Y. Airborne particulate matter (PM 2.5) triggers cornea inflammation and pyroptosis via NLRP3 activation. Ecotoxicol. Environ. Saf. 2021, 207, 111306. [Google Scholar] [CrossRef]
- Colarusso, C.; Terlizzi, M.; Molino, A.; Pinto, A.; Sorrentino, R. Role of the inflammasome in chronic obstructive pulmonary disease (COPD). Oncotarget 2017, 8, 81813–81824. [Google Scholar] [CrossRef] [PubMed]
- Theofani, E.; Semitekolou, M.; Morianos, I.; Samitas, K.; Xanthou, G. Targeting NLRP3 inflammasome activation in severe asthma. J. Clin. Med. 2019, 8, 1615. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.Y.; Yao, R.-Q.; Li, Y.-X.; Zhao, P.Y.; Ren, C.; Du, X.-H.; Yao, Y.-M. Lysosomal quality control of cell fate: A novel therapeutic target for human diseases. Cell Death Dis. 2020, 11, 817. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Ding, Y.; Zhong, L.; Jiang, L.; Geng, C.; Yao, X.; Cao, J. Tacrine induces apoptosis through lysosome- and mitochondria-dependent pathway in HepG2 cells. Toxicol. Vitro 2014, 28, 667–674. [Google Scholar] [CrossRef]
- Stoka, V.; Turk, V.; Turk, B. Lysosomal cathepsins and their regulation in aging and neurodegeneration. Ageing Res. Rev. 2016, 32, 22–37. [Google Scholar] [CrossRef]
- Zou, J.; Kawai, T.; Tsuchida, T.; Kozaki, T.; Tanaka, H.; Shin, K.-S.; Kumar, H.; Akira, S. Poly IC triggers a cathepsin D- and IPS-1-dependent pathway to enhance cytokine production and mediate dendritic cell necroptosis. Immunity 2013, 38, 717–728. [Google Scholar] [CrossRef]
- Chen, R.; Jäättelä, M.; Liu, B. Lysosome as a central hub for rewiring PH homeostasis in tumors. Cancers 2020, 12, 2437. [Google Scholar] [CrossRef]
- Nääv, Å.; Erlandsson, L.; Isaxon, C.; Frostner, E.; Ehinger, J.; Sporre, M.; Krais, A.; Strandberg, B.; Lundh, T.; Elmér, E.; et al. Urban PM2.5 Induces Cellular Toxicity, Hormone Dysregulation, Oxidative Damage, Inflammation, and Mitochondrial Interference in the HRT8 Trophoblast Cell Line. Front. Endocrinol. 2020, 11, 75. [Google Scholar] [CrossRef]
- Wang, S.; Liu, F.; Zeng, Z.; Yang, H.; Jiang, H. The protective effect of bafilomycin A1 Against cobalt nanoparticle-induced cytotoxicity and aseptic inflammation in macrophages in vitro. Biol. Trace Elem. Res. 2016, 169, 94–105. [Google Scholar] [CrossRef]
- Miyayama, T.; Matsuoka, M. Involvement of lysosomal dysfunction in silver nanoparticle-induced cellular damage in A549 human lung alveolar epithelial cells. J. Occup. Med. Toxicol. 2016, 11, 20616. [Google Scholar] [CrossRef]
- Schütz, I.; Hernandez, T.L.; Gao, Q.; Puchkov, D.; Jabs, S.; Nordmeyer, D.; Schmudde, M.; Rühl, E.; Graf, C.M.; Haucke, V. Lysosomal dysfunction caused by cellular accumulation of silica nanoparticles. J. Biol. Chem. 2016, 291, 14170–14184. [Google Scholar] [CrossRef] [PubMed]
- Hannafon, B.; Ding, W. Intercellular communication by exosome-derived microRNAs in cancer. Int. J. Mol. Sci. 2013, 14, 14240–14269. [Google Scholar] [CrossRef] [PubMed]
- Sipos, A.; Kim, K.-J.; Chow, R.H.; Flodby, P.; Borok, Z.; Crandall, E.D.; Sipos, A.; Kim, K.-J.; Chow, R.H.; Flodby, P.; et al. Alveolar epithelial cell processing of nanoparticles activates autophagy and lysosomal exocytosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 315, L286–L300. [Google Scholar] [CrossRef] [PubMed]
- Yanes, R.E.; Tarn, D.; Hwang, A.A.; Ferris, D.P.; Sherman, S.P.; Thomas, C.R.; Lu, J.; Pyle, A.D.; Zink, J.I.; Tamanoi, F. Involvement of lysosomal exocytosis in the excretion of mesoporous silica nanoparticles and enhancement of drug delivery effect by exocytosis inhibition. Small 2013, 9, 697–704. [Google Scholar] [CrossRef]
- Buratta, S.; Tancini, B.; Sagini, K.; Delo, F.; Chiaradia, E.; Urbanelli, L.; Emiliani, C. Lysosomal exocytosis, exosome release and secretory autophagy: The autophagic- and endo-lysosomal systems go extracellular. Int. J. Mol. Sci. 2020, 21, 2576. [Google Scholar] [CrossRef]
- Lee, C.; Lamech, L.; Johns, E.; Overholtzer, M. Selective Lysosome Membrane Turnover Is Induced by Nutrient Starvation. Dev. Cell 2020, 55, 289–297. [Google Scholar] [CrossRef]
- Samie, M.; Xu, H. Lysosomal exocytosis and lipid storage disorders. J. Lipid. Res. 2014, 55, 995–1009. [Google Scholar] [CrossRef]
- Kopacz, A.; Kloska, D.; Forman, H.J.; Jozkowicz, A.; Przeczek, A.G. Beyond repression of Nrf2: An update on Keap1. Free Radic. Biol. Med. 2020, 157, 63–74. [Google Scholar] [CrossRef]
- Liu, E.; Lu, X.; Wang, D. A Systematic Review of Carbon Capture, Utilization and Storage: Status, Progress and Challenges. Energies 2023, 16, 2865. [Google Scholar] [CrossRef]
- Shibata, Y.; Morikawa, T. Review of the JCAP/JATOP Air Quality Model Study in Japan. Atmosphere 2021, 12, 943. [Google Scholar] [CrossRef]
- Walsh, C.; Rayner, P.; Simmons, J.; Fiddes, S.; Schofield, R.; Bridgman, H.; Beaupark, S.; Broome, R.; Chambers, S.; Chang, L.; et al. A Clean Air Plan for Sydney: An Overview of the Special Issue on Air Quality in New South Wales. Atmosphere 2019, 10, 774. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onodera, A.; Shimomura, T.; Ochi, H.; Sunada, R.; Fukutomi, E.; Hidaka, K.; Kawai, Y. The Cellular Accumulation of Vehicle Exhaust Particulates Changes the Acidic pH Environment of Lysosomes in BEAS-2B Airway Epithelial Cells. J. Xenobiot. 2023, 13, 653-661. https://doi.org/10.3390/jox13040042
Onodera A, Shimomura T, Ochi H, Sunada R, Fukutomi E, Hidaka K, Kawai Y. The Cellular Accumulation of Vehicle Exhaust Particulates Changes the Acidic pH Environment of Lysosomes in BEAS-2B Airway Epithelial Cells. Journal of Xenobiotics. 2023; 13(4):653-661. https://doi.org/10.3390/jox13040042
Chicago/Turabian StyleOnodera, Akira, Takuya Shimomura, Hirohisa Ochi, Ryuto Sunada, Eiko Fukutomi, Koushi Hidaka, and Yuichi Kawai. 2023. "The Cellular Accumulation of Vehicle Exhaust Particulates Changes the Acidic pH Environment of Lysosomes in BEAS-2B Airway Epithelial Cells" Journal of Xenobiotics 13, no. 4: 653-661. https://doi.org/10.3390/jox13040042
APA StyleOnodera, A., Shimomura, T., Ochi, H., Sunada, R., Fukutomi, E., Hidaka, K., & Kawai, Y. (2023). The Cellular Accumulation of Vehicle Exhaust Particulates Changes the Acidic pH Environment of Lysosomes in BEAS-2B Airway Epithelial Cells. Journal of Xenobiotics, 13(4), 653-661. https://doi.org/10.3390/jox13040042