Plastic Contamination in Seabass and Seabream from Off-Shore Aquaculture Facilities from the Mediterranean Sea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Preparation
2.3. Quantification and Characterization of Particles Using µFT-IR
2.4. Statistical Analysis
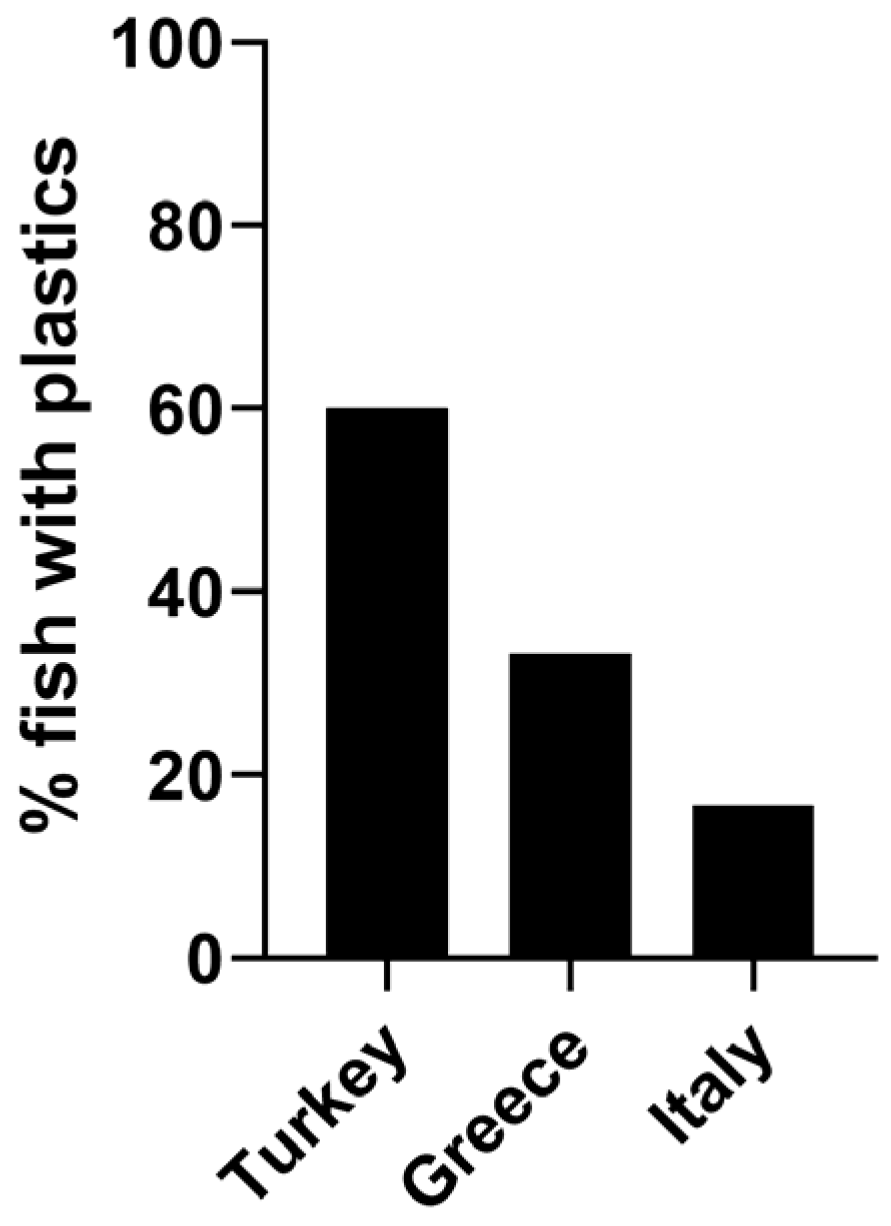
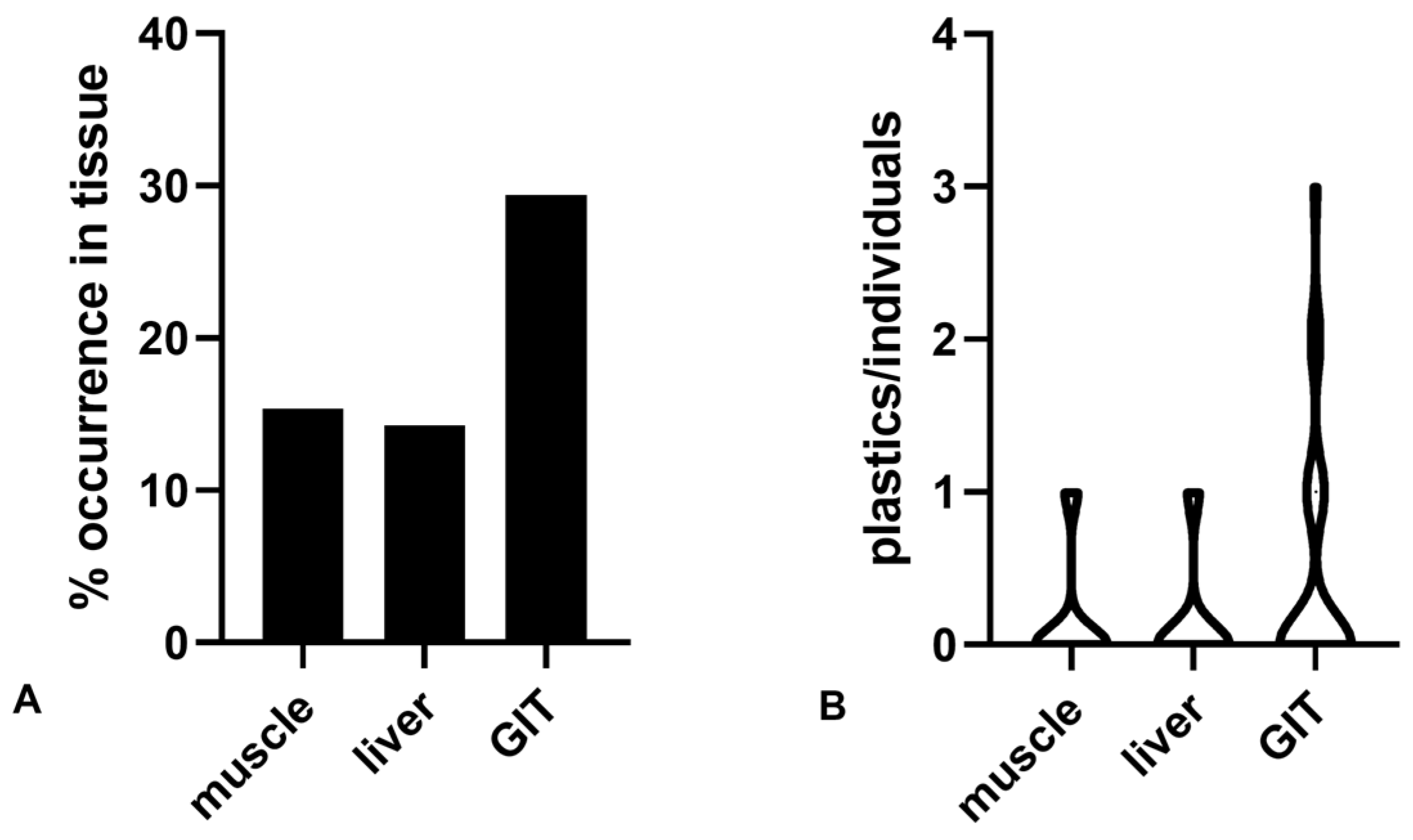
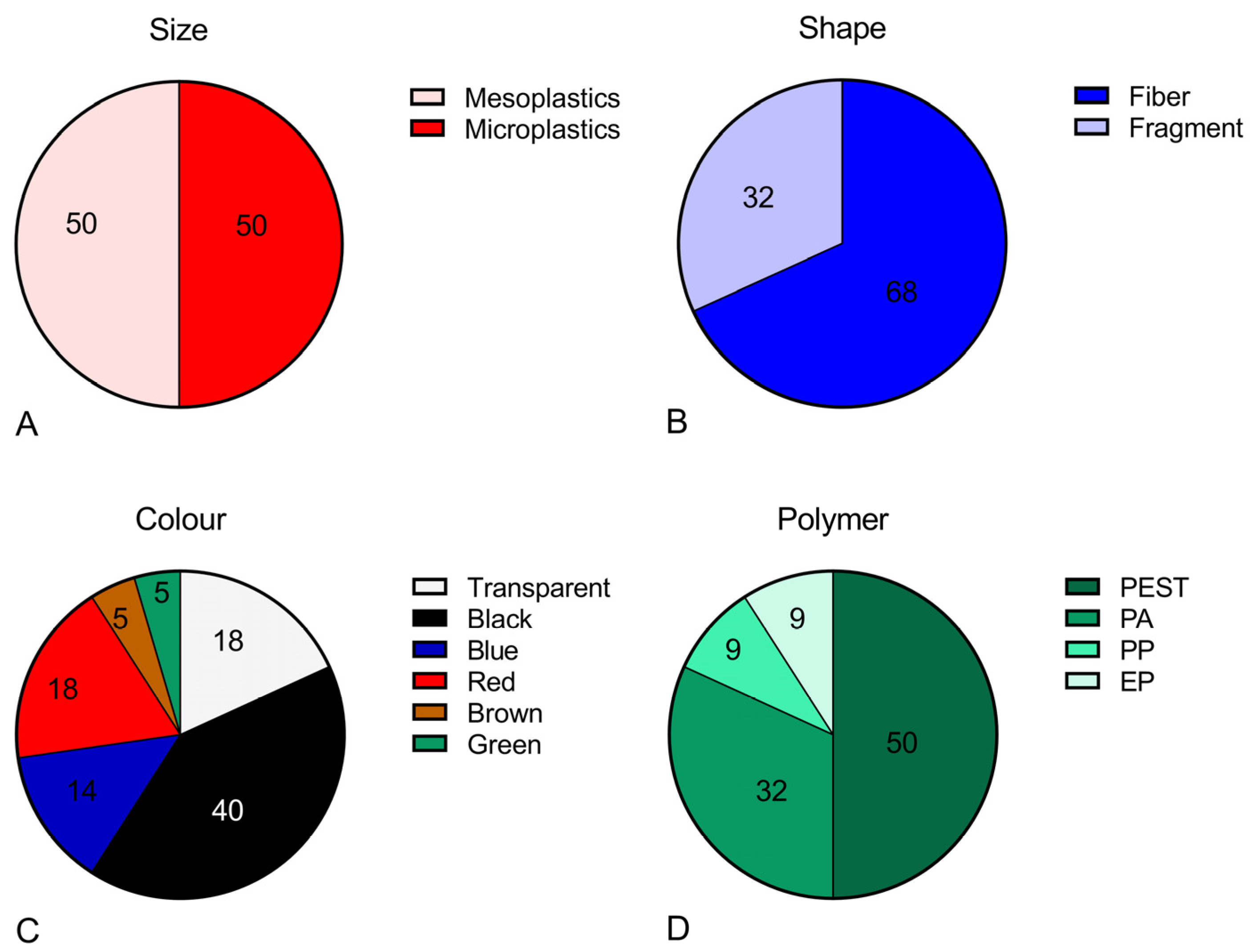
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petersen, F.; Hubbart, J.A. The occurrence and transport of microplastics: The state of the science. Sci. Total Environ. 2021, 758, 143936. [Google Scholar] [CrossRef] [PubMed]
- Thiele, C.J.; Hudson, M.D.; Russell, A.E.; Saluveer, M.; Sidaoui-Haddad, G. Microplastics in fish and fishmeal: An emerging environmental challenge? Sci. Rep. 2021, 11, 2045. [Google Scholar] [CrossRef] [PubMed]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in seafood and the implications for human health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Garrido Gamarro, E.; Ryder, J.; Elvevoll, E.O.; Olsen, R.L. Microplastics in fish and shellfish—A threat to seafood safety? J. Aquat. Food Prod. 2020, 29, 417–425. [Google Scholar] [CrossRef]
- Galloway, T.S. Micro-and nano-plastics and human health. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: New York, NY, USA, 2015; pp. 343–366. [Google Scholar]
- Giani, D.; Baini, M.; Galli, M.; Casini, S.; Fossi, M.C. Microplastics occurrence in edible fish species (Mullus barbatus and Merluccius merluccius) collected in three different geographical sub-areas of the Mediterranean Sea. Mar. Pollut. Bull. 2019, 140, 129–137. [Google Scholar] [CrossRef]
- Walkinshaw, C.; Lindeque, P.K.; Thompson, R.; Tolhurst, T.; Cole, M. Microplastics and seafood: Lower trophic organisms at highest risk of contamination. Ecotox. Environ. Safe. 2020, 190, 110066. [Google Scholar] [CrossRef]
- Vázquez-Rowe, I.; Ita-Nagy, D.; Kahhat, R. Microplastics in fisheries and aquaculture: Implications to food sustainability and safety. Curr. Opin. Green Sustain. Chem. 2021, 29, 100464. [Google Scholar] [CrossRef]
- Catarino, A.I.; Macchia, V.; Sanderson, W.G.; Thompson, R.C.; Henry, T.B. Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibres fallout during a meal. Environ. Pollut. 2018, 237, 675–684. [Google Scholar] [CrossRef]
- Prata, J.C. Microplastics in wastewater: State of the knowledge on sources, fate and solutions. Mar. Pollut. Bull. 2018, 129, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human consumption of microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [PubMed]
- Ageel, H.K.; Harrad, S.; Abdallah, M.A.E. Occurrence, human exposure, and risk of microplastics in the indoor environment. Environ. Sci. Process. Impacts 2022, 24, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Daviglus, M.; Sheeshka, J.; Murkin, E. Health benefits from eating fish. Comments Toxicol. 2002, 8, 345–374. [Google Scholar] [CrossRef]
- European Market Observatory for Fisheries and Aquaculture Products. The EU Fish Market 2020 Edition. Available online: https://www.eumofa.eu/documents/20178/415635/EN_The+EU+fish+market_2020.pdf (accessed on 18 May 2023).
- European Market Observatory for Fisheries and Aquaculture Products. Available online: https://www.eumofa.eu/documents/20178/121372/PTAT+Case+Study+-+Seabass+in+the+EU.pdf (accessed on 18 May 2023).
- Llorente García, I.; Fernández Polanco, J.M.; Odriozola Zamanillo, M.D.; Bjørndal, T.; Asche, F.; Guillen, J.; Avdelas, L.; Nielsen, R.; Cozzolino, M.; Luna, M.; et al. Assessment of the economic performance of the seabream and seabass aquaculture industry in the European Union. Mar. Policy 2020, 117, 103876. [Google Scholar] [CrossRef]
- European Market Observatory for Fisheries and Aquaculture Products. Case Study—Price Structure in the Supply Chain for Fresh Seabream in Italy. Available online: https://www.eumofa.eu/documents/20178/0/Price+structure+Seabream+in+Italy/f76598c2-50d3-4bc4-b98b-b13926430066 (accessed on 18 May 2023).
- European Market Observatory for Fisheries and Aquaculture Products. Available online: https://www.eumofa.eu/documents/20178/477018/EN_The+EU+fish+market_2021.pdf/27a6d912-a758-6065-c973-c1146ac93d30?t=1636964632989 (accessed on 18 May 2023).
- Food and Agriculture Organization. Available online: http://www.fao.org/fishery/species/2291/en (accessed on 18 May 2023).
- Food and Agriculture Organization. Available online: https://www.fao.org/fishery/en/aqspecies/sbg (accessed on 18 May 2023).
- Sánchez-Almeida, R.; Hernández-Sánchez, C.; Villanova-Solano, C.; Díaz-Peña, F.J.; Clemente, S.; González-Sálamo, J.; González-Pleiter, M.; Hernández-Borges, J. Microplastics Determination in Gastrointestinal Tracts of European Sea Bass (Dicentrarchus labrax) and Gilt-Head Sea Bream (Sparus aurata) from Tenerife (Canary Islands, Spain). Polymers 2022, 14, 1931. [Google Scholar] [CrossRef]
- Ferrante, M.; Pietro, Z.; Allegui, C.; Maria, F.; Antonio, C.; Pulvirenti, E.; Favara, C.; Chiara, C.; Grasso, A.; Omayma, M.; et al. Microplastics in fillets of Mediterranean seafood. A risk assessment study. Environ. Res. 2022, 204, 112247. [Google Scholar] [CrossRef]
- Reinold, S.; Herrera, A.; Saliu, F.; Hernández-González, C.; Martinez, I.; Lasagni, M.; Gómez, M. Evidence of microplastic ingestion by cultured European sea bass (Dicentrarchus labrax). Mar. Pollut. Bull. 2021, 168, 112450. [Google Scholar] [CrossRef]
- Bessa, F.; Barría, P.; Neto, J.M.; Frias, J.P.; Otero, V.; Sobral, P.; Marques, J.C. Occurrence of microplastics in commercial fish from a natural estuarine environment. Mar. Pollut. Bull. 2018, 128, 575–584. [Google Scholar] [CrossRef]
- Kılıç, E. Microplastic ingestion evidence by economically important farmed fish species from Turkey. Mar. Pollut. Bull. 2022, 183, 114097. [Google Scholar] [CrossRef]
- Akoueson, F.; Sheldon, L.M.; Danopoulos, E.; Morris, S.; Hotten, J.; Chapman, E.; Li, J.; Rotchell, J.M. A preliminary analysis of microplastics in edible versus non-edible tissues from seafood samples. Environ. Pollut. 2020, 263, 114452. [Google Scholar] [CrossRef] [PubMed]
- Savoca, S.; Matanović, K.; D’Angelo, G.; Vetri, V.; Anselmo, S.; Bottari, T.; Mancuso, M.; Kužir, S.; Spanò, N.; Capillo, G.; et al. Ingestion of plastic and non-plastic microfibers by farmed gilthead sea bream (Sparus aurata) and common carp (Cyprinus carpio) at different life stages. Sci. Total Environ. 2021, 782, 146851. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 2020, 717, 134625. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.A.; Ibrahim, M.I.; Shabaka, S.; Ghobashy, M.M.; Shreadah, M.A.; Ghani, S.A.A. Microplastics contamination in commercial fish from Alexandria City, the Mediterranean Coast of Egypt. Environ. Pollut. 2022, 313, 120044. [Google Scholar] [CrossRef]
- Binelli, A.; Della Torre, C.; Nigro, L.; Riccardi, N.; Magni, S. A realistic approach for the assessment of plastic contamination and its ecotoxicological consequences: A case study in the metropolitan city of Milan (N. Italy). Sci. Total Environ. 2022, 806, 150574. [Google Scholar] [CrossRef]
- Magni, S.; Della Torre, C.; Nigro, L.; Binelli, A. Can COVID-19 pandemic change plastic contamination? The Case study of seven watercourses in the metropolitan city of Milan (N. Italy). Sci. Total Environ. 2022, 831, 154923. [Google Scholar] [CrossRef]
- Lusher, A.L.; Tirelli, V.; O’Connor, I.; Officer, R. Microplastics in Arctic polar waters: The first reported values of particles in surface and sub-surface samples. Sci. Rep. 2015, 5, 14947. [Google Scholar] [CrossRef]
- Magni, S.; Binelli, A.; Pittura, L.; Avio, C.G.; Della Torre, C.; Parenti, C.C.; Gorbi, S.; Regoli, F. The fate of microplastics in an Italian Wastewater Treatment Plant. Sci. Total Environ. 2019, 652, 602–610. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Huffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef]
- Chen, G.; Li, Y.; Wang, J. Occurrence and ecological impact of microplastics in aquaculture ecosystems. Chemosphere 2021, 274, 129989. [Google Scholar] [CrossRef]
- Zhou, A.; Zhang, Y.; Xie, S.; Chen, Y.; Li, X.; Wang, J.; Zou, J. Microplastics and their potential effects on the aquaculture systems: A critical review. Rev. Aquac. 2021, 13, 719–733. [Google Scholar] [CrossRef]
- Wu, H.; Hou, J.; Wang, X. A review of microplastic pollution in aquaculture: Sources, effects, removal strategies and prospects. Ecotox. Environ. Safe. 2023, 252, 114567. [Google Scholar] [CrossRef] [PubMed]
- Welden, N.A.; Cowie, P.R. Long-term microplastic retention causes reduced body condition in the langoustine, Nephrops norvegicus. Environ. Pollut. 2016, 218, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.D.; Weinstein, J.E. Size-and shape-dependent effects of microplastic particles on adult daggerblade grass shrimp (Palaemonetes pugio). Environ. Toxicol. Chem. 2017, 36, 3074–3080. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Vethaak, A.D.; Lavorante, B.R.; Lundebye, A.K.; Guilhermino, L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef]
- De Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef]
- Abihssira-García, I.S.; Kögel, T.; Gomiero, A.; Kristensen, T.; Krogstad, M.; Olsvik, P.A. Distinct polymer-dependent sorption of persistent pollutants associated with Atlantic salmon farming to microplastics. Mar. Pollut. Bull. 2022, 180, 113794. [Google Scholar] [CrossRef]
- Cholewińska, P.; Moniuszko, H.; Wojnarowski, K.; Pokorny, P.; Szeligowska, N.; Dobicki, W.; Polechoński, R.; Górniak, W. The occurrence of microplastics and the formation of biofilms by pathogenic and opportunistic bacteria as threats in aquaculture. Int. J. Environ. Res. 2022, 19, 8137. [Google Scholar] [CrossRef]
- Tsangaris, C.; Digka, N.; Valente, T.; Aguilar, A.; Borrell, A.; de Lucia, G.A.; Gambaiani, D.; Garcia-Garin, O.; Kaberi, H.; Martin, J.; et al. Using Boops boops (osteichthyes) to assess microplastic ingestion in the Mediterranean Sea. Mar. Pollut. Bull. 2020, 158, 111397. [Google Scholar] [CrossRef]
- Güven, O.; Gökdağ, K.; Jovanović, B.; Kıdeyş, A.E. Microplastic litter composition of the Turkish territorial waters of the Mediterranean Sea, and its occurrence in the gastrointestinal tract of fish. Environ. Pollut. 2017, 223, 286–294. [Google Scholar] [CrossRef]
- Mistri, M.; Sfriso, A.A.; Casoni, E.; Nicoli, M.; Vaccaro, C.; Munari, C. Microplastic accumulation in commercial fish from the Adriatic Sea. Mar. Pollut. Bul. 2022, 174, 113279. [Google Scholar] [CrossRef] [PubMed]
- Capillo, G.; Savoca, S.; Panarello, G.; Mancuso, M.; Branca, C.; Romano, V.; D’Angelo, G.; Bottari, T.; Spanò, N. Quali-quantitative analysis of plastics and synthetic microfibers found in demersal species from Southern Tyrrhenian Sea (Central Mediterranean). Mar. Pollut. Bull. 2020, 150, 110596. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, S.; Perez-Rubín, C.; Gavara, R.; Handcock, R.N.; Rivas, M.L. Presence and implications of plastics in wild commercial fishes in the Alboran Sea (Mediterranean Sea). Sci. Total Environ. 2022, 850, 158025. [Google Scholar] [CrossRef] [PubMed]
- Savoca, S.; Capillo, G.; Mancuso, M.; Bottari, T.; Crupi, R.; Branca, C.; Romano, V.; Faggio, C.; D’Angelo, G.; Spanò, N. Microplastics occurrence in the Tyrrhenian waters and in the gastrointestinal tract of two congener species of seabreams. Environ. Toxicol. Pharmacol. 2019, 67, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Cimmaruta, R.; Giovannini, S.; Bianchi, J.; Matiddi, M.; Bellisario, B.; Nascetti, G. Microplastics occurrence in fish with different habits from the central Tyrrhenian Sea. Reg. Stud. Mar. Sci. 2022, 52, 102251. [Google Scholar] [CrossRef]
- Kılıç, E.; Yücel, N. Microplastic occurrence in the gastrointestinal tract and gill of bioindicator fish species in the northeastern Mediterranean. Mar. Pollut. Bull. 2022, 177, 113556. [Google Scholar] [CrossRef]
- Sayed, A.E.D.H.; Hamed, M.; Badrey, A.E.; Ismail, R.F.; Osman, Y.A.; Osman, A.G.; Soliman, H.A. Microplastic distribution, abundance, and composition in the sediments, water, and fishes of the Red and Mediterranean seas, Egypt. Mar. Pollut. Bull. 2021, 173, 112966. [Google Scholar] [CrossRef]
- Savoca, M.S.; McInturf, A.G.; Hazen, E.L. Plastic ingestion by marine fish is widespread and increasing. Glob. Chang. Biol. 2021, 27, 2188–2199. [Google Scholar] [CrossRef]
- Abbasi, S.; Soltani, N.; Keshavarzi, B.; Moore, F.; Turner, A.; Hassanaghaei, M. Microplastics in different tissues of fish and prawn from the Musa Estuary, Persian Gulf. Chemosphere 2018, 205, 80–87. [Google Scholar] [CrossRef]
- Ramsperger, A.F.R.M.; Narayana, V.K.B.; Gross, W.; Mohanraj, J.; Thelakkat, M.; Greiner, A.; Schmalz, H.; Kress, H.; Laforsch, C. Environmental exposure enhances the internalization of microplastic particles into cells. Sci. Adv. 2020, 6, eabd1211. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016, 14, e04501. [Google Scholar]
- Liu, L.; Xu, K.; Zhang, B.; Ye, Y.; Zhang, Q.; Jiang, W. Cellular internalization and release of polystyrene microplastics and nanoplastics. Sci. Total Environ. 2021, 779, 146523. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization. Available online: https://www.fao.org/3/ca9231en/CA9231EN.pdf (accessed on 20 September 2023).
- Lebreton, L.C.; Van Der Zwet, J.; Damsteeg, J.W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef] [PubMed]
- Serranti, S.; Palmieri, R.; Bonifazi, G.; Cózar, A. Characterization of microplastic litter from oceans by an innovative approach based on hyperspectral imaging. Waste Manag. 2018, 76, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Alvito, A.; Bellodi, A.; Cau, A.; Moccia, D.; Mulas, A.; Palmas, F.; Pesci, P.; Follesa, M.C. Amount and distribution of benthic marine litter along Sardinian fishing grounds (CW Mediterranean Sea). Waste Manag. 2018, 75, 131–140. [Google Scholar] [CrossRef]
- Liubartseva, S.; Coppini, G.; Lecci, R.; Clementi, E. Tracking plastics in the Mediterranean: 2D Lagrangian model. Mar. Pollut. Bull. 2018, 129, 151–162. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S.; Shetti, N.P.; Nadagouda, M.N.; Aminabhavi, T.M. Microplastics in the environment: Occurrence, perils, and eradication. Chem. Eng. J. 2021, 408, 127317. [Google Scholar] [CrossRef]
- Chan, H.S.H.; Dingle, C.; Not, C. Evidence for non-selective ingestion of microplastic in demersal fish. Mar. Pollut. Bull. 2019, 149, 110523. [Google Scholar] [CrossRef]
- Wu, J.; Ye, Q.; Sun, L.; Liu, J.; Huang, M.; Wang, T.; Wu, P.; Zhu, N. Impact of persistent rain on microplastics distribution and plastisphere community: A field study in the Pearl River, China. Sci. Total Environ. 2023, 879, 163066. [Google Scholar] [CrossRef]
- Skirtun, M.; Sandra, M.; Strietman, W.J.; van den Burg, S.W.; De Raedemaecker, F.; Devriese, L.I. Plastic pollution pathways from marine aquaculture practices and potential solutions for the North-East Atlantic region. Mar. Pollut. Bull. 2022, 174, 113178. [Google Scholar] [CrossRef]
- Wang, W.; Do, A.T.N.; Kwon, J.H. Ecotoxicological effects of micro-and nanoplastics on terrestrial food web from plants to human beings. Sci. Total Environ. 2022, 834, 155333. [Google Scholar] [CrossRef] [PubMed]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef] [PubMed]
- De Falco, F.; Di Pace, E.; Cocca, M.; Avella, M. The contribution of washing processes of synthetic clothes to microplastic pollution. Sci. Rep. 2019, 9, 6633. [Google Scholar] [CrossRef] [PubMed]
- Magni, S.; Nigro, L.; Della Torre, C.; Binelli, A. Characterization of plastics and their ecotoxicological effects in the Lambro River (N. Italy). J. Hazard. Mater. 2021, 412, 125204. [Google Scholar] [CrossRef]
- Rochman, C.M.; Brookson, C.; Bikker, J.; Djuric, N.; Earn, A.; Bucci, K.; Athey, S.; Huntington, A.; McIlwraith, H.; Munno, K.; et al. Rethinking microplastics as a diverse contaminant suite. Environ. Toxicol. Chem. 2019, 38, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Dibke, C.; Fischer, M.; Scholz-Böttcher, B.M. Microplastic mass concentrations and distribution in German bight waters by pyrolysis–gas chromatography–mass spectrometry/thermochemolysis reveal potential impact of marine coatings: Do ships leave skid marks? Environ. Sci. Technol. 2021, 55, 2285–2295. [Google Scholar] [CrossRef]
- Lusher, A.L.; Mchugh, M.; Thompson, R.C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef]
- Remy, F.; Collard, F.; Gilbert, B.; Compère, P.; Eppe, G.; Lepoint, G. When microplastic is not plastic: The ingestion of artificial cellulose fibers by macrofauna living in seagrass macrophytodetritus. Environ. Sci. Technol. 2015, 49, 11158–11166. [Google Scholar] [CrossRef]
- Compa, M.; Ventero, A.; Iglesias, M.; Deudero, S. Ingestion of microplastics and natural fibres in Sardina pilchardus (Walbaum, 1792) and Engraulis encrasicolus (Linnaeus, 1758) along the Spanish Mediterranean coast. Mar. Pollut. Bull. 2018, 128, 89–96. [Google Scholar] [CrossRef]
- Gago, J.; Carretero, O.; Filgueiras, A.V.; Viñas, L. Synthetic microfibers in the marine environment: A review on their occurrence in seawater and sediments. Mar. Pollut. Bull. 2018, 127, 365–376. [Google Scholar] [CrossRef]
- Acharya, S.; Rumi, S.S.; Hu, Y.; Abidi, N. Microfibers from synthetic textiles as a major source of microplastics in the environment: A review. Text. Res. J. 2021, 91, 2136–2156. [Google Scholar] [CrossRef]
- Athey, S.N.; Erdle, L.M. Are we underestimating anthropogenic microfiber pollution? A critical review of occurrence, methods, and reporting. Environ. Toxicol. Chem. 2022, 41, 822–837. [Google Scholar] [CrossRef] [PubMed]
| Matrix | Geographic Region | Extraction Technique | Instrumental Analysis | Limits of the Method | Range Concentration | Ref. |
|---|---|---|---|---|---|---|
| GIT of D. labrax and S. aurata (aquaculture) | Canary Islands Spain | Digestion in 10% KOH (w/v) at 60 °C for 24 h, filtration (50 µm mesh stainless-steel filters) | Visual sorting with microscope. MP characterization with FTIR | 50 µm | Items/ind. Range: 0–23 Items length range: 69 µm–12.4 mm | [23] |
| Muscle of wild and farmed S. aurata | Tunisia | Mineralization with 65% nitric acid, extraction with dichloromethane (DCM), and dispersion in aluminum stubs for scanning electron microscope: “SEM Specimen Stubs” | Scanning electron microscopy coupled to a X energy dispersion detector (SEM-EDX) | <10 µm | Smallest and biggest median (IQR) diameter of MPs (1.8 and 2.5 μm). In S. aurata farmed mean MPs ± SD (p/g) 9.50 × 104 ± 6.64 × 104. Min-Max (p/g) 4.97 × 104–21.20 × 104. Median (IQR) 7.38 × 104 (5.53–14.54 × 104). Mean diameter ± SD (μm) 2.04 ± 0.30. Min-Max (μm) 1.7–2.4. Median (IQR) 1.9 (1.8–2.35) | [24] |
| GIT of D. labrax (aquaculture) | Canary Islands Spain | Digestion with 10% KOH (2 weeks, room temperature), filtration (25 μm mesh stainless-steel filters), stock in 10% EDTA (1 day) | Visual sorting with microscope. Larger particles (>200 μm) analyzed with FT-IR. Smaller particles (<200 μm) analyzed with a μ-FTIR | 10 µm | 0.6 ± 0.8–2.7 ± 1.85 particles/ind | [25] |
| GIT of D. labrax (aquaculture) | Mondego estuary Portugal | Digestion with 10% KOH (5 days, 60 °C), filtration (1.2 μm filter papers) desiccation at 60 °C | Visual sorting with stereomicroscope. MPs characterization with μ-FTIR | ≤1 mm | 38% of the fish ingested MP average of 1.67 ± 0.27 (SD) particles/ind. Average 3.41 ± 2.91 (SD) microplastics/ind of the individuals that had ingested MPs | [26] |
| GIT of S. aurata and D. labrax (aquaculture) | Turkey | Digestion with 20 mL of 30% H2O2 per gram under heating, addition of 400 mL NaCl solution (1.2 g/mL NaCl), and filtration (50 μm pore size filters) | Visual sorting with microscope. MP characterization with FTIR | 50 µm (mesh size) | 50% of seabream and 52% of seabass contaminated. Mean MP abundance in the GIT 1 ± 1.6 particles/ind. MP abundance in seabream 0.8 ± 1.1 particles/ind and 0.95 ± 1.1 particles/ind in seabass. Mean length of MPs 1.4 ± 1.3 mm | [27] |
| Flesh, gills and GIT of D. labrax (aquaculture) | Greece | Digestion with 30% H2O2 (24 h, 65 °C at 80 rpm, followed by 24–48 h at room temperature), filtration (5 μm pore size, 47 mm diameter cellulose membrane filter) | Visual sorting with stereomicroscope MP characterization with µ-FT-IR. | 5 μm (pore size) | Incidence of contaminated fish 17% | [28] |
| GIT of S. aurata (aquaculture) | Italy and Croatia | Digestion with 10% KOH (48 h, 50 °C with oscillation), addition of hypersaline NaCl solution (15%), and filtration (glass fiber membrane with 1.5 mm and 0.7 mm pore size and 47 mm diameter). | Visual sorting with stereomicroscope. MP characterization with μ-FT-IR | 240 μm (smallest detected) | 0.21 (Fry) and 1.3 (adult) items/ind., respectively. 0.48 items/ind (Fry and adults). Fibers, ranging in size from 0.24 to 8.86 mm. | [29] |
| Flesh, gills, and GIT D. labrax (wild) | Portugal | Digestion with 10% KOH (24 h, 60 °C for flesh and GIT, 72 h, 40 °C for gills), filtration (glass-microfiber filter with 1.2 µm pore size). | Visual sorting with stereomicroscope. MP characterization with μ-FT-IR | <100 μm | 42% of fish contaminated; 1.3 ± 2.5 MP items/ind. in the GIT 0.8 ± 1.4 MP items/ind. in gills and 0.4 ± 0.7 MP items/g in the dorsal muscle | [30] |
| GIT S. aurata (wild) | Egypt | Digestion with 10% potassium hydroxide (KOH), incubation 40 °C; filtration on 20 μm and nitrocellulose filter. | Visual sorting with stereomicroscope. MPs characterization with differential scanning calorimetry (DSC) and thermal gravimetric analysis (TGA). | >20 μm | 93.3% of fish contaminated 38.3 ± 28.4 items/ind | [31] |
| Origin | Species | Organ | Shape | Dimension (mm) | Color | Chemical Composition |
|---|---|---|---|---|---|---|
| Turkey | S. aurata | GIT | Fragment | 1.30 | Green | PP |
| D. labrax | GIT | Fiber | 0.58 | Black | PEST | |
| D. labrax | GIT | Fiber | 0.28 | Blue | EP | |
| S. aurata | GIT | Fiber | 0.23 | Black | PA | |
| D. labrax | GIT | Fiber | 2.30 | Black | PA | |
| S. aurata | muscle | Fiber | 1.01 | Black | PEST | |
| D. labrax | GIT | Fragment | 0.27 | Brown | PA | |
| D. labrax | GIT | Fragment | 0.12 | Black | PP | |
| Greece | S. aurata | GIT | Fiber | 1.62 | Transparent | PEST |
| S. aurata | GIT | Fiber | 0.85 | Transparent | PA | |
| S. aurata | GIT | Fiber | 1.88 | Transparent | PA | |
| S. aurata | liver | Fiber | 1.30 | Blue | PA | |
| S. aurata | GIT | Fiber | 2.08 | Black | PEST | |
| S. aurata | GIT | Fiber | 1.09 | Red | PA | |
| S. aurata | GIT | Fiber | 1.59 | Black | PEST | |
| D. labrax | muscle | Fragment | 0.53 | Blue | EP | |
| D. labrax | GIT | Fiber | 0.50 | Black | PEST | |
| Italy | D. labrax | GIT | Fiber | 3.34 | Transparent | PEST |
| Matrix | Geographic Region | Extraction Technique | Instrumental Analysis | Limits of the Method | Range Concentration | Ref. |
|---|---|---|---|---|---|---|
| GIT of Boops boops | Spain, France, Italy, and Greece | Digestion with hydrogen peroxide (H2O2 15%), filtration under vacuum on fiber glass filters (pore size 1.2 μm) | Visual sorting with stereomicroscope. MP characterization with FTIR | 1.2 μm | 46.8% of positiveness, 1.17 ± 0.07 items/ind. 1–14 items per fish | [46] |
| GIT of 28 different species | Turkey | Digestion with 35% H2O2. Filtration with 26 μm zooplankton mesh | Visual sorting with stereomicroscope. MP characterization with FTIR | 26 μm | 58% of positiveness with average 2.36 items/ind. | [47] |
| GIT of 4 different demersal fish | Adriatic Sea | Digestion with 10% KOH, 48 h at 50 °C; separation with NaCl hypersaline solution; filtration under vacuum on GF/F fiber glass filters (0.7 μm). Staining with Nile red (9-diethylamino-5H-benzo[α]phenoxazine-5-one) | Visual sorting with stereomicroscope. MP characterization with μ-Raman spectroscopy | 0.7 μm | 57.5% of positiveness and up to 2.47 ± 2.99 items/ind. | [48] |
| GIT and gills of five demersal fish species | Southern Tyrrhenian Sea | No extraction. The GIT and the gills were inspected with the aid of a dissecting stereomicroscope | Visual sorting with stereomicroscope. MP characterization with ATR-FTIR and μ-Raman spectroscopy. | 1 μm (Raman spatial resolution) | 16.8% of positiveness with average 0.24 items/ind. | [49] |
| GIT of Mullus barbatus and Merluccius merluccius | North Tyrrhenian Sea, Adriatic Sea, and Ionian Sea | Extraction with 10% of KOH solution 1/3 v.v. incubated at 60 °C for 6 h after 15 min sonication, filtration on glass fiber filters (1.6 μm mesh) | Visual sorting with stereomicroscope. Test with hot needle technique. MP characterization with FTIR | >100 μm | 23.3% of positiveness, range 8.3–48% Average 1.38 plastics/ind. | [8] |
| GIT of Scyliorinus canicula and Mullus barbatus | Alboran Sea | Digestion with 10% KOH, homogenization, filtration on 150 μm sieve | Visual sorting with stereomicroscope (ultraviolet light or through a scanning electron microscope). MP characterization with μFTIR | 150 μm | 9.8 and 32.7% of positiveness (7 and 24 fibers/ind) | [50] |
| GIT of two congener species of seabreams: Pagellus erythrinus and P. bogarave | Tyrrhenian Sea | Manual extraction | Visual sorting with stereomicroscope. MP characterization with FTIR and Raman spectroscopy | Not reported | 12.5% of positiveness | [51] |
| GIT of Mullus barbatus and Umbrina cirrosa | Central Tyrrhenian Sea | Digestion with 5% HNO3 + 15% H2O2, incubation 40 °C; filtration on 2.7 μm glass microfiber membrane | Visual sorting with stereomicroscope. MP characterization with FTIR | 2.7 μm | 90% of positiveness, 3.4 ± 1.9 items/ind. and 1–8 as range | [52] |
| GIT of commercial fish | Egypt | Digestion with 10% potassium hydroxide (KOH), incubation 40 °C; filtration on 20 μm and nitrocellulose filter | Visual sorting with stereomicroscope. MP characterization with differential scanning calorimetry (DSC) and thermal gravimetric analysis (TGA) | >20 μm | 91.8 ± 8.4% of positiveness and an average of 11.7 ± 9.5 items/ind. | [31] |
| GIT and gills of demersal fish | Turkey | Digestion with 30% H2O2, filtration on 50 μm pore size filter | Visual sorting with microscope. MP characterization with FTIR | >50 μm | 85% of positiveness | [53] |
| Different species | Egypt | Digestion with 10% H2O2, incubation 50 °C; second digestion with 30% H2O2, filtration on 1 mm and 300 μm sieve | Visual sorting with microscope. MP characterization with ATR-FTIR. | >300 μm | 58% of positiveness, 2.36 items/ind. | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosconi, G.; Panseri, S.; Magni, S.; Malandra, R.; D’Amato, A.; Carini, M.; Chiesa, L.; Della Torre, C. Plastic Contamination in Seabass and Seabream from Off-Shore Aquaculture Facilities from the Mediterranean Sea. J. Xenobiot. 2023, 13, 625-640. https://doi.org/10.3390/jox13040040
Mosconi G, Panseri S, Magni S, Malandra R, D’Amato A, Carini M, Chiesa L, Della Torre C. Plastic Contamination in Seabass and Seabream from Off-Shore Aquaculture Facilities from the Mediterranean Sea. Journal of Xenobiotics. 2023; 13(4):625-640. https://doi.org/10.3390/jox13040040
Chicago/Turabian StyleMosconi, Giacomo, Sara Panseri, Stefano Magni, Renato Malandra, Alfonsina D’Amato, Marina Carini, Luca Chiesa, and Camilla Della Torre. 2023. "Plastic Contamination in Seabass and Seabream from Off-Shore Aquaculture Facilities from the Mediterranean Sea" Journal of Xenobiotics 13, no. 4: 625-640. https://doi.org/10.3390/jox13040040
APA StyleMosconi, G., Panseri, S., Magni, S., Malandra, R., D’Amato, A., Carini, M., Chiesa, L., & Della Torre, C. (2023). Plastic Contamination in Seabass and Seabream from Off-Shore Aquaculture Facilities from the Mediterranean Sea. Journal of Xenobiotics, 13(4), 625-640. https://doi.org/10.3390/jox13040040








