Microbiome: The Next Frontier in Psychedelic Renaissance
Abstract
1. Introduction
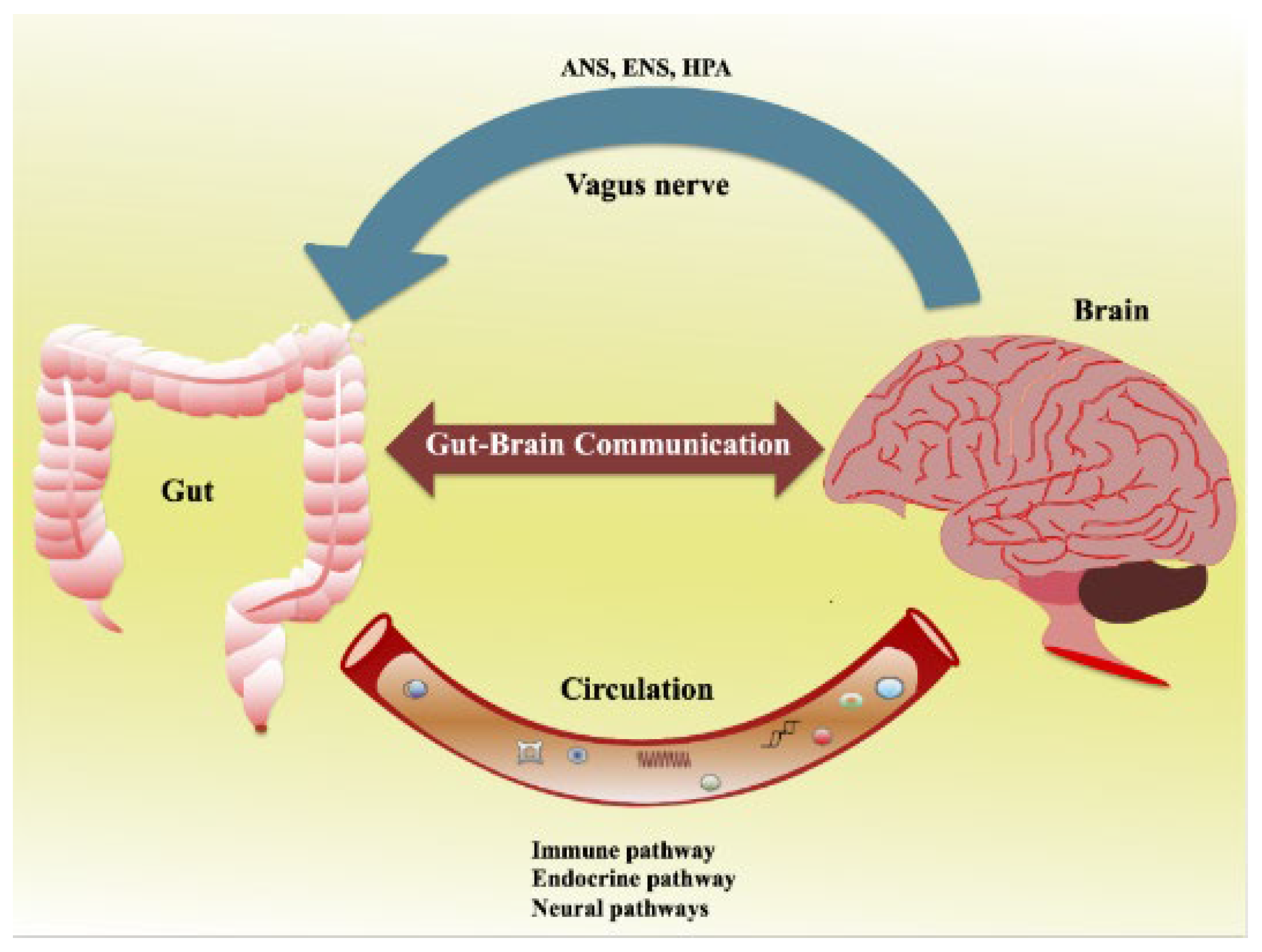
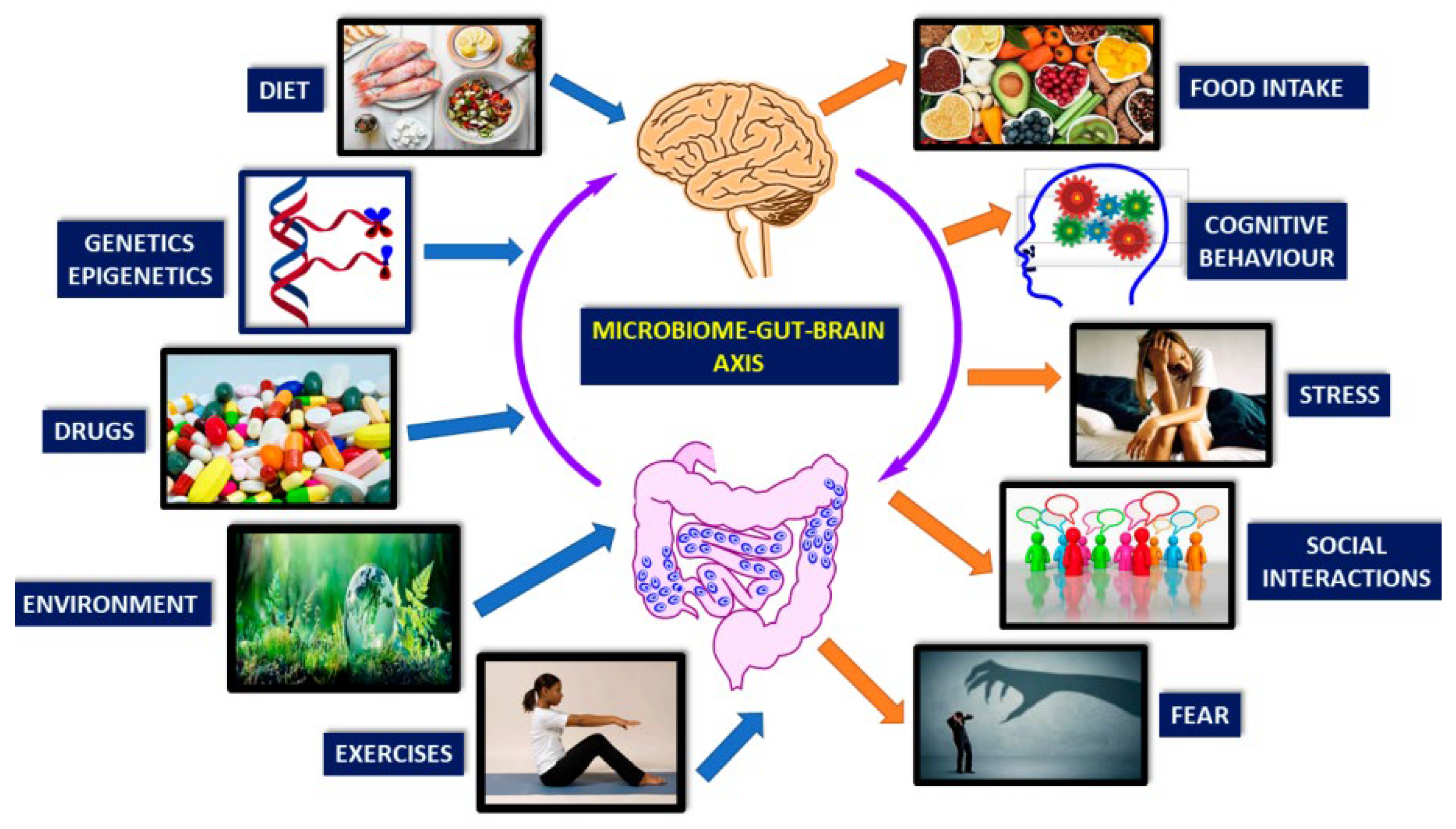
2. Understanding the Gut–Brain Axis
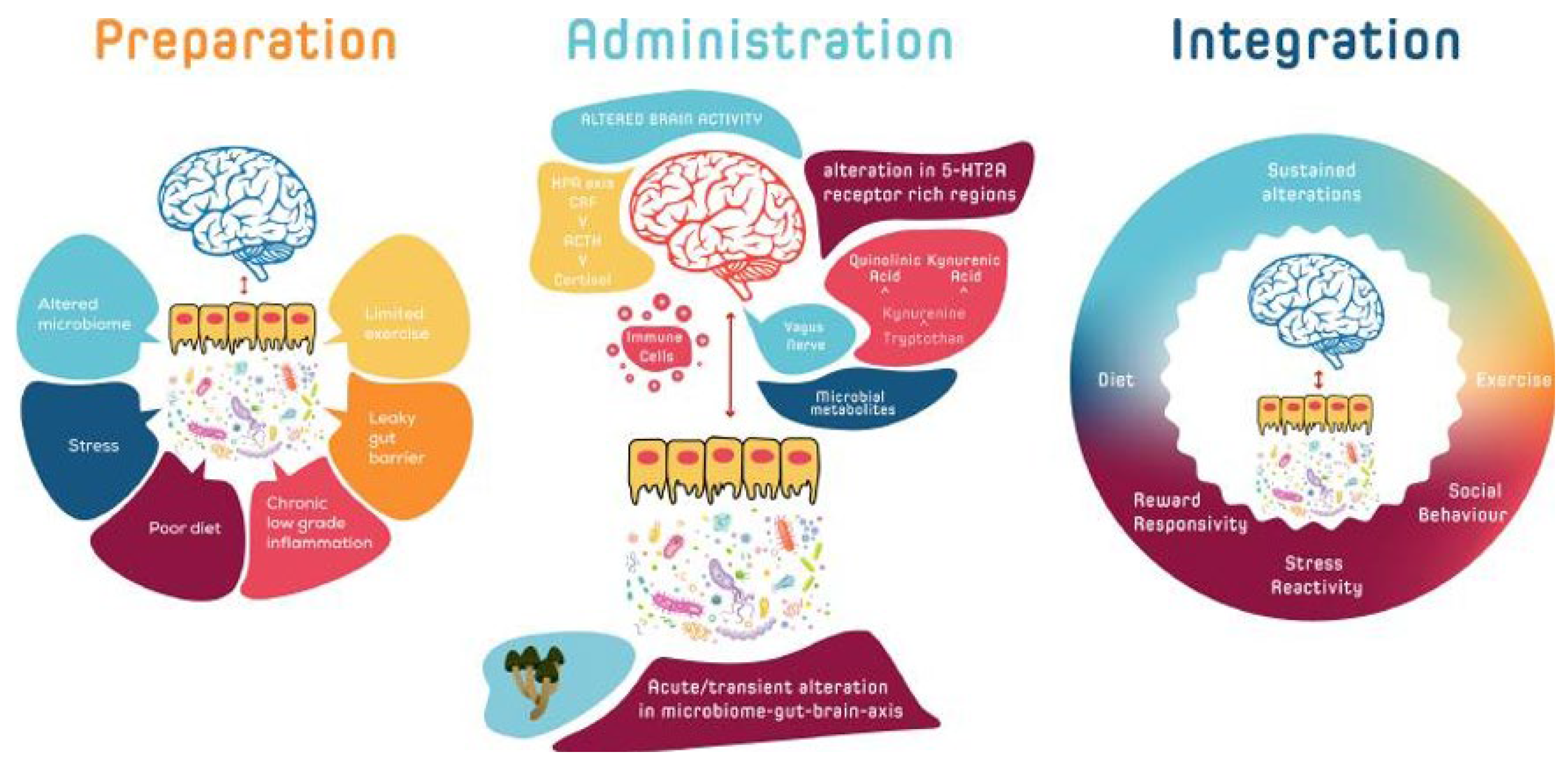
3. Microbiome and Psychedelic Interaction
4. Interpersonal Variability in Psychedelic Response
5. Implications for Mental Health Treatment
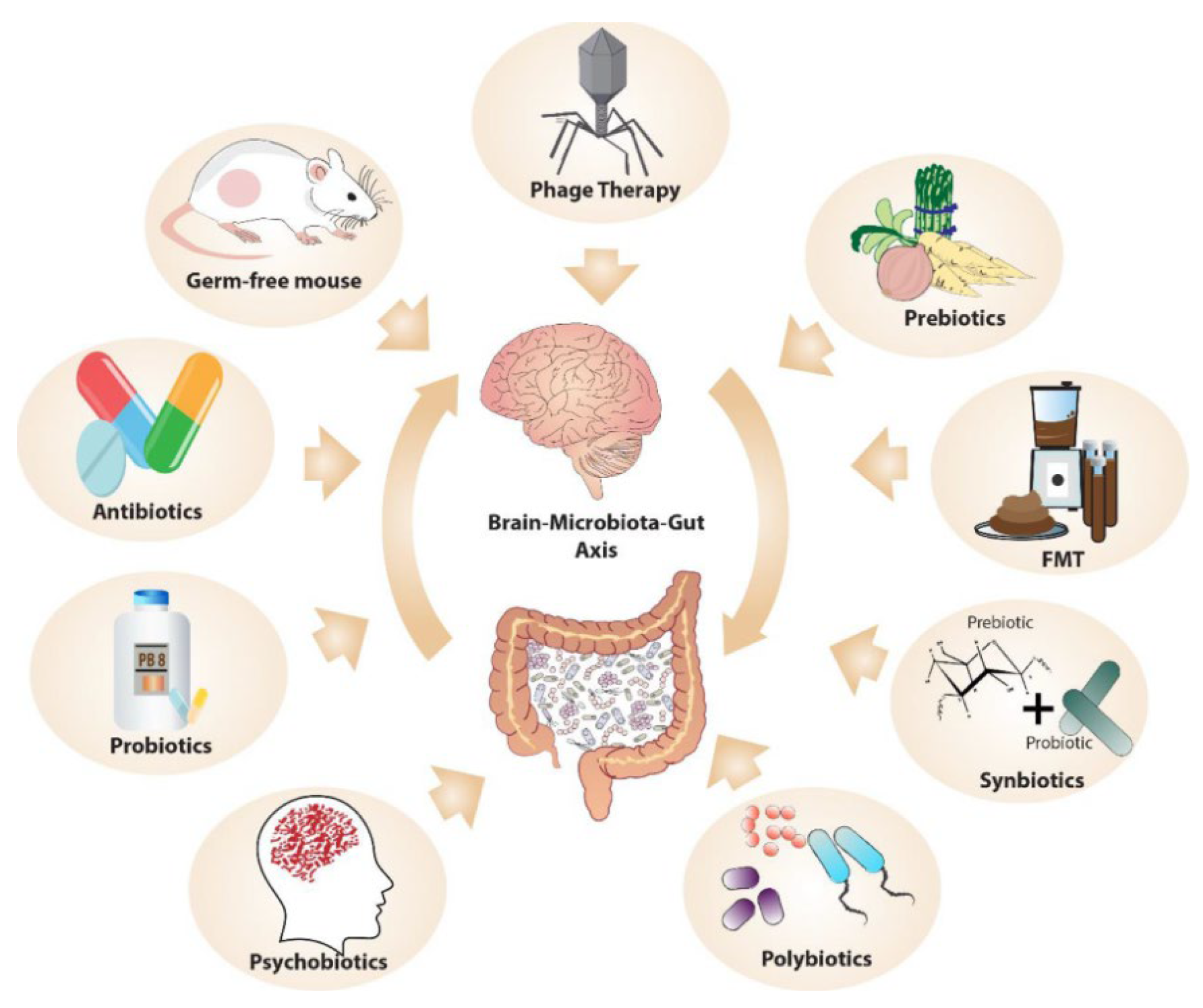
6. Microbiome-Targeted Interventions in Psychedelic Therapy
7. Personalized Medicine and Psychedelics
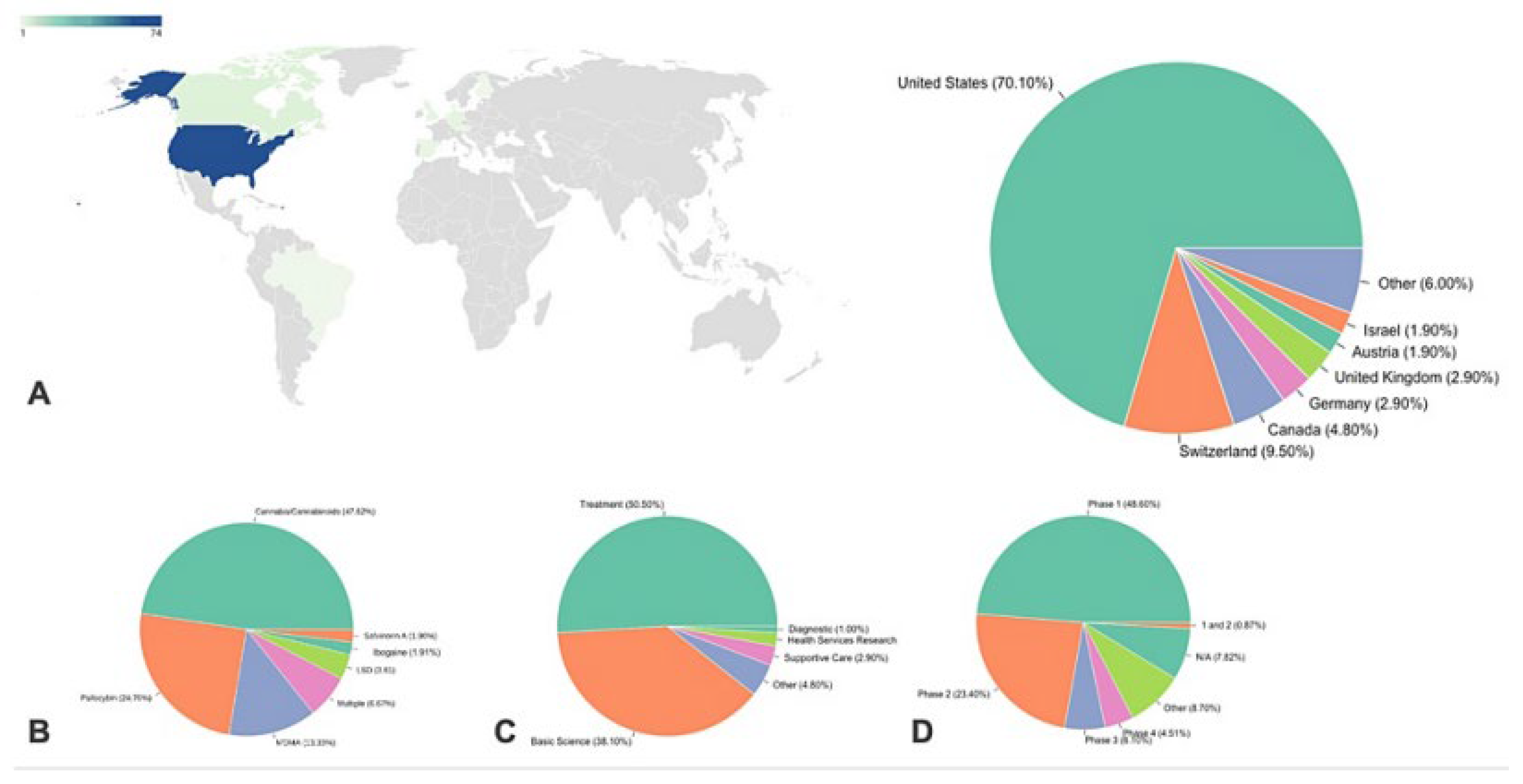
8. Limitations and Future Research Directions
9. Summary and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, B.L.; Gumpper, R.H. Psychedelics as Transformative Therapeutics. Am. J. Psychiatry 2023, 180, 340–347, Psychedelic history. Available online: https://blog.retreat.guru/the-history-of-psychedelics; https://www.portasophia.org/timelines/addiction-timeline.html (accessed on 27 May 2023). [CrossRef]
- Heal, D.J.; Smith, S.L.; Belouin, S.J.; Henningfield, J.E. Psychedelics: Threshold of a Therapeutic Revolution. Neuropharmacology 2023, 236, 109610. [Google Scholar] [CrossRef] [PubMed]
- Feduccia, A.; Agin-Liebes, G.; Price, C.M.; Grinsell, N.; Paradise, S.; Rabin, D.M. The need for establishing best practices and gold standards in psychedelic medicine. J. Affect Disord. 2023, 332, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Misera, A.; Łoniewski, I.; Palma, J.; Kulaszyńska, M.; Czarnecka, W.; Kaczmarczyk, M.; Liśkiewicz, P.; Samochowiec, J. Clinical significance of microbiota changes under the influence of psychotropic drugs. An updated narrative review. Front. Microbiol. 2023, 14, 1125022. [Google Scholar] [CrossRef]
- Kargbo, R.B. Microbiome-Gut-Brain Axis Modulation: New Approaches in Treatment of Neuropsychological and Gastrointestinal Functional Diorders. ACS Med. Chem. Lett. 2023, 14, 692. [Google Scholar] [CrossRef] [PubMed]
- This review is based on a comprehensive literature search performed in PubMed and SciFinder databases. The search strategy included a combination of the following keywords: “psychedelic renaissance”, “mental health”, “therapeutic potential”, “gut microbiome”, “psychedelic substances”, “personalized medicine”, “gut-brain axis”, “mood regulation”, “microbiome-targeted interventions”, and “psychopharmacology”. The databases were searched without a date limit up until May 2023. Additional relevant articles were identified from the reference lists of articles obtained in the initial search. Notably, the list of keywords is not exhaustive, and other related terms were used in the search process to ensure a thorough review.
- Angoa-Pérez, M.; Kuhn, D.M. Evidence for Modulation of Substance Use Disorders by the Gut Microbiome: Hidden in Plain Sight. Pharmacol. Rev. 2021, 73, 571–596. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Bolstridge, M.; Day, C.M.J.; Rucker, J.; Watts, R.; Erritzoe, D.E.; Kaelen, M.; Giribaldi, B.; Bloomfield, M.; Pilling, S.; et al. Psilocybin with psychological support for treatment-resistant depression: Six-month follow-up. Psychopharmacology 2018, 235, 399–408. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Casciati, A.; Mancuso, M.; Vitali, R.; Pazzaglia, S. Gut-brain axis: Does intestinal inflammation affect hippocampal neurogenesis and medulloblastoma development? Neural. Regen. Res. 2023, 18, 2381–2382. [Google Scholar] [CrossRef]
- Longo, S.; Rizza, S.; Federici, M. Microbiota-gut-brain axis: Relationships among the vagus nerve, gut microbiota, obesity, and diabetes. Acta. Diabetol. 2023, 60, 1007–1017. [Google Scholar] [CrossRef]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, diet and stress as modulators of gut microbiota: Implications for neurodegenerative diseases. Neurobiol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Guo, K.; Zeng, L.; Zeng, B.; Huo, R.; Luo, Y.; Wang, H.; Dong, M.; Zheng, P.; Zhou, C.; et al. Metabolite identification in fecal microbiota transplantation mouse livers and combined proteomics with chronic unpredictive mild stress mouse livers. Transl. Psychiatry 2018, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Cawthon, C.R.; Ihde, B.T.; Hajnal, A.; DiLorenzo, P.M.; de La Serre, C.B.; Czaja, K. Diet-driven microbiota dysbiosis is associated with vagal remodeling and obesity. Physiol. Behav. 2017, 173, 305–317. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stilling, R.M.; Stanton, C.; Cryan, J.F. Collective unconscious: How gut microbes shape human behavior. J. Psychiatr. Res. 2015, 63, 1–9. [Google Scholar] [CrossRef]
- Gao, M.; Tu, H.; Liu, P.; Zhang, Y.; Zhang, R.; Jing, L.; Zhang, K. Association analysis of gut microbiota and efficacy of SSRIs antidepressants in patients with major depressive disorder. J. Affect. Disord. 2023, 330, 40–47. [Google Scholar] [CrossRef]
- Bahr, S.M.; Tyler, B.C.; Wooldridge, N.; Butcher, B.D.; Burns, T.L.; Teesch, L.M.; Oltman, C.L.; Azcarate-Peril, M.A.; Kirby, J.R.; Calarge, C.A. Use of the second-generation antipsychotic, risperidone, and secondary weight gain are associated with an altered gut microbiota in children. Transl. Psychiatry 2015, 5, 652. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Muthukumaraswamy, S.; Roseman, L.; Kaelen, M.; Droog, W.; Murphy, K.; Tagliazucchi, E.; Schenberg, E.E.; Nest, T.; Orban, C.; et al. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc. Natl. Acad. Sci. USA 2016, 113, 4853–4858. [Google Scholar] [CrossRef]
- Nichols, D.E.; Johnson, M.W.; Nichols, C.D. Psychedelics as Medicines: An Emerging New Paradigm. Clin. Pharmacol. Ther. 2017, 101, 209–219. [Google Scholar] [CrossRef]
- Flanagan, T.W.; Nichols, C.D. Psychedelics as anti-inflammatory agents. Int. Rev. Psychiatry 2018, 30, 363–375. [Google Scholar] [CrossRef]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut–Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Mayer, E.A. Gut feelings: The emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef]
- Cui, J.J.; Huang, Z.Y.; Xie, Y.H.; Wu, J.B.; Xu, G.H.; Li, C.F.; Zhang, M.M.; Yi, L.T. Gut microbiota mediated inflammation, neuroendocrine and neurotrophic functions involved in the antidepressant-like effects of diosgenin in chronic restraint stress. J. Affect. Disord. 2023, 321, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- Gao, N.; Yang, Y.; Liu, S.; Fang, C.; Dou, X.; Zhang, L.; Shan, A. Gut-Derived Metabolites from Dietary Tryptophan Supplementation Quench Intestinal Inflammation through the AMPK-SIRT1-Autophagy Pathway. J. Agric. Food Chem. 2022, 70, 16080–16095. [Google Scholar] [CrossRef]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.B.; Xavier, R.J. Conditioning of the immune system by the microbiome. Trends Immunol. 2023, 44, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Lukáčová, I.; Ambro, Ľ.; Dubayová, K.; Mareková, M. The gut microbiota, its relationship to the immune system, and possibilities of its modulation. Epidemiol. Mikrobiol. Imunol. 2023, 72, 40–53. [Google Scholar]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef]
- Angthong, P.; Chaiyapechara, S.; Rungrassamee, W. Shrimp microbiome and immune development in the early life stages. Dev. Comp. Immunol. 2023, 147, 104765. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef]
- Suganya, K.; Koo, B. Gut–Brain Axis: Role of Gut Microbiota on Neurological Disorders and How Probiotics/Prebiotics Beneficially Modulate Microbial and Immune Pathways to Improve Brain Functions. Int. J. Mol. Sci. 2020, 21, 7551. [Google Scholar] [CrossRef] [PubMed]
- Asbjornsdottir, B.; Miranda-Ribera, A.; Fiorentino, M.; Konno, T.; Cetinbas, M.; Lan, J.; Sadreyev, R.I.; Gudmundsson, L.S.; Gottfredsson, M.; Lauth, B.; et al. Prophylactic Effect of Bovine Colostrum on Intestinal Microbiota and Behavior in Wild-Type and Zonulin Transgenic Mice. Biomedicines 2022, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Shadab Mehr, N.; Mohamadi, M.H.; Shokri, F.; Heidary, M.; Sadeghifard, N.; Khoshnood, S. Obesity and gut-microbiota-brain axis: A narrative review. J. Clin. Lab. Anal. 2022, 36, e24420. [Google Scholar] [CrossRef]
- Wang, J.; He, L.; Wang, S.; Zhao, H.; Chen, J.; Dong, Y.; Yasen, S.; Wang, L.; Zou, H. Therapeutic effect of the total saponin from Panax Japonicus on experimental autoimmune encephalomyelitis by attenuating inflammation and regulating gut microbiota in mice. J. Ethnopharmacol. 2023, 315, 116681. [Google Scholar] [CrossRef]
- Luczynski, P.; Whelan, S.O.; O’Sullivan, C.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. Adult microbiota-deficient mice have distinct dendritic morphological changes: Differential effects in the amygdala and hippocampus. Eur. J. Neurosci. 2016, 44, 2654–2666. [Google Scholar] [CrossRef]
- Zhu, M.; Ouyang, J.; Zhou, F.; Zhao, C.; Zhu, W.; Liu, C.; Huang, P.; Li, J.; Tang, J.; Zhang, Z.; et al. Polysaccharides from Fu brick tea ameliorate obesity by modulating gut microbiota and gut microbiota-related short chain fatty acid and amino acid metabolism. J. Nutr. Biochem. 2023, 118, 109356. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Wang, X.; Huang, S.; Zhang, M.; Su, Y.; Pan, Z.; Liang, J.; Xie, X.; Wang, Q.; Chen, J.; Zhou, L.; et al. Gegen Qinlian Decoction Activates AhR/IL-22 to Repair Intestinal Barrier by Modulating Gut Microbiota-Related Tryptophan Metabolism in Ulcerative Colitis Mice. J. Ethnopharmacol. 2023, 302, 115919. [Google Scholar] [CrossRef]
- Lamas, B.; Richard, M.L.; Leducq, V.; Pham, H.-P.; Michel, M.-L.; Da Costa, G.; Bridonneau, C.; Jegou, S.; Hoffmann, T.W.; Natividad, J.M.; et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 2016, 22, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Maccauro, V.; Airola, C.; Santopaolo, F.; Gasbarrini, A.; Ponziani, F.R.; Pompili, M. Gut Microbiota and Infectious Complications in Advanced Chronic Liver Disease: Focus on Spontaneous Bacterial Peritonitis. Life 2023, 13, 991. [Google Scholar] [CrossRef] [PubMed]
- Söderholm, J.D.; Yates, D.A.; Gareau, M.G.; Yang, P.C.; MacQueen, G.; Perdue, M.H. Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Fairlie, T.; Shah, A.; Talley, N.J.; Chey, W.D.; Koloski, N.; Yeh Lee, Y.; Gwee, K.A.; Jones, M.P.; Holtmann, G. Overlap of disorders of gut-brain interaction: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Kubera, M.; Leunis, J.C. The gut-brain barrier in major depression: Intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol. Lett. 2008, 29, 117–124. [Google Scholar]
- Zhu, G.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Gut Microbiota and its Metabolites: Bridge of Dietary Nutrients and Alzheimer’s Disease. Adv. Nutr. 2023, 14, 819–839. [Google Scholar] [CrossRef]
- Calder, A.; Mock, S.; Friedli, N.; Pasi, P.; Hasler, G. Psychedelics in the Treatment of Eating Disorders: Rationale and Potential Mechanisms. Eur. Neuropsychopharmacol. 2023, 75, 1–14. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Nutt, D.J. Serotonin and brain function: A tale of two receptors. J. Psychopharmacol. 2017, 31, 1091–1120. [Google Scholar] [CrossRef]
- Sicignano, D.; Snow-Caroti, K.; Hernandez, A.V.; White, C.M. The Impact of Psychedelic Drugs on Anxiety and Depression in Advanced Cancer or other Life-threatening Disease: A Systematic Review with Meta-analysis. Am. J. Clin. Oncol. 2023, 46, 236–245. [Google Scholar] [CrossRef]
- Bogenschutz, M.P.; Forcehimes, A.A.; Pommy, J.A.; Wilcox, C.E.; Barbosa, P.C.R.; Strassman, R.J. Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. J. Psychopharmacol. 2015, 29, 289–299. [Google Scholar] [CrossRef]
- Kelly, J.R.; Clarke, G.; Harkin, A.; Corr, S.C.; Galvin, S.; Pradeep, V.; Cryan, J.F.; Dinan, T.G. Seeking the Psilocybiome: Psychedelics meet the microbiota-gut-brain axis. Int. J. Clin. Health Psychol. 2023, 23, 100349. [Google Scholar] [CrossRef] [PubMed]
- Dinis-Oliveira, R.J. Metabolism of psilocybin and psilocin: Clinical and forensic toxicological relevance. Drug Metab. Rev. 2017, 49, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Milani, C.; Lauretani, F.; Nouvenne, A.; Mancabelli, L.; Lugli, G.A.; Turroni, F.; Duranti, S.; Mangifesta, M.; Viappiani, A.; et al. Gut microbiota composition is associated with polypharmacy in elderly hospitalized patients. Sci. Rep. 2017, 7, 11102. [Google Scholar] [CrossRef] [PubMed]
- Cussotto, S.; Strain, C.R.; Fouhy, F.; Strain, R.G.; Peterson, V.L.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology 2019, 236, 1671–1685. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Asif, H.; Dai, L.; He, Y.; Zheng, W.; Wang, D.; Ren, H.; Tang, J.; Li, C.; Jin, K.; et al. Alteration of the gut microbiome in first-episode drug-naïve and chronic medicated schizophrenia correlate with regional brain volumes. J. Psychiatr. Res. 2020, 123, 136–144. [Google Scholar] [CrossRef]
- Baslam, A.; Aitbaba, A.; Lamrani Hanchi, A.; Tazart, Z.; Aboufatima, R.; Soraa, N.; Ait-El-Mokhtar, M.; Boussaa, S.; Baslam, M.; Chait, A. Modulation of Gut Microbiome in Ecstasy/MDMA-Induced Behavioral and Biochemical Impairment in Rats and Potential of Post-Treatment with Anacyclus pyrethrum L. Aqueous Extract to Mitigate Adverse Effects. Int. J. Mol. Sci. 2023, 24, 9086. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef]
- Hirschfeld, T.; Prugger, J.; Majić, T.; Schmidt, T.T. Dose-response relationships of LSD-induced subjective experiences in humans. Neuropsychopharmacology 2023, in press. [Google Scholar] [CrossRef]
- Moujaes, F.; Preller, K.H.; Ji, J.L.; Murray, J.D.; Berkovitch, L.; Vollenweider, F.X.; Anticevic, A. Toward Mapping Neurobehavioral Heterogeneity of Psychedelic Neurobiology in Humans. Biol. Psychiatry 2023, 93, 1061–1070. [Google Scholar] [CrossRef]
- Hartogsohn, I. Set and setting, psychedelics and the placebo response: An extra-pharmacological perspective on psychopharmacology. J. Psychopharmacol. 2016, 30, 1259–1267. [Google Scholar] [CrossRef]
- St Arnaud, K.O.; Sharpe, D. Contextual Parameters Associated with Positive and Negative Mental Health in Recreational Psychedelic Users. J. Psychoact. Drugs 2023, 55, 30–39. [Google Scholar] [CrossRef]
- Dahan, O. Navigating intensive altered states of consciousness: How can the set and setting key parameters promote the science of human birth? Front. Psychiatry 2023, 14, 1072047. [Google Scholar] [CrossRef] [PubMed]
- Winkelman, M.J. The Evolved Psychology of Psychedelic Set and Setting: Inferences Regarding the Roles of Shamanism and Entheogenic Ecopsychology. Front. Pharmacol. 2021, 12, 619890. [Google Scholar] [CrossRef] [PubMed]
- Aday, J.S.; Davis, A.K.; Mitzkovitz, C.M.; Bloesch, E.K.; Davoli, C.C. Predicting Reactions to Psychedelic Drugs: A Systematic Review of States and Traits Related to Acute Drug Effects. ACS Pharmacol. Transl. Sci. 2021, 4, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Biskupska, J.; Borowiak, K.S.; Karlin-Grazewicz, K.; Janus, T.; Waloszczyk, P.; Potocka-Banas, B.; Machoy-Mokrzynska, A.; Ossowski, A.; Ciechanowicz, A. Estimation of BDNF Gene Polymorphism and Predisposition to Dependence Development for Selected Psychoactive Compounds: Genetic Aspects of Addiction with the Selected Drugs, Amphetamine, Tetrahydrocannabinol and Opiates. Hum. Exp. Toxicol. 2013, 32, 236–240. [Google Scholar] [CrossRef]
- O’Brien, C.P.; Childress, A.R.; Ehrman, R.; Robbins, S.J. Conditioning factors in drug abuse: Can they explain compulsion? J. Psychopharmacol. 1998, 12, 15–22. [Google Scholar] [CrossRef]
- Johnson, M.W.; Griffiths, R.R. Potential therapeutic effects of psilocybin. Neurotherapeutics 2017, 14, 734–740. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Goodwin, G.M. The therapeutic potential of psychedelic drugs: Past, present, and future. Neuropsychopharmacology 2017, 42, 2105–2113. [Google Scholar] [CrossRef]
- Mithoefer, M.C.; Mithoefer, A.T.; Feduccia, A.A.; Jerome, L.; Wagner, M.; Wymer, J.; Holland, J.; Hamilton, S.; Yazar-Klosinski, B.; Emerson, A.; et al. 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: A randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry 2018, 5, 486–497. [Google Scholar] [CrossRef]
- Skosnik, P.D.; Cortes-Briones, J.A. Targeting the ecology within: The role of the gut-brain axis and human microbiota in drug addiction. Med. Hypotheses 2016, 93, 77–80. [Google Scholar] [CrossRef]
- Kurtz, J.S.; Patel, N.A.; Gendreau, J.L.; Yang, C.; Brown, N.; Bui, N.; Picton, B.; Harris, M.; Hatter, M.; Beyer, R.; et al. The Use of Psychedelics in the Treatment of Medical Conditions: An Analysis of Currently Registered Psychedelics Studies in the American Drug Trial Registry. Cureus 2022, 14, 29167. [Google Scholar] [CrossRef]
- Qu, W.; Chen, Z.; Hu, X.; Zou, T.; Huang, Y.; Zhang, Y.; Hu, Y.; Tian, S.; Wan, J.; Liao, R.; et al. Profound Perturbation in the Metabolome of a Canine Obesity and Metabolic Disorder Model. Front. Endocrinol. 2022, 13, 849060. [Google Scholar] [CrossRef] [PubMed]
- Luna, R.A.; Foster, J.A. Gut brain axis: Diet microbiota interactions and implications for modulation of anxiety and depression. Curr. Opin. Biotechnol. 2015, 32, 35–41. [Google Scholar] [CrossRef]
- Teixeira, P.J.; Johnson, M.W.; Timmermann, C.; Watts, R.; Erritzoe, D.; Douglass, H.; Kettner, H.; Carhart-Harris, R.L. Psychedelics and Health Behaviour Change. J. Psychopharmacol. 2022, 36, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.I.; Mörkl, S.; Sandhu, K.V.; Cryan, J.F.; Dinan, T.G. The Gut Microbiome and Mental Health: What Should We Tell Our Patients? Can. J. Psychiatry 2019, 64, 747–760. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017, 66, 569–580. [Google Scholar] [CrossRef]
- Kirchherr, H.; Kühn-Velten, W.N. Quantitative determination of forty-eight antidepressants and antipsychotics in human serum by HPLC tandem mass spectrometry: A multi-level, single-sample approach. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006, 843, 100–113. [Google Scholar] [CrossRef]
- Nichols, C.D.; Sanders-Bush, E. A phenylisopropylamine derivative of metabotropic glutamate receptor antagonist produces a hallucinogen-specific-like discriminative stimulus in mice. Pharmacol. Biochem. Behav. 2004, 79, 739–746. [Google Scholar] [CrossRef]
- Gonzalez-Maeso, J.; Weisstaub, N.V.; Zhou, M.; Chan, P.; Ivic, L.; Ang, R.; Lira, A.; Bradley-Moore, M.; Ge, Y.; Zhou, Q.; et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 2007, 53, 439–452. [Google Scholar] [CrossRef]
- Hartogsohn, I. Constructing drug effects: A history of set and setting. Drug Sci. Policy Law 2017, 3, 2050324516683325. [Google Scholar] [CrossRef]
- Kelly, J.R.; Gillan, C.M.; Prenderville, J.; Kelly, C.; Harkin, A.; Clarke, G. Psychedelic Therapy’s Transdiagnostic Effects: A Research Domain Criteria (RDoC) Perspective. Front. Psychiatry 2020, 12, 800072. [Google Scholar] [CrossRef] [PubMed]
- Bonnieux, J.N.; VanderZwaag, B.; Premji, Z.; Garcia-Romeu, A.; Garcia-Barrera, M.A. Psilocybin’s effects on cognition and creativity: A scoping review. J. Psychopharmacol. 2023, 37, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Palitsky, R.; Kaplan, D.M.; Peacock, C.; Zarrabi, A.J.; Maples-Keller, J.L.; Grant, G.H.; Dunlop, B.W.; Raison, C.L. Importance of Integrating Spiritual, Existential, Religious, and Theological Components in Psychedelic-Assisted Therapies. JAMA Psychiatry 2023, 80, 743–749. [Google Scholar] [CrossRef]
- Nigam, K.; Curseen, K.A.; Beaussant, Y. Psychedelics and Related Pharmacotherapies as Integrative Medicine for Older Adults in Palliative Care. Clin. Geriatr. Med. 2023, 39, 423–436. [Google Scholar] [CrossRef]
- Watts, R.; Day, C.; Krzanowski, J.; Nutt, D.; Carhart-Harris, R. Patients’ accounts of increased “connectedness” and “acceptance” after psilocybin for treatment-resistant depression. J. Humanist Psychol. 2017, 57, 520–564. [Google Scholar] [CrossRef]
- Spriggs, M.J.; Murphy-Beiner, A.; Murphy, R.; Bornemann, J.; Thurgur, H.; Schlag, A.K. ARC: A Framework for Access, Reciprocity and Conduct in Psychedelic Therapies. Front. Psychol. 2023, 14, 1119115. [Google Scholar] [CrossRef] [PubMed]
- Koenig, J.; Parzer, P.; Reichl, C.; Andritzky, B.; Brunner, R.; Resch, F.; Kaess, M. Cortisol and self-harm in adolescents: A community study. J. Psychiatr. Res. 2016, 83, 78–85. [Google Scholar] [CrossRef]
- Aytac, H.M.; Oyaci, Y.; Aydin, P.C.; Pehlivan, M.; Pehlivan, S. COMTVal158Met Polymorphism Is Associated with Ecstasy (MDMA)-Induced Psychotic Symptoms in the Turkish Population. Neurosciences 2022, 27, 24–30. [Google Scholar] [CrossRef]
- Karakan, T.; Ozkul, C.; Küpeli Akkol, E.; Bilici, S.; Capasso, R. Gut-Brain-Microbiota Axis: Antibiotics and Functional Gastrointestinal Disorders. Nutrients 2021, 13, 389. [Google Scholar] [CrossRef]
- Ko, K.; Kopra, E.I.; Cleare, A.J.; Rucker, J.J. Psychedelic Therapy for Depressive Symptoms: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2023, 322, 194–204. [Google Scholar] [CrossRef]
- Van der Meer, P.B.; Fuentes, J.J.; Kaptein, A.A.; Schoones, J.W.; de Waal, M.M.; Goudriaan, A.E.; Kramers, K.; Schellekens, A.; Somers, M.; Bossong, M.G.; et al. Therapeutic Effect of Psilocybin in Addiction: A Systematic Review. Front. Psychiatry 2023, 14, 1134454. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.W.; Garcia-Romeu, A.; Griffiths, R.R. Long-term follow-up of psilocybin-facilitated smoking cessation. Am. J. Drug Alcohol Abuse 2017, 43, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Fineberg, N.A.; Pellegrini, L.; Clarke, A.; Perera, U.; Drummond, L.M.; Albert, U.; Laws, K.R. Meta-Analysis of Cognitive Behaviour Therapy and Selective Serotonin Reuptake Inhibitors for the Treatment of Hypochondriasis: Implications for Trial Design. Compr. Psychiatry 2022, 118, 152334. [Google Scholar] [CrossRef] [PubMed]
- Aqil, M.; Roseman, L. More than meets the eye: The role of sensory dimensions in psychedelic brain dynamics, experience, and therapeutics. Neuropharmacology 2023, 223, 109300. [Google Scholar] [CrossRef] [PubMed]
- Halpern, J.H.; Lerner, A.G.; Passie, T. Behavioral Neurobiology of Psychedelic Drugs; Halberstadt, A.L., Vollenweider, F.X., Nichols, D.E., Eds.; Current Topics in Behavioral Neurosciences, Volume 36; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kargbo, R.B. Microbiome: The Next Frontier in Psychedelic Renaissance. J. Xenobiot. 2023, 13, 386-401. https://doi.org/10.3390/jox13030025
Kargbo RB. Microbiome: The Next Frontier in Psychedelic Renaissance. Journal of Xenobiotics. 2023; 13(3):386-401. https://doi.org/10.3390/jox13030025
Chicago/Turabian StyleKargbo, Robert B. 2023. "Microbiome: The Next Frontier in Psychedelic Renaissance" Journal of Xenobiotics 13, no. 3: 386-401. https://doi.org/10.3390/jox13030025
APA StyleKargbo, R. B. (2023). Microbiome: The Next Frontier in Psychedelic Renaissance. Journal of Xenobiotics, 13(3), 386-401. https://doi.org/10.3390/jox13030025





