Ecosystem Protection through Myco-Remediation of Chromium and Arsenic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Estimation of Physicochemical Properties of Soil Samples
2.2.1. Physical Properties
2.2.2. Chemical Properties
- The pH of the samples was estimated using a digital pH meter.
- The digital conductivity meter was used to estimate the electrical conductivity of the samples.
- Carbon content in the samples was determined by the wet-digestion method [41].
- The total nitrogen content was determined by Kjeldahl’s method [42].
- The method that [43] used was to determine the total phosphorus of the samples.
- The total potassium of the samples was estimated on a flame photometer by direct feeding.
- The total chromium and arsenic levels in soil samples were determined by the method described by McGrath and Cunliffe [44].
2.3. Isolation of Fungal Isolates and Their Maintenance
2.4. Screening of Fungal Isolates
2.5. Characterization of Fungal Isolates
2.6. Optimization of Cultural Conditions
2.7. Heavy Metals Removal Efficacy
2.8. Molecular Characterization of Fungal Isolates
3. Results and Discussion
3.1. Sample Collection and Analysis of Physico-Chemical Properties
3.2. Isolation of Cultures and Their Minimum Inhibitory Concentration (MIC)
3.3. Morphological and Biochemical Characterization
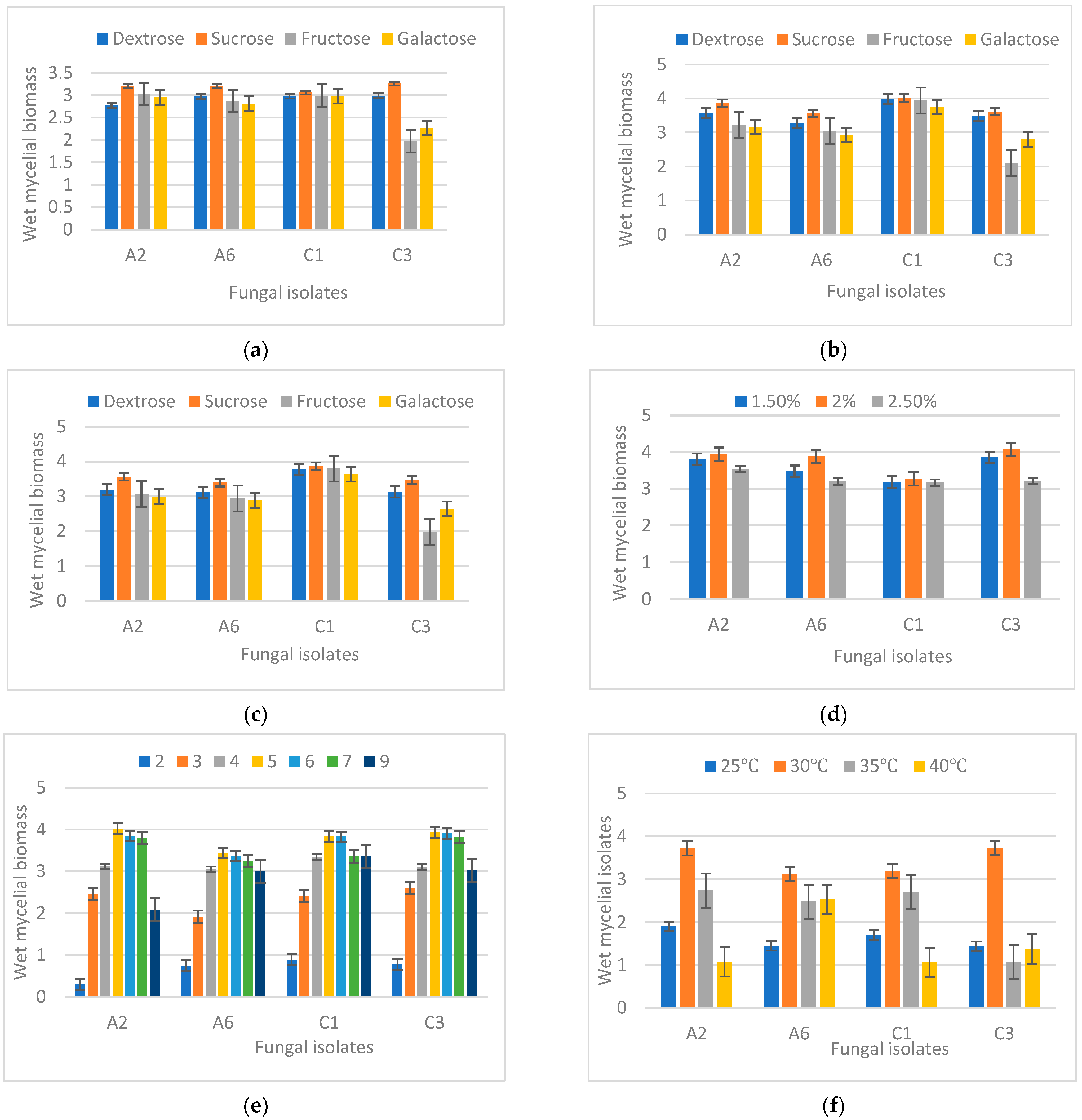
3.4. Optimization of Cultural Conditions
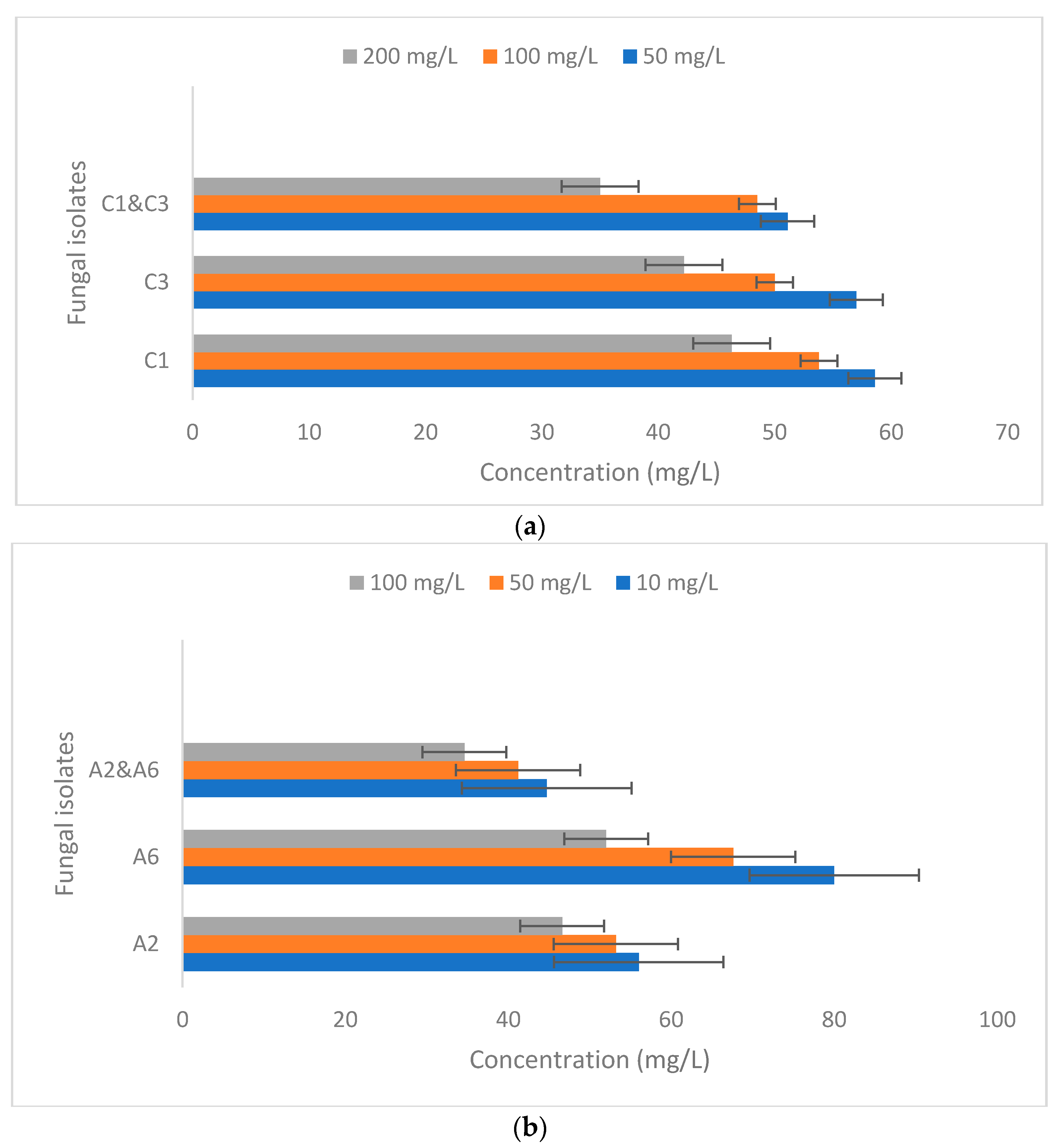
3.5. Heavy Metals Removal Efficacy of Fungal Isolates
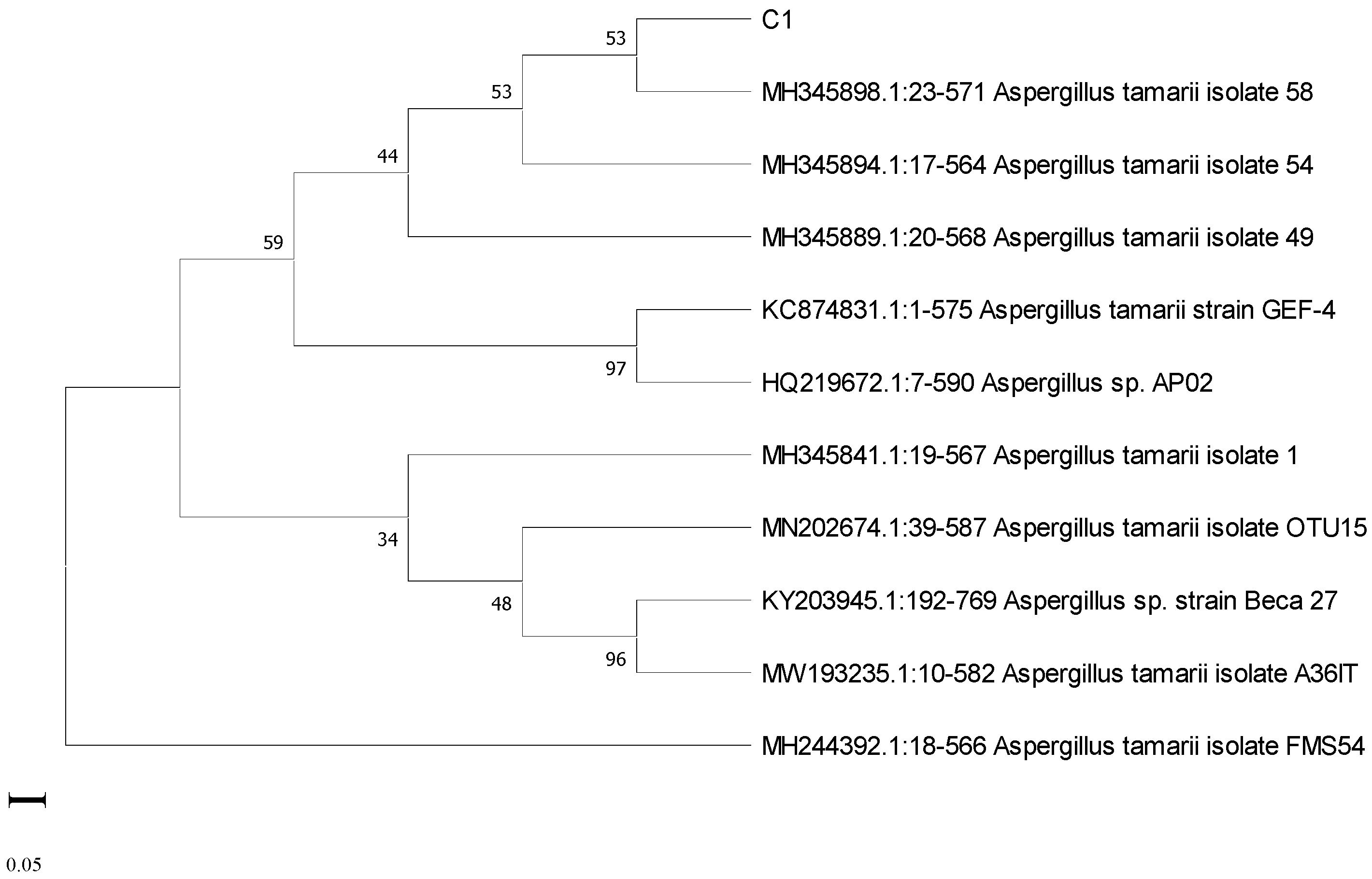
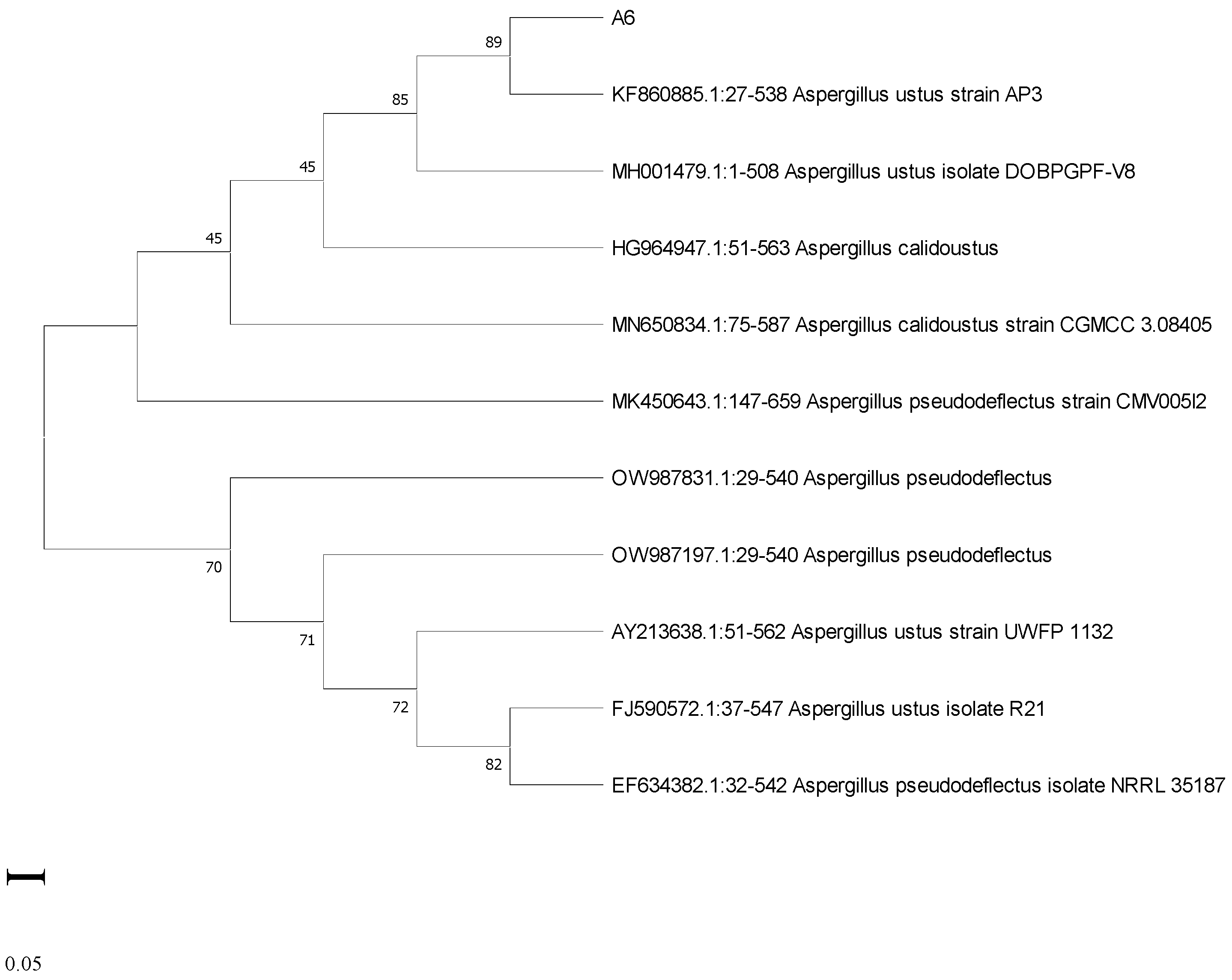
3.6. Molecular Characterization of Potent Isolates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lenin, M.; Kumar, M.S.; Mycin, T.R. Effect of sago factory effluent on seed germination and seedling growth of gingelly (Sesamum indicum L.) varieties. Int. J. Life Sci. Biotechnol. Pharm. Res. 2014, 3, 2250–3137. [Google Scholar]
- Kumar, V.; Thakur, R.K. Health risk assessment of heavy metals via dietary intake of vegetables grown in wastewater irrigated areas of Jagjeetpur, Haridwar India. Arch. Agric. Environ. Sci. 2018, 3, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Yadav, A.K.; Kumar, N.; Sreekrishnan, T.R.; Satya, S.; Bishnoi, N.R. Removal of chromium and nickel from aqueous solution in constructed wetland: Mass balance, adsorption–desorption and FTIR study. Chem. Eng. J. 2010, 160, 122–128. [Google Scholar] [CrossRef]
- Ma, H.W.; Hung, M.L.; Chen, P.C. A systemic health risk assessment for the chromium cycle in Taiwan. Environ. Int. 2007, 33, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Evelyne, R.J.; Ravinskar, V. Bioremediation of Chromium Contamination—A Review. Int. J. Res. Earth Environ. Sci. 2014, 2, 20–26. [Google Scholar]
- Cheng, G.J.; Li, X.H. Bioreduction of Chromium (VI) by Bacillus sp. Isolated from Soils of Iron Mineral Area. Eur. J. Soil Biol. 2009, 45, 483–487. [Google Scholar] [CrossRef]
- Kaur, H.; Kumar, A. Bioremediation of Hexavalent Chromium in Wastewater Effluent by Pseudomonas putida (MTCC 102). Int. J. Res. 2014, 1, 18–24. [Google Scholar]
- Khan, M.Z.H.; Hasan, M.R.; Khan, M.; Aktar, S.; Fatema, K. Distribution of heavy metals in surface sediments of the Bay of Bengal Coast. J. Toxicol. 2017, 2017, 9235764. [Google Scholar] [CrossRef]
- Rafi, S.; Shoaib, A.; Awan, Z.A.; Rizvi, N.B.; Shafiq, M. Chromium tolerance, oxidative stress response, morphological characteristics, and FTIR studies of phytopathogenic fungus Sclerotium rolfsii . Folia Microbiol. 2017, 62, 207–219. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sengupta, M.K.; Hossain, M.A.; Ahamed, S.; Das, B.; Nayak, B.; Lodh, D.; Rahman, M.M.; Chakraborti, D. Arsenic contamination in groundwater: A global perspective with emphasis on the Asian scenario. J. Health Population Nut. 2006, 24, 142–163. [Google Scholar]
- Wang, Y.; Le Pape, P.; Morin, G.; Asta, M.P.; King, G.; Bártová, B.; Suvorova, E.; Frutschi, M.; Ikogou, M.; Pham, V.H.C.; et al. Arsenic speciation in mekong delta sediments depends on their depositional environment. Environ. Sci. Technol. 2018, 52, 3431–3439. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.F. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002, 133, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Int. Sci. Res. Notices 2011, 2011, 402647. [Google Scholar] [CrossRef] [Green Version]
- Modaihsh, A.; Al-Swailem, M.; Mahjoub, M. Heavy metal contents of commercial inorganic fertilizer used in the Kingdom of Saudi Arabia. J. Agric. Mar. Sci. 2004, 9, 21–25. [Google Scholar] [CrossRef] [Green Version]
- Chehregani, A.; Malayeri, B.E. Removal of heavy metals by native accumulator plants. Int. J. Agric. Biol. 2007, 9, 462–465. [Google Scholar]
- Fulekar, M.; Singh, A.; Bhaduri, A.M. Genetic engineering strategies for enhancing phytoremediation of heavy metals. Afr. J. Biotechnol. 2009, 8, 529–535. [Google Scholar]
- Sabiha-Javied, M.T.; Chaudhry, M.M.; Tufai, M.; Irfan, N. Heavy metal pollution from phosphate rock used for the production of fertilizer in Pakistan. Microchem. J. 2009, 91, 94–99. [Google Scholar] [CrossRef]
- DeSesso, J.; Jacobson, C.; Scialli, A.; Farr, C.; Holson, J. An assessment of the developmental toxicity of inorganic arsenic. Reprod. Toxicol. 1998, 2, 385–433. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Environmental Health Criteria 224: Arsenic and Arsenic Compounds, 2nd ed.; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Gunatilake, S.K. Methods of removing heavy metals from industrial wastewater. Methods 2015, 1, 14. [Google Scholar]
- Rani, A.; Saharan, B.S. Optimization of cultural conditions for anaerobically treated distillery effluent bioremediation by an isolate Pseudomonas putida SAG. J. Appl. Nat. Sci. 2009, 1, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Ranga, P.; Sharma, D.; Saharan, B.S. Bioremediation of azo dye and textile effluents using Pseudomonas putida MTCC2445. Asian J. Microbiol. Biotechnol. Environ. Sci. 2020, 22, 88–94. [Google Scholar]
- Ranga, P.; Sharma, D.; Saharan, B.S. Bioremediation of Textile Effluent using Bacterial Consortium Obtained from Industrial Polluted Site. Ecol. Environ. Conserv. 2020, 26, S247–S254. [Google Scholar]
- Ranga, P.; Yogita; Saharan, B.S.; Mehta, S. Microbial Enzyme and Process Involved in Bioremediation. Int. J. Pharm. Technol. Biotechnol. 2021, 8, 17–26. [Google Scholar]
- Verma, S.; Saharan, B.S. 16S rRNA Phylogenetic Analysis of Heavy Metal Tolerant Plant Growth Promoting Rhizobacteria. Nat. Environ. Pollut. Technol. 2020, 19, 1763–1766. [Google Scholar]
- Tyagi, S.; Dhiman, V.K.; Dhiman, V.K.; Pandey, H.; Singh, D.; Kumar, R.; Singhal, R.K.; Javed, T.; Lee, K.J.; Saharan, B.; et al. Plant Defense Strategies and Biomarkers against Heavy Metal Stress. Environ. Res. 2023. (under publication). [Google Scholar]
- Saharan, B.S.; Brar, B.; Duhan, J.S.; Kumar, R.; Marwaha, S.; Rajput, V.D.; Minkina, T. Molecular and Physiological Mechanisms to Mitigate Abiotic Stress Conditions in Plants. Life 2022, 12, 1634. [Google Scholar] [CrossRef]
- Saharan, B.S.; Nehra, V. Plant growth promoting Rhizobacteria: A critical review. Life Sci. Med. Res. 2011, 21, 30. [Google Scholar]
- Singh, M.; Srivastava, P.K.; Verma, P.C.; Kharwar, R.N.; Singh, N.; Tripathi, R.D. Soil fungi for mycoremediation of arsenic pollution in agriculture soils. J. Appl. Microbiol. 2015, 119, 1278–1290. [Google Scholar] [CrossRef] [Green Version]
- Maheswari, S.; Murugesan, A.G. Remediation of arsenic in soil by Aspergillus nidulans isolated from an arsenic-contaminated site. Environ. Technol. 2009, 30, 921–926. [Google Scholar] [CrossRef]
- Anahid, S.; Yaghmaei, S.; Ghobadinejad, Z. Heavy metal tolerance of fungi. Sci. Iran. 2011, 18, 502–508. [Google Scholar] [CrossRef] [Green Version]
- Ryan, D.R.; Leukes, W.D.; Burton, S.G. Fungal bioremediation of phenolic wastewaters in an airlift reactor. Biotechnol. Prog. 2005, 21, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Tanvi, D.A.; Pratam, K.M.; Lohit, R.T.; Vijayalakshmi, B.K.; Devaraja, T.N.; Vasudha, M.; Ramesh, A.; Chakra, P.S.; Gayathri, D. Biosorption of heavy metal arsenic from Industrial Sewage of Davangere District, Karnataka, India, using indigenous fungal isolates. SN Appl. Sci. 2020, 2, 1860. [Google Scholar] [CrossRef]
- Kumar, V.; Dwivedi, S.K. Hexavalent chromium reduction ability and bioremediation potential of Aspergillus flavus CR500 isolated from electroplating wastewater. Chemosphere 2019, 237, 124567. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Hamayun, M.; Rahman, H.; Iqbal, A.; Shah, M.; Irshad, M.; Qasim, M.; Bibi, S.; Islam, B. Bioremediation of hexavalent chromium by endophytic fungi; safe and improved production of Lactuca sativa L. Chemosphere 2018, 211, 653–663. [Google Scholar]
- Gola, D.; Dey, P.; Bhattacharya, A.; Mishra, A.; Malik, A.; Namburath, M.; Ahammad, S.Z. Multiple heavy metal removal using an entomopathogenic fungi Beauveria bassiana . Biores. Technol. 2016, 218, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Shazia, I.; Uzma, S.G.; Talat, A. Bioremediation of heavy metals using isolates of filamentous fungus Aspergillus fumigatus collected from polluted soil of Kasur, Pakistan. Int. J. Biol. Sci. 2013, 2, 66–73. [Google Scholar]
- Vala, A.K. Tolerance and removal of arsenic by a facultative marine fungus Aspergillus candidus . Biores. Technol. 2010, 101, 2565–2567. [Google Scholar] [CrossRef]
- Joshi, P.K.; Swarup, A.; Maheshwari, S.; Kumar, R.; Singh, N. Bioremediation of heavy metals in liquid media through fungi isolated from contaminated sources. Indian J. Microbiol. 2011, 51, 482–487. [Google Scholar] [CrossRef] [Green Version]
- Walkley, A.; Black, I.A. An examination of the method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen–Total. In Methods of Soil Analysis, 2nd ed.; Paged, A.L., Ed.; Agronomy Monograph 9, Part 2; American Society of Agronomy: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- John, M.K. Calorimetric determination of phosphorus in soil and plant materials with ascorbic acid. Soil Sci. 1970, 109, 214–220. [Google Scholar] [CrossRef]
- McGrath, H.C.; Cunliffe, C.H. Simplified methods for extraction of metals (Fe, Cu, Ni, Cd, Pb, Cr, Co and Mn) from soil/sewage sludges. J. Sci. Food Agric. 1985, 36, 794–798. [Google Scholar] [CrossRef]
- Leck, A. Preparation of lactophenol cotton blue slide mounts. Community Eye Health. 1999, 12, 24. [Google Scholar] [PubMed]
- Mohanty, S.; Ghosh, S.; Nayak, S.; Das, A.P. Isolation, identification and screening of manganese solubilizing fungi from low-grade manganese ore deposits. Geomicrobiol. J. 2017, 34, 309–316. [Google Scholar] [CrossRef]
- Teather, R.M.; Wood, P.J. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl. Environ. Microbiol. 1982, 43, 77–780. [Google Scholar] [CrossRef] [Green Version]
- Senthivelan, T.; Kanagaraj, J.; Panda, R.C.; Narayani, T. Screening and production of a potential extracellular fungal laccase from Penicillium chrysogenum: Media optimization by response surface methodology (RSM) and central composite rotatable design (CCRD). Biotechnol. Rep. 2019, 23, e00344. [Google Scholar] [CrossRef]
- Sethi, S.; Gupta, S. Isolation, characterization and optimization of cultural conditions for amylase production from fungi. J. Glob. Sci. 2015, 4, 3356–3363. [Google Scholar]
- Bibi, N.; Ali, S.; Tabassum, R. Isolation and identification of novel indigenous bacterial strain as a low-cost pectinase source. Braz. Arch. Biol. Technol. 2018, 61. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Chadetrik, R.; Kumar, R.; Bishnoi, K.; Bhatia, D.; Kumar, A.; Bishnoi, N.R.; Singh, N. Biosorption optimization of lead (II), cadmium (II) and copper (II) using response surface methodology and applicability in isotherms and thermodynamics modeling. J. Hazard. Mater. 2010, 174, 623–634. [Google Scholar] [CrossRef]
- Angin, I.; Yaganoglu, A.V.; Turan, M. Effects of long-term wastewater irrigation on soil properties. J. Sustain. Agric. 2005, 26, 31–42. [Google Scholar] [CrossRef]
- Qing, X.; Yutong, Z.; Shenggao, L. Assessment of heavy metal pollution and human health risk in urban soils of steel industrial city (Anshan), Liaoning, Northeast China. Ecotoxicol. Environ. Saf. 2015, 120, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Oguh, C.E.; Obiwulu, E.N.O. Human Risk on Heavy Metal Pollution and Bioaccumulation Factor in Soil and Some Edible Vegetables around Active Auto-Mechanic Workshop in Chanchaga Minna Niger State, Nigeria. Ann. Ecol. Environ. Sci. 2020, 4, 12–22. [Google Scholar]
- Gadd, G.M. Microbial influence on metal mobility and application for bioremediation. Geoderma 2004, 122, 109–119. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Vaish, A.; Dwivedi, S.; Chakrabarty, D.; Singh, N.; Tripathi, R.D. Biological removal of arsenic pollution by soil fungi. Sci. Total Environ. 2011, 409, 2430–2442. [Google Scholar] [CrossRef]
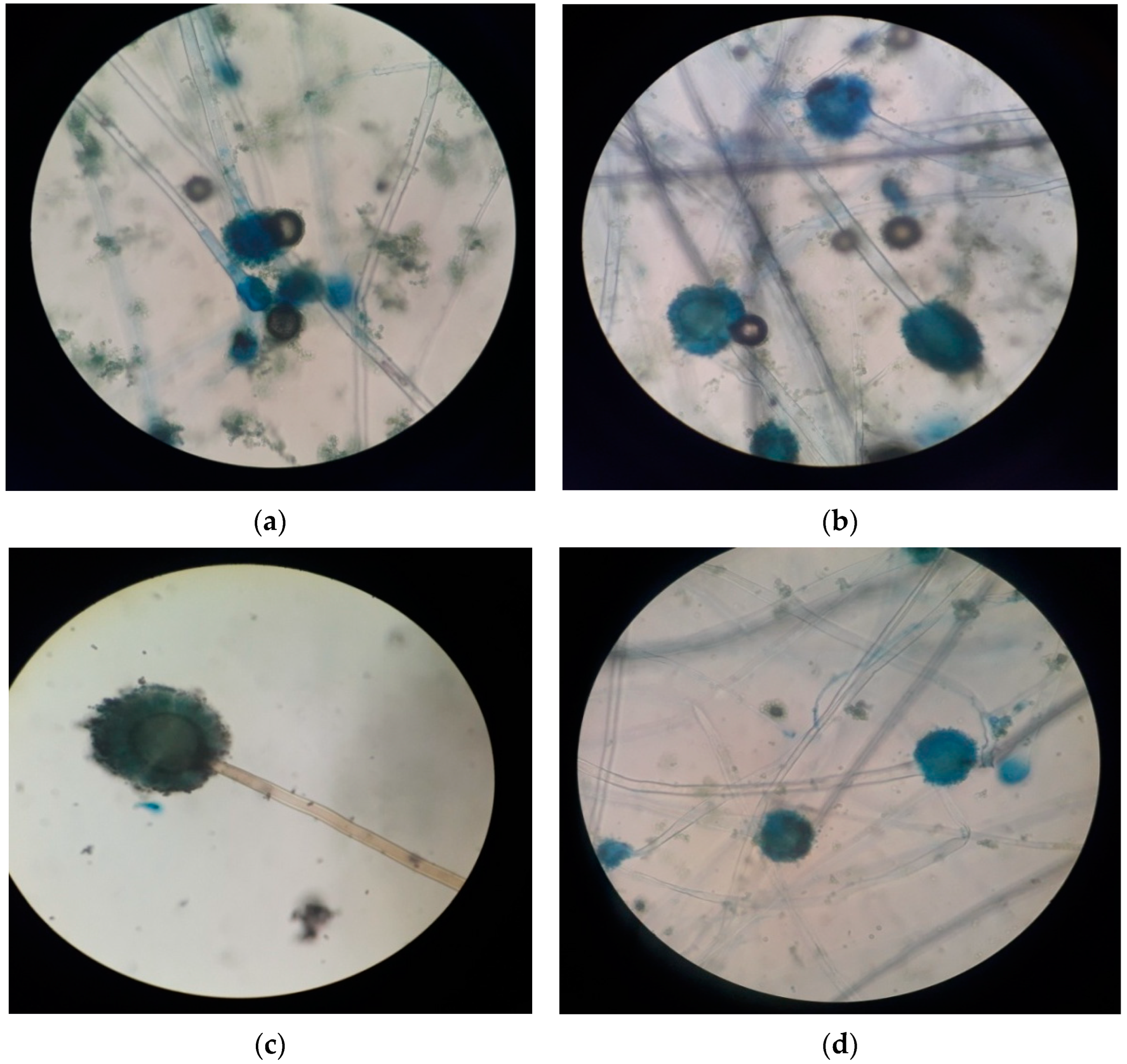
| Site No. | Site (s) | Soil Texture/Type | pH | EC (mS/cm) | Total C (%) | Total N (%) | Total P (%) | Total K (%) | Heavy Metals (mg/L) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cr | As | |||||||||
| 1 | Automarket, Hisar Phase I | Loam | 7.0 | 1.03 | 0.32 | 0.11 | 0.03 | 1.3 | 0.80 | 0.23 |
| 2 | Jindal Steel Industry, Hisar | Silty clay loam | 8.2 | 1.14 | 0.16 | 0.08 | 0.15 | 1.0 | 0.23 | 0.14 |
| 3 | Ludas Outlet No. I | Loam | 7.3 | 0.52 | 0.61 | 0.26 | 0.12 | 1.5 | 0.47 | 0.08 |
| 4 | Auto-market, Hisar | Sand | 7.1 | 1.18 | 0.21 | 0.07 | 0.06 | 1.1 | 1.53 | 0.10 |
| 5 | Ludas Outlet No. II | Loam | 8.4 | 0.66 | 0.68 | 0.31 | 0.28 | 2.1 | 0.22 | 0.20 |
| 6 | Auto-market, Hisar | Sand | 7.5 | 0.98 | 0.25 | 0.10 | 0.07 | 0.8 | 1.06 | 0.69 |
| 7 | Farmer’s fields of village Dabra, Hisar | Sand | 8.3 | 0.51 | 0.37 | 0.15 | 0.13 | 1.4 | 0.58 | 0.15 |
| 8 | Farmer’s fields of village Kaimari, Hisar | Sandy loam | 8.0 | 0.56 | 0.48 | 0.25 | 0.21 | 1.2 | 0.39 | 0.17 |
| 9 | Farmer’s fields of village Shahpur, Hisar | Clay loam | 8.2 | 0.60 | 0.49 | 0.22 | 0.24 | 1.5 | 0.45 | 0.18 |
| 10 | Textile site of Panipat | Sandy | 8.5 | 2.50 | 0.64 | 0.22 | 0.18 | 2.2 | 1.80 | 0.12 |
| 11 | Tannery site of Panipat | Clay loam | 8.8 | 1.45 | 0.59 | 0.26 | 0.13 | 2.7 | 2.23 | 0.11 |
| Metal(s) | Isolate (s) | MIC (mg/L) |
|---|---|---|
| Chromium | C1 | >5000 |
| C2 | 800 | |
| C3 | >5000 | |
| C4 | 800 | |
| C5 | 2300 | |
| C6 | 900 | |
| C7 | 1200 | |
| C8 | 900 | |
| C9 | 1200 | |
| C10 | 800 | |
| Arsenic | A1 | 400 |
| A2 | >5000 | |
| A3 | 2200 | |
| A4 | 200 | |
| A5 | 1400 | |
| A6 | >5000 | |
| A7 | 100 | |
| A8 | 400 | |
| A9 | 200 |
| Fungal Isolate (s) | Morphological Characteristics | Microscopic Characteristics | |||
|---|---|---|---|---|---|
| Colour | Margin | Elevation | Appearance | ||
| C1 | Olive green with white margin | Filliform | Raised | Cottony | Hyphae were hyaline, smooth, and septate, conidiophore unbranched, globular vesicle present and smooth globose spores were present |
| C3 | Green | Entire | Flat | Powdery | Clear, smooth, septate hyphae, conidiospores present, uniseriate, spores globular in shape |
| A2 | Olive green with white margin | Filliform | Raised | Cottony | Septate and hyaline hyphae, long unbranched conidiophores, globular vesicle |
| A6 | Grey with white margin | Filliform | Raised | Cottony | Septate and smooth hyphae, long and unbranched conidiophores, uniseriate. |
| Fungal Isolate (s) | Carbohydrate | Cellulase | Amylase | Laccase | Pectinase |
|---|---|---|---|---|---|
| C1 | + | + | − | − | + |
| C3 | + | − | − | + | + |
| A2 | + | + | − | − | + |
| A6 | + | − | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamal, N.; Parshad, J.; Saharan, B.S.; Kayasth, M.; Mudgal, V.; Duhan, J.S.; Mandal, B.S.; Sadh, P.K. Ecosystem Protection through Myco-Remediation of Chromium and Arsenic. J. Xenobiot. 2023, 13, 159-171. https://doi.org/10.3390/jox13010013
Kamal N, Parshad J, Saharan BS, Kayasth M, Mudgal V, Duhan JS, Mandal BS, Sadh PK. Ecosystem Protection through Myco-Remediation of Chromium and Arsenic. Journal of Xenobiotics. 2023; 13(1):159-171. https://doi.org/10.3390/jox13010013
Chicago/Turabian StyleKamal, Neel, Jagdish Parshad, Baljeet Singh Saharan, Monika Kayasth, Vishal Mudgal, Joginder Singh Duhan, Balwan Singh Mandal, and Pardeep Kumar Sadh. 2023. "Ecosystem Protection through Myco-Remediation of Chromium and Arsenic" Journal of Xenobiotics 13, no. 1: 159-171. https://doi.org/10.3390/jox13010013
APA StyleKamal, N., Parshad, J., Saharan, B. S., Kayasth, M., Mudgal, V., Duhan, J. S., Mandal, B. S., & Sadh, P. K. (2023). Ecosystem Protection through Myco-Remediation of Chromium and Arsenic. Journal of Xenobiotics, 13(1), 159-171. https://doi.org/10.3390/jox13010013







