Abstract
The use of antibiotics in ornamental fish is not regulated, as they are not intended for human consumption. Although antibiotic resistant bacteria have been detected in ornamental fish worldwide, there have been no studies to look at the situation in Hong Kong. Therefore, the present study was conducted to investigate the use of antibiotics in ornamental fish. Ornamental fish were purchased from five local pet fish shops and the antibiotics in carriage water were quantified using liquid chromatography tandem mass spectrometry. Moreover, Aeromonas and Pseudomonas spp. present in carriage water were isolated and their minimum inhibitory concentrations against selected antibiotics were determined. Results indicated that among the twenty antibiotics screened, doxycycline (0.0155–0.0836 µg L−1), oxytetracycline (0.0102–29.0 µg L−1), tetracycline (0.0350–0.244 µg L−1), enrofloxacin (0.00107–0.247 µg L−1), and oxalinic acid (n.d.−0.514 µg L−1) were detected in all sampled shops. Additionally, MIC results revealed that some of the Aeromonas and Pseudomonas spp. isolates were highly resistant to all antibiotics selected. Our findings confirmed that multiple antibiotics are being used in ornamental fish and the associated bacteria are resistant to selected antibiotics, suggesting that this could be a significant transmission route of antibiotic resistant bacteria to household indoor environments.
1. Introduction
The live ornamental fish trade is a rapidly growing sector of the aquaculture industry worldwide. The market size for the industry was estimated at USD 5.4 billion in 2021, and it has been predicted to increase by 8.5% from 2022 to 2030 [1]. To maximize productivity, intensive cultivation systems have been adopted in ornamental fish farms, creating stressful conditions such as high stocking density and suboptimal hygiene, which would weaken host defenses and increase incidences of microbial diseases such as columnaris and furunculosis [2,3]. Antibiotics have become the primary means of reducing loss caused by bacterial infection [4]. Antibiotics have been commonly administered in the form of medicated feed or baths as growth promotors, disease treatment, or prophylactic measures to prevent opportunistic pathogens during cultivation or transportation [5,6]. A wide range of antibiotics and chemotherapeutics have been indiscriminately applied in Chinese aquaculture, such as fluoroquinolones, macrolides, sulfonamides, and tetracyclines [7,8]. To our knowledge, rather limited studies have been conducted to investigate antibiotic use in the ornamental fish industry. A recent study carried out in Sri Lanka reported the heavy use of antibiotics in a local ornamental fish farm [9]. Antibiotic usage in food fish has been extensively studied and reported. Oxytetracycline, sulfonamides, and quinolones are some of the most common antibiotic and chemotherapeutic agents used worldwide in food fish [10]. Similar antibiotics are utilized in ornamental fish farming as well as food fish because of their broad-spectrum bacteriostatic properties against gram-negative bacteria [7,8,11,12]. Two studies on antibiotic residues in the muscle tissue of cultured fish in China revealed a high prevalence and residual concentrations of tetracyclines, with similar concentrations detected [8,12]. As the regulations on food fish do not apply to ornamental fish, it is reasonable to predict that the use of antibiotics in the ornamental sector is even more prevalent.
It has been suggested that ingested antimicrobial agents are poorly absorbed by fish and are eventually excreted with their metabolites in feces into the environment with their antimicrobial activity intact [13,14,15]. The discharge of these antimicrobials into the aquatic environment exerts selective pressures, creating reservoirs of antibiotic-resistant bacteria (ARB) and transferrable resistance genes in both fish pathogens and normal flora in the water body. As a result, the indigenous microbial community in the environment will be disrupted and selected, resulting in a decrease in microbial diversity [16,17,18]. Apart from microbial selection, antibiotic resistance genes have been exchanged between bacteria of terrestrial and aquatic origin [19]. The acquisition of antibiotic resistance through horizontal gene transfer events increases the incidence of drug-resistance, thereby posing a significant threat to human health [20]. Numerous investigations from mainland China and other countries have shed light on the occurrence and persistence of antibiotic resistance in aquaculture systems, including those for pet and food fish [21,22,23,24,25,26]. On the contrary, in Hong Kong, there are no environmental surveillance programs or regulations regarding antimicrobial use in the ornamental fish industry. To our best knowledge, the application of antibiotics in ornamental species is largely unknown, and there is no published study to investigate the types and concentrations of antibiotics. Therefore, the amount of antimicrobial use in ornamental fish is indiscriminate, often without prescription. Abuse of antimicrobials exposes the farming environment to various antimicrobials and poses a huge challenge to the fish and microbial communities [27].
Zoonotic pathogens isolated in ornamental fish that exhibit resistance to various antibiotics have been reported [5,22,27,28]. Given that zoonotic diseases associated with ornamental fish have been reported in humans, there is a possibility of infection from ornamental fish to humans, as well as a risk of ARB transmission to the general public [5]. Certain classes of antibiotics, which include fluoroquinolones and macrolides, are regarded as crucial for disease control in humans [29]. Increased exposure to antibiotics used only in human medicine potentiates the emergence of drug-resistant bacteria, which in turn compromises human health by reducing the number of available and effective treatments [30]. Therefore, the situation of antibiotic use and resistant bacteria in the ornamental fish industry should be investigated. It is also important for the authorities, the public, and the industry to recognize the extent to which antibiotic use associated with ornamental fish has expedited the spread of antibiotic resistance.
This work aims to identify and quantify the antibiotics used in ornamental fish available in Hong Kong and to study the resistance of zoonotic pathogens associated with ornamental fish to selected antibiotics. Given the historical use of antibiotics such as oxytetracycline and oxalinic acid in aquaculture, it is likely that high levels of antibiotic residues contribute to the development of antibiotic tolerance and the dissemination of antibiotic resistance in the ornamental fish industry [22]. Aeromonas and Pseudomonas spp. are zoonotic bacteria and ubiquitous in aquatic environments, and exposure to these bacteria could be a potential transmission route of drug-resistant bacteria between humans and ornamental fish [31,32]. Therefore, their susceptibilities to selected antibiotics were also studied in the form of minimum inhibitory concentrations (MIC).
2. Materials and Methods
2.1. Chemicals and Reagents
Methanol (MeOH) and acetonitrile (ACN) were of HPLC grade and purchased from Duksan Company (Ansan, Republic of Korea). Analytical-grade formic acid, sodium hydroxide and ammonium acetate were provided by Fisher Scientific (Waltham, WA, USA) and Fluka (Buchs, Switzerland), respectively. Milli-Q water from Merk Millipore was used throughout the study when deionized water was needed. Twenty native antibiotic standards with purity > 98% used in solid-phase extraction-liquid chromatography-tandem mass spectrometry (SPE-LC-MS/MS) analysis belonged to five groups of antibiotic, including four tetracyclines [tetracycline (TC), chlortetracycline (CTC), oxytetracycline (OCT), tetracycline (TC)], six fluoroquinolones (ciprofloxacin (CFX), enrofloxacin (EFX), ofloxacin (OFX), oxalinic acid (OA), sparfloxacin (SAR), and sarafloxacin (SFX)], three macrolides [clarithromycin (CTM), roxithromycin (RTM), and tylosin (TYL)], and six sulfonamides [sulfadiazine (SDZ), sulfamethazine (STZ), sulfamonomethoxine (SMM), sulfathiazole (SAZ), sulfamethoxazole (SMX), and sulfamerazine (SMZ)], and a diaminopyrimidines [trimethoprim (TMP)], were purchased from Sigma-Aldrich (St. Louis, MO, USA) and J&K Chemical Ltd. (Beijing, China). The internal standards, including sulfamethoxazole-13C6, ciprofloxacin-d8, roxithromycin-d7, and caffeine-13C3, were purchased from HPC Standard GmbH (Cunnersdorf, Germany) and Cambridge Isotope Laboratory (Tewksbury, MA, USA). The stock solution of individual compounds and internal standards (1 g L−1) was prepared by dissolving 10 mg in 10 mL of methanol for all antibiotics except fluoroquinolones, which was dissolved in methanol with sodium hydroxide. All stock solutions were stored at −80 °C. A 0.5 mg L−1 mixture of working standard containing all native compounds was freshly prepared by diluting the stock solution with methanol.
All antibiotics used in microbiological analyses were of high purity (>95%). Tetracycline hydrochloride, doxycycline hydrochloride, oxytetracycline hydrochloride, enrofloxacin, and oxalinic acid were purchased from Santa Cruz Biotechnology (Heidelberg, Germany) and MedChemExpress (Monmouth Junction, NJ, USA). Bacteria culturing media used in this study include nutrient broth (BD Difco, Sparks, MD, USA), nutrient agar (Oxoid, Hampshire, UK) and cation-adjusted Mueller Hinton broth (CAMHB) (Sigma-Aldrich, St. Louis, MO, USA). In addition, glutamate starch phenol red agar (GSP agar) was prepared according to the recipe provided by Sigma-Aldrich [33].
2.2. Research Premises and Sampling
Freshwater ornamental fish were purchased at a monthly interval evenly split between August and September 2022 from five different pet fish shops (S1–S5) in Goldfish Market, Kowloon. In each shop, three packs of ornamental fish (together with the carriage water) were purchased each time, and thirty samples were purchased for the study. Out of the thirty samples collected, twelve each were of zebrafish (Danio rerio) and southern platyfish (Xiphophorus maculatus), and six were of koi carp (Cyprinus rubrofuscus). Samples were immediately transported to the laboratory. Fish carriage water was divided into two portions for antibiotic concentration analysis (300 mL) and bacterial analysis (1 mL), according to Section 2.3 and Section 2.4, respectively.
2.3. Antibiotic Analysis
2.3.1. Sample Extraction
Antibiotics in 30 carriage water subsamples were extracted and quantified using solid-phase extraction (SPE) coupled with liquid chromatography-tandem mass spectrometry (LC-MS/MS) according to Chen and Zhou with some modifications [34]. Briefly, 300 mL of carriage water samples were adjusted to pH 3.5 with 50% formic acid and spiked with 50 µL of 1000 µg L−1 internal standards. Acidified samples were cleaned up and concentrated by SPE using an Oasis HLB cartridge (6 cc, 200 mg sorbent, Waters®, Milford, MA, USA). SPE cartridge was preconditioned with 6 mL methanol, followed by 12 mL Milli-Q water and 6 mL Milli-Q water adjusted to pH 3.5 ± 0.05 with 50% formic acid, before the water samples were percolated at a flow rate of 5 mL min−1. After extraction, antibiotics were eluted from the SPE cartridge with 8 mL of methanol, followed by concentrating 0.2 mL under a gentle nitrogen stream. Finally, the extracts were reconstituted to 1 mL using water and methanol (8:2, v/v).
2.3.2. Instrumental Analysis
An Agilent 1290 infinity LC system coupled with an Agilent 6460 triple quadrupole mass spectrometer (Agilent, Palo Alto, CA, USA) equipped with positive electrospray ionization (ESI) mode was used to determine all target antibiotics. The mass spectrometric condition was as follows: capillary voltage: 4000 V, nebulizer pressure: 45 PSI, drying gases temperature: 350 °C, and source gas flow: 10 L min−1. Analyte separation was achieved by injecting 10 µL sample into an Agilent InfinityLab Poroshell 120 EC-C18 (3.0 150 mm 2.7 µm) with guard filter. The mobile phases used in the chromatography included phase A: 0.1% formic acid with 5 mM ammonia acetate in Milli-Q water and phase B: ACN: MeOH 80:20 (v/v). The mobile phase gradient was performed at a flow rate of 0.4 mL min−1 from 95% A for 2 min, 60% A for 5 min, 55% A for 2 min, 40% A for 1 min, 95% A for 2 min, and finally, hold 95% A for 0.5 min. Each run lasted for 12.5 min and was followed by a 3-min post-run. The protonated ion ([M+H]+) was selected for the mass spectrometer analysis. LC-MSMS method was established after extensive optimization. The detailed optimization conditions, including retention time, precursor ion, product ions, and fragmentation are shown in Table A1.
2.3.3. Method Validation and Quality Control
In order to evaluate the accuracy and sensitivity of the analytical method, recovery, limit of detection (LOD), and limit of quantification (LOQ) were studied. Recoveries were calculated by spiking both native antibiotics and internal standards into Milli-Q water in triplicate at concentrations of 50 and 100 ng L−1. The recoveries of the target compounds were between 65% and 118% and 58.9% and 99.3%, respectively. The limits of quantification were calculated based on the standard deviation of response and the slope of each compound, ranged from 0.03 to 0.95 ng L−1, while the limits of detection ranged from 0.01 to 0.31 ng L−1. (Details are shown in Table A2). The concentration of target antibiotics in water samples was quantified using the internal standard method in order to compensate for the matrix effects between the samples. Isotope labeled internal standards were spiked in samples and the calibration curve was set at the same concentration. A calibration curve was constructed from seven points spiked with internal standards (1, 5, 10, 20, 50, 75, 100 µg L−1). The determination coefficients of the calibration curve varied from 0.98 to 0.99. Furthermore, the procedural blank was run to check for contamination, and a quality control sample was run every ten samples, followed by an injection of the solvent blank.
2.4. Bacterial Analysis
2.4.1. Bacterial Isolation
One hundred microliters of water samples (100, 10−1 and 10−2) were spread on GSP agar in duplicate to isolate Aeromonas and Pseudomonas spp. The agar plates were incubated aerobically at 30 °C for 48–72 h. Subsequently, plates were inspected, and colony numbers were counted based on morphological characteristics, according to pigmentation, colony form, and surface appearance. Yellow colonies surrounded by a yellow zone were presumed to be Aeromonas spp., while the blue-violet colonies surrounded by a red-violet zone were presumed to be Pseudomonas spp. Four colonies of each target bacterium isolated from each shop in two months were randomly selected for antibiotic susceptibility test. Reference strains Aeromonas hydrophila ATCC 7966, Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 10145 were used as quality control.
2.4.2. Antibiotic Susceptibility Test
Antimicrobial susceptibility testing was performed by a standard two-fold serial broth microdilution method using CAMHB according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) [35,36]. Prior to sampling in August and September 2022, preliminary work was conducted to determine the most commonly used antibiotics. The preliminary results (data not shown) suggested that DC, OTC, TC, EFX, and OA were the major antibiotics detected in the carriage water samples. These antibiotics were also selected for the subsequent antibiotic susceptibility study.
Four single colonies of presumptive Aeromonas spp. and Pseudomonas spp. isolated from GSP agar were selected randomly. The minimum inhibitory concentrations (MICs) of these colonies were determined against five antibiotics: DC, TC, OTC, EFX and OA. Each colony was suspended in 3 mL phosphate-buffered saline and adjusted to 0.5 McFarland standard with a nephelometer (V3011, Thermo Scientific, MA, USA). The bacterial suspension containing about 1 × 108 CFU mL−1 was diluted to 1 × 106 CFU mL−1 with CAMHB. Fifty microliters of the bacterial suspension were added to each well of a 96-well plate (Jet Biofil, Guangzhou, China). Each well of the 96-well plate contained antibiotic solution at final concentrations as follows: doxycycline, 8–128 mg L−1; tetracycline 2–256 mg L−1; oxytetracycline 2–256 mg L−1; enrofloxacin 0.5–64 mg L−1; and oxalinic acid 0.5–32 mg L−1. The plate was incubated at 37 °C for 18 h and the absorbance at 600 nm was measured.
The MIC of each isolate for each compound was determined as the lowest concentration of an antimicrobial that inhibited the growth of a given culture. Each isolate was examined in triplicate, and each batch of media was checked with E. coli ATCC 25922. The susceptibility of isolates was interpreted following the guidelines of CLSI and the breakpoint of each antibiotic as follows: doxycycline, 8 mg L−1; oxytetracycline, 64 mg L−1; tetracycline, 16 mg L−1; enrofloxacin, 0.5 mg L−1; and oxalinic acid, 0.5 mg L−1 [36,37,38,39]. The MIC values obtained were used to determine the mean MIC values, ranges of MIC, and resistant prevalence. Resistant prevalence was only conducted for Aeromonas spp., owing to the availability of epidemiological cut-off values and antibiotic susceptibility studies related to aquaculture [36,40,41,42].
2.5. Data Analysis
The mean MIC values of Aeromonas and Pseudomonas spp. isolated from the five sampled shops in two months were determined to represent the overall antibiotic resistance. If the MIC of the isolate was lower or higher than the corresponding test range of each antibiotic, a two-fold decrease or increase of the minimum or maximum test point was used in the calculation, respectively. The relationship between antibiotic concentrations in carriage water and the mean MIC value of Aeromonas and Pseudomonas spp. isolates from the same sample was examined by Pearson correlation. All analyses were performed in Prism 9.4.1 for Mac (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Concentrations of Antibiotics in Carriage Water Samples
Concentrations of antibiotics detected in the carriage water samples are summarized in Table 1. Of the twenty antibiotics tested, all MLs, SAs, one TCs (CTC), and five FQs (CFX, OFX, NFX, SAR and SFX) were not detected in all carriage water samples. Five antibiotic compounds, including three TCs (DR, OTC and TC) and two FQs (EFX and OA), were detected in carriage water samples collected in August. Meanwhile, for the samples collected in September, three TCs (DC, OTC, and TC) and one FQs (EFX) were detected. In general, OTC was observed to be the most dominant compound from all carriage water samples collected in two months from five shops, with the concentration ranging from 0.102 to 29.0 µg L−1.

Table 1.
The concentrations of antibiotics detected in carriage water samples collected from Goldfish Market in two months (mean ± SD, n = 15).
3.2. Antibiotic Resistance Prevalence and MIC of Aeromonas and Pseudomonas spp.
Table 2 and Table 3 show the prevalence of antibiotic resistant isolates and MIC ranges and mean MIC concentrations of each sample collected from two sampling months in five shops for the five antibiotics for Aeromonas and Pseudomonas spp., respectively. All Aeromonas isolates displayed high resistance to OTC, TC and OA, with a resistance prevalence rate above 90%. This result indicates that most of the Aeromonas spp. isolates were resistant to OTC, TC, and OA. The resistance rates for DC and EFX ranged from 55% to 70%, respectively, indicating a moderate susceptibility to these two compounds. As there are no reported MIC cut-off values for Pseudomonas spp., the prevalence of susceptibility could not be assessed. Resistance of the Pseudomonas isolates to OTC and TC was high, with the MIC levels not less than 32 mg L−1, whereas resistance to DC, EFX, and OA was low to moderate. Overall, the resistance levels of the two target bacteria among sampling shops differed considerably, with the exception of the consistently high MIC levels of OTC and TC, and the MICs of isolates from the two months displaying similar levels. It should be noted that the highest MIC for OTC and TC against isolated Aeromonas spp. and Pseudomonas spp. were ≥256 mg L−1.

Table 2.
Antibiotic resistance prevalence and levels of Aeromonas spp.

Table 3.
Antibiotic resistance prevalence and levels of Pseudomonas spp.
3.3. Correlations of Concentrations of Antibiotics and the Corresponding MIC Levels of Aeromonas and Pseduomonas spp.
Figure 1 shows the relationship between mean MIC values of bacterial isolates and the mean concentrations of the antibiotics detected in the carriage water samples. The values of Aeromonas spp. Figure 1a–e utilized were obtained by excluding values below the breakpoints, whereas those of Pseudomonas spp. Figure 1f–j were calculated using the means after excluding values below the test range.
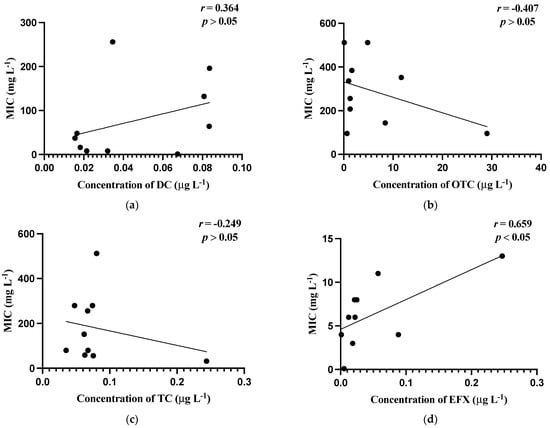
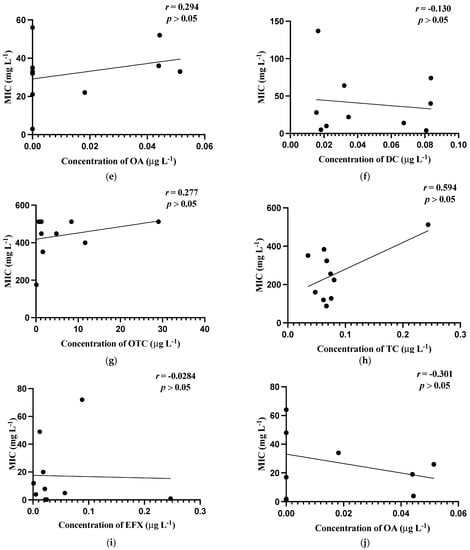
Figure 1.
Correlations of minimum inhibitory concentrations (MICs, mg L−1) against the residual concentrations of antibiotics (µg L−1). (a) MIC (mg L−1) of Aeromonas spp. against concentration (µg L−1) of doxycycline; (b) MIC (mg L−1) of Aeromonas spp. against concentration (µg L−1) of oxytetracycline; (c) MIC (mg L−1) of Aeromonas spp. against concentration (µg L−1) of tetracycline; (d) MIC (mg L−1) of Aeromonas spp. against concentrations (µg L−1) of enrofloxacin; (e) MIC (mg L−1) of Aeromonas spp. against concentrations (µg L−1) of oxalinic acid; (f) MIC (mg L−1) of Pseudomonas spp. against concentrations (µg L−1) of doxycycline; (g) MIC (mg L−1) of Pseudomonas spp. against concentrations (µg L−1) of oxytetracycline; (h) MIC (mg L−1) of Pseudomonas s spp. against concentrations (µg L−1) of tetracycline; (i) MIC (mg L−1) of Pseudomonas spp. against concentrations (µg L−1) of enrofloxacin; (j) MIC (mg L−1) of Pseudomonas spp. against concentrations (µg L−1) of oxalinic acid.
In general, weak relationships were observed between MIC levels and the concentrations of antibiotics in carriage water samples, which were reflected by the low correlation coefficient (r) values obtained. Noticeably, only the correlation between the MIC values of Aeromonas isolates and the concentration of EFX in the carriage water samples was the strongest and significant (p ≤ 0.05).
4. Discussion
To our knowledge, this is the first study that has investigated antibiotic consumption in ornamental fish and antibiotic resistance in Aeromonas and Pseudomonas spp. in Hong Kong. The Goldfish Market is a popular tourist spot, and it is also a place where ornamental fish shops are concentrated, with 40 to 50 shops selling a large variety of ornamental fish. Most of the ornamental fish are imported from other regions and immediately after arrival, the fish will be introduced to fish tanks. Fish sold in the Goldfish Market are either pre-packaged in plastic bags and then hung at the entrance of stores or displayed in fish tanks for selection by customers. These display methods are stressful to fish, creating favorable conditions for disease prevalence. Therefore, it is unsurprising that antibiotics are added to rearing or carriage water to ensure the fish’s survival before sale. Among the 20 antibiotics analyzed, DC, OTC, TC, EFX, and OA were most commonly detected during the study period. Except for OA, four antibiotics (DC, OTC, TC, and EFX) were detected in all carriage water samples, suggesting that antibiotics were being supplemented regularly in fish tanks in all shops.
In Hong Kong, the regulations on antibiotic use only apply to food animals. For example, no more than 100 µg kg−1 of oxytetracycline is permitted [43]. Restrictions regarding the ornamental fish industry have yet to be established by the government, as well as the antibiotic use and susceptibility study for the industry. Currently, no research regarding antibiotic use and the corresponding susceptibility has been established for the ornamental fish industry, nor has the government implemented any surveillance programs or regulations in order to understand and monitor the local situation.
The concentrations of antibiotic residues in carriage water samples from the ornamental fish market varied by shops and months. The quantitative variation in the occurrence of antibiotics in the carriage water samples can be attributed to different practices and needs in the rearing of ornamental fish with regard to the use of antibiotics. For instance, oxytetracycline and enrofloxacin had the highest concentrations in the class of tetracyclines and fluoroquinolones, ranging from 0.102 to 29.0 µg L−1 and 0.00107 to 0.247 µg L−1, respectively. In addition, the widespread use of oxytetracycline and enrofloxacin in the industry can also be observed. This observation, in terms of the use of antibiotics in rearing ornamental fish, is similar to the findings of a previous investigation conducted in Sri Lanka [9].
Due to the large proportion of antibiotic compounds that can enter and persist in aquatic environments, they will have the potential to select for resistant bacteria in the environment over time [4]. The transmission of antibiotic resistance genes from microorganisms in the aquatic environment to terrestrial microbial species will eventually reach human consumers. Food fish have been recognized as one of the major reservoirs of ARB and resistance genes [44]. Knowing that a large variety of antibiotic residues have been recorded in aquatic products, the microbiome of aquatic products may have undergone selection and developed resistance to different antibiotics [7]. Among the twenty antibiotics targeted in this study, OA and EFX are exclusively used for veterinary purposes, while DC, OTC and TC are used in both humans and animals [7,29]. The high frequency of detection of bacteria that are resistant to OTC and TC in the present study suggested that the ornamental fish industry may also be a hotspot for the emergence of antibiotic resistance. Previous studies also reported a high prevalence of resistance to common antibiotics, including OTC and TC used in ornamental fish available in different locations, demonstrating that the ornamental fish industry is a potential reservoir of multi-drug resistant bacteria [45,46,47,48]. In addition to ARB, the prevalence of resistance genes has been investigated worldwide [10]. The wastewater discharged from an ornamental fish market in China was found to contain high levels and a wide range of resistance genes, showing the potential of ornamental fish to be a reservoir of antibiotic residues [21].
Nevertheless, the present study confirmed that antibiotics are being used by the ornamental fish sellers in Hong Kong, and rather high resistance was observed in the bacteria present in the carriage water samples. The bacteria associated with fish could also be the hotspots of ARB. Moreover, Figure 1 shows weak correlations established between the majority of antibiotic residues and corresponding MIC levels in the present study. The presence of high levels of ARB with low concentrations of antibiotics was detected. One reason is probably that antibiotic resistance was developed in ornamental fish farms prior to their distribution to the retail market.
The detection of zoonotic pathogens with high resistance to multiple antibiotics in fish carriage water is rather alarming. The prophylactic use of antibiotics in ornamental fish should be discontinued to reduce the development of ARB. Reducing the stocking density of fish, for example, is one of the simple but effective ways to reduce the stress experienced by fish in the display tanks, which in turn reduces the need for antibiotics. Although ornamental fish are not intended for human consumption, the bacteria could persist in household fish tanks for an extended period and be transmitted to humans. Furthermore, aeration by an air pump in a household aquarium can be a means to transmit pathogens from one tank to another via aerosols [49,50]. Aerosolized ARB will contaminate the surrounding environment and potentially be inhaled by humans [51]. As fishkeeping is one of the most popular hobbies in Hong Kong, introducing fish that carry ARB could pose potential health risks to citizens. The potential role of fish tanks in the dissemination of ARB will be investigated in the future.
5. Conclusions
In this work, the quantity of antibiotics and ARB prevalence in the carriage water of ornamental fish were studied. OTC had the highest concentration of antibiotics detected during the two-month sampling period in all sampled shops. Moreover, high levels of antibiotic resistance were found in the susceptibility tests of Aeromonas and Pseudomonas spp. against OTC and TC. There were low or no positive correlations between the MIC levels of the two target bacteria and concentrations of antibiotic residues, suggesting that additional variables may contribute to the development of antibiotic resistance in the local ornamental fish industry. It is reasonable to assume that high levels of antibiotic resistance were developed before arriving the sampled shops.
Author Contributions
Conceptualization, C.A.-Y., K.-L.L. and W.-Y.M.; methodology, C.A.-Y., K.-L.L. and K.-W.C.; validation, C.A.-Y. and K.-L.L.; formal analysis, C.A.-Y. and K.-L.L.; investigation, C.A.-Y. and K.-W.C.; resources, W.-Y.M.; data curation, C.A.-Y. and K.-W.C.; writing—original draft preparation, C.A.-Y.; writing—review and editing, W.-Y.M.; supervision, W.-Y.M.; funding acquisition, W.-Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
The work described in this paper was fully supported by Hong Kong Metropolitan University Research Grant (No. PFDS/2021/06).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Optimized LC-MS/MS parameters including retention time, precursor, product ions, and fragmentation.
Table A1.
Optimized LC-MS/MS parameters including retention time, precursor, product ions, and fragmentation.
| Target Antibiotics | Abbreviation | Retention Time (min) | Precursor Ion | Product Ion 1 | Product Ion 2 | Fragmentation (v) |
|---|---|---|---|---|---|---|
| Ciprofloxacin | CFX | 3.85 | 332.1 | 231.1 | 314.1 | 135 |
| Enrofloxacin | EFX | 4.013 | 360.2 | 342.2 | 316.2 | 135 |
| Ofloxacin | OFX | 3.815 | 362.2 | 318.2 | 261.1 | 145 |
| Oxalinic acid | OA | 6.213 | 262.1 | 216 | 244.1 | 85 |
| Sparfloxacin | SAR | 4.328 | 393.2 | 349.2 | 251.1 | 150 |
| Sarafloxacin | SFX | 4.314 | 386.1 | 368.2 | 342.2 | 150 |
| Norfloxacin | NFX | 3.80 | 320.1 | 302.2 | 231.1 | 105 |
| Chlortetracycline | CTC | 4.926 | 479.1 | 462.2 | 444.1 | 150 |
| Doxycycline | DC | 5.152 | 445.2 | 428.1 | 98 | 125 |
| Oxytetracycline | OTC | 3.919 | 461.2 | 426.1 | 443.2 | 140 |
| Tetracycline | TC | 4.089 | 445.1 | 410.1 | 154 | 120 |
| Tylosin tartrate | TYL | 8.625 | 916.5 | 174.1 | 101.1 | 185 |
| Roxithromycin | RTM | 11.545 | 839.5 | 158.1 | 116.1 | 190 |
| Clarithromycin | CTM | 11.382 | 748.5 | 158.1 | 116 | 200 |
| sulfamethazine | STZ | 4.496 | 279.1 | 186 | 124.1 | 115 |
| sulfamonomethoxine | SMM | 4.915 | 281.1 | 156 | 108 | 105 |
| sulfathiazole | SAZ | 3.898 | 256 | 156 | 92.1 | 110 |
| sulfamethoxazole | SMX | 5.564 | 254.1 | 156 | 92 | 110 |
| Sulfamerazine | SMZ | 4.193 | 265.1 | 108 | 92.1 | 110 |
| Sulfadiazine | SDZ | 3.865 | 251.1 | 108.1 | 92.1 | 120 |
| Trimethoprim | TMP | 5.784 | 291.2 | 230.1 | 123 | 155 |
| Sulfamethoxazole-13C6 | SMZ-13C6 | 5.557 | 261.1 | 162 | 98 | 87 |
| Ciprofloxacin-d8 | CFX-d8 | 3.865 | 340.2 | 322.2 | 235 | 113 |
| Roxithromyxcin-d7 | RTM-d7 | 11.48 | 845.6 | 158.1 | 116 | 136 |
| Caffeine-13C3 | Caffeine-13C3 | 3.733 | 198.1 | 140 | 112.1 | 84 |

Table A2.
Description of recovery, limits of quantification (LOQ) and detection (LOD).
Table A2.
Description of recovery, limits of quantification (LOQ) and detection (LOD).
| Group | Compound | Recovery (%) | LOD (ng L−1) | LOQ (ng L−1) | |
|---|---|---|---|---|---|
| 50 ng L−1 | 100 ng L−1 | ||||
| Fluoroquinolones | CFX | 65.0 ± 11.4 | 72.8 ± 13.0 | 0.040 | 0.122 |
| EFX | 70.8 ± 10.5 | 58.9 ± 6.8 | 0.316 | 0.957 | |
| OFX | 70.7 ± 14.0 | 79.4 ± 7.2 | 0.023 | 0.069 | |
| OA | 96.0 ± 1.6 | 75.9 ± 11.6 | 0.008 | 0.024 | |
| SAR | 89.9 ± 7.0 | 92.7 ± 15.3 | 0.024 | 0.072 | |
| SFX | 78.7 ± 9.1 | 95.1 ± 15.4 | 0.028 | 0.084 | |
| NFX | 66.2 ± 13.7 | 65.9 ± 7.6 | 0.067 | 0.203 | |
| Tetracycline | CTC | 118.2 ± 16.2 | 91.5 ± 14.5 | 0.210 | 0.640 |
| DC | 91.03 ± 7.1 | 76.41 ± 15.9 | 0.226 | 0.685 | |
| OTC | 107.6 + 13.9 | 63.69 + 9.1 | 0.201 | 0.610 | |
| TC | 89.27 + 6.7 | 72.79 + 21.3 | 0.230 | 0.696 | |
| Macrolides | TYL | 80.17 + 4.3 | 65.18 + 11.6 | 0.013 | 0.041 |
| RTM | 68.0 + 5.4 | 60.33 + 6.1 | 0.041 | 0.126 | |
| CTM | 82.35 + 1.3 | 76.5 + 4.1 | 0.091 | 0.275 | |
| Sulfonamides | STZ | 90.2 ± 19.3 | 76.8 ± 12.3 | 0.018 | 0.056 |
| SMM | 98.4 ± 5.4 | 91.8 ± 10.6 | 0.108 | 0.328 | |
| SAZ | 70.0 ± 7.0 | 87.6 ± 3.5 | 0.110 | 0.334 | |
| SMX | 96.2 ± 2.0 | 90.0 ± 5.3 | 0.056 | 0.170 | |
| SMZ | 80.2 ± 6.7 | 80.7 ± 14.8 | 0.017 | 0.052 | |
| SDZ | 82.4 ± 6.4 | 99.3 ± 10.7 | 0.051 | 0.155 | |
| Diaminopyrimidines | TMP | 95.1 ± 4.7 | 99.8 ± 7.8 | 0.011 | 0.033 |
References
- Grand View Research. Ornamental Fish Market Size, Share & Trends Analysis Report By Product (Tropical Freshwater, Temperate, Marine), By Application (Commercial, Household), By Region, And Segment Forecasts, 2022–2030; Grand View Research: San Francisco, CA, USA, 2021; p. 85. [Google Scholar]
- Austin, B.; Austin, D.A. Bacterial Fish Pathogens: Disease of Farmed and Wild Fish; Springer International Publishing AG: Cham, Switzerland, 2016. [Google Scholar]
- Magnadottir, B. Immunological Control of Fish Diseases. Mar. Biotechnol. 2010, 12, 361–379. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.P.; Tomova, A.; Ivanova, L.; Dölz, H.; Millanao, A.; Buschmann, A.H. Antimicrobial use in aquaculture re-examined: Its relevance to antimicrobial resistance and to animal and human health. Environ. Microbiol. 2013, 15, 1917–1942. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.; RajiĆ, A.; Dutil, L.; Cernicchiaro, N.; Uhland, F.C.; Mercier, B.; TuŠEvljak, N. Zoonotic bacteria, antimicrobial use and antimicrobial resistance in ornamental fish: A systematic review of the existing research and survey of aquaculture-allied professionals. Epidemiol. Infect. 2012, 140, 192–206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ Microbiol 2006, 8, 7. [Google Scholar] [CrossRef]
- Liu, X.; Steele, J.C.; Meng, X.-Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review. Environ. Pollut. 2017, 223, 161–169. [Google Scholar] [CrossRef]
- He, X.; Deng, M.; Wang, Q.; Yang, Y.; Yang, Y.; Nie, X. Residues and health risk assessment of quinolones and sulfonamides in cultured fish from Pearl River Delta, China. Aquaculture 2016, 458, 38–46. [Google Scholar] [CrossRef]
- Guruge, K.S.; Goswami, P.; Tanoue, R.; Nomiyama, K.; Wijesekara, R.G.S.; Dharmaratne, T.S. First nationwide investigation and environmental risk assessment of 72 pharmaceuticals and personal care products from Sri Lankan surface waterways. Sci. Total Environ. 2019, 690, 683–695. [Google Scholar] [CrossRef]
- Hemamalini, N.; Shanmugam, S.A.; Kathirvelpandian, A.; Deepak, A.; Kaliyamurthi, V.; Suresh, E. A critical review on the antimicrobial resistance, antibiotic residue and metagenomics-assisted antimicrobial resistance gene detection in freshwater aquaculture environment. Aquac. Res. 2022, 53, 344–366. [Google Scholar] [CrossRef]
- Hossain, S.; Heo, G.J. Ornamental fish: A potential source of pathogenic and multidrug-resistant motile Aeromonas spp. Lett. Appl. Microbiol. 2021, 72, 2–12. [Google Scholar] [CrossRef]
- Liu, S.; Dong, G.; Zhao, H.; Chen, M.; Quan, W.; Qu, B. Occurrence and risk assessment of fluoroquinolones and tetracyclines in cultured fish from a coastal region of northern China. Environ. Sci. Pollut. Res. Int. 2018, 25, 8035–8043. [Google Scholar] [CrossRef]
- Iwu, C.D.; Korsten, L.; Okoh, A.I. The incidence of antibiotic resistance within and beyond the agricultural ecosystem: A concern for public health. MicrobiologyOpen 2020, 9, e1035. [Google Scholar] [CrossRef] [PubMed]
- Elmund, G.K.; Morrison, S.M.; Grant, D.W.; Nevins, M.P. Role of excreted chlortetracycline in modifying the decomposition process in feedlot waste. Bull. Environ. Contam. Toxicol. 1971, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Cabello, F.C.P.; Godfrey, H.P.P.; Buschmann, A.H.P.; Dölz, H.J.P. Aquaculture as yet another environmental gateway to the development and globalisation of antimicrobial resistance. Lancet Infect. Dis. 2016, 16, e127–e133. [Google Scholar] [CrossRef] [PubMed]
- Heuer, H.; Schmitt, H.; Smalla, K. Antibiotic resistance gene spread due to manure application on agricultural fields. Curr. Opin. Microbiol. 2011, 14, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Heuer, H.; Solehati, Q.; Zimmerling, U.; Kleineidam, K.; Schloter, M.; Müller, T.; Focks, A.; Thiele-Bruhn, S.; Smalla, K. Accumulation of Sulfonamide Resistance Genes in Arable Soils Due to Repeated Application of Manure Containing Sulfadiazine. Appl. Environ. Microbiol. 2011, 77, 2527–2530. [Google Scholar] [CrossRef]
- Nunes, O.C.; Manaia, C.M.; Kolvenbach, B.A.; Corvini, P.F.X. Living with sulfonamides: A diverse range of mechanisms observed in bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 10389–10408. [Google Scholar] [CrossRef]
- Ryu, S.-H.; Park, S.-G.; Choi, S.-M.; Hwang, Y.-O.; Ham, H.-J.; Kim, S.-U.; Lee, Y.-K.; Kim, M.-S.; Park, G.-Y.; Kim, K.-S.; et al. Antimicrobial resistance and resistance genes in Escherichia coli strains isolated from commercial fish and seafood. Int. J. Food Microbiol. 2012, 152, 14–18. [Google Scholar] [CrossRef]
- Lupo, A.; Coyne, S.; Berendonk, T.U. Origin and evolution of antibiotic resistance: The common mechanisms of emergence and spread in water bodies. Front. Microbiol. 2012, 3, 18. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Zhao, H. Prevalence of antibiotic resistance genes in wastewater collected from ornamental fish market in northern China. Environ. Pollut. 2021, 271, 116316. [Google Scholar] [CrossRef]
- Verner-Jeffreys, D.W.; Welch, T.J.; Schwarz, T.; Pond, M.J.; Woodward, M.J.; Haig, S.J.; Rimmer, G.S.E.; Roberts, E.; Morrison, V.; Baker-Austin, C. High prevalence of multidrug-tolerant bacteria and associated antimicrobial resistance genes isolated from ornamental fish and their carriage water. PLoS ONE 2009, 4, e8388. [Google Scholar] [CrossRef]
- Hossain, S.; De Silva, B.C.J.; Wimalasena, S.H.M.P.; Pathirana, H.N.K.S.; Dahanayake, P.S.; Heo, G.-J. Distribution of Antimicrobial Resistance Genes and Class 1 Integron Gene Cassette Arrays in Motile Aeromonas spp. Isolated from Goldfish (Carassius auratus). Microb. Drug Resist. 2018, 24, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- De Silva, L.A.D.S.; Wickramanayake, M.V.K.S.; Heo, G.J. Virulence and antimicrobial resistance potential of Aeromonas spp. associated with shellfish. Lett. Appl. Microbiol. 2021, 73, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Wickramanayake, M.V.K.S.; Dahanayake, P.S.; Hossain, S.; Heo, G.J. Antimicrobial resistance of pathogenic Aeromonas spp. isolated from marketed Pacific abalone (Haliotis discus hannai) in Korea. J. Appl. Microbiol. 2020, 128, 606–617. [Google Scholar] [CrossRef]
- Dobiasova, H.; Kutilova, I.; Piackova, V.; Vesely, T.; Cizek, A.; Dolejska, M. Ornamental fish as a source of plasmid-mediated quinolone resistance genes and antibiotic resistance plasmids. Vet. Microbiol. 2014, 171, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Sicuro, B.; Pastorino, P.; Barbero, R.; Barisone, S.; Dellerba, D.; Menconi, V.; Righetti, M.; De Vita, V.; Prearo, M. Prevalence and antibiotic sensitivity of bacteria isolated from imported ornamental fish in Italy: A translocation of resistant strains? Prev. Vet. Med. 2020, 175, 104880. [Google Scholar] [CrossRef] [PubMed]
- Levings, R.S.; Lightfoot, D.; Hall, R.M.; Djordjevic, S.P. Aquariums as reservoirs for multidrug-resistant Salmonella Paratyphi B. Emerg. Infect. Dis. 2006, 12, 507–510. [Google Scholar] [CrossRef]
- World Health Organization. Critically Important Antimicrobials for Human Medicine; World Health Organization: Geneva, Switzerland, 2018.
- González-Candelas, F.; Comas, I.; Martínez, J.L.; Galán, J.C.; Baquero, F. The Evolution of Antibiotic Resistance; Elsevier: Amsterdam, The Netherlands, 2017; pp. 257–284. [Google Scholar]
- Lowry, T.; Smith, A.S. Aquatic zoonoses associated with food, bait, ornamental, and tropical fish. J. Am. Vet. Med. Assos. 2007, 231, 876–880. [Google Scholar] [CrossRef]
- Roberts, H.E.; Palmeiro, B.; Scott, W.E. Bacterial and Parasitic Diseases of Pet Fish. Vet. Clin. N. Am. Exot. Anim. Pract. 2009, 12, 609–638. [Google Scholar] [CrossRef]
- Sigma-Aldrich. GSP Agar. Available online: https://www.sigmaaldrich.com/HK/en/product/sial/50875?gclid=Cj0KCQjwnP-ZBhDiARIsAH3FSRcOqH28PThGc0zQIca2E7wosTqJ3EDHwv8IbntmvIrGcm-sTeV_7CEaApyJEALw_wcB&gclsrc=aw.ds (accessed on 7 October 2022).
- Chen, K.; Zhou, J.L. Occurrence and behavior of antibiotics in water and sediments from the Huangpu River, Shanghai, China. Chemosphere 2014, 95, 604–612. [Google Scholar] [CrossRef]
- CLSI. VET04-A2: Methods for Broth Dilution Susceptibility Testing of Bacteria Isolated From Aquatic Animals; CLSI: Wayne, PA, USA, 2014. [Google Scholar]
- CLSI. M45: Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria; CLSI: Wayne, PA, USA, 2016. [Google Scholar]
- CLSI. VET03/VET04-S2: Performance Standards for Antimicrobial Susceptibility Testing of Bacteria Isolated From Aquatic Animals; Second Informational Supplement; VET03/VET04-S2; CLSI: Wayne, PA, USA, 2014. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Broth Dilution Susceptibility Testing of Bacteria Isolated From Aquatic Animals; Approved Guideline—Second Edition; CLSI: Wayne, PA, USA, 2014. [Google Scholar]
- CLSI. M100: Performance Standards for Antimicrobial Susceptibility Testing; CLSI: Wayne, PA, USA, 2020. [Google Scholar]
- Baron, S.; Granier, S.A.; Larvor, E.; Jouy, E.; Cineux, M.; Wilhelm, A.; Gassilloud, B.; Bouquin, S.L.; Kempf, I.; Chauvin, C. Aeromonas diversity and antimicrobial susceptibility in freshwater-An attempt to set generic epidemiological cut-off values. Front. Microbiol. 2017, 8, 503. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, J.; Wu, Z.; Zhang, Q.; Wang, S.; Hao, J.; Ouyang, L.; Li, A. Establishment of Epidemiological Resistance Cut-Off Values of Aquatic Aeromonas to Eight Antimicrobial Agents. Microorganisms 2022, 10, 776. [Google Scholar] [CrossRef] [PubMed]
- Cizek, A.; Dolejska, M.; Sochorova, R.; Strachotova, K.; Piackova, V.; Vesely, T. Antimicrobial resistance and its genetic determinants in aeromonads isolated in ornamental (koi) carp (Cyprinus carpio koi) and common carp (Cyprinus carpio). Vet. Microbiol. 2010, 142, 435–439. [Google Scholar] [CrossRef] [PubMed]
- The Government of the Hong Kong Special Administrative Region. Harmful Substances in Food Regulations (Cap. 132 Sub. Leg. AF); The Government of the Hong Kong Special Administrative Region: Hong Kong, China, 2012.
- Gao, P.; Mao, D.; Luo, Y.; Wang, L.; Xu, B.; Xu, L. Occurrence of sulfonamide and tetracycline-resistant bacteria and resistance genes in aquaculture environment. Water Res. 2012, 46, 2355–2364. [Google Scholar] [CrossRef]
- Hossain, S.; Dahanayake, P.S.; De Silva, B.C.J.; Wickramanayake, M.V.K.S.; Wimalasena, S.H.M.P.; Heo, G.J. Multidrug resistant Aeromonas spp. isolated from zebrafish (Danio rerio): Antibiogram, antimicrobial resistance genes and class 1 integron gene cassettes. Lett. Appl. Microbiol. 2019, 68, 370–377. [Google Scholar] [CrossRef]
- Hossain, S.; De Silva, B.C.J.; Dahanayake, P.S.; De Zoysa, M.; Heo, G.-J. Phylogenetic characteristics, virulence properties and antibiogram profile of motile Aeromonas spp. isolated from ornamental guppy (Poecilia reticulata). Arch. Microbiol. 2020, 202, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Hill, R.; Bermudez, L.E.; Miller-Morgan, T. Imported ornamental fish are colonized with antibiotic-resistant bacteria. J. Fish Dis. 2013, 36, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Preena, P.G.; Arathi, D.; Raj, N.S.; Arun Kumar, T.V.; Arun Raja, S.; Reshma, R.N.; Raja Swaminathan, T. Diversity of antimicrobial-resistant pathogens from a freshwater ornamental fish farm. Lett. Appl. Microbiol. 2020, 71, 108–116. [Google Scholar] [CrossRef]
- Roberts-Thomson, A.; Barnes, A.; Fielder, D.S.; Lester, R.J.G.; Adlard, R.D. Aerosol dispersal of the fish pathogen, Amyloodinium ocellatum. Aquaculture 2006, 257, 118–123. [Google Scholar] [CrossRef]
- Wooster, G.A.; Bowser, P.R. The Aerobiological Pathway of a Fish Pathogen: Survival and Dissemination of Aeromonas salmonicida in Aerosols and its Implications in Fish Health Management. J. World Aquac. Soc. 1996, 27, 7–14. [Google Scholar] [CrossRef]
- Gołaś, I.; Szmyt, M.; Glińska-Lewczuk, K. Water as a Source of Indoor Air Contamination with Potentially Pathogenic Aeromonas hydrophila in Aquaculture. Int. J. Environ. Res. Public Health 2022, 19, 2379. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).