Research Progress of the Endocrine-Disrupting Effects of Disinfection Byproducts
Abstract
1. Introduction
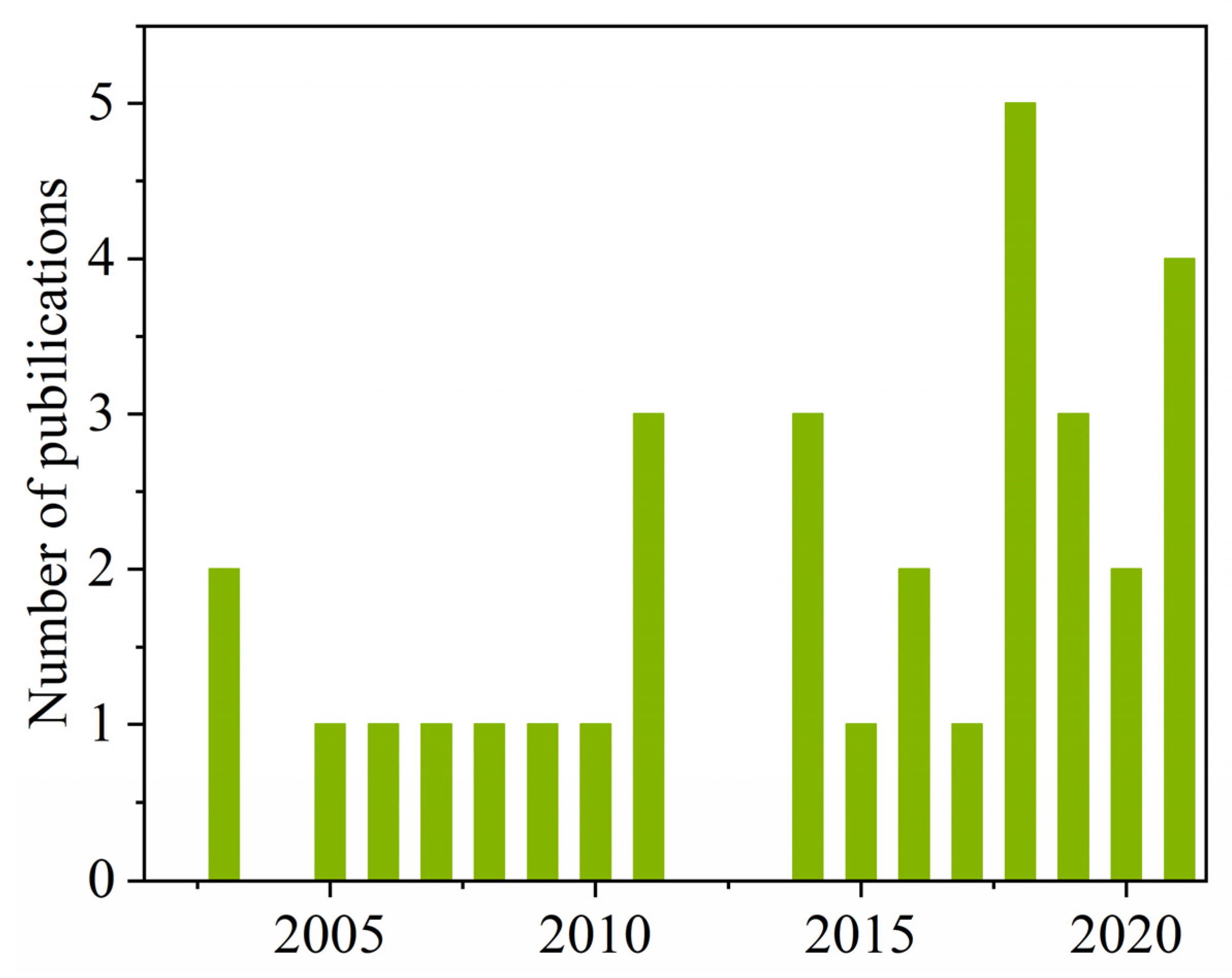
2. Performance of Publications
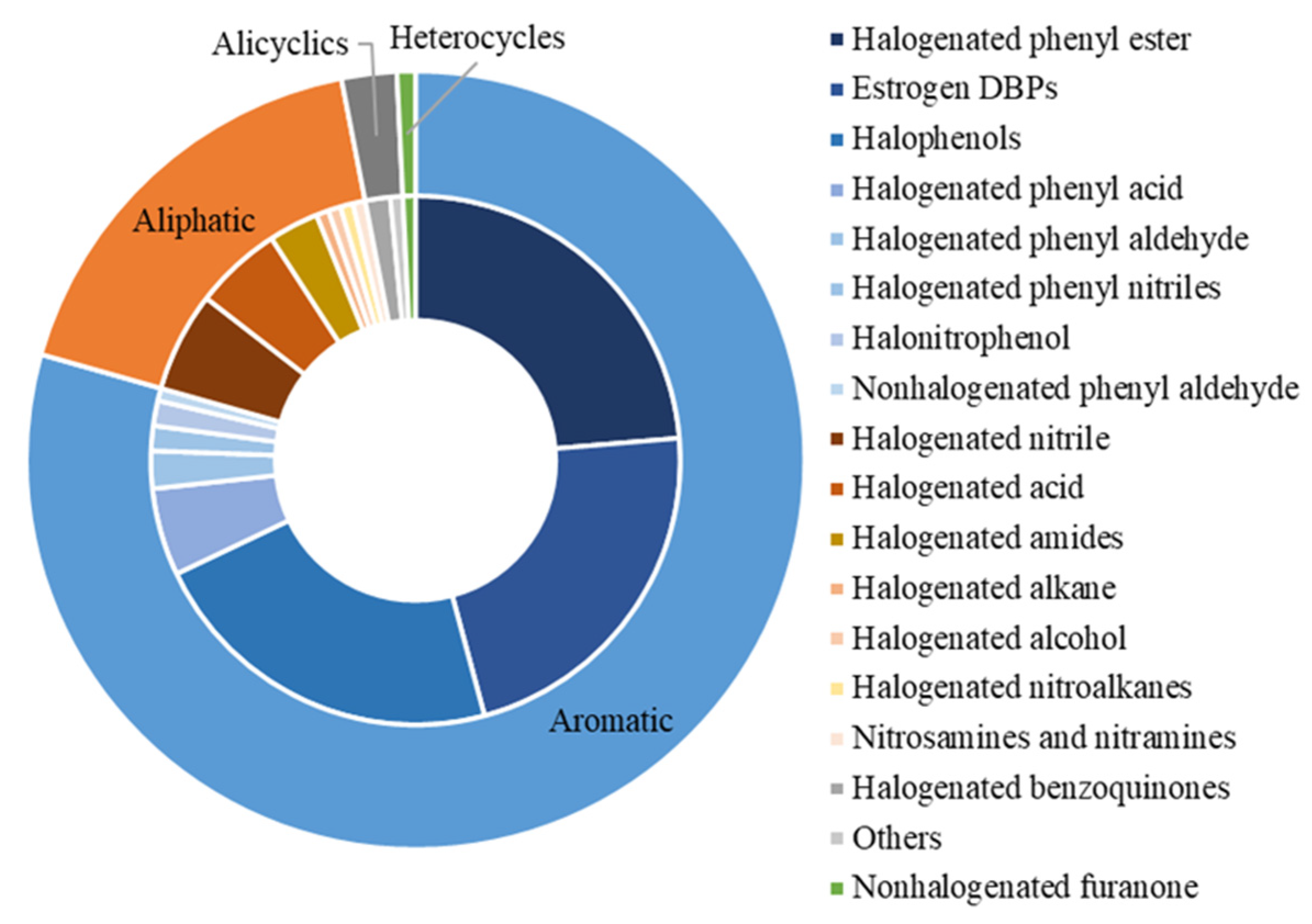
3. Characterization of DBPs with Endocrine-Disrupting Data
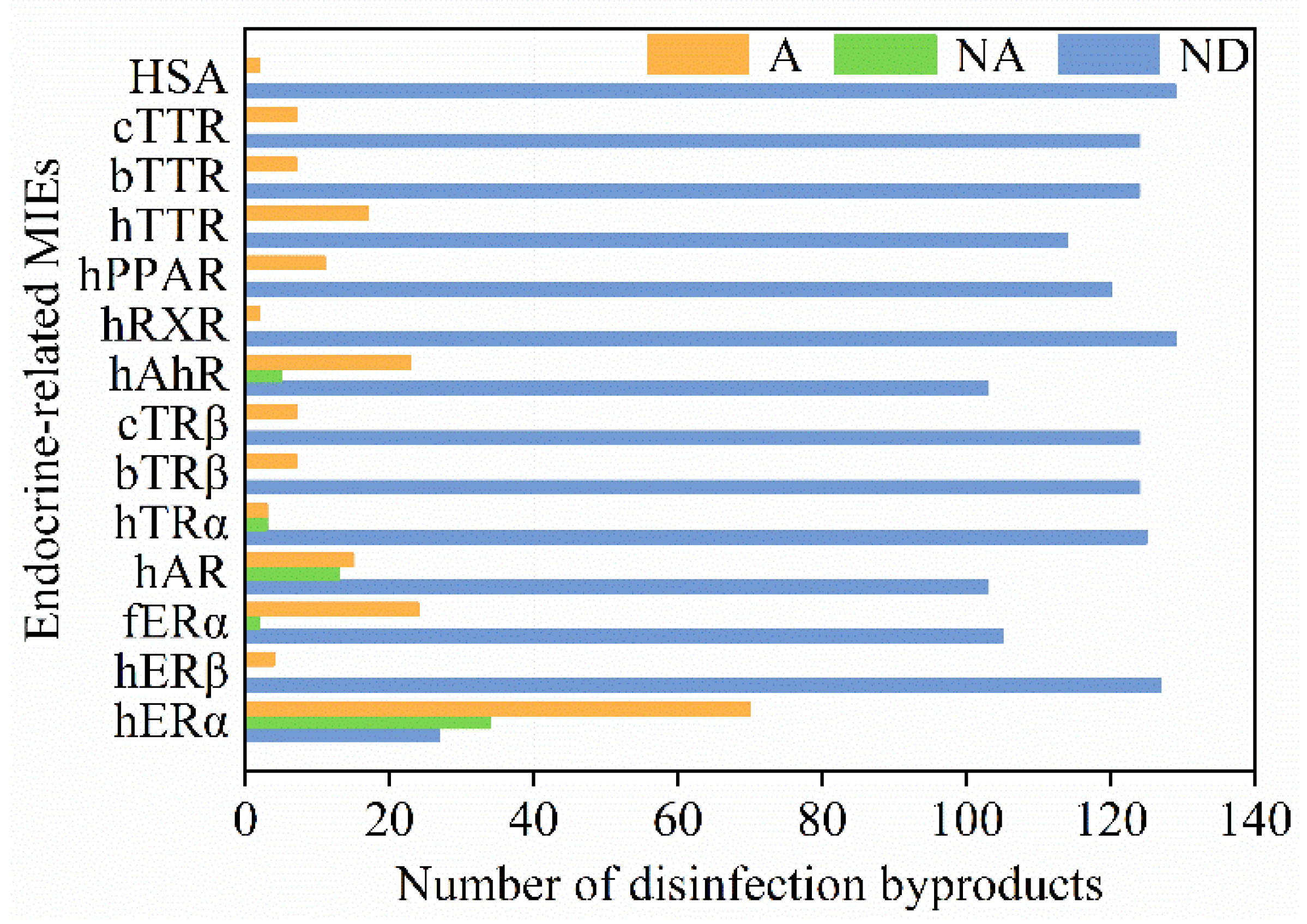
4. Endocrine-Related MIEs of DBPs
4.1. Hormone Receptor-Mediated Mechanism of Endocrine Disruption
4.2. Non-Receptor-Mediated Mechanism of Endocrine Disruption
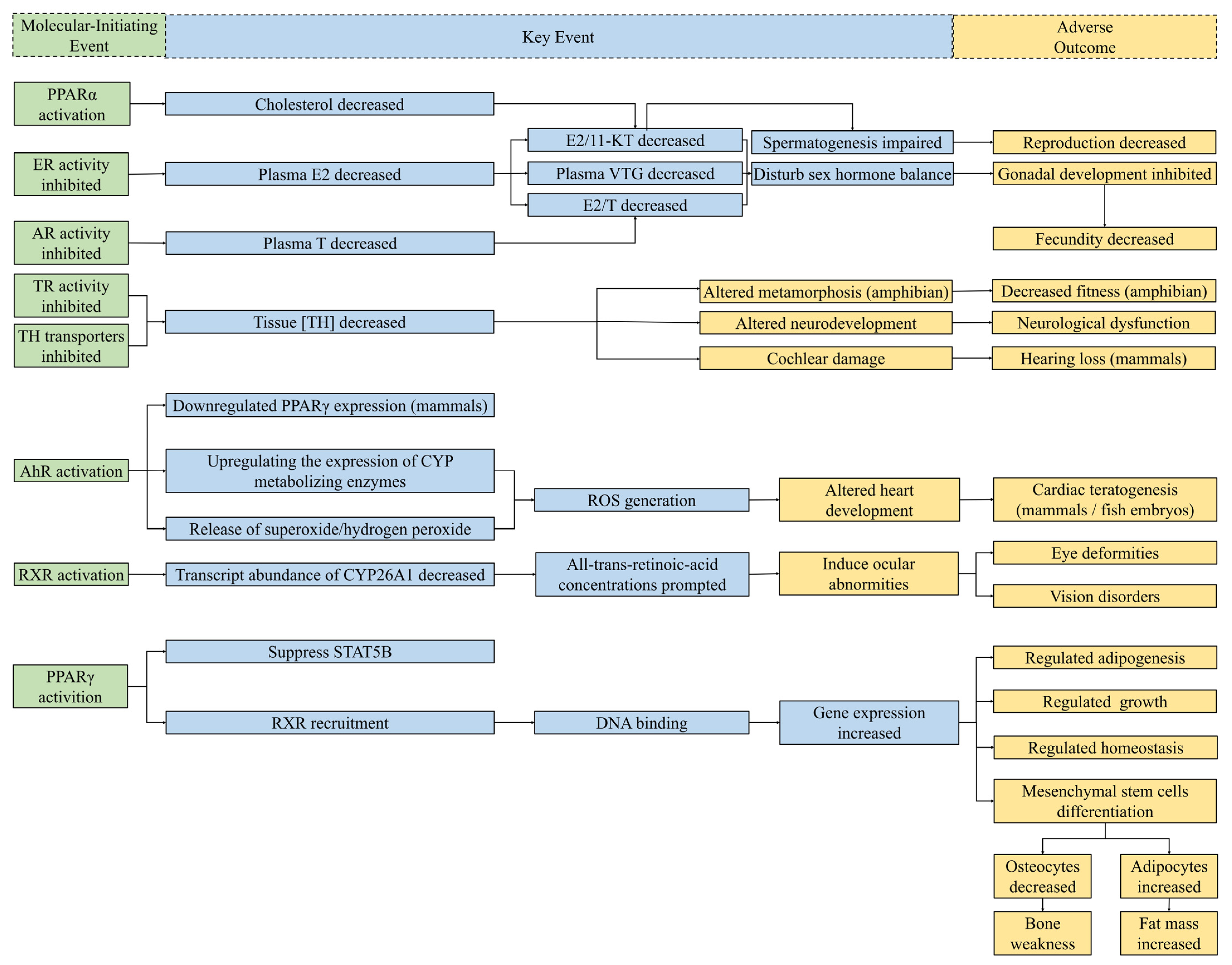
5. Potential Endocrine Adverse Outcome Pathways of DBPs
6. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, X.; Yang, M.; Zhu, Q.; Wagner, E.D.; Plewa, M.J. Comparative Quantitative Toxicology and QSAR Modeling of the Haloacetonitriles: Forcing Agents of Water Disinfection Byproduct Toxicity. Environ. Sci. Technol. 2020, 54, 8909–8918. [Google Scholar] [CrossRef] [PubMed]
- Plewa, M.J.; Richardson, S.D. Disinfection By-Products in Drinking Water, Recycled Water and Wastewater: Formation, Detection, Toxicity and Health Effects: Preface. J. Environ. Sci. 2017, 58, 1. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.D. Disinfection by-products and other emerging contaminants in drinking water. TrAC Trends Anal. Chem. 2003, 22, 666–684. [Google Scholar] [CrossRef]
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; Demarini, D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat. Res. Rev. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, Y.; Ruan, T.; Jiang, G. Identification of N-Nitrosamines and Nitrogenous Heterocyclic Byproducts during Chloramination of Aromatic Secondary Amine Precursors. Environ. Sci. Technol. 2020, 54, 12949–12958. [Google Scholar] [CrossRef]
- Bellar, T.A.; Lichtenberg, J.J.; Kroner, R.C. The occurrence of organohalides in chlorinated drinking waters. J. Am. Water Works Assoc. 1974, 66, 703–706. [Google Scholar] [CrossRef]
- Čulin, J.; Mustać, B. Environmental risks associated with ballast water management systems that create disinfection by-products (DBPs). Ocean Coast. Manag. 2015, 105, 100–105. [Google Scholar] [CrossRef]
- Luan, X.; Liu, X.; Fang, C.; Chu, W.; Xu, Z. Ecotoxicological effects of disinfected wastewater effluents: A short review of in vivo toxicity bioassays on aquatic organisms. Environ. Sci. Water Res. Technol. 2020, 6, 2275–2286. [Google Scholar] [CrossRef]
- Li, C.; Wang, D.; Xu, X.; Wang, Z. Formation of known and unknown disinfection by-products from natural organic matter fractions during chlorination, chloramination, and ozonation. Sci. Total Environ. 2017, 587, 177–184. [Google Scholar] [CrossRef]
- Richardson, S.D.; DeMarini, D.M.; Kogevinas, M.; Fernandez, P.; Marco, E.; Lourencetti, C.; Ballesté, C.; Heederik, D.; Meliefste, K.; McKague, A.B.; et al. What’s in the pool? A comprehensive identification of disinfection by-products and assessment of mutagenicity of chlorinated and brominated swimming pool water. Environ. Health Perspect. 2010, 118, 1523–1530. [Google Scholar] [CrossRef]
- Tang, H.; Zhong, H.; Pan, Y.; Zhou, Q.; Huo, Z.; Chu, W.; Xu, B. A New Group of Heterocyclic Nitrogenous Disinfection Byproducts (DBPs) in Drinking Water: Role of Extraction pH in Unknown DBP Exploration. Environ. Sci. Technol. 2021, 55, 6764–6772. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, H.; Zhou, Q.; Li, A.; Shuang, C.; Xian, Q.; Xu, B.; Pan, Y. New phenolic halogenated disinfection byproducts in simulated chlorinated drinking water: Identification, decomposition, and control by ozone-activated carbon treatment. Water Res. 2018, 146, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Lu, Y.; Du, Y.; Wang, W.L.; Yang, L.L.; Wu, Q.Y. Comprehensive GCxGC-qMS with a mass-to-charge ratio difference extraction method to identify new brominated byproducts during ozonation and their toxicity assessment. J. Hazard. Mater. 2021, 403, 124103. [Google Scholar] [CrossRef]
- Chaves, R.S.; Guerreiro, C.S.; Cardoso, V.V.; Benoliel, M.J.; Santos, M.M. Toxicological assessment of seven unregulated drinking water Disinfection By-products (DBPs) using the zebrafish embryo bioassay. Sci. Total Environ. 2020, 742, 140522. [Google Scholar] [CrossRef]
- Chowdhury, S.; Alhooshani, K.; Karanfil, T. Disinfection byproducts in swimming pool: Occurrences, implications and future needs. Water Res. 2014, 53, 68–109. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zeng, Q.; Wang, L.; Huang, Y.H.; Lu, Z.W.; Wang, P.; He, M.J.; Huang, X.; Lu, W.Q. Temporal variability in urinary levels of drinking water disinfection byproducts dichloroacetic acid and trichloroacetic acid among men. Environ. Res. 2014, 135, 126–132. [Google Scholar] [CrossRef]
- Cao, W.C.; Zeng, Q.; Luo, Y.; Chen, H.X.; Miao, D.Y.; Li, L.; Cheng, Y.H.; Li, M.; Wang, F.; You, L.; et al. Blood Biomarkers of Late Pregnancy Exposure to Trihalomethanes in Drinking Water and Fetal Growth Measures and Gestational Age in a Chinese Cohort. Environ. Health Perspect. 2016, 124, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.D.; Plewa, M.J. CHO cell cytotoxicity and genotoxicity analyses of disinfection by-products: An updated review. J. Environ. Sci. 2017, 58, 64–76. [Google Scholar] [CrossRef]
- Wendel, F.M.; Ternes, T.A.; Richardson, S.D.; Duirk, S.E.; Pals, J.A.; Wagner, E.D.; Plewa, M.J. Comparative Toxicity of High-Molecular Weight Iopamidol Disinfection Byproducts. Environ. Sci. Technol. Lett. 2016, 3, 81–84. [Google Scholar] [CrossRef]
- Lan, J.; Rahman, S.M.; Gou, N.; Jiang, T.; Plewa, M.J.; Alshawabkeh, A.; Gu, A.Z. Genotoxicity Assessment of Drinking Water Disinfection Byproducts by DNA Damage and Repair Pathway Profiling Analysis. Environ. Sci. Technol. 2018, 52, 6565–6575. [Google Scholar] [CrossRef]
- Kali, S.; Khan, M.; Ghaffar, M.S.; Rasheed, S.; Waseem, A.; Iqbal, M.M.; Bilal Khan Niazi, M.; Zafar, M.I. Occurrence, influencing factors, toxicity, regulations, and abatement approaches for disinfection by-products in chlorinated drinking water: A comprehensive review. Environ. Pollut. 2021, 281, 116950. [Google Scholar] [CrossRef]
- Jia, X.; Jin, J.; Gao, R.; Feng, T.; Huang, Y.; Zhou, Q.; Li, A. Degradation of benzophenone-4 in a UV/chlorine disinfection process: Mechanism and toxicity evaluation. Chemosphere 2019, 222, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Liberatore, H.K.; Plewa, M.J.; Wagner, E.D.; VanBriesen, J.M.; Burnett, D.B.; Cizmas, L.H.; Richardson, S.D. Identification and Comparative Mammalian Cell Cytotoxicity of New Iodo-Phenolic Disinfection Byproducts in Chloraminated Oil and Gas Wastewaters. Environ. Sci. Technol. Lett. 2017, 4, 475–480. [Google Scholar] [CrossRef]
- Li, C.; Wang, D.; Xu, X.; Xu, M.; Wang, Z. Spatial variations in the occurrence of potentially genotoxic disinfection by-products in drinking water distribution systems in China. Environ. Pollut. 2017, 231, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, Q.; Huang, C.; Yang, M.; Li, J.; Chen, Y.; Yang, B.; Zhao, X. Comparative cytotoxicity of halogenated aromatic DBPs and implications of the corresponding developed QSAR model to toxicity mechanisms of those DBPs: Binding interactions between aromatic DBPs and catalase play an important role. Water Res. 2020, 170, 115283. [Google Scholar] [CrossRef]
- Gonsioroski, A.; Mourikes, V.E.; Flaws, J.A. Endocrine Disruptors in Water and Their Effects on the Reproductive System. Int. J. Mol. Sci. 2020, 21, 1929. [Google Scholar] [CrossRef]
- Uyak, V.; Koyuncu, I.; Oktem, I.; Cakmakci, M.; Toroz, I. Removal of trihalomethanes from drinking water by nanofiltration membranes. J. Hazard. Mater. 2008, 152, 789–794. [Google Scholar] [CrossRef]
- Plattard, N.; Dupuis, A.; Migeot, V.; Haddad, S.; Venisse, N. An overview of the literature on emerging pollutants: Chlorinated derivatives of Bisphenol A (ClxBPA). Environ. Int. 2021, 153, 106547. [Google Scholar] [CrossRef]
- Diana, M.; Felipe-Sotelo, M.; Bond, T. Disinfection byproducts potentially responsible for the association between chlorinated drinking water and bladder cancer: A review. Water Res. 2019, 162, 492–504. [Google Scholar] [CrossRef]
- Mazhar, M.A.; Khan, N.A.; Ahmed, S.; Khan, A.H.; Hussain, A.; Rahisuddin; Changani, F.; Yousefi, M.; Ahmadi, S.; Vambol, V. Chlorination disinfection by-products in municipal drinking water—A review. J. Clean. Prod. 2020, 273, 123159. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. National Interim Primary Drinking Water Regulations. Am. Water Work. Assoc. 1976, 68, 57–68. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking Water Quality, 4th ed.; WHO Press: Geneva, Switzerland, 2011; pp. 307–434. [Google Scholar]
- Han, J.; Zhang, X.; Jiang, J.; Li, W. How Much of the Total Organic Halogen and Developmental Toxicity of Chlorinated Drinking Water Might Be Attributed to Aromatic Halogenated DBPs? Environ. Sci. Technol. 2021, 55, 5906–5916. [Google Scholar] [CrossRef]
- Liu, X.; Chen, L.; Yang, M.; Tan, C.; Chu, W. The occurrence, characteristics, transformation and control of aromatic disinfection by-products: A review. Water Res. 2020, 184, 116076. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.D.; Mitch, W.A. Halonitroalkanes, halonitriles, haloamides, and N-nitrosamines: A critical review of nitrogenous disinfection byproduct formation pathways. Environ. Sci. Technol. 2012, 46, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Mian, H.R.; Hu, G.; Hewage, K.; Rodriguez, M.J.; Sadiq, R. Prioritization of unregulated disinfection by-products in drinking water distribution systems for human health risk mitigation: A critical review. Water Res. 2018, 147, 112–131. [Google Scholar] [CrossRef]
- Du, P.; Zhao, H.; Cao, H.; Huang, C.H.; Liu, W.; Li, Y. Transformation of halobenzoquinones with the presence of amino acids in water: Products, pathways and toxicity. Water Res. 2017, 122, 299–307. [Google Scholar] [CrossRef]
- Chen, Y.H.; Qin, L.T.; Mo, L.Y.; Zhao, D.N.; Zeng, H.H.; Liang, Y.P. Synergetic effects of novel aromatic brominated and chlorinated disinfection byproducts on Vibrio qinghaiensis sp.-Q67. Environ. Pollut. 2019, 250, 375–385. [Google Scholar] [CrossRef]
- Cui, H.; Chen, B.; Jiang, Y.; Tao, Y.; Zhu, X.; Cai, Z. Toxicity of 17 Disinfection By-products to Different Trophic Levels of Aquatic Organisms: Ecological Risks and Mechanisms. Environ. Sci. Technol. 2021, 55, 10534–10541. [Google Scholar] [CrossRef]
- Delacroix, S.; Vogelsang, C.; Tobiesen, A.; Liltved, H. Disinfection by-products and ecotoxicity of ballast water after oxidative treatment--results and experiences from seven years of full-scale testing of ballast water management systems. Mar. Pollut. Bull. 2013, 73, 24–36. [Google Scholar] [CrossRef]
- Ding, X.; Zhu, J.; Zhang, J.; Dong, T.; Xia, Y.; Jiao, J.; Wang, X.; Zhou, W. Developmental toxicity of disinfection by-product monohaloacetamides in embryo-larval stage of zebrafish. Ecotoxicol. Environ. Saf. 2020, 189, 110037. [Google Scholar] [CrossRef]
- Fisher, D.; Yonkos, L.; Ziegler, G.; Friedel, E.; Burton, D. Acute and chronic toxicity of selected disinfection byproducts to Daphnia magna, Cyprinodon variegatus, and Isochrysis galbana. Water Res. 2014, 55, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Hanigan, D.; Truong, L.; Simonich, M.; Tanguay, R.; Westerhoff, P. Zebrafish embryo toxicity of 15 chlorinated, brominated, and iodinated disinfection by-products. J. Environ. Sci. 2017, 58, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zhou, D.; Yu, S.; Chen, W. The removal process of 2,2-dichloroacetamide (DCAcAm), a new disinfection by-product, in drinking water treatment process and its toxicity on zebrafish. Chemosphere 2016, 159, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.; Ferreira, C.; Ferreira, I.; Mansilha, C. Acute and chronic toxicity assessment of haloacetic acids using Daphnia magna. J. Toxicol. Environ. Health A 2019, 82, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Teixidó, E.; Piqué, E.; Gonzalez-Linares, J.; Llobet, J.M.; Gómez-Catalán, J. Developmental effects and genotoxicity of 10 water disinfection by-products in zebrafish. J. Water. Health. 2015, 13, 54–66. [Google Scholar] [CrossRef]
- Yu, S.; Lin, T.; Chen, W.; Tao, H. The toxicity of a new disinfection by-product, 2,2-dichloroacetamide (DCAcAm), on adult zebrafish (Danio rerio) and its occurrence in the chlorinated drinking water. Chemosphere 2015, 139, 40–46. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Yang, X.; Wang, L. Aquatic toxicity and aquatic ecological risk assessment of wastewater-derived halogenated phenolic disinfection byproducts. Sci. Total Environ. 2022, 809, 151089. [Google Scholar] [CrossRef]
- Lu, L.; Zhan, T.; Ma, M.; Xu, C.; Wang, J.; Zhang, C.; Liu, W.; Zhuang, S. Thyroid Disruption by Bisphenol S Analogues via Thyroid Hormone Receptor beta: In Vitro, in Vivo, and Molecular Dynamics Simulation Study. Environ. Sci. Technol. 2018, 52, 6617–6625. [Google Scholar] [CrossRef]
- Holmes, B.E.; Smeester, L.; Fry, R.C.; Weinberg, H.S. Identification of endocrine active disinfection by-products (DBPs) that bind to the androgen receptor. Chemosphere 2017, 187, 114–122. [Google Scholar] [CrossRef]
- Long, K.; Sha, Y.; Mo, Y.; Wei, S.; Wu, H.; Lu, D.; Xia, Y.; Yang, Q.; Zheng, W.; Wei, X. Androgenic and Teratogenic Effects of Iodoacetic Acid Drinking Water Disinfection Byproduct in Vitro and in Vivo. Environ. Sci. Technol. 2021, 55, 3827–3835. [Google Scholar] [CrossRef]
- Liu, H.; Yang, X.; Yin, C.; Wei, M.; He, X. Development of predictive models for predicting binding affinity of endocrine disrupting chemicals to fish sex hormone-binding globulin. Ecotoxicol. Environ. Saf. 2017, 136, 46–54. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Peng, T.; Yang, X.; Liu, H. Development of QSAR models for predicting the binding affinity of endocrine disrupting chemicals to eight fish estrogen receptor. Ecotoxicol. Environ. Saf. 2018, 148, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Colborn, T.; vom Saal, F.S.; Soto, A.M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspect. 1993, 101, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ou, W.; Zhao, S.; Xi, Y.; Wang, L.; Liu, H. Rapid Screening of Human Transthyretin Disruptors through a Tiered in Silico Approach. ACS Sustain. Chem. Eng. 2021, 9, 5661–5672. [Google Scholar] [CrossRef]
- Yamauchi, K.; Ishihara, A.; Fukazawa, H.; Terao, Y. Competitive interactions of chlorinated phenol compounds with 3,3′,5-triiodothyronine binding to transthyretin: Detection of possible thyroid-disrupting chemicals in environmental waste water. Toxicol. Appl. Pharmacol. 2003, 187, 110–117. [Google Scholar] [CrossRef]
- Takemura, H.; Ma, J.; Sayama, K.; Terao, Y.; Zhu, B.T.; Shimoi, K. In vitro and in vivo estrogenic activity of chlorinated derivatives of bisphenol A. Toxicology 2005, 207, 215–221. [Google Scholar] [CrossRef]
- Nakamura, H.; Shiozawa, T.; Terao, Y.; Shiraishi, F.; Fukazawa, H. By-products produced by the reaction of estrogens with hypochlorous acid and their estrogen activities. J. Health Sci. 2006, 52, 124–131. [Google Scholar] [CrossRef][Green Version]
- Terasaki, M.; Kosaka, K.; Kunikane, S.; Makino, M.; Shiraishi, F. Assessment of thyroid hormone activity of halogenated bisphenol A using a yeast two-hybrid assay. Chemosphere 2011, 84, 1527–1530. [Google Scholar] [CrossRef]
- Riu, A.; le Maire, A.; Grimaldi, M.; Audebert, M.; Hillenweck, A.; Bourguet, W.; Balaguer, P.; Zalko, D. Characterization of novel ligands of ERalpha, Erbeta, and PPARgamma: The case of halogenated bisphenol A and their conjugated metabolites. Toxicol. Sci. 2011, 122, 372–382. [Google Scholar] [CrossRef]
- Riu, A.; Grimaldi, M.; le Maire, A.; Bey, G.; Phillips, K.; Boulahtouf, A.; Perdu, E.; Zalko, D.; Bourguet, W.; Balaguer, P. Peroxisome proliferator-activated receptor gamma is a target for halogenated analogs of bisphenol A. Environ. Health Perspect. 2011, 119, 1227–1232. [Google Scholar] [CrossRef]
- Terasaki, M.; Yasuda, M.; Makino, M.; Shimoi, K. Aryl hydrocarbon receptor potency of chlorinated parabens in the aquatic environment. Environ. Sci. Water Res. Technol. 2015, 1, 375–382. [Google Scholar] [CrossRef]
- Li, N.; Jiang, W.; Ma, M.; Wang, D.; Wang, Z. Chlorination by-products of bisphenol A enhanced retinoid X receptor disrupting effects. J. Hazard. Mater. 2016, 320, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Shi, J.C.; Hu, J.Y.; Hu, W.X.; Zhang, J.; Shao, B. Chlorination of bisphenol F and the estrogenic and peroxisome proliferator-activated receptor gamma effects of its disinfection byproducts. Water Res. 2016, 107, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Shi, J.; Zhang, J.; Yang, Y.; Hu, J.; Shao, B. Identification of the disinfection byproducts of bisphenol S and the disrupting effect on peroxisome proliferator-activated receptor gamma (PPARgamma) induced by chlorination. Water Res. 2018, 132, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Holmes, B.E.; Smeester, L.; Fry, R.C.; Weinberg, H.S. Disinfection Byproducts Bind Human Estrogen Receptor-alpha. Environ. Toxicol. Chem. 2019, 38, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ou, W.; Xi, Y.; Chen, J.; Liu, H. Emerging Polar Phenolic Disinfection Byproducts Are High-Affinity Human Transthyretin Disruptors: An in Vitro and in Silico Study. Environ. Sci. Technol. 2019, 53, 7019–7028. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, M.; Yi, J.; Zhu, Q.; Huang, C.; Chen, Y.; Li, J.; Yang, B.; Zhao, X. Comprehensive Insights into the Interactions of Two Emerging Bromophenolic DBPs with Human Serum Albumin by Multispectroscopy and Molecular Docking. ACS Omega 2019, 4, 563–572. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, C.G.; Kim, Y.J. Characterizing the potential estrogenic and androgenic activities of two disinfection byproducts, mono-haloacetic acids and haloacetamides, using in vitro bioassays. Chemosphere 2020, 242, 125198. [Google Scholar] [CrossRef]
- Gouukon, Y.; Yasuda, M.T.; Yasukawa, H.; Terasaki, M. Occurrence and AhR activity of brominated parabens in the Kitakami River, North Japan. Chemosphere 2020, 249, 126152. [Google Scholar] [CrossRef]
- Park, C.G.; Jung, K.C.; Kim, D.H.; Kim, Y.J. Monohaloacetonitriles induce cytotoxicity and exhibit different mode of action in endocrine disruption. Sci. Total Environ. 2021, 761, 143316. [Google Scholar] [CrossRef]
- Hu, J.Y.; Cheng, S.J.; Aizawa, T.; Terao, Y.; Kunikane, S. Products of aqueous chlorination of 17 beta-estradiol and their estrogenic activities. Environ. Sci. Technol. 2003, 37, 5665–5670. [Google Scholar] [CrossRef] [PubMed]
- Bila, D.; Montalvão, A.F.; Azevedo Dde, A.; Dezotti, M. Estrogenic activity removal of 17beta-estradiol by ozonation and identification of by-products. Chemosphere 2007, 69, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Maniero, M.G.; Bila, D.M.; Dezotti, M. Degradation and estrogenic activity removal of 17beta-estradiol and 17alpha-ethinylestradiol by ozonation and O3/H2O2. Sci. Total Environ. 2008, 407, 105–115. [Google Scholar] [PubMed]
- Wu, Q.-Y.; Hu, H.-Y.; Zhao, X.; Sun, Y.-X. Effect of Chlorination on the Estrogenic/Antiestrogenic Activities of Biologically Treated Wastewater. Environ. Sci. Technol. 2009, 43, 4940–4945. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Hu, H.Y.; Zhao, X.; Li, Y.; Liu, Y. Characterization and identification of antiestrogenic products of phenylalanine chlorination. Water Res. 2010, 44, 3625–3634. [Google Scholar] [CrossRef]
- Shang, G.; Xue, J.; Li, M.; Hu, H.Y.; Lu, Y. Estrogen receptor affinity chromatography: A new method for characterization of novel estrogenic disinfection by-products. Chemosphere 2014, 104, 251–257. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Tang, X.; Huang, H.; Li, Y.; Hu, H.Y.; Ding, Y.N.; Shao, Y.R. Antiestrogenic activity and related disinfection by-product formation induced by bromide during chlorine disinfection of sewage secondary effluent. J. Hazard. Mater. 2014, 273, 280–286. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, S.; Zhao, F.; Zhang, H.; An, W.; Yang, M.; Zhang, Z.; Hu, J. Byproducts of aqueous chlorination of equol and their estrogenic potencies. Chemosphere 2018, 212, 393–399. [Google Scholar] [CrossRef]
- Sasaki, K.; Terasaki, M. Estrogen agonistic/antagonistic activity of brominated parabens. Environ. Sci. Pollut Res. Int. 2018, 25, 21257–21266. [Google Scholar] [CrossRef]
- Jakopin, Ž. Assessment of the endocrine-disrupting potential of halogenated parabens: An in silico approach. Chemosphere 2021, 264, 128447. [Google Scholar] [CrossRef]
- Xia, Y.; Mo, Y.; Yang, Q.; Yu, Y.; Jiang, M.; Wei, S.; Lu, D.; Wu, H.; Lu, G.; Zou, Y.; et al. Iodoacetic Acid Disrupting the Thyroid Endocrine System in Vitro and in Vivo. Environ. Sci. Technol. 2018, 52, 7545–7552. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Liang, D.; Liang, Y.; Chen, M.; Wang, F.; Wang, H.; Jiang, G. Assessing developmental toxicity and estrogenic activity of halogenated bisphenol A on zebrafish (Danio rerio). Chemosphere 2014, 112, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Stanojević, M.; Vračko Grobelšek, M.; Sollner Dolenc, M. Computational evaluation of endocrine activity of biocidal active substances. Chemosphere 2021, 267, 129284. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Ji, C.; Ren, F.; Aniagu, S.; Tong, J.; Jiang, Y.; Chen, T. AHR-mediated oxidative stress contributes to the cardiac developmental toxicity of trichloroethylene in zebrafish embryos. J. Hazard. Mater. 2020, 385, 121521. [Google Scholar] [CrossRef]
- Li, Y.; Ma, H.; Chen, R.; Zhang, H.; Nakanishi, T.; Hu, J. Maternal Transfer of 2-Ethylhexyl Diphenyl Phosphate Leads to Developmental Toxicity Possibly by Blocking the Retinoic Acid Receptor and Retinoic X Receptor in Japanese Medaka (Oryzias latipes). Environ. Sci. Technol. 2021, 55, 5056–5064. [Google Scholar] [CrossRef]
- Kirk, A.B.; Michelsen-Correa, S.; Rosen, C.; Martin, C.F.; Blumberg, B. PFAS and Potential Adverse Effects on Bone and Adipose Tissue Through Interactions with PPARgamma. Endocrinology 2021, 162, bqab194. [Google Scholar] [CrossRef]
- Chen, P.; Yang, J.; Chen, G.; Yi, S.; Liu, M.; Zhu, L. Thyroid-Disrupting Effects of 6:2 and 8:2 Polyfluoroalkyl Phosphate Diester (diPAPs) at Environmentally Relevant Concentrations from Integrated In Silico and In Vivo Studies. Environ. Sci. Technol. Lett. 2020, 7, 330–336. [Google Scholar] [CrossRef]
- Paul Friedman, K.; Watt, E.D.; Hornung, M.W.; Hedge, J.M.; Judson, R.S.; Crofton, K.M.; Houck, K.A.; Simmons, S.O. Tiered High-Throughput Screening Approach to Identify Thyroperoxidase Inhibitors Within the ToxCast Phase I and II Chemical Libraries. Toxicol. Sci. 2016, 151, 160–180. [Google Scholar] [CrossRef]
- Park, C.B.; Kim, G.E.; On, J.; Pyo, H.; Park, J.W.; Cho, S.H. Sex-specific effects of bisphenol S with tissue-specific responsiveness in adult zebrafish: The antiandrogenic and antiestrogenic effects. Ecotoxicol. Environ. Saf. 2022, 229, 113102. [Google Scholar] [CrossRef]
- Zorn, K.M.; Foil, D.H.; Lane, T.R.; Hillwalker, W.; Feifarek, D.J.; Jones, F.; Klaren, W.D.; Brinkman, A.M.; Ekins, S. Comparison of Machine Learning Models for the Androgen Receptor. Environ. Sci. Technol. 2020, 54, 13690–13700. [Google Scholar] [CrossRef] [PubMed]
- Paul-Friedman, K.; Martin, M.; Crofton, K.M.; Hsu, C.W.; Sakamuru, S.; Zhao, J.; Xia, M.; Huang, R.; Stavreva, D.A.; Soni, V.; et al. Limited Chemical Structural Diversity Found to Modulate Thyroid Hormone Receptor in the Tox21 Chemical Library. Environ. Health Perspect. 2019, 127, 097009. [Google Scholar] [CrossRef] [PubMed]
- Swedenborg, E.; Rüegg, J.; Mäkelä, S.; Pongratz, I. Endocrine disruptive chemicals: Mechanisms of action and involvement in metabolic disorders. J. Mol. Endocrinol. 2009, 43, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; De Luca, L.M. Therapeutic Potential of "Rexinoids" in Cancer Prevention and Treatment. Cancer Res. 2009, 69, 4945–4947. [Google Scholar] [CrossRef]
- Henriksen, A.D.; Andrade, A.; Harris, E.P.; Rissman, E.F.; Wolstenholme, J.T. Bisphenol A Exposure in Utero Disrupts Hypothalamic Gene Expression Particularly Genes Suspected in Autism Spectrum Disorders and Neuron and Hormone Signaling. Int. J. Mol. Sci. 2020, 21, 3129. [Google Scholar] [CrossRef]
- Ponzi, D.; Gioiosa, L.; Parmigiani, S.; Palanza, P. Effects of Prenatal Exposure to a Low-Dose of Bisphenol A on Sex Differences in Emotional Behavior and Central Alpha2-Adrenergic Receptor Binding. Int. J. Mol. Sci. 2020, 21, 3269. [Google Scholar] [CrossRef]
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-A and the Great Divide: A Review of Controversies in the Field of Endocrine Disruption. Endocr. Rev. 2009, 30, 75–95. [Google Scholar] [CrossRef]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- Fic, A.; Žegura, B.; Gramec, D.; Mašič, L.P. Estrogenic and androgenic activities of TBBA and TBMEPH, metabolites of novel brominated flame retardants, and selected bisphenols, using the XenoScreen XL YES/YAS assay. Chemosphere 2014, 112, 362–369. [Google Scholar] [CrossRef]
- Xi, Y.; Yang, X.; Zhang, H.; Liu, H.; Watson, P.; Yang, F. Binding interactions of halo-benzoic acids, halo-benzenesulfonic acids and halo-phenylboronic acids with human transthyretin. Chemosphere 2020, 242, 125135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kamstra, J.H.; Ghorbanzadeh, M.; Weiss, J.M.; Hamers, T.; Andersson, P.L. In Silico Approach to Identify Potential Thyroid Hormone Disruptors among Currently Known Dust Contaminants and Their Metabolites. Environ. Sci. Technol. 2015, 49, 10099–10107. [Google Scholar] [CrossRef] [PubMed]
- Grimm, F.A.; Lehmler, H.J.; He, X.; Robertson, L.W.; Duffel, M.W. Sulfated metabolites of polychlorinated biphenyls are high-affinity ligands for the thyroid hormone transport protein transthyretin. Environ. Health Perspect. 2013, 121, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Meerts, I.; van Zanden, J.J.; Luijks, E.A.C.; van Leeuwen-Bol, I.; Marsh, G.; Jakobsson, E.; Bergman, A.; Brouwer, A. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol. Sci. 2000, 56, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.M.; Andersson, P.L.; Zhang, J.; Simon, E.; Leonards, P.E.; Hamers, T.; Lamoree, M.H. Tracing thyroid hormone-disrupting compounds: Database compilation and structure-activity evaluation for an effect-directed analysis of sediment. Anal. Bioanal. Chem. 2015, 407, 5625–5634. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Wang, C.; Zhang, Q.; Yang, X.; Huang, S.; Luo, Y.; Feng, Y.; Zheng, Q. Transcriptomic analysis of adult zebrafish heart and brain in response to 2, 6-dichloro-1, 4-benzoquinone exposure. Ecotoxicol. Environ. Saf. 2021, 226, 112835. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, X.; Zheng, Q.; Moe, B.; Li, X.F. Halobenzoquinone-Induced Developmental Toxicity, Oxidative Stress, and Apoptosis in Zebrafish Embryos. Environ. Sci. Technol. 2018, 52, 10590–10598. [Google Scholar] [CrossRef]
- Mansouri, K.; Kleinstreuer, N.; Abdelaziz, A.M.; Alberga, D.; Alves, V.M.; Andersson, P.L.; Andrade, C.H.; Bai, F.; Balabin, I.; Ballabio, D.; et al. CoMPARA: Collaborative Modeling Project for Androgen Receptor Activity. Environ. Health Perspect. 2020, 128, 027002. [Google Scholar] [CrossRef]
| Endpoints | Groups of DBPs |
|---|---|
| hERα | Halogenated phenyl esters (31); estrogen DBPs (29); halophenols (14); halogenated nitriles (8); halogenated acids (7); halogenated amides (4); halogenated phenyl nitriles (2); halogenated benzoquinones (2); halogenated alcohols (1); halogenated alkanes (1); halogenated nitroalkanes (1); nitrosamines and nitramines (1); nonhalogenated phenyl aldehydes (1); nonhalogenated furanone (1); others (1) |
| hERβ | Halophenols (4) |
| fERα | Estrogen DBPs (4) |
| hAR | Halogenated nitrile (8); halogenated acids (7); halogenated amides (4); halophenols (2); halogenated benzoquinones (2); halogenated alcohols (1); halogenated alkanes (1); halogenated nitroalkanes (1); nitrosamines and nitramines (1); nonhalogenated furanone (1) |
| hTRα | Halophenols (6) |
| bTRβ | Halophenols (7) |
| cTRβ | Halophenols (7) |
| hAhR | Halogenated phenyl ester (28) |
| hRXR | Halophenols (2) |
| hPPAR | Halophenols (11) |
| hTTR | Halogenated phenyl acid (7); halophenols (5); halogenated phenyl aldehydes (3); halonitrophenols (2) |
| bTTR | Halophenols (7) |
| cTTR | Halophenols (7) |
| hHSA | Halophenols (2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, S.; Liu, H.; Yang, X. Research Progress of the Endocrine-Disrupting Effects of Disinfection Byproducts. J. Xenobiot. 2022, 12, 145-157. https://doi.org/10.3390/jox12030013
Sui S, Liu H, Yang X. Research Progress of the Endocrine-Disrupting Effects of Disinfection Byproducts. Journal of Xenobiotics. 2022; 12(3):145-157. https://doi.org/10.3390/jox12030013
Chicago/Turabian StyleSui, Shuxin, Huihui Liu, and Xianhai Yang. 2022. "Research Progress of the Endocrine-Disrupting Effects of Disinfection Byproducts" Journal of Xenobiotics 12, no. 3: 145-157. https://doi.org/10.3390/jox12030013
APA StyleSui, S., Liu, H., & Yang, X. (2022). Research Progress of the Endocrine-Disrupting Effects of Disinfection Byproducts. Journal of Xenobiotics, 12(3), 145-157. https://doi.org/10.3390/jox12030013







