Comparative Study of Δ9-Tetrahydrocannabinol and Cannabidiol on Melanogenesis in Human Epidermal Melanocytes from Different Pigmentation Phototypes: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
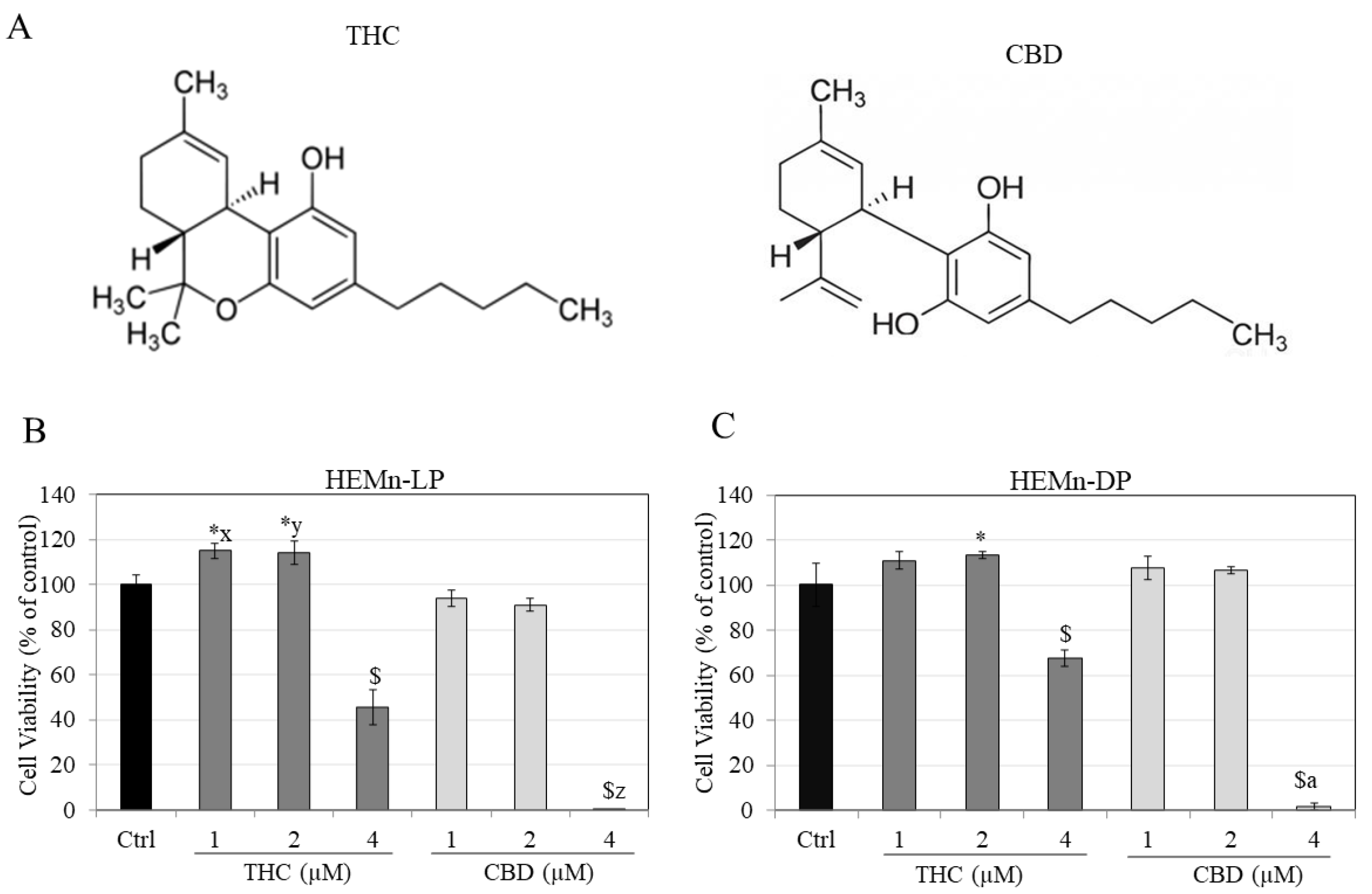
2.3. Cytotoxicity Assay
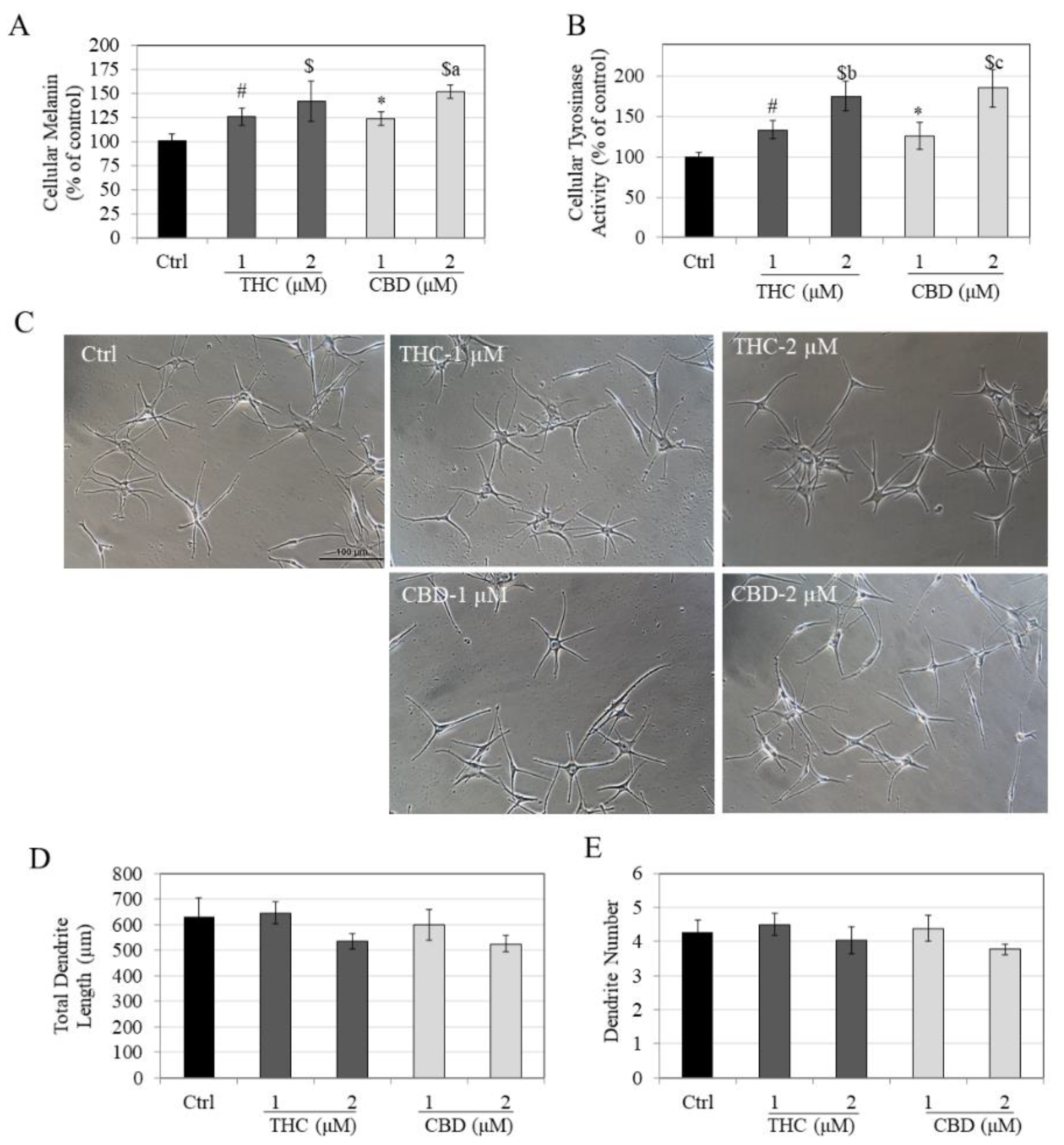
2.4. Intracellular Melanin Assay
2.5. Intracellular Tyrosinase Activity
2.6. Measurement of Melanocyte Dendricity
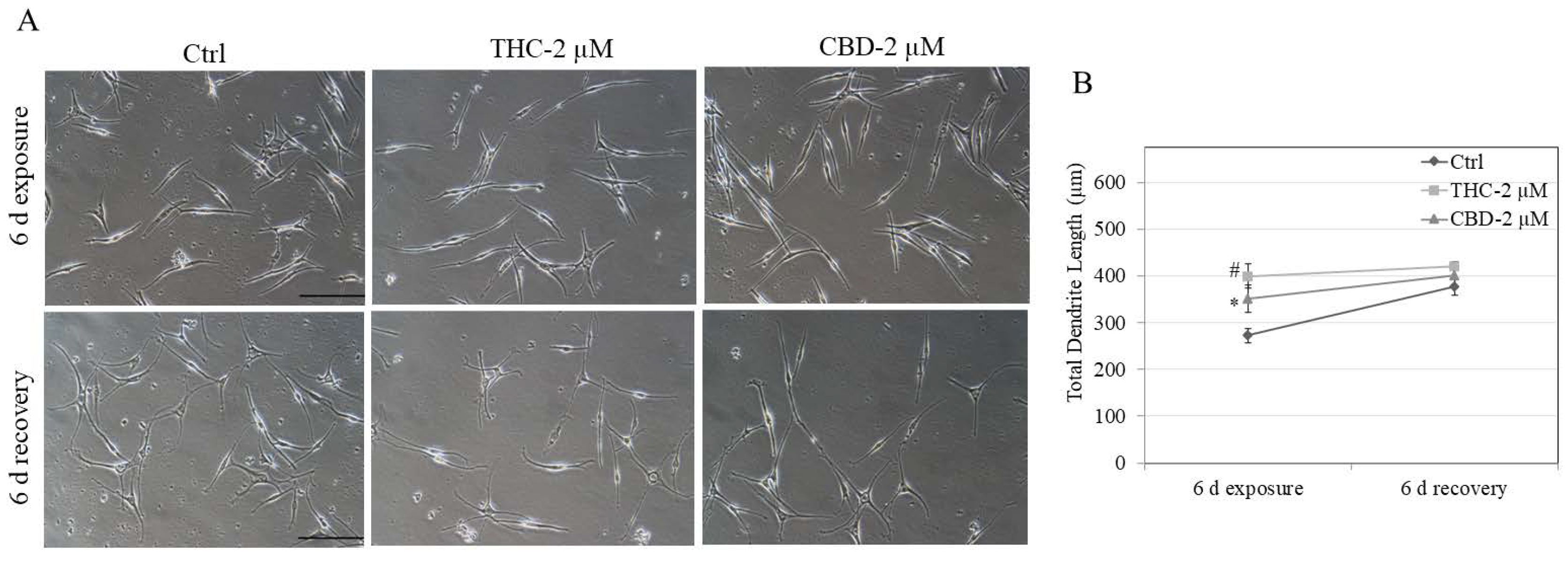
2.7. Recovery Study of Dendricity
2.8. Intracellular Reactive Oxygen Species (ROS)
2.9. Statistical Analysis
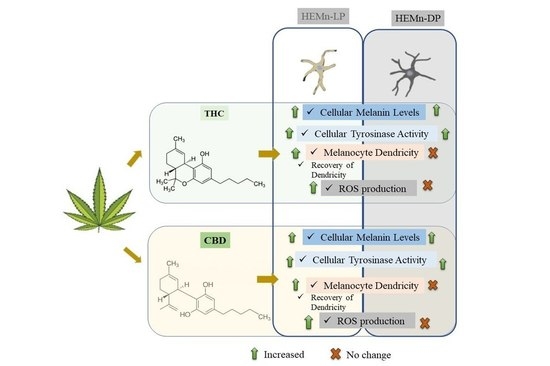
3. Results
3.1. Effects of Compounds on Viability of HEMn-LP and HEMn-DP Cells
3.2. Effects of Compounds on Melanin Synthesis, Tyrosinase Activity and Melanocyte Dendricity in HEMn-LP Cells
3.3. Effects of Compounds on Melanin Synthesis, Tyrosinase Activity and Melanocyte Dendricity in HEMn-DP Cells
3.4. Effects of Compounds on Recovery of Melanocyte Dendricity in HEMn-LP Cells
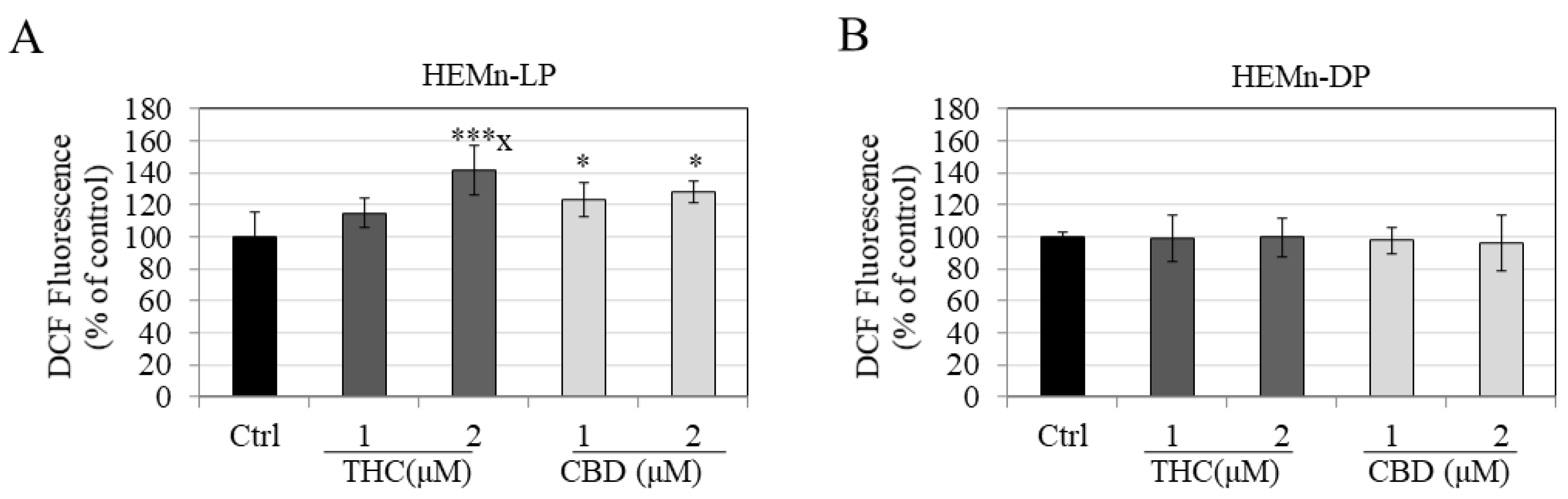
3.5. Effects of Compounds on Intracellular ROS Generation in HEMn-LP and HEMn-DP Cells
4. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urits, I.; Charipova, K.; Gress, K.; Li, N.; Berger, A.A.; Cornett, E.M.; Kassem, H.; Ngo, A.L.; Kaye, A.D.; Viswanath, O. Adverse Effects of Recreational and Medical Cannabis. Psychopharmacol. Bull. 2021, 51, 94–109. [Google Scholar]
- Kichloo, A.; Albosta, M.; Aljadah, M.; El-Amir, Z.; Goldar, G.; Khan, M.Z.; Dahiya, D.S.; Vallabhaneni, S.; Wani, F.; Singh, J. Marijuana: A systems-based primer of adverse effects associated with use and an overview of its therapeutic utility. SAGE Open Med. 2021, 9, 20503121211000909. [Google Scholar] [CrossRef]
- Izzo, A.A.; Borrelli, F.; Capasso, R.; Di Marzo, V.; Mechoulam, R. Non-psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 2009, 30, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.L.; Mason, M.J.; Langenderfer, J. The shifting landscape of cannabis legalization: Potential benefits and regulatory perspectives. J. Consum. Aff. 2021, 55, 1169–1177. [Google Scholar] [CrossRef]
- Barrus, D.G.; Capogrossi, K.L.; Cates, S.C.; Gourdet, C.K.; Peiper, N.C.; Novak, S.P.; Lefever, T.W.; Wiley, J.L. Tasty THC: Promises and challenges of cannabis edibles. Methods Rep. 2016, 2016, 1611. [Google Scholar]
- Kögel, C.C.; Balcells-Olivero, M.M.; López-Pelayo, H.; Miquel, L.; Teixidó, L.; Colom, J.; Nutt, D.J.; Rehm, J.; Gual, A. The standard joint unit. Drug Alcohol Depend. 2017, 176, 109–116. [Google Scholar] [CrossRef]
- Huestis, M.A.; Gustafson, R.A.; Moolchan, E.T.; Barnes, A.; Bourland, J.A.; Sweeney, S.A.; Hayes, E.F.; Carpenter, P.M.; Smith, M.L. Cannabinoid concentrations in hair from documented cannabis users. Forensic Sci. Int. 2007, 169, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Bonn-Miller, M.O.; Loflin, M.J.; Thomas, B.F.; Marcu, J.P.; Hyke, T.; Vandrey, R. Labeling accuracy of cannabidiol extracts sold online. JAMA 2017, 318, 1708–1709. [Google Scholar] [CrossRef]
- Czégény, Z.; Nagy, G.; Babinszki, B.; Bajtel, Á.; Sebestyén, Z.; Kiss, T.; Csupor-Löffler, B.; Tóth, B.; Csupor, D. CBD, a precursor of THC in e-cigarettes. Sci. Rep. 2021, 11, 8951. [Google Scholar]
- Cichorek, M.; Wachulska, M.; Stasiewicz, A. Heterogeneity of neural crest-derived melanocytes. Open Life Sci. 2013, 8, 315–330. [Google Scholar] [CrossRef]
- Cichorek, M.; Wachulska, M.; Stasiewicz, A.; Tymińska, A. Skin melanocytes: Biology and development. Adv. Dermatol. Allergol./Postȩpy Dermatol. I Alergol. 2013, 30, 30. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B.; Breathnach, A.S. The epidermal melanin unit system. Dermatol. Wochenschr. 1963, 147, 481–489. [Google Scholar]
- Nordlund, J.J. The melanocyte and the epidermal melanin unit: An expanded concept. Dermatol. Clin. 2007, 25, 271–281. [Google Scholar] [CrossRef]
- Sulaimon, S.S.; Kitchell, B.E. The biology of melanocytes. Vet. Dermatol. 2003, 14, 57–65. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [Green Version]
- Delijewski, M.; Beberok, A.; Otręba, M.; Wrześniok, D.; Rok, J.; Buszman, E. Effect of nicotine on melanogenesis and antioxidant status in HEMn-LP melanocytes. Environ. Res. 2014, 134, 309–314. [Google Scholar] [CrossRef]
- Delijewski, M.; Wrześniok, D.; Otręba, M.; Beberok, A.; Rok, J.; Buszman, E. Nicotine impact on melanogenesis and antioxidant defense system in HEMn-DP melanocytes. Mol. Cell. Biochem. 2014, 395, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, M.; Ueda, Y.; Hayashi, M.; Kato, H.; Furuhashi, T.; Morita, A. Tobacco smoke–induced skin pigmentation is mediated by the aryl hydrocarbon receptor. Exp. Dermatol. 2013, 22, 556–558. [Google Scholar] [CrossRef]
- Sinclair, C.F.; Foushee, H.R.; Scarinci, I.; Carroll, W.R. Perceptions of harm to health from cigarettes, blunts, and marijuana among young adult African American men. J. Health Care Poor Underserved 2013, 24, 1266–1275. [Google Scholar] [CrossRef]
- Wu, T.-C.; Tashkin, D.P.; Djahed, B.; Rose, J.E. Pulmonary hazards of smoking marijuana as compared with tobacco. N. Engl. J. Med. 1988, 318, 347–351. [Google Scholar] [CrossRef]
- Pucci, M.; Pasquariello, N.; Battista, N.; Di Tommaso, M.; Rapino, C.; Fezza, F.; Zuccolo, M.; Jourdain, R.; Finazzi Agro, A.; Breton, L.; et al. Endocannabinoids stimulate human melanogenesis via type-1 cannabinoid receptor. J. Biol. Chem. 2012, 287, 15466–15478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magina, S.; Esteves-Pinto, C.; Moura, E.; Serrao, M.P.; Moura, D.; Petrosino, S.; Di Marzo, V.; Vieira-Coelho, M.A. Inhibition of basal and ultraviolet B-induced melanogenesis by cannabinoid CB(1) receptors: A keratinocyte-dependent effect. Arch Dermatol. Res. 2011, 303, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Telek, A.; Biro, T.; Bodo, E.; Toth, B.I.; Borbiro, I.; Kunos, G.; Paus, R. Inhibition of human hair follicle growth by endo- and exocannabinoids. FASEB J. 2007, 21, 3534–3541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, Y.S.; Kim, Y.-J.; Kim, M.O.; Kang, M.; Oh, S.W.; Nho, Y.H.; Park, S.-H.; Lee, J. Cannabidiol upregulates melanogenesis through CB1 dependent pathway by activating p38 MAPK and p42/44 MAPK. Chem. -Biol. Interact. 2017, 273, 107–114. [Google Scholar] [CrossRef]
- Yamamoto, I.; Watanabe, K.; Matsunaga, T.; Kimura, T.; Funahashi, T.; Yoshimura, H. Pharmacology and toxicology of major constituents of marijuana—on the metabolic activation of cannabinoids and its mechanism. J. Toxicol. Toxin Rev. 2003, 22, 577–589. [Google Scholar] [CrossRef]
- Stout, S.M.; Cimino, N.M. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: A systematic review. Drug Metab. Rev. 2014, 46, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.D. Potential adverse drug events with tetrahydrocannabinol (THC) due to drug–drug interactions. J. Clin. Med. 2020, 9, 919. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.R.; Goldstein, J.A.; Pieper, J.A. Cytochrome P450 2C9 polymorphisms: A comprehensive review of the in-vitro and human data. Pharm. Genom. 2002, 12, 251–263. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.; Huang, S.-Q.; Su, H.-H.; Zhou, S.-F. Genetic polymorphism of the human cytochrome P450 2C9 gene and its clinical significance. Curr. Drug Metab. 2009, 10, 781–834. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Novel Chemically Modified Curcumin (CMC) Analogs Exhibit Anti-Melanogenic Activity in Primary Human Melanocytes. Int. J. Mol. Sci. 2021, 22, 6043. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Asoprisnil, A Selective progesterone receptor modulator (Sprm), inhibits melanosome export in B16f10 cells and Hemn-Dp melanocytes. Molecules 2020, 25, 3581. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S. Effects of serotype and species dependency of bacterial lipopolysaccharides in human melanocytes from lightly and darkly-pigmented skin. BBA Adv. 2022, 2, 100042. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. A Novel Pro-Melanogenic Effect of Standardized Dry Olive Leaf Extract on Primary Human Melanocytes from Lightly Pigmented and Moderately Pigmented Skin. Pharmaceuticals 2021, 14, 252. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Effects of Fluoride Exposure on Primary Human Melanocytes from Dark and Light Skin. Toxics 2020, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Khare, M.; Taylor, A.H.; Konje, J.C.; Bell, S.C. Δ9-Tetrahydrocannabinol inhibits cytotrophoblast cell proliferation and modulates gene transcription. MHR: Basic Sci. Reprod. Med. 2006, 12, 321–333. [Google Scholar]
- Cherlet, T.; Scott, J. Tetrahydrocannabinol (THC) alters synthesis and release of surfactant-related material in isolated fetal rabbit type II cells. Drug Chem. Toxicol. 2002, 25, 171–190. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-Tetrahydrocannabinol, cannabidiol and Δ9-Tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goenka, S.; Simon, S.R. Comparative study of doxycycline, sancycline, and 4-dedimethylamino sancycline (CMT-3) on epidermal melanogenesis. Arch. Dermatol. Res. 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Simon, S.R. Cmt-308, a nonantimicrobial chemically-modified tetracycline, exhibits anti-melanogenic activity by suppression of melanosome export. Biomedicines 2020, 8, 411. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Simon, S.R. Inhibitory effects of the bioactive thermorubin isolated from the fungus thermoactinomyces antibioticus on melanogenesis. Cosmetics 2020, 7, 61. [Google Scholar] [CrossRef]
- Kawasaki, A.; Kumasaka, M.; Satoh, A.; Suzuki, M.; Tamura, K.; Goto, T.; Asashima, M.; Yamamoto, H. Mitf contributes to melanosome distribution and melanophore dendricity. Pigment. Cell Melanoma Res. 2008, 21, 56–62. [Google Scholar] [CrossRef]
- Chiaverini, C.; Beuret, L.; Flori, E.; Abbe, P.; Bille, K.; Bahadoran, P.; Ortonne, J.-P.; Bertolotto, C.; Ballotti, R. Microphthalmia-associated transcription factor regulates RAB27A gene expression and controls melanosome transport. J. Biol. Chem. 2008, 283, 12635–12642. [Google Scholar] [CrossRef] [Green Version]
- Scott, G. Rac and rho: The story behind melanocyte dendrite formation. Pigment. Cell Res. 2002, 15, 322–330. [Google Scholar] [CrossRef]
- Kim, M.; Lee, C.-S.; Lim, K.-M. Rhododenol activates melanocytes and induces morphological alteration at sub-cytotoxic levels. Int. J. Mol. Sci. 2019, 20, 5665. [Google Scholar] [CrossRef] [Green Version]
- Toson, E.-S.A. Impact of marijuana smoking on liver and sex hormones: Correlation with oxidative stress. Nat. Sci. 2011, 9, 76–87. [Google Scholar]
- Sarafian, T.A.; Habib, N.; Oldham, M.; Seeram, N.; Lee, R.-P.; Lin, L.; Tashkin, D.P.; Roth, M.D. Inhaled marijuana smoke disrupts mitochondrial energetics in pulmonary epithelial cells in vivo. Am. J. Physiol. -Lung Cell. Mol. Physiol. 2006, 290, L1202–L1209. [Google Scholar] [CrossRef] [PubMed]
- Sarafian, T.A.; Magallanes, J.A.M.; Shau, H.; Tashkin, D.; Roth, M.D. Oxidative stress produced by marijuana smoke: An adverse effect enhanced by cannabinoids. Am. J. Respir. Cell Mol. Biol. 1999, 20, 1286–1293. [Google Scholar] [CrossRef] [Green Version]
- O’Llenecia, S.W.; Gurm, H.; Sharma, R.; Verma, N.; May, L.L.; Raha, S. Delta-9-tetrahydrocannabinol inhibits invasion of HTR8/SVneo human extravillous trophoblast cells and negatively impacts mitochondrial function. Sci. Rep. 2021, 11, 4029. [Google Scholar]
- Massi, P.; Vaccani, A.; Ceruti, S.; Colombo, A.; Abbracchio, M.P.; Parolaro, D. Antitumor effects of cannabidiol, a nonpsychoactive cannabinoid, on human glioma cell lines. J. Pharmacol. Exp. Ther. 2004, 308, 838–845. [Google Scholar] [CrossRef] [Green Version]
- Trouba, K.J.; Hamadeh, H.K.; Amin, R.P.; Germolec, D.R. Oxidative stress and its role in skin disease. Antioxid. Redox Signal. 2002, 4, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Pavel, S.; van Nieuwpoort, F.; van der Meulen, H.; Out, C.; Pizinger, K.; Cetkovská, P.; Smit, N.P.; Koerten, H.K. Disturbed melanin synthesis and chronic oxidative stress in dysplastic naevi. Eur. J. Cancer 2004, 40, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-T.; Choi, B.; Tang, M.-S. Melanocytes are deficient in repair of oxidative DNA damage and UV-induced photoproducts. Proc. Natl. Acad. Sci. USA 2010, 107, 12180–12185. [Google Scholar] [CrossRef] [Green Version]
- Maresca, V.; Flori, E.; Briganti, S.; Mastrofrancesco, A.; Fabbri, C.; Mileo, A.M.; Paggi, M.G.; Picardo, M. Correlation between melanogenic and catalase activity in in vitro human melanocytes: A synergic strategy against oxidative stress. Pigment. Cell Melanoma Res. 2008, 21, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Yohn, J.J.; Norris, D.A.; Yrastorza, D.G.; Buno, I.J.; Leff, J.A.; Hake, S.S.; Repine, J.E. Disparate antioxidant enzyme activities in cultured human cutaneous fibroblasts, keratinocytes, and melanocytes. J. Investig. Dermatol. 1991, 97, 405–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kvam, E.; Tyrrell, R.M. The role of melanin in the induction of oxidative DNA base damage by ultraviolet A irradiation of DNA or melanoma cells. J. Investig. Dermatol. 1999, 113, 209–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kipp, C.; Young, A.R. The soluble eumelanin precursor 5,6-dihydroxyindole-2-carboxylic acid enhances oxidative damage in human keratinocyte DNA after UVA irradiation. Photochem. Photobiol. 1999, 70, 191–198. [Google Scholar] [CrossRef]
- Bustamante, J.; Bredeston, L.; Malanga, G.; Mordoh, J. Role of melanin as a scavenger of active oxygen species. Pigment. Cell Res. 1993, 6, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.; Li, K.; Liu, L.; Zhang, Y.; Zhou, Z.; Li, C.; Gao, T. Heme oxygenase-1 protects human melanocytes from H2O2-induced oxidative stress via the Nrf2-ARE pathway. J. Investig. Dermatol. 2011, 131, 1420–1427. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, M.; Böckmann, S.; Hinz, B. Up-regulation of heme oxygenase-1 expression and inhibition of disease-associated features by cannabidiol in vascular smooth muscle cells. Oncotarget 2018, 9, 34595. [Google Scholar] [CrossRef] [Green Version]
- Casares, L.; García, V.; Garrido-Rodríguez, M.; Millán, E.; Collado, J.A.; García-Martín, A.; Peñarando, J.; Calzado, M.A.; de la Vega, L.; Muñoz, E. Cannabidiol induces antioxidant pathways in keratinocytes by targeting BACH1. Redox Biol. 2020, 28, 101321. [Google Scholar] [CrossRef]
- Musshoff, F.; Madea, B. Review of biologic matrices (urine, blood, hair) as indicators of recent or ongoing cannabis use. Ther. Drug Monit. 2006, 28, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khajuria, H.; Nayak, B.P. Detection of Δ9-Tetrahydrocannabinol (THC) in hair using GC–MS. Egypt. J. Forensic Sci. 2014, 4, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Magina, S.; Vieira-Coelho, M.A.; Kozmus, C.E.P.; Serrão, M.P.; Moura, D. Cannabinoid Receptors in Human Sk-Mel-1 Melanoma Cells and Their Role on Melanogenesis. In Proceedings of the 48th Annual Meeting of the American Society of Cell Biology, San Francisco, CA, USA, 12–17 December 2008. [Google Scholar]
- Han, J.; Colditz, G.A.; Hunter, D.J. Risk factors for skin cancers: A nested case–control study within the Nurses’ Health Study. Int. J. Epidemiol. 2006, 35, 1514–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noonan, F.P.; Zaidi, M.R.; Wolnicka-Glubisz, A.; Anver, M.R.; Bahn, J.; Wielgus, A.; Cadet, J.; Douki, T.; Mouret, S.; Tucker, M.A. Melanoma induction by ultraviolet A but not ultraviolet B radiation requires melanin pigment. Nat. Commun. 2012, 3, 884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tada, M.; Kohno, M.; Niwano, Y. Scavenging or quenching effect of melanin on superoxide anion and singlet oxygen. J. Clin. Biochem. Nutr. 2010, 46, 224–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goenka, S. Comparative Study of Δ9-Tetrahydrocannabinol and Cannabidiol on Melanogenesis in Human Epidermal Melanocytes from Different Pigmentation Phototypes: A Pilot Study. J. Xenobiot. 2022, 12, 131-144. https://doi.org/10.3390/jox12020012
Goenka S. Comparative Study of Δ9-Tetrahydrocannabinol and Cannabidiol on Melanogenesis in Human Epidermal Melanocytes from Different Pigmentation Phototypes: A Pilot Study. Journal of Xenobiotics. 2022; 12(2):131-144. https://doi.org/10.3390/jox12020012
Chicago/Turabian StyleGoenka, Shilpi. 2022. "Comparative Study of Δ9-Tetrahydrocannabinol and Cannabidiol on Melanogenesis in Human Epidermal Melanocytes from Different Pigmentation Phototypes: A Pilot Study" Journal of Xenobiotics 12, no. 2: 131-144. https://doi.org/10.3390/jox12020012
APA StyleGoenka, S. (2022). Comparative Study of Δ9-Tetrahydrocannabinol and Cannabidiol on Melanogenesis in Human Epidermal Melanocytes from Different Pigmentation Phototypes: A Pilot Study. Journal of Xenobiotics, 12(2), 131-144. https://doi.org/10.3390/jox12020012