Effects of Greek Honey and Propolis on Oxidative Stress and Biochemical Parameters in Regular Blood Donors
Abstract
:1. Introduction
2. Materials and Methods
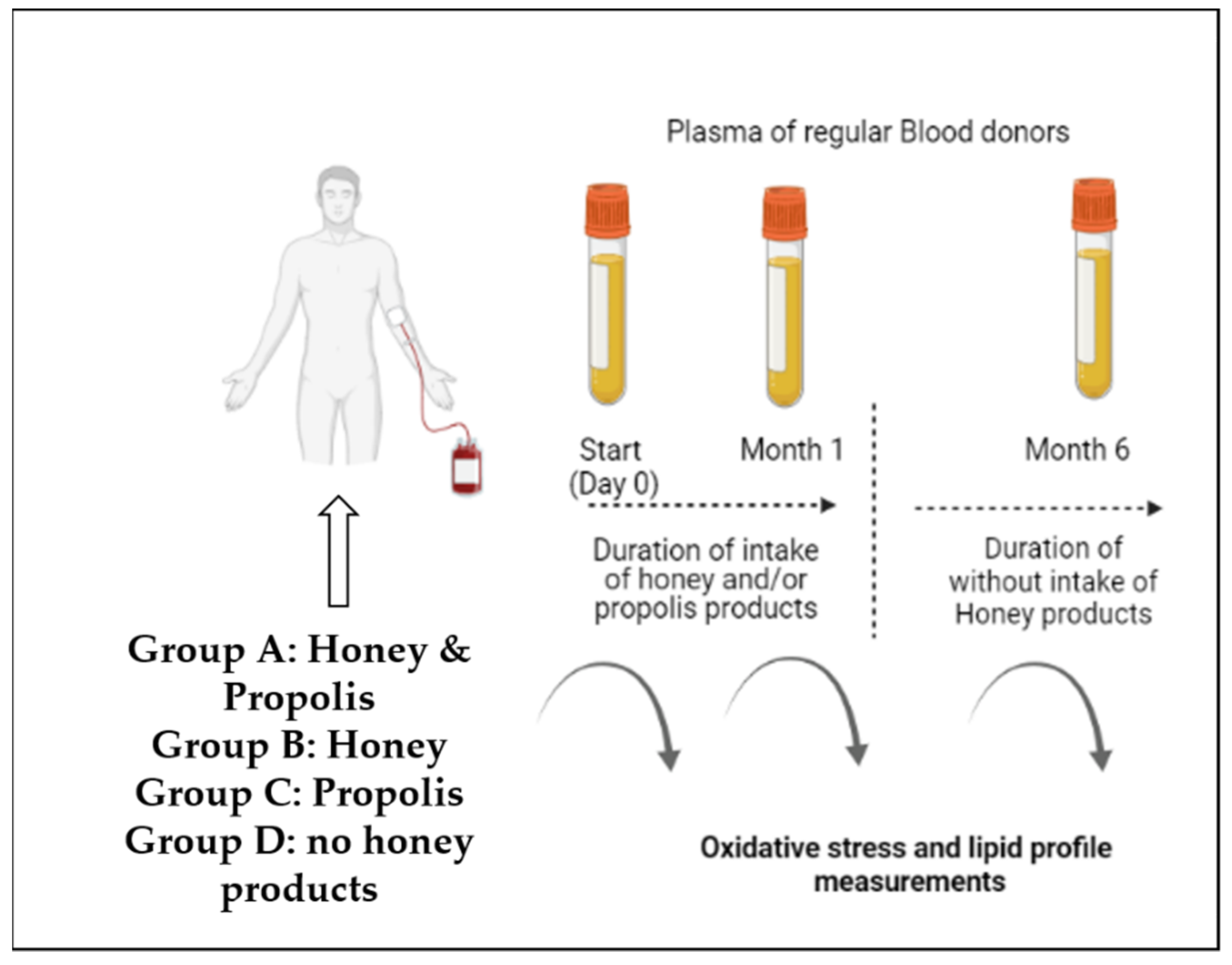
2.1. Study Design
2.2. Honey Product Samples
2.3. Ethical Statement
2.4. Analysis of ROS Values
2.5. Analysis of Blood Lipids and Ferritin
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ajibola, A.; Chamunorwa, J.P.; Erlwanger, K.H. Nutraceutical values of natural honey and its contribution to human health and wealth. Nutr. Metab. 2012, 9, 61. [Google Scholar] [CrossRef]
- Pita-Calvo, C.; Vázquez, M. Differences between honeydew and blossom honeys: A review. Trends Food Sci. Technol. 2017, 59, 79–87. [Google Scholar] [CrossRef]
- Ball, D.W. The chemical composition of honey. J. Chem. Educ. 2007, 84, 1643–1646. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; González-Paramás, A.M. Chemical composition of honey. In Bee Products—Chemical and Biological Properties; Springer: Berlin/Heidelberg, Germany, 2017; pp. 43–82. ISBN 9783319596891. [Google Scholar]
- Dzugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant activity as biomarker of honey variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef]
- Ahangari, Z.; Naseri, M. Chemical Composition and Its Applications in Endodontics. Iran. Endod. J. 2018, 13, 285–292. [Google Scholar]
- Gupta, S.; Kundabala, M.; Acharya, S.R. A comparative evaluation of the antibacterial efficacy of propolis 30% sodium hypochlorite and 02% chlorhexidine gluconate against enterococcus faecalis-An in vitro study. Endodontology 2007, 19, 31–38. [Google Scholar]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- D’Oria, R.; Schipani, R.; Leonardini, A.; Natalicchio, A.; Perrini, S.; Cignarelli, A.; Laviola, L.; Giorgino, F. The Role of Oxidative Stress in Cardiac Disease: From Physiological Response to Injury Factor. Oxid. Med. Cell. Longev. 2020, 2020, 5732956. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The Role of Reactive Oxygen Species in the Pathogenesis of Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease: A Mini Review. Oxid. Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidatives stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Fibach, E.; Rachmilewitz, E. The Role of Oxidative Stress in Hemolytic Anemia. Curr. Mol. Med. 2008, 8, 609–619. [Google Scholar] [CrossRef]
- Tsamesidis, I.; Lymperaki, E.; Pantaleoa, A.; Vagdatli, E.; Nikza, P.; Lettas, A.; Satta, A.; Oggiano, M.; Fozza, C. Hematological, biochemical and antioxidant indices variations in regular blood donors among Mediterranean regions. Transfus. Apher. Sci. 2020, 58, 102659. [Google Scholar] [CrossRef]
- Majid, M.; Younis, M.A.; Naveed, A.K.; Shah, M.U.; Azeem, Z.; Tirmizi, S.H. Effects of natural honey on blood glucose and lipid profile in young healthy Pakistani males. J. Ayub Med. Coll. Abbottabad 2013, 25, 44–47. [Google Scholar]
- Rasad, H.; Entezari, M.H.; Ghadiri, E.; Mahaki, B.; Pahlavani, N. The effect of honey consumption compared with sucrose on lipid profile in young healthy subjects (randomized clinical trial). Clin. Nutr. ESPEN 2018, 26, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.I.; Sulaiman, S.A.; Boukraa, L. Antioxidant Properties of Honey and Its Role in Preventing Health Disorder. Open Nutraceuticals J. 2014, 3, 6–16. [Google Scholar] [CrossRef]
- Azman, K.F.; Zakaria, R. Honey as an antioxidant therapy to reduce cognitive ageing. Iran. J. Basic Med. Sci. 2019, 22, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Anthimidou, E.; Mossialos, D. Antibacterial activity of greek and cypriot honeys against staphylococcus aureus and pseudomonas aeruginosa in comparison to manuka honey. J. Med. Food 2013, 16, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Stagos, D.; Soulitsiotis, N.; Tsadila, C.; Papaeconomou, S.; Arvanitis, C.; Ntontos, A.; Karkanta, F.; Adamou-Androulaki, S.; Petrotos, K.; Spandidos, D.A.; et al. Antibacterial and antioxidant activity of different types of honey derived from Mount Olympus in Greece. Int. J. Mol. Med. 2018, 42, 726–734. [Google Scholar] [CrossRef]
- Bodega, G.; Alique, M.; Puebla, L.; Carracedo, J.; Ramírez, R.M. Microvesicles: ROS scavengers and ROS producers. J. Extracell. Vesicles 2019, 8, 1626654. [Google Scholar] [CrossRef]
- Rao, P.V.; Krishnan, K.T.; Salleh, N.; Gan, S.H. Biological and therapeutic effects of honey produced by honey bees and stingless bees: A comparative review. Rev. Bras. Farm. 2016, 26, 657–664. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. Advances in the analytical methods for determining the antioxidant properties of honey: A review. Afr. J. Tradit Complement. Altern. Med. 2012, 9, 36–42. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Tulipani, S.; Romandini, S.; Bertoli, E.; Battino, M. Contribution of honey in nutrition and human health: A review. Mediterr. J. Nutr. Metab. 2010, 3, 15–23. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Functional properties of honey, propolis, and royal jelly. J. Food Sci. 2008, 73, R117–R124. [Google Scholar] [CrossRef] [PubMed]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.A. Effects of honey and its mechanisms of action on the development and progression of cancer. Molecules 2014, 21, 2497–2522. [Google Scholar] [CrossRef] [PubMed]
- Al-Tamimi, A.M. Changes in plasma lipoproteins during low-fat, high-carbohydrate diets: Effects of energy intake Honey does not adversely impact blood lipids of adult men and women: A randomized cross-over trial. Nutr. Res. 2020, 74, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Mohammadimanesh, A.; Vahidiniya, A.A.; Doaei, S.; Gholamalizadeh, M.; Shahvegharasl, Z.; Salehi, I.; Fayyaz, N.; Khosravi, H.M. The effect of different types of honey on the lipid profile of streptozotocin-induced diabetic rats. Arch. Med. Sci. Atheroscler. Dis. 2019, 4, 113–118. [Google Scholar] [CrossRef]
- Al-Waili, N.S. Effects of daily consumption of honey solution on hematological indices and blood levels of minerals and enzymes in normal individuals. J. Med. Food 2003, 6, 135–140. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef]
| Blood Groups (n = 80) | ||||
|---|---|---|---|---|
| A (n = 20) Mean (±SD) | B (n = 20) Mean (±SD) | C (n = 20) Mean (±SD) | D (n = 20) Mean (±SD) | |
| Sex, Male (%) | 50 | 50 | 50 | 50 |
| Age | 44 (±11.35) | 45 (±7.52) | 47 (±7.31) | 42 (±3.17) |
| BMI | 24.8 (±2.11) | 24.3 (±1.65) | 23.4 (±1.94) | 24.2 (±1.41) |
| Group A (Honey and Propolis) | Group B (Honey) | Group C (Propolis) | Group D (No Honey Products) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biochemical Parameters | Day 0 | Month 1 | Month 6 | Day 0 | Month 1 | Month 6 | Day 0 | Month 1 | Month 6 | Day 0 | Month 1 | Month 6 |
| Total Cholesterol (mg/dL) | 185.5 ± 25 | 185.3 ± 21 | 176.2 ± 19 | 212.1 ± 17 | 215.5 ± 18 | 193.5 ± 16 | 176 ± 14 | 182.4 ± 15 | 181.4 ± 19 | 181.2 ± 21 | 184.3 ± 15 | 183.5 ± 19 |
| HDL (mg/dL) | 47.1 ± 6 | 45.1 ± 5 | 39.6 ± 4 | 45.5 ± 7 | 44.2 ± 3 | 43.2 ± 6 | 48.6 ± 4 | 52 ± 5 | 47.5 ± 3 | 43.2 ± 4 | 45.5 ± 6 | 46.2 ± 7 |
| LDL (mg/dL) | 114.7 ± 15 | 116.1 ± 14 | 104.1 ± 16 | 137.8 ± 18 | 136.2 ± 13 | 116.7 ± 19 | 118.3 ± 15 | 111.2 ± 18 | 108.2 ± 11 | 125.5 ± 18 | 130.4 ± 17 | 129.2 ± 21 |
| Trig. (mg/dL) | 118.7 ± 11 | 122.3 ± 16 | 163.7 * ± 15 | 144 ± 12 | 157.7 ± 16 | 167.4 * ± 12 | 92.8 ± 9 | 90.4 ± 15 | 145.5 * ± 12 | 114.2 ± 15 | 123.5 ± 11 | 121.4 ± 17 |
| Ferritin (ng/mL) | 53.8 ± 28 | 62.5 ± 23 | 38.3 ± 18 | 96.9 ± 35 | 114.6 ± 45 | 65.4 ± 35 | 67.7 ± 28 | 68.7 ± 23 | 101.3 * ± 32 | 62.1 ± 25 | 73.5 ± 29 | 67.5 ± 18 |
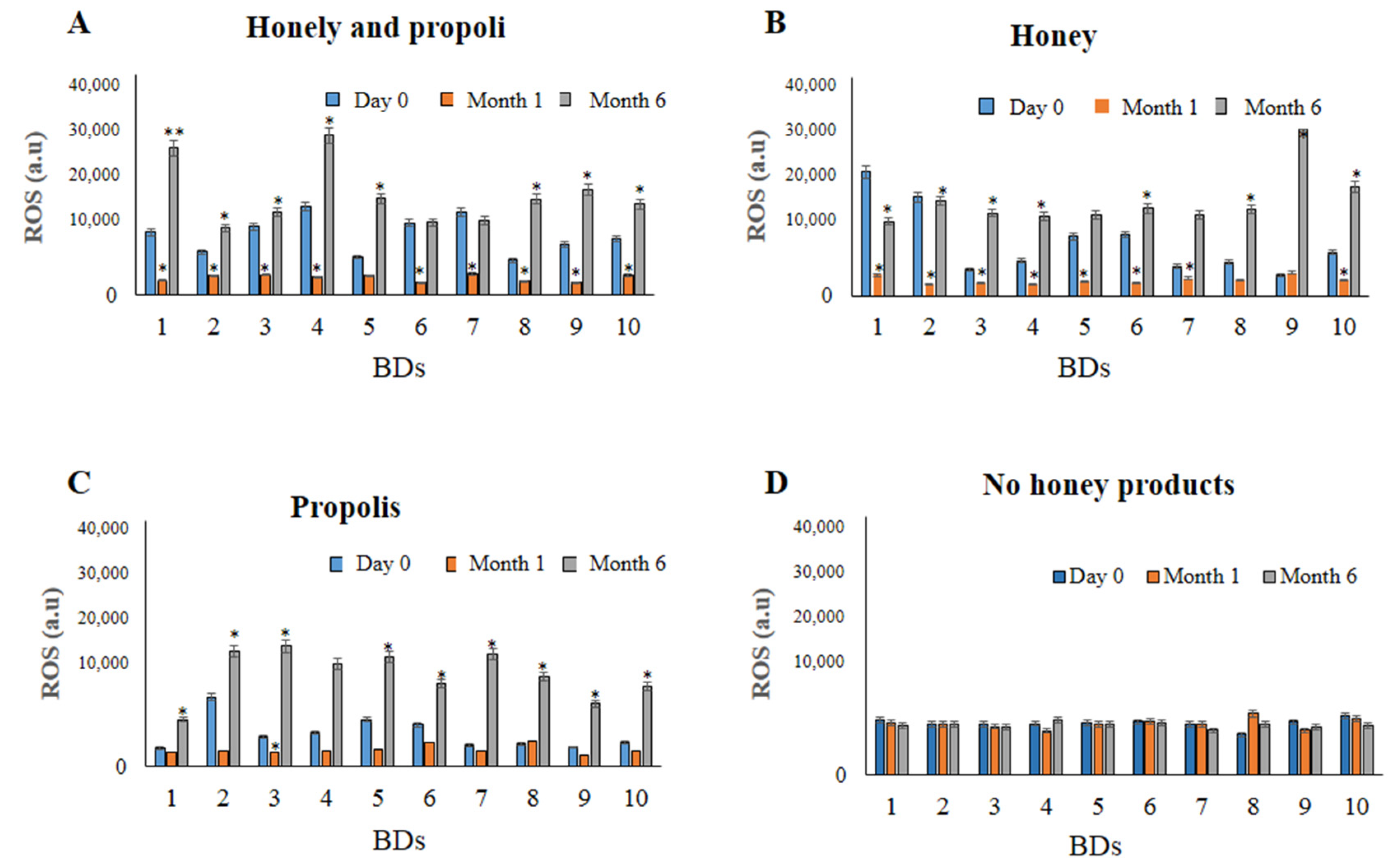
| ROS (a.u) | 10,880 ± 254 | 3159 * ± 352 | 18,024 ** ± 340 | 7854 ± 158 | 2760 * ± 325 | 19,863 * ± 124 | 5374 ± 305 | 2793 * ± 205 | 15,013 * ± 167 | 7856 ± 158 | 8647 ± 114 | 8025 ± 201 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsamesidis, I.; Egwu, C.O.; Samara, D.; Vogiatzi, D.; Lettas, A.; Lymperaki, E. Effects of Greek Honey and Propolis on Oxidative Stress and Biochemical Parameters in Regular Blood Donors. J. Xenobiot. 2022, 12, 13-20. https://doi.org/10.3390/jox12010002
Tsamesidis I, Egwu CO, Samara D, Vogiatzi D, Lettas A, Lymperaki E. Effects of Greek Honey and Propolis on Oxidative Stress and Biochemical Parameters in Regular Blood Donors. Journal of Xenobiotics. 2022; 12(1):13-20. https://doi.org/10.3390/jox12010002
Chicago/Turabian StyleTsamesidis, Ioannis, Chinedu O. Egwu, Diana Samara, Dimitra Vogiatzi, Athanasios Lettas, and Evgenia Lymperaki. 2022. "Effects of Greek Honey and Propolis on Oxidative Stress and Biochemical Parameters in Regular Blood Donors" Journal of Xenobiotics 12, no. 1: 13-20. https://doi.org/10.3390/jox12010002
APA StyleTsamesidis, I., Egwu, C. O., Samara, D., Vogiatzi, D., Lettas, A., & Lymperaki, E. (2022). Effects of Greek Honey and Propolis on Oxidative Stress and Biochemical Parameters in Regular Blood Donors. Journal of Xenobiotics, 12(1), 13-20. https://doi.org/10.3390/jox12010002








