Development, Reliability and Validity of Engagement in Exercise Rehabilitation Scale for Patients with Stroke
Abstract
1. Introduction
2. Methods
2.1. Conceptual Framework
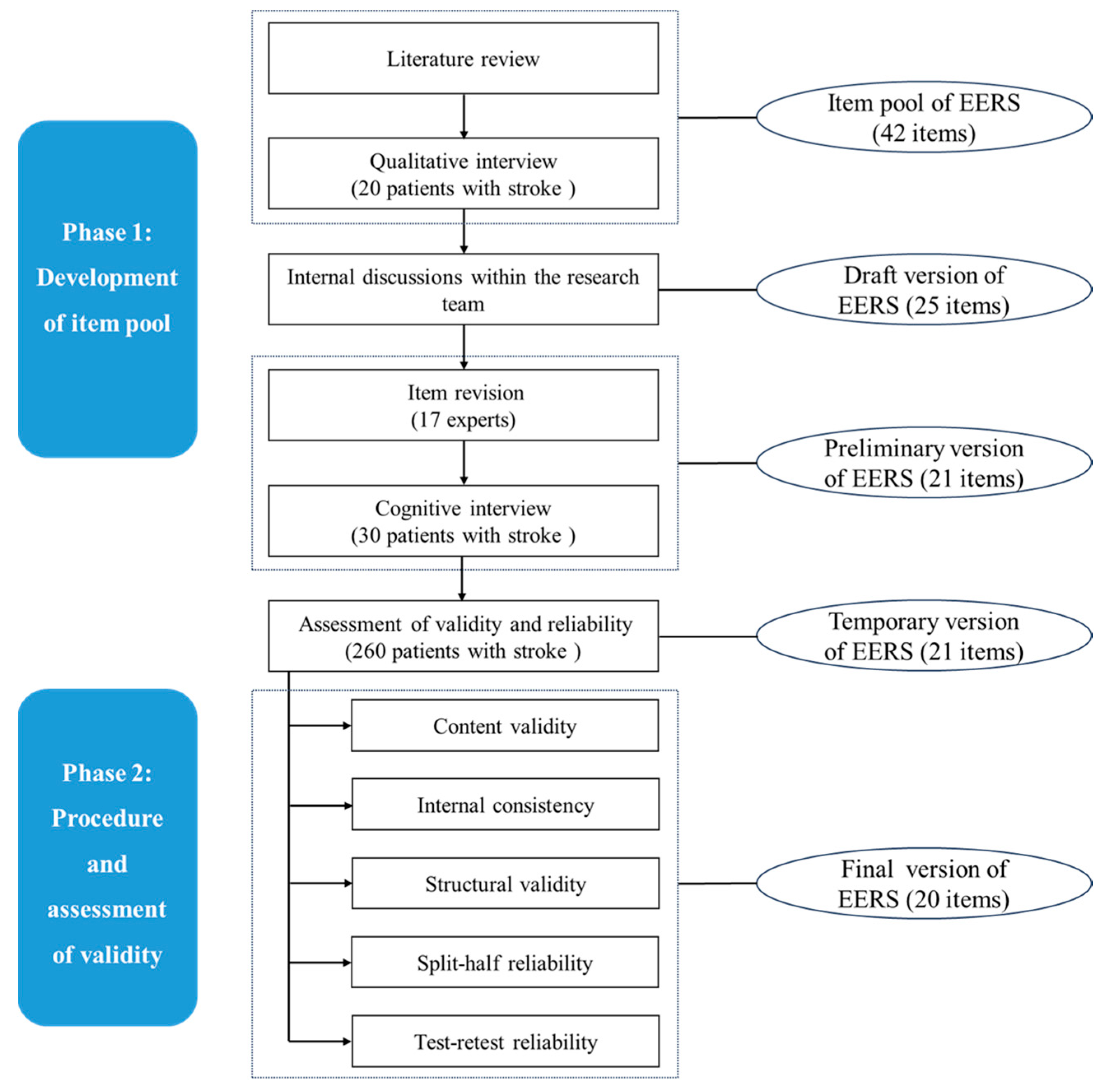
2.2. Phase 1: Development of Item Pool
2.2.1. Literature Review
2.2.2. Qualitative Interview
2.2.3. Formulation of the Draft EERS
2.2.4. Item Revision
2.2.5. Cognitive Interview
2.3. Phase 2: Procedure and Assessment of Validity
2.3.1. Sample Size
2.3.2. Participants
2.3.3. Measurements
2.3.4. Data Collection
2.4. Ethical Considerations
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Participants
3.2. Item Analysis
3.3. Validity
3.4. Reliability
3.5. Analysis of Demographic Data
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Public Involvement Statement
Guidelines and Standards Statement
Use of Artificial Intelligence
Conflicts of Interest
References
- Feigin, V.L.; Norrving, B.; Mensah, G.A. Global Burden of Stroke. Circ. Res. 2017, 120, 439–448. [Google Scholar] [CrossRef]
- Collaborators GBDS. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Kim, J.; Thayabaranathan, T.; Donnan, G.A.; Howard, G.; Howard, V.J.; Rothwell, P.M.; Feigin, V.; Norrving, B.; Owolabi, M.; Pandian, J.; et al. Global Stroke Statistics 2019. Int. J. Stroke 2020, 15, 819–838. [Google Scholar] [CrossRef]
- Hatem, S.M.; Saussez, G.; Della Faille, M.; Prist, V.; Zhang, X.; Dispa, D.; Bleyenheuft, Y. Rehabilitation of Motor Function after Stroke: A Multiple Systematic Review Focused on Techniques to Stimulate Upper Extremity Recovery. Front. Hum. Neurosci. 2016, 10, 442. [Google Scholar] [CrossRef]
- Stinear, C.M.; Lang, C.E.; Zeiler, S.; Byblow, W.D. Advances and challenges in stroke rehabilitation. Lancet Neurol. 2020, 19, 348–360. [Google Scholar] [CrossRef]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef] [PubMed]
- Busk, H.; Ahler, J.; Bricca, A.; Mikal Holm, P.; Varning Poulsen, D.; Skou, S.T.; Tang, L.H. Exercise-based rehabilitation in and with nature: A scoping review mapping available interventions. Ann. Med. 2023, 55, 2267083. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. 3), 1–72. [Google Scholar] [CrossRef]
- de Diego-Alonso, C.; Alegre-Ayala, J.; Blasco-Abadía, J.; Doménech-García, V.; Bellosta-López, P. Associations between objective and self-perceived physical activity and participation in everyday activities in mild stroke survivors. PLoS ONE 2025, 20, e0321047. [Google Scholar] [CrossRef]
- Saunders, D.H.; Sanderson, M.; Hayes, S.; Johnson, L.; Kramer, S.; Carter, D.D.; Jarvis, H.; Brazzelli, M.; Mead, G.E. Physical fitness training for stroke patients. Cochrane Database Syst. Rev. 2020, 3, CD003316. [Google Scholar] [PubMed]
- Whitehead, S.; Baalbergen, E. Post-stroke rehabilitation. S. Afr. Med. J. 2019, 109, 81. [Google Scholar] [CrossRef]
- Neil, H.P. Stroke Rehabilitation. Crit. Care Nurs. Clin. N. Am. 2023, 35, 95–99. [Google Scholar] [CrossRef]
- Wei, X.; Sun, S.; Zhang, M.; Zhao, Z. A systematic review and meta-analysis of clinical efficacy of early and late rehabilitation interventions for ischemic stroke. BMC Neurol. 2024, 24, 91. [Google Scholar] [CrossRef]
- Young, B.M.; Holman, E.A.; Cramer, S.C. Rehabilitation Therapy Doses Are Low After Stroke and Predicted by Clinical Factors. Stroke 2023, 54, 831–839. [Google Scholar] [CrossRef]
- Kringle, E.A.; Terhorst, L.; Butters, M.A.; Skidmore, E.R. Clinical Predictors of Engagement in Inpatient Rehabilitation Among Stroke Survivors with Cognitive Deficits: An Exploratory Study. J. Int. Neuropsychol. Soc. 2018, 24, 572–583. [Google Scholar] [CrossRef]
- Sánchez González, M.L.; Nitsch, K.; Carnahan, N.; Aaron, R.V.; Hosey, M.M.; Schechter, N. Patient engagement in inpatient rehabilitation: A scoping review of measures and evolving conceptualizations. Rehabil. Psychol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Blank, L.; Cantrell, A.; Sworn, K.; Booth, A. Factors which facilitate or impede patient engagement with pulmonary and cardiac rehabilitation: A rapid evaluation mapping review. Health Soc. Care Deliv. Res. 2023, 11, 1–59. [Google Scholar] [CrossRef]
- Man, C.; Liu, T.; Yan, S.; Xie, Q.; Liu, H. Research status and hotspots of patient engagement: A bibliometric analysis. Patient Educ. Couns. 2024, 125, 108306. [Google Scholar] [CrossRef] [PubMed]
- Imms, C.; Granlund, M.; Wilson, P.H.; Steenbergen, B.; Rosenbaum, P.L.; Gordon, A.M. Participation, both a means and an end: A conceptual analysis of processes and outcomes in childhood disability. Dev. Med. Child. Neurol. 2017, 59, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Horton, S.; Howell, A.; Humby, K.; Ross, A. Engagement and learning: An exploratory study of situated practice in multi-disciplinary stroke rehabilitation. Disabil. Rehabil. 2011, 33, 270–279. [Google Scholar] [CrossRef]
- Lequerica, A.H.; Kortte, K. Therapeutic engagement: A proposed model of engagement in medical rehabilitation. Am. J. Phys. Med. Rehabil. 2010, 89, 415–422. [Google Scholar] [CrossRef]
- Kortte, K.B.; Falk, L.D.; Castillo, R.C.; Johnson-Greene, D.; Wegener, S.T. The Hopkins Rehabilitation Engagement Rating Scale: Development and psychometric properties. Arch. Phys. Med. Rehabil. 2007, 88, 877–884. [Google Scholar] [CrossRef]
- Bright, F.A.; Kayes, N.M.; Worrall, L.; McPherson, K.M. A conceptual review of engagement in healthcare and rehabilitation. Disabil. Rehabil. 2015, 37, 643–654. [Google Scholar] [CrossRef]
- Wu, T.Y.; Lien, B.Y.; Lequerica, A.H.; Lu, W.S.; Hsieh, C.L. Development and Validation of the Occupational Therapy Engagement Scale for Patients with Stroke. Occup. Ther. Int. 2019, 2019, 3164254. [Google Scholar] [CrossRef]
- Jiang, H.; Lin, B.; Liu, Z.; Mei, Y.; Li, X.; Ma, L.; Zhang, Z. Patient engagement in rehabilitation: An evolutionary concept analysis. Clin. Rehabil. 2025, 39, 224–235. [Google Scholar] [CrossRef]
- Forbrigger, S.; DePaul, V.G.; Davies, T.C.; Morin, E.; Hashtrudi-Zaad, K. Home-based upper limb stroke rehabilitation mechatronics: Challenges and opportunities. Biomed. Eng. Online 2023, 22, 67. [Google Scholar] [CrossRef]
- Kayola, G.; Mataa, M.M.; Asukile, M.; Chishimba, L.; Chomba, M.; Mortel, D.; Nutakki, A.; Zimba, S.; Saylor, D. Stroke Rehabilitation in Low- and Middle-Income Countries: Challenges and Opportunities. Am. J. Phys. Med. Rehabil. 2023, 102 (Suppl. 1), S24–S32. [Google Scholar] [CrossRef]
- Markle-Reid, M.; Valaitis, R.; Bartholomew, A.; Fisher, K.; Fleck, R.; Ploeg, J.; Salerno, J. An integrated hospital-to-home transitional care intervention for older adults with stroke and multimorbidity: A feasibility study. J. Comorb. 2020, 10, 2235042X19900451. [Google Scholar] [CrossRef]
- Adeoye, O.; Nystrom, K.V.; Yavagal, D.R.; Luciano, J.; Nogueira, R.G.; Zorowitz, R.D.; Khalessi, A.A.; Bushnell, C.; Barsan, W.G.; Panagos, P.; et al. Recommendations for the Establishment of Stroke Systems of Care: A 2019 Update. Stroke 2019, 50, e187–e210. [Google Scholar] [CrossRef] [PubMed]
- Prvu Bettger, J.; McCoy, L.; Smith, E.E.; Fonarow, G.C.; Schwamm, L.H.; Peterson, E.D. Contemporary trends and predictors of postacute service use and routine discharge home after stroke. J. Am. Heart Assoc. 2015, 4, e001038. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.; John-Baptiste, A.; Meyer, M.; Richardson, M.; Speechley, M.; Ure, D.; Markle-Reid, M.; Teasell, R. Assessing the impact of a home-based stroke rehabilitation programme: A cost-effectiveness study. Disabil. Rehabil. 2019, 41, 2060–2065. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chou, W.; Hong, R.B.; Lee, J.H.; Chang, J.H. Home-based rehabilitation versus hospital-based rehabilitation for stroke patients in post-acute care stage: Comparison on the quality of life. J. Formos. Med. Assoc. 2023, 122, 862–871. [Google Scholar] [CrossRef]
- Nascimento, L.R.; Gaviorno, L.F.; de Souza Brunelli, M.; Gonçalves, J.V.; Arêas, F. Home-based is as effective as centre-based rehabilitation for improving upper limb motor recovery and activity limitations after stroke: A systematic review with meta-analysis. Clin. Rehabil. 2022, 36, 1565–1577. [Google Scholar] [CrossRef] [PubMed]
- Lenze, E.J.; Munin, M.C.; Quear, T.; Dew, M.A.; Rogers, J.C.; Begley, A.E.; Reynolds, C.F., 3rd. The Pittsburgh Rehabilitation Participation Scale: Reliability and validity of a clinician-rated measure of participation in acute rehabilitation. Arch. Phys. Med. Rehabil. 2004, 85, 380–384. [Google Scholar] [CrossRef]
- Lequerica, A.H.; Rapport, L.J.; Whitman, R.D.; Millis, S.R.; Vangel, S.J.; Hanks, R.A.; Axelrod, B.N. Psychometric properties of the Rehabilitation Therapy Engagement Scale when used among individuals with acquired brain injury. Rehabil. Psychol. 2006, 51, 331–337. [Google Scholar] [CrossRef]
- Carrozzino, D.; Patierno, C.; Guidi, J.; Berrocal Montiel, C.; Cao, J.; Charlson, M.E.; Christensen, K.S.; Concato, J.; De Las Cuevas, C.; de Leon, J.; et al. Clinimetric Criteria for Patient-Reported Outcome Measures. Psychother. Psychosom. 2021, 90, 222–232. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, X.; Zhao, Z.; Lin, B.; Mei, Y.; Chen, S.; Zhang, Z. Exploring Engagement in Exercise-Based Rehabilitation for Patients with Stroke: A Qualitative Study Using the Patient Health Engagement Model. Nurs. Health Sci. 2025, 27, e70148. [Google Scholar] [CrossRef]
- Almanasreh, E.; Moles, R.; Chen, T.F. Evaluation of methods used for estimating content validity. Res. Soc. Adm. Pharm. 2019, 15, 214–221. [Google Scholar] [CrossRef]
- DeVellis, R.F. Scale Development: Theory and Applications; Sage Publications, Inc.: Thousand Oaks, CA, USA, 1991. [Google Scholar]
- de Winter, J.C.; Dodou, D.; Wieringa, P.A. Exploratory Factor Analysis with Small Sample Sizes. Multivar. Behav. Res. 2009, 44, 147–181. [Google Scholar] [CrossRef]
- Bernhardt, J.; Hayward, K.S.; Kwakkel, G.; Ward, N.S.; Wolf, S.L.; Borschmann, K.; Krakauer, J.W.; Boyd, L.A.; Carmichael, S.T.; Corbett, D.; et al. Agreed definitions and a shared vision for new standards in stroke recovery research: The Stroke Recovery and Rehabilitation Roundtable taskforce. Int. J. Stroke 2017, 12, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.L.; Zheng, Z.X.; Sun, Y.M.; Mei, Y.X.; Xie, J.F.; Zhang, Y.Q. Development and reliability and validity test of Functional Exercise Compliance Scale for community stroke patients. Chin. J. Rehabil. Med. 2013, 28, 574–578. [Google Scholar]
- Zhang, D.; Zheng, H.; Zeng, Y.; Yu, X.; Zhao, Y.; Gan, Y.; Chai, X.; Cheng, W.; Chen, Z.; Zhou, Y. Psychometric properties of the Chinese version of the Quiet Quitting Scale. BMC Nurs. 2025, 24, 270. [Google Scholar] [CrossRef]
- Abma, I.L.; Rovers, M.; van der Wees, P.J. Appraising convergent validity of patient-reported outcome measures in systematic reviews: Constructing hypotheses and interpreting outcomes. BMC Res. Notes 2016, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Boateng, G.O.; Neilands, T.B.; Frongillo, E.A.; Melgar-Quiñonez, H.R.; Young, S.L. Best Practices for Developing and Validating Scales for Health, Social, and Behavioral Research: A Primer. Front. Public Health 2018, 6, 149. [Google Scholar] [CrossRef]
- Cerny, B.A.; Kaiser, H.F. A Study Of A Measure Of Sampling Adequacy For Factor-Analytic Correlation Matrices. Multivar. Behav. Res. 1977, 12, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.C. A Review of Exploratory Factor Analysis Decisions and Overview of Current Practices: What We Are Doing and How Can We Improve? Int. J. Hum.–Comput. Interact. 2016, 32, 51–62. [Google Scholar] [CrossRef]
- Souza, A.C.; Alexandre, N.M.C.; Guirardello, E.B. Psychometric properties in instruments evaluation of reliability and validity. Epidemiol. Serv. Saude 2017, 26, 649–659. [Google Scholar] [CrossRef]
- Cortina, J.M. What Is Coefficient Alpha? An Examination of Theory and Applications. J. Appl. Psychol. 1993, 78, 98–104. [Google Scholar] [CrossRef]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lai, B.; Mehta, T.; Thirumalai, M.; Padalabalanarayanan, S.; Rimmer, J.H.; Motl, R.W. Exercise Training Guidelines for Multiple Sclerosis, Stroke, and Parkinson Disease: Rapid Review and Synthesis. Am. J. Phys. Med. Rehabil. 2019, 98, 613–621. [Google Scholar] [CrossRef]
- Rasmussen, R.S.; Ostergaard, A.; Kjaer, P.; Skerris, A.; Skou, C.; Christoffersen, J.; Seest, L.S.; Poulsen, M.B.; Ronholt, F.; Overgaard, K. Stroke rehabilitation at home before and after discharge reduced disability and improved quality of life: A randomised controlled trial. Clin. Rehabil. 2016, 30, 225–236. [Google Scholar] [CrossRef]
- Schmidt, R.; Geisler, D.; Urban, D.; Pries, R.; Franzisket, C.; Voigt, C.; Ivanova, G.; Neumuth, T.; Classen, J.; Wagner, M.; et al. Stroke survivors’ preferences on assessing patient-reported outcome measures. J. Patient Rep. Outcomes 2023, 7, 124. [Google Scholar] [CrossRef]
- Long, Z.; Huang, L. Development and reliability and validity test of the sleep health literacy scale for college students. BMC Public. Health 2025, 25, 1257. [Google Scholar] [CrossRef] [PubMed]
- Cappelleri, J.C.; Jason Lundy, J.; Hays, R.D. Overview of classical test theory and item response theory for the quantitative assessment of items in developing patient-reported outcomes measures. Clin. Ther. 2014, 36, 648–662. [Google Scholar] [CrossRef]
- Andrich, D. Controversy and the Rasch model: A characteristic of incompatible paradigms? Med Care 2004, 42 (Suppl. 1), I7–I16. [Google Scholar] [CrossRef]
- King, G.; Chiarello, L.A.; Thompson, L.; McLarnon, M.J.W.; Smart, E.; Ziviani, J.; Pinto, M. Development of an observational measure of therapy engagement for pediatric rehabilitation. Disabil. Rehabil. 2019, 41, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liang, X.; Yin, S. Validity and Reliability of a Simplified Chinese Version of Cancer Survivors’ Unmet Needs Scale (CaSUN). Psychooncology 2024, 33, e70008. [Google Scholar] [CrossRef] [PubMed]
- Higgins, T.; Larson, E.; Schnall, R. Unraveling the meaning of patient engagement: A concept analysis. Patient Educ. Couns. 2017, 100, 30–36. [Google Scholar] [CrossRef]
- Hickmann, E.; Richter, P.; Schlieter, H. All together now—Patient engagement, patient empowerment, and associated terms in personal healthcare. BMC Health Serv. Res. 2022, 22, 1116. [Google Scholar] [CrossRef]
- Bright, F.A.; Kayes, N.M.; Cummins, C.; Worrall, L.M.; McPherson, K.M. Co-constructing engagement in stroke rehabilitation: A qualitative study exploring how practitioner engagement can influence patient engagement. Clin. Rehabil. 2017, 31, 1396–1405. [Google Scholar] [CrossRef]
- Laut, J.; Cappa, F.; Nov, O.; Porfiri, M. Increasing patient engagement in rehabilitation exercises using computer-based citizen science. PLoS ONE 2015, 10, e0117013. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.J.; McKay, V.R.; Hansen, P.E.; Barco, P.P.; Jones, K.; Lee, Y.; Patel, R.D.; Chen, D.; Heinemann, A.W.; Lenze, E.J.; et al. Using Implementation Science to Guide the Process of Adapting a Patient Engagement Intervention for Inpatient Spinal Cord Injury/Disorder Rehabilitation. Arch. Phys. Med. Rehabil. 2022, 103, 2180–2188. [Google Scholar] [CrossRef] [PubMed]
| Demographic Characteristics | Total Sample | Retest Group | ||
|---|---|---|---|---|
| n/Mean | %/SD | n/Mean | %/SD | |
| Age | 66.46 | 9.75 | 65.5 | 8.34 |
| Gender | ||||
| Men | 191 | 73.5 | 26 | 86.67 |
| Women | 69 | 26.5 | 4 | 13.33 |
| Education level | ||||
| Middle school or lower | 133 | 51.2 | 10 | 33.33 |
| High school/technical secondary school | 81 | 31.2 | 12 | 40.00 |
| College or above | 46 | 17.7 | 8 | 26.67 |
| Marital status | ||||
| Married | 227 | 87.3 | 28 | 93.33 |
| Unmarried/Divorced/Widowed | 33 | 12.7 | 2 | 6.67 |
| Number of strokes | ||||
| 1 | 115 | 44.2 | 14 | 46.67 |
| 2 | 86 | 33.1 | 9 | 30.00 |
| 3 | 41 | 15.8 | 5 | 16.67 |
| ≥4 | 18 | 6.9 | 2 | 6.67 |
| Type of stroke | ||||
| Ischemic stroke | 238 | 91.5 | 29 | 96.67 |
| Hemorrhagic stroke | 6 | 2.3 | 1 | 3.33 |
| Mixed-type stroke | 16 | 6.2 | 0 | 0.00 |
| Modified Rankin score | ||||
| 0 | 97 | 37.3 | 13 | 43.33 |
| 1 | 107 | 41.2 | 13 | 43.33 |
| 2 | 36 | 13.8 | 2 | 6.67 |
| 3 | 20 | 7.7 | 2 | 6.67 |
| Item | Factor 1 |
|---|---|
| 1. I believe that exercise rehabilitation is a dynamic and continuous process that requires ongoing investment. | 0.913 |
| 2. I have some knowledge and skills related to exercise rehabilitation. | 0.840 |
| 3. I have clear goals for my exercise rehabilitation. | 0.893 |
| 4. I can correctly evaluate the effectiveness of exercise rehabilitation. | 0.899 |
| 5. I can apply the knowledge I have learned in exercise rehabilitation to real-life situations. | 0.894 |
| 6. I can recognize my strengths and weaknesses in the exercise rehabilitation process. | 0.892 |
| 7. I can make timely decisions to optimize my exercise rehabilitation plan. | 0.909 |
| 8. I am willing to invest time and energy in exercise rehabilitation. | 0.931 |
| 9. I have a positive attitude towards participating in exercise rehabilitation. | 0.940 |
| 10. I can effectively manage negative emotions during the exercise rehabilitation process. | 0.864 |
| 11. I feel proud and satisfied with the progress I make in the exercise rehabilitation process. | 0.908 |
| 12. During the exercise rehabilitation process, I feel understood and supported by others (medical staff, family members, etc.). | 0.825 |
| 13. I actively participate in the formulation of the exercise rehabilitation plan. | 0.886 |
| 14. I have a regular exercise habit. | 0.938 |
| 15. I interact and collaborate effectively with medical staff regarding exercise rehabilitation. | 0.764 |
| 16. I learn methods and skills for exercise rehabilitation. | 0.847 |
| 17. I concentrate on completing each exercise rehabilitation session. | 0.924 |
| 18. I complete my exercise rehabilitation plan on time. | 0.939 |
| 19. I adjust the intensity or content of exercise rehabilitation according to my own condition. | 0.908 |
| 20. I monitor my exercise data independently. | 0.884 |
| Demographic Characteristics | Mean | SD | Statistic | p |
|---|---|---|---|---|
| Age | 2.247 | 0.026 | ||
| <65 | 59.34 | 21.25 | ||
| ≥65 | 53.26 | 21.30 | ||
| Gender | 0.295 | 0.864 | ||
| Men | 55.76 | 21.83 | ||
| Women | 55.25 | 20.48 | ||
| Type of stroke | 0.164 | 0.849 | ||
| Ischemic stroke | 55.85 | 21.66 | ||
| Hemorrhagic stroke | 52.17 | 21.40 | ||
| Mixed-type stroke | 53.56 | 19.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Wang, X.; Wang, W.; Mei, Y.; Lin, B.; Chen, J.; Zhang, Z. Development, Reliability and Validity of Engagement in Exercise Rehabilitation Scale for Patients with Stroke. Nurs. Rep. 2025, 15, 303. https://doi.org/10.3390/nursrep15080303
Jiang H, Wang X, Wang W, Mei Y, Lin B, Chen J, Zhang Z. Development, Reliability and Validity of Engagement in Exercise Rehabilitation Scale for Patients with Stroke. Nursing Reports. 2025; 15(8):303. https://doi.org/10.3390/nursrep15080303
Chicago/Turabian StyleJiang, Hu, Xiaoxuan Wang, Wenna Wang, Yongxia Mei, Beilei Lin, Jing Chen, and Zhenxiang Zhang. 2025. "Development, Reliability and Validity of Engagement in Exercise Rehabilitation Scale for Patients with Stroke" Nursing Reports 15, no. 8: 303. https://doi.org/10.3390/nursrep15080303
APA StyleJiang, H., Wang, X., Wang, W., Mei, Y., Lin, B., Chen, J., & Zhang, Z. (2025). Development, Reliability and Validity of Engagement in Exercise Rehabilitation Scale for Patients with Stroke. Nursing Reports, 15(8), 303. https://doi.org/10.3390/nursrep15080303






