Association between Caffeine Intake and All-Cause and Cause-Specific Mortality: An Analysis of the National Health and Nutrition Examination Survey (NHANES) 1999–2014 Database
Abstract
:1. Introduction
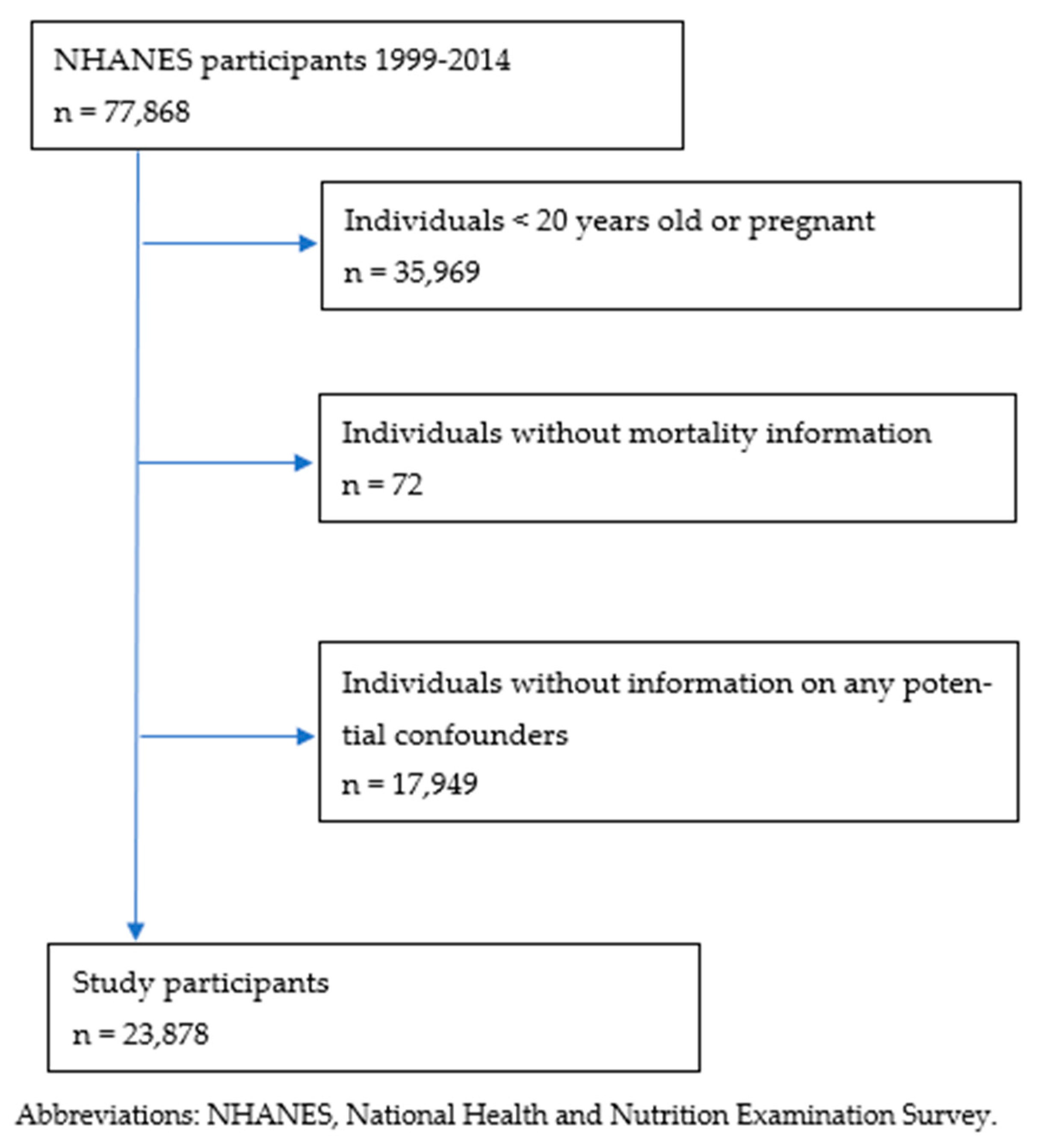
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- National Coffee Association. NCA National Coffee Data Trends. 2019. Available online: https://nationalcoffee.blog/2019/03/09/national-coffee-drinking-trends-2019/ (accessed on 2 February 2021).
- Heckman, M.A.; Weil, J.; De Mejia, E.G. Caffeine (1, 3, 7-trimethylxanthine) in foods: A comprehensive review on consumption, functionality, safety, and regulatory matters. J. Food Sci. 2010, 5, R77–R87. [Google Scholar] [CrossRef]
- Koloverou, E.; Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Georgousopoulou, E.N.; Laskaris, A.; on behalf of the ATTICA Study Group. The evaluation of inflammatory and oxidative stress biomarkers on coffee-diabetes association: Results from the 10-year follow-up of the ATTICA study (2002–2012). Eur. J. Clin. Nutr. 2015, 69, 1220–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedman, N.D.; Park, Y.; Abnet, C.C.; Hollenbeck, A.R.; Sinha, R. Association of coffee drinking with total and cause-specific mortality. N. Engl. J. Med. 2012, 366, 1891–1904. [Google Scholar] [CrossRef] [PubMed]
- Loftfield, E.; Cornelis, M.C.; Caporaso, N.; Yu, K.; Sinha, R.; Freedman, N. Association of coffee drinking with mortality by genetic variation in caffeine metabolism: Findings from the UK Biobank. JAMA Int. Med. 2018, 178, 1086–1097. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Je, Y.; Giovannucci, E. Coffee consumption and all-cause and cause-specific mortality: A meta-analysis by potential modifiers. Eur. J. Epidemiol. 2019, 34, 731–752. [Google Scholar] [CrossRef] [PubMed]
- Frary, C.D.; Johnson, R.K.; Wang, M.Q. Food sources and intakes of caffeine in the diets of persons in the United States. J. Am. Diet. Assoc. 2005, 105, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Rehm, C.D. Sources of Caffeine in Diets of US Children and Adults: Trends by Beverage Type and Purchase Location. Nutrients 2016, 8, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, D.C.; Knight, C.A.; Hockenberry, J.; Teplansky, R.; Hartman, T.J. Beverage caffeine intakes in the U.S. Food Chem. Toxicol. 2014, 63, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Tsujimoto, T.; Kajio, H.; Sugiyama, T. Association between Caffeine Intake and All-Cause and Cause-Specific Mortality: A Population-Based Prospective Cohort Study. Mayo Clin. Proc. 2017, 92, 1190–1202. [Google Scholar] [CrossRef] [Green Version]
- National Center for Health Statistics. National Health and Nutrition Examination Survey. 2017. Available online: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed on 2 February 2021).
- Raper, N.; Perloff, B.; Ingwersen, L.; Steinfeldt, L.; Anand, J. An overview of USDA’s Dietary Intake Data System. J. Food Compos. Anal. 2004, 17, 545–555. [Google Scholar] [CrossRef]
- US Department of Agriculture, Agricultural Research Service. USDA Food and Nutrient Database for Dietary Studies, 5.0. Food Surveys Research Group. 2012. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fndds-versions-and-citations/ (accessed on 2 February 2021).
- US Department of Health and Human Services and Department of Agriculture. 2015–2020 Dietary Guidelines for Americans; US Government Printing Office: Washington, DC, USA, 2015. Available online: https://health.gov/our-work/food-and-nutrition/2015-2020-dietary-guidelines/ (accessed on 2 February 2021).
- National Center for Health Statistics. 2015 Public-Use Linked Mortality Files. 2020. Available online: https://www.cdc.gov/nchs/data-linkage/mortality-public.htm (accessed on 2 February 2021).
- American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2020 update: A report from the American Heart Association. Circulation 2020, 141, e139–e151. [Google Scholar]
- Van Dongen, L.H.; Mölenberg, F.J.; Soedamah-Muthu, S.S.; Kromhout, D.; Geleijnse, J.M. Coffee consumption after myocardial infarction and risk of cardiovascular mortality: A prospective analysis in the Alpha Omega Cohort. Am. J. Clin. Nutr. 2017, 106, 1113–1120. [Google Scholar] [CrossRef] [Green Version]
- Crippa, A.; Discacciati, A.; Larsson, S.; Wolk, A.; Orsini, N. Coffee Consumption and Mortality from All Causes, Cardiovascular Disease, and Cancer: A Dose-Response Meta-Analysis. Am. J. Epidemiol. 2014, 180, 763–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Garcia, E.; van Dam, R.M.; Li, T.Y.; Rodriguez-Artalejo, F.; Hu, F.B. The relationship of coffee consumption with mortality. Ann. Int. Med. 2008, 148, 904–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, M.; Kurahashi, N.; Iwasaki, M.; Shimazu, T.; Tanaka, Y.; Mizokami, M.; Tsugane, S. Effect of Coffee and Green Tea Consumption on the Risk of Liver Cancer: Cohort Analysis by Hepatitis Virus Infection Status. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1746–1753. [Google Scholar] [CrossRef] [Green Version]
- Bøhn, S.; Blomhoff, R.; Paur, I. Coffee and cancer risk, epidemiological evidence, and molecular mechanisms. Mol. Nutr. Food Res. 2014, 58, 915–930. [Google Scholar] [CrossRef]
- Harris, H.R.; Bergkvist, L.; Wolk, A. Coffee and black tea consumption and breast cancer mortality in a cohort of Swedish women. Br. J. Cancer 2012, 107, 874–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botelho, F.; Lunet, N.; Barros, H. Coffee and gastric cancer: Systematic review and meta-analysis. Cad. Saude Publica 2006, 22, 889–900. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Sui, X.; Lavie, C.J.; Hebert, J.R.; Earnest, C.P.; Zhang, J.; Blair, S.N. Association of coffee consumption with all-cause and cardiovascular disease mortality. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2013; Volume 88, pp. 1066–1074. [Google Scholar]
- Kamimori, G.H.; Somani, S.M.; Knowlton, R.G.; Perkins, R.M. The effects of obesity and exercise on the pharmacokinetics of caffeine in lean and obese volunteers. Eur. J. Clin. Pharmacol. 1987, 31, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Ale-Agha, N.; Goy, C.; Jakobs, P.; Spyridopoulos, I.; Gonnissen, S.; Dyballa-Rukes, N.; Aufenvenne, K.; von Ameln, F.; Zurek, M.; Spannbrucker, T.; et al. CDKN1B/p27 is localized in mitochondria and improves respiration-dependent processes in the cardiovascular system-new mode of action for caffeine. PLoS Biol. 2018, 16, e2004408. [Google Scholar] [CrossRef]
- Spyridopoulos, I.; Fichtlscherer, S.; Popp, R.; Toennes, S.W.; Fisslthaler, B.; Trepels, T.; Zernecke, A.; Liehn, E.A.; Weber, C.; Zeiher, A.M.; et al. Caffeine Enhances Endothelial Repair by an AMPK-Dependent Mechanism. Arter. Thromb. Vasc. Biol. 2008, 28, 1967–1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renner, B.; Clarke, G.; Grattan, T.; Beisel, M.A.; Mueller, C.; Werner, U.; Kobal, G.; Brune, K. Caffeine Accelerates Absorption and Enhances the Analgesic Effect of Acetaminophen. J. Clin. Pharmacol. 2007, 47, 715–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, H.; Simmons, M.S.; Tashkin, D.P.; Hui, K.K.; Lee, E.Y. Bronchodilator effects of caffeine in coffee: A dose-response study of asthmatic subjects. Chest 1986, 89, 335–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, M.; Mirzaei, F.; Pan, A.; Okereke, O.I.; Willett, W.C.; O’Reilly, É.J.; Koenen, K.; Ascherio, A. Coffee, caffeine, and risk of depression among women. Arch Intern Med. 2011, 171, 1571–1578. [Google Scholar] [CrossRef] [Green Version]
- Palatini, P.; Ceolotto, G.; Ragazzo, F.; Dorigatti, F.; Saladini, F.; Papparella, I.; Mos, L.; Zanata, G.; Santonastaso, M. CYP1A2 genotype modifies the association between coffee intake and the risk of hypertension. J. Hypertens. 2009, 27, 1594–1601. [Google Scholar] [CrossRef] [Green Version]
- Cano-Marquina, A.; Tarín, J.; Cano, A. The impact of coffee on health. Maturitas 2013, 75, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.S.; Aggarwal, S.; Arora, R.R. The effect of coffee and quantity of consumption on specific cardiovascular and all-cause mortality: Coffee consumption does not affect mortality. Am. J. Ther. 2016, 23, e232–e237. [Google Scholar] [CrossRef]
- Andersen, L.F.; Jacobs, D.R., Jr.; Carlsen, M.H.; Blomhoff, R. Consumption of coffee is associated with reduced risk of death attributed to inflammatory and cardiovascular diseases in the Iowa Women’s Health Study. Am. J Clin. Nutr. 2006, 83, 1039–1046. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, J.A.; Dunbar, C.C.; Schnoll, R.; Kokolis, R.; Kokolis, S.; Kassotis, J. Caffeinated beverage intake and the risk of heart disease mortality in the elderly: A prospective analysis. Am. J. Clin. Nutr. 2007, 85, 392–398. [Google Scholar] [CrossRef] [Green Version]
- Slimani, N.; Ferrari, P.; Ocké, M.; Welch, A.; Boeing, H.; van Liere, M.; Pala, V.; Amiano, P.; Lagiou, A.; Mattisson, I.; et al. Standardization of the 24-hour diet recall calibration method used in the European Prospective Investigation into Cancer and Nutrition (EPIC): General concepts and preliminary results. Eur. J. Clin. Nutr. 2000, 54, 900–917. [Google Scholar] [CrossRef]
| Characteristics | Caffeine Intake (mg/Day) | p-Value b | ||
|---|---|---|---|---|
| <100 N (%) | 100–200 N (%) | >200 N (%) | ||
| Age | <0.001 | |||
| 20–34 | 4959 (28.7) | 1732 (22.3) | 1426 (15.7) | |
| 35–50 | 4036 (23.4) | 1966 (25.3) | 2807 (30.9) | |
| 50+ | 8271 (47.9) | 4075 (52.4) | 4843 (53.4) | |
| Sex | <0.001 | |||
| Male | 9477 (54.9) | 4009 (51.6) | 3879 (42.7) | |
| Female | 7789 (45.1) | 3764 (48.4) | 5197 (57.3) | |
| Race | <0.001 | |||
| White | 6174 (35.8) | 3967 (51.0) | 6348 (69.9) | |
| Black | 4960 (28.7) | 1286 (16.5) | 768 (8.5) | |
| Hispanic | 4945 (28.6) | 2002 (25.8) | 1530 (16.9) | |
| Other | 1187 (6.9) | 518 (6.7) | 430 (4.7) | |
| Education | <0.001 | |||
| <High school | 5402 (31.3) | 2043 (26.3) | 1944 (21.4) | |
| High school | 3826 (22.2) | 1855 (23.9) | 2276 (25.1) | |
| Some college | 3336 (19.4) | 1726 (22.3) | 2150 (23.7) | |
| Postgraduate | 4678 (27.1) | 2138 (27.5) | 2698 (29.8) | |
| Income (dollars/year) | <0.001 | |||
| <15,000 | 4826 (29.7) | 1865 (25.2) | 1986 (22.7) | |
| 15,000–25,000 | 2065 (12.7) | 870 (11.8) | 941 (10.8) | |
| 25,000–35,000 | 2103 (12.9) | 967 (13.1) | 1026 (11.8) | |
| 35,000–55,000 | 2713 (16.7) | 1332 (18.0) | 1611 (18.4) | |
| 55,000–75,000 | 1609 (9.9) | 762 (10.3) | 1027 (11.8) | |
| >75,000 | 2954 (18.1) | 1600 (21.6) | 2143 (24.5) | |
| BMI (kg/m2) | <0.001 | |||
| <18.5 | 284 (1.7) | 122 (1.6) | 126 (1.4) | |
| 18.5–24.9 | 4853 (28.7) | 2166 (28.3) | 2467 (27.5) | |
| 25–29.9 | 5644 (33.4) | 2658 (34.7) | 3202 (35.7) | |
| ≥30 | 6134 (36.2) | 2706 (35.4) | 3167 (35.4) | |
| Smoke | ||||
| Yes | 6480 (37.6) | 3762 (48.4) | 5745 (63.3) | <0.001 |
| Hypertension c | ||||
| Yes | 6115 (35.9) | 2726 (35.2) | 3082 (34.1) | 0.01 |
| Diabetes d | ||||
| Yes | 2088 (12.3) | 913 (12.0) | 983 (11.1) | <0.001 |
| Cancer e | ||||
| Yes | 1491 (8.7) | 775 (10.0) | 1007 (11.1) | <0.001 |
| Nutrition | ||||
| Energy (kcal) | 1824 ± 826 | 2121 ± 901 | 2294 ± 956 | <0.001 |
| Carbohydrate (g) | 238 ± 110 | 266 ± 121 | 279 ± 131 | <0.001 |
| Protein (g) | 68 ± 35 | 80 ± 37 | 86 ± 39 | <0.001 |
| Fat (g) | 67 ± 34 | 78 ± 37 | 87 ± 40 | <0.001 |
| Mortality Cause | All Participants | Caffeine Intake (mg/Day) | ||
|---|---|---|---|---|
| <100 (n = 12,006) | 100–200 (n = 5624) | >200 (n = 6248) | ||
| All-cause mortality | ||||
| No. of cases (%) | 2206 | 1150 (52.1) | 503 (22.8) | 553 (25.1) |
| Age-adjusted HR (95% CI) | 1.0 | 0.77 (0.69–0.86) * | 0.69 (0.63–0.75) * | |
| Multivariable-adjusted HR (95% CI) | 1.0 | 0.78 (0.67–0.91) * | 0.68 (0.60–0.78) * | |
| CVD mortality | ||||
| No. of cases (%) | 394 | 207 (52.5) | 75 (19.1) | 112 (28.4) |
| Age-adjusted HR (95% CI) | 1.0 | 0.70 (0.56–0.88) * | 0.72 (0.58–0.89) * | |
| Multivariable-adjusted HR (95% CI) | 1.0 | 0.63 (0.45–0.88) * | 0.67 (0.50–0.88) * | |
| Multivariable-adjusted cause-specific HR (95% CI) | 1.0 | 0.67 (0.47–0.94) | 0.77 (0.57–1.04) | |
| Cancer mortality | ||||
| No. of cases (%) | 525 | 243 (46.3) | 115 (21.9) | 167 (31.8) |
| Age-adjusted HR (95% CI) | 1.0 | 0.85 (0.71–1.03) | 0.87 (0.74–1.02) | |
| Multivariable-adjusted HR (95% CI) | 1.0 | 0.78 (0.58–1.05) | 0.94 (0.72–1.21) | |
| Multivariable-adjusted cause-specific HR (95% CI) | 1.0 | 0.80 (0.59–1.08) * | 0.98 (0.76–1.27) * | |
| Stratification Variable | Caffeine Intake (mg/Day) | ||||
|---|---|---|---|---|---|
| <100 | 100–200 | >200 | |||
| Referent | HR | 95% CI | HR | 95% CI | |
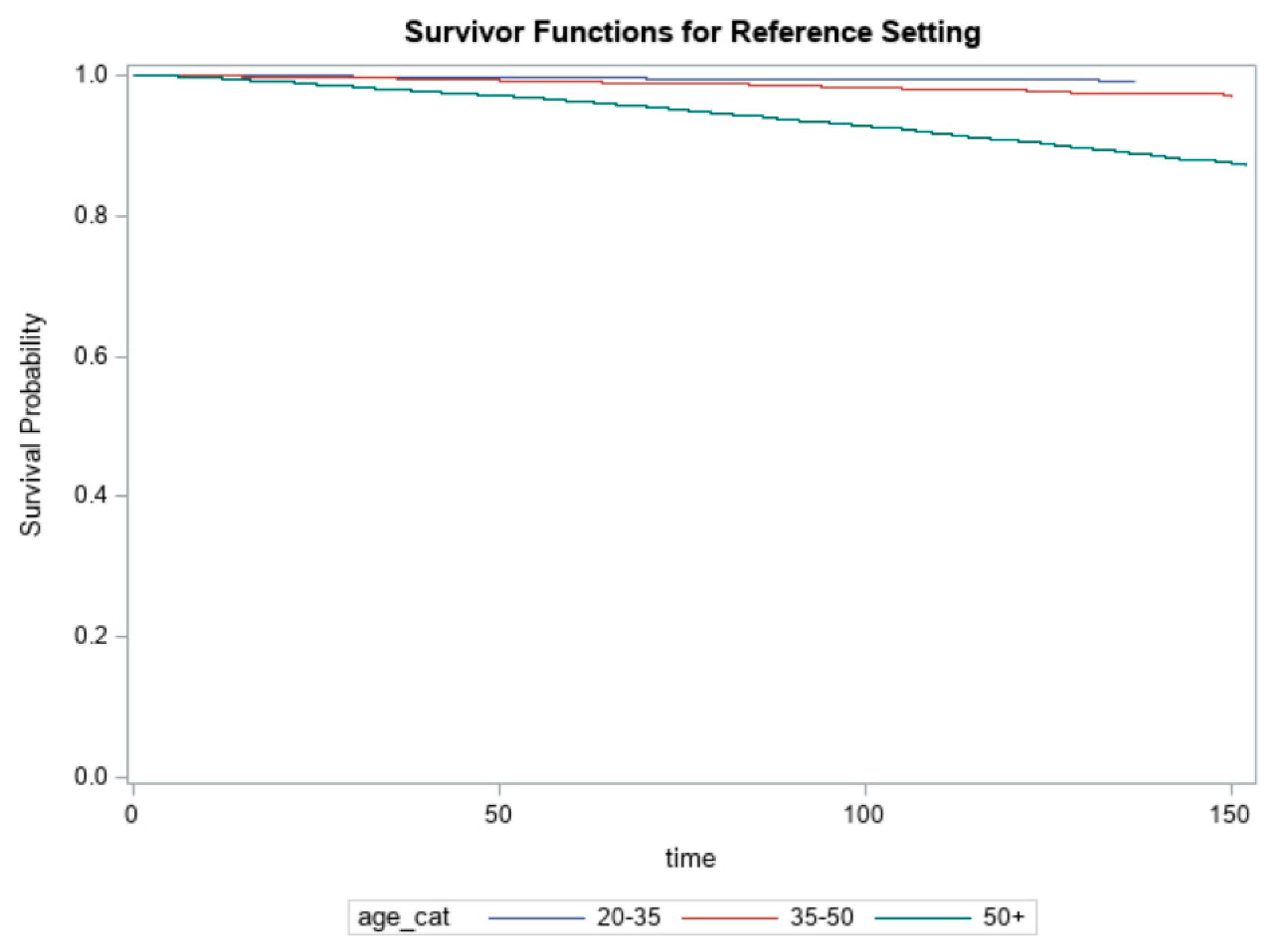
| Age | |||||
| 20–35 | 2.03 | 1.03–3.99 * | 1.45 | 0.74–2.85 | |
| 35–50 | 0.55 | 0.37–0.84 * | 0.89 | 0.63–1.24 | |
| 50+ | 0.80 | 0.71–0.89 * | 0.67 | 0.61–0.74 * | |
| BMI | |||||
| <18.5 | 0.76 | 0.33–1.76 | 1.35 | 0.67–2.71 | |
| 18.5–24.9 | 0.75 | 0.62–0.90 * | 0.66 | 0.55–0.80 * | |
| 25–29.9 | 0.87 | 0.72–1.05 | 0.66 | 0.56–0.78 * | |
| ≥30 | 0.77 | 0.64–0.94 * | 0.75 | 0.63–0.91 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Wang, J.; Jose, M.; Seo, Y.; Feng, L.; Ge, S. Association between Caffeine Intake and All-Cause and Cause-Specific Mortality: An Analysis of the National Health and Nutrition Examination Survey (NHANES) 1999–2014 Database. Nurs. Rep. 2021, 11, 901-912. https://doi.org/10.3390/nursrep11040083
Feng J, Wang J, Jose M, Seo Y, Feng L, Ge S. Association between Caffeine Intake and All-Cause and Cause-Specific Mortality: An Analysis of the National Health and Nutrition Examination Survey (NHANES) 1999–2014 Database. Nursing Reports. 2021; 11(4):901-912. https://doi.org/10.3390/nursrep11040083
Chicago/Turabian StyleFeng, Juan, Jing Wang, Mini Jose, Yaewon Seo, Li Feng, and Song Ge. 2021. "Association between Caffeine Intake and All-Cause and Cause-Specific Mortality: An Analysis of the National Health and Nutrition Examination Survey (NHANES) 1999–2014 Database" Nursing Reports 11, no. 4: 901-912. https://doi.org/10.3390/nursrep11040083
APA StyleFeng, J., Wang, J., Jose, M., Seo, Y., Feng, L., & Ge, S. (2021). Association between Caffeine Intake and All-Cause and Cause-Specific Mortality: An Analysis of the National Health and Nutrition Examination Survey (NHANES) 1999–2014 Database. Nursing Reports, 11(4), 901-912. https://doi.org/10.3390/nursrep11040083






