Abstract
Background/Objectives: Chronic anemia and iron overload in thalassemia lead to organ failures, including the heart, liver, endocrine glands, and spleen. Comprehensive multidisciplinary management is pivotal in improving patients’ clinical outcomes and well-being. The report aims to present a rare case of improved clinical condition in a transfusion-dependent thalassemia patient. Methods: Medical summaries were collected to compare patients’ conditions at various time frames. Furthermore, the report was composed in chronological order. Results: A 31-year-old male diagnosed with beta-thalassemia major and multiple comorbidities, including diabetes with a history of diabetic ketoacidosis, heart failure with a history of cardiac arrest, hepatomegaly, severe thoracolumbar scoliosis, and depression, exhibited a high physical endurance. The patient managed to maintain a strong treatment adherence and undergo intensive regular multidisciplinary follow-ups. The patient gained cardiac function improvement and metabolic stabilization, leading to completing a 5 k marathon without complication. Conclusions: Intensive management of iron overload through double oral chelation allows organ function improvement. Better mental health attenuates the patient’s overall well-being and is attributed to the ability to gain high physical endurance.
1. Introduction
Thalassemia is one of the most prevalent monogenic disorders, characterized by reduced hemoglobin chain synthesis and chronic hemolytic anemia. It follows an autosomal recessive inheritance pattern. β-thalassemia is highly prevalent in the “Thalassemia Belt”, spanning the Mediterranean Basin, the Middle East, and Southeast Asia. Clinically, it is classified by severity (minor, intermedia, or major) and transfusion dependency, distinguishing non-transfusion-dependent thalassemia (NTDT) from transfusion-dependent thalassemia (TDT) [1,2].
β-Thalassemia, a prevalent form of thalassemia, results from a pathogenic variant of β-globin gene, causing an imbalance with excess α-globin chains. These unpaired α-globin chains aggregate into insoluble inclusions within erythroid precursors, leading to ineffective erythropoiesis and hemolysis, which collectively cause anemia. In response, erythropoietin production increases, stimulating erythroid marrow hyperplasia in medullary and extramedullary sites. Extramedullary erythropoiesis can result in craniofacial deformities (Cooley’s facies), skeletal abnormalities, cortical bone thinning, and splenomegaly. Chronic anemia may further lead to cardiac enlargement, heart failure, growth retardation, cognitive impairment, and osteoporosis [1,2].
Managing thalassemia major patients involves regular and life-long blood transfusions to maintain adequate hemoglobin levels. However, the human body lacks a physiological mechanism to excrete the excess iron from these transfusions. Labile iron will be released into the plasma, where it binds to transferrin [3]. Once the transferrin binding capacity is saturated, non-transferrin-bound iron (NTBI) is transported into various tissues, including the hepatocytes, cardiac myocytes, and endocrine glands, leading to cardiac siderosis, liver fibrosis, and endocrine abnormalities (growth retardation, hypothyroidism, hypoparathyroidism, hypogonadism, osteoporosis, and diabetes mellitus). Therefore, iron chelation therapy is crucial to prevent iron overload and facilitate iron removal from tissues [4]. Thalassemia patients with splenomegaly due to extramedullary hematopoiesis may be indicated for splenectomy to reduce transfusion requirements.
Apart from physical burden, thalassemia can pose a significant psychological burden to patients. Notably, in thalassemia major, both deteriorating functional health and multiple treatment regimens can bring a great burden to the patient early in life, leading to stress or anxiety, and more if left untreated. Therefore, comprehensive management should not only address the systemic complications but also integrate mental health support for patients, which clinicians may overlook. This case report aims to describe a TDT β-thalassemia patient with multiple comorbidities arising from iron overload complications. It highlights the critical role of comprehensive management strategies in improving the patient’s well-being and clinical outcomes, emphasizing the importance of an individualized, multidisciplinary approach in addressing the complex challenges associated with this condition.
2. Case Presentation
The patient is a 31-year-old Indonesian male diagnosed with beta-thalassemia major based on genetic testing at 8 months of age due to prolonged skin pallor. The patient is categorized as transfusion-dependent thalassemia (TDT). He underwent a splenectomy at the age of 9 months. His hemoglobin levels were maintained between 7 and 8 g/dL. Since he started exercising regularly in 2023, the pre-transfusion hemoglobin levels have risen and ranged between 9 and 10 g/dL, maintained through routine blood transfusions administered every three weeks. Family history reveals that the patient’s parents and sibling have been diagnosed with β-Thalassemia minor. The patient had a history of syncope in 2019 due to exhaustion while cycling. The syncope resulted in a falling accident, which caused a right arm fracture. In 2020, the patient experienced recurrent episodes of pulseless ventricular tachycardia (VT) and diabetic ketoacidosis (DKA), which were complicated by cardiogenic shock presenting with hypotension (blood pressure 70/50 mmHg) and cold extremities. One day before hospitalization, the patient reported progressive dyspnea without associated fever, cough, or wheezing. Additionally, there was a two-day history of nausea, vomiting, and reduced appetite, though left-sided chest pain or referred pain was absent. The patient also reported fatigue exacerbated by prolonged walking or standing without prior triggers. During hospitalization, the patient underwent cardiopulmonary resuscitation (CPR), resulting in a return of spontaneous circulation (ROSC), and was subsequently managed in the intensive care unit (ICU) for seven days. During ICU management, the patient received tailored pharmacological interventions. Inotropic and vasopressor support included dobutamine (10 mcg/kg/min) and norepinephrine (0.5 mcg/kg/min). Antiarrhythmic therapy was administered with amiodarone. Electrolyte management involved magnesium sulfate (20%) and calcium gluconate. Glucose regulation utilized an insulin drip (2 U/hour, titrated per diabetic ketoacidosis protocol), transitioning to basal–bolus insulin therapy with Lantus (8 U SC daily) and Novorapid (6 U SC three times daily). Acid–base correction was achieved with sodium bicarbonate (125 mEq IV over 1 h, then 125 mEq over 4 h). Iron chelation included deferoxamine (2000 mg SC, three times weekly) and deferiprone (500 mg PO three times daily). Leukodepleted PRC transfusions were administered at Hb < 9.2 g/dL, targeting 12 g/dL. Anticoagulation involved heparin (10,000 units/24 h IV) transitioning to warfarin (1 mg PO daily). Following stabilization, he was transferred to general care and later discharged. Despite clinical improvement, the patient continued to experience symptoms of exertional dyspnea and fatigue post-discharge.
The most recent physical examination results are as follows: the patient weighed 39 kg and had a height of 166 cm, resulting in a body mass index (BMI) of 14.2 kg/m², indicating underweight status. Cooley’s facies and icteric sclerae were noted. Jugular venous pressure (JVP) was within normal limits. Cardiac and lung examinations were unremarkable, including inspection, auscultation, and percussion. An abdominal examination identified hepatomegaly with no palpable spleen.
The patient presented with multiple comorbidities, including heart failure with preserved ejection fraction (HFpEF) secondary to myocardial hemosiderosis, diabetes mellitus due to pancreatic hemosiderosis, which was well controlled with insulin, and osteoporosis. The patient was also diagnosed with thoracolumbar scoliosis in 2018, as seen from his chest X-ray projection taken in 2019 (Figure 1). These findings were compounded by the patient’s low BMI, suggesting underweight status.

Figure 1.
The patient’s chest X-ray with a posteroanterior (PA) projection (2019) depicting an illustration of thoracic scoliosis with a rightward curvature that may be seen in a patient with thoracolumbar scoliosis (R indicates the right side).
To address the patient’s heart failure, the patient is managed with 80 mg acetylsalicylic acid once daily, 5 mg Bisoprolol once daily, 25 mg Spironolactone once daily, and 1 mg Warfarin once daily to manage symptoms and prevent thromboembolic events. Diabetes mellitus management includes administering 12 units insulin glargine subcutaneously once daily and 6 units insulin aspart subcutaneously three times daily for diabetes control. In β-thalassemia major, iron overload is managed with dual chelation therapy. The patient receives 1000 mg Deferasirox orally once daily and 1000 mg Deferiprone orally three times daily, three times per week. Previously, for 1500 mg Deferasirox, the dosage was adjusted based on clinical condition. Osteoporosis management includes 35 mg risedronate once weekly to reduce bone resorption and calcium supplementation using 500 mg calcium carbonate (CaCO3) twice daily. The patient’s latest calcium ion level was normal at 1.09 mmol/L.
The patient also receives regular blood transfusion once every three weeks with pre-transfusion Hb level of 9–10 g/dL. Prior to transfusion, blood donors underwent nucleic acid testing (NAT) screening for HBV, HCV, HIV, and syphilis; additionally selected donors are also screened for malaria. Leukocyte reduction is performed with pre-storage filter or bedside filter. However, due to the limited availability of pre-storage filtration, bedside filters are more often used, and as a result, the patient regularly receives leuko-reduced units.
The patient’s treatment plan incorporates regular self-monitoring of blood glucose to enable dynamic adjustment of insulin doses for optimal diabetes management. Routine follow-ups include annual echocardiography, which has demonstrated right ventricular remodeling, concentric left ventricular remodeling, global normokinesia, mild tricuspid regurgitation (TR), preserved systolic function of both ventricles, and normal diastolic function. Over the past six years of follow-up, the patient’s ejection fraction (EF) has shown improvement, as depicted in Figure 2. For the monitoring of beta-thalassemia major, the patient undergoes routine laboratory assessments, including complete blood counts before transfusion, and ferritin levels, transferrin saturation, liver function, and kidney function tests every three months. Additionally, annual T2 magnetic resonance imaging (MRI)* is performed to evaluate iron overload, particularly in the heart, liver, and pancreas. Figure 2 illustrates the relationship between iron overload markers—cardiac MRI T2*, mean ferritin, and transferrin saturation levels—and cardiac function, as assessed by ejection fraction (EF) via echocardiography.
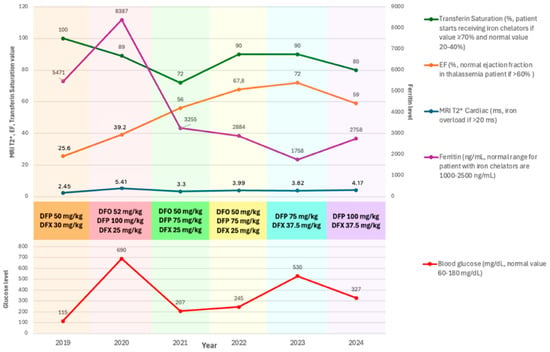
Figure 2.
Markers of iron overload, including mean serum ferritin levels, ferritin saturation, and cardiac MRI T2 measurements, alongside left ventricular ejection fraction (LVEF), mean blood glucose, and the iron chelation therapies employed between 2019 and 2024. DFO: deferoxamine (administered subcutaneously); DFP: deferiprone (oral); DFX: Deferasirox (oral).
The patient was advised to undertake low-intensity cardiovascular exercise following comprehensive evaluations by specialists in physical rehabilitation, cardiology, and hematology–oncology. Initiating marathon running in 2017, the patient successfully participated in multiple 5 K and 10 K events. However, deteriorating health in 2020 necessitated the cessation of all physical activities. By 2023, the patient exhibited strong adherence to treatment and regular follow-up, evidenced by significant metabolic stabilization and improved cardiac function. This progress enabled a gradual return to low-intensity cardiovascular exercises, such as walking and jogging. Current exercise prescription involves jogging 1–2 sessions per week, each lasting 1–2 h, performed at vigorous intensity based on heart rate (146–175 beats per minute corresponds to approximately 77–93% of maximum heart rate). Notably, in 2024, the patient completed a 5 K marathon without experiencing significant exertional dyspnea and fatigue.
The patient previously consumed an unrestricted, high-fat, iron-rich diet consisting of junk food, red meat, seafood, and sugary beverages (e.g., milk tea, milk coffee). Recently, the patient adopted a structured diet of three daily meals: breakfast with fruits and oats; lunch and dinner with rice, chicken or fish, and vegetables. High-sugar beverages were discontinued following a diabetes mellitus diagnosis. The patient intermittently supplemented his diet with omega-3 and 1000 IU of vitamin D3 daily; baseline serum 25(OH)D was 13 ng/mL, indicating vitamin D deficiency.
During treatment, the patient exhibited severe depression, indicated by a PHQ-9 (Patient Health Questionnaire-9) score of 20, attributed to their underlying health condition. The attending physician referred the patient to the psychiatry department for further evaluation and management involving antidepressants and cognitive behavioral therapy. At times, the patient experienced intense fear that led to sleeping difficulties and distressing flashbacks, including memories of a near-death experience and defibrillator use. He developed overwhelming anxiety, became more emotional, and frequently expressed negative thoughts regarding his condition. The death of a fellow thalassemia patient further impacted his emotional state. Despite these challenges, following psychiatric intervention (antidepressants and cognitive behabioral therapy), the patient demonstrated significant improvement in depressive symptoms (PHQ-9 score of 10), accompanied by increased self-confidence and the use of personal experience to raise thalassemia awareness on social media.
3. Discussion
Iron overload is a significant complication of thalassemia, arising from both intrinsic and extrinsic disruptions to iron homeostasis. Key contributors include ineffective erythropoiesis, increased gastrointestinal iron absorption, and frequent blood transfusions. Ineffective erythropoiesis induces chronic anemia, which suppresses hepcidin production via erythroferrone signaling. Hepcidin, a liver-derived hormone that regulates iron balance, inhibits intestinal iron absorption and iron release from macrophages. In thalassemia, low hepcidin levels result in unregulated dietary iron absorption, compounding iron overload. Additionally, each blood transfusion introduces 200–250 mg of iron, creating a cumulative iron burden that cannot be excreted, further exacerbating systemic iron overload. Excess iron primarily accumulates in the liver, the body’s main site for iron storage and detoxification. Chronic hepatic iron deposition can lead to fibrosis, cirrhosis, and hepatocellular carcinoma. Iron also deposits in extrahepatic organs, including the endocrine glands and heart. Endocrine iron overload may impair pancreatic function, causing diabetes, and disrupt pituitary function, leading to growth retardation and delayed puberty. Cardiac iron overload is particularly severe, increasing the risk of cardiomyopathy, heart failure, and arrhythmias, which are major causes of morbidity and mortality in thalassemia patients [5].
Considering the complications mentioned above, various methods exist for monitoring iron overload. The most-used method was measuring serum ferritin (SF) as it is inexpensive, easier, and correlates with body iron storage, with various studies having identified a lower risk of cardiac disease if SF is maintained below 2.500 µg/L for a decade or more [1]. However, serum ferritin, along with liver iron concentration and myocardial biopsy was found to be unrelated to myocardial iron deposition. Thus, MRI T2* sequences are routinely used to monitor iron overload in cardiac, hepatic, and pancreatic tissue [1]. A study by Chaosuwannakit demonstrated that a decline in cardiac MRI T2* correlates with LVEF deterioration, followed by ventricular dilation and hypertrophy. The findings emphasize the importance of routine cardiac T2* MRI monitoring, which may help reduce heart failure rates over five years in patients with severe cardiac T2* values (<25 ms) by enabling early intervention and aggressive chelation therapy optimization [6].
3.1. Cardiac Function in TDT Patients
It should be noted that the cardiac function or EF of thalassemia patients are different from that of normal subjects. In TDT patients, routine blood transfusion leads to increased end-diastolic volume and decreased end-systolic volume, leading to large stroke volume, increased EF, and cardiac output [7]. This hyperdynamic circulation leads to increased left ventricular (LV) mass, and the lower limit of LVEF is higher compared to non-thalassemia patients [7]. In this case, even though the EF showed a normal range (59%) and was classified as HFpEF, the patient still had severe iron overload from MRI T2*, which may still lead to a dire prognosis. Myocardial iron overload (T2* < 20 ms) has a high risk of acute decompensation and rhythm disorders [1]. Kirk et al. [8] observed that 47% of thalassemia major patients went through heart failure, and 14% of them had arrhythmia within 1 year after cardiac MRI T2* value falls <6 ms (severe). Employing cardiac MRI T2* to identify and treat patients at risk is a practical approach to mitigating the significant cardiac mortality associated with myocardial siderosis [8].
Other than myocardial iron overload that can be controlled by monitoring with cardiac MRI T2*, factors such as myocardial fibrosis and previous cardiac complications (i.e., ventricular dysfunction and arrhythmias) are important predictors of heart failure [9]. Myocardial fibrosis was shown to be correlated with age, negative cardiac remodeling, hepatitis C virus (HCV) infection, diabetes mellitus (DM) for adults, and myocardial iron overload in pediatrics [9]. Although arrhythmia could not be seen from the cardiac MRI T2* value, the risk was found to be reduced after the improvement of iron chelation therapy [9]. In this case, the patient received appropriate therapeutic interventions and was closely monitored to address their glucose dysregulation or diabetes mellitus (DM) through the use of both long-acting and short-acting insulin regimens. Despite this, the patient had a history of severe cardiac complications and significantly elevated cardiac MRI T2* values. Although it is theoretically anticipated that such patients would have an increased risk of heart failure-related mortality, the patient demonstrated enhanced physical tolerance compared to their condition immediately post-hospital discharge (2020). Currently, the patient is capable of participating in a 5 km marathon without experiencing dyspnea or fatigue, despite no observable improvement in their cardiac MRI T2* values. This observation underscores the influence of additional factors beyond cardiac function in modulating exercise capacity.
The relationship between cardiac health and exercise performance becomes evident when considering the impact of cardiac iron accumulation on physical activity. Iron accumulation in cardiac muscles can lead to cardiac dysfunction, which is a critical factor in determining exercise capacity. A study by Piatti et al. [10] indicates that cardiac iron overload is positively associated with reduced peak oxygen uptake (V’O2 peak), one of the key measurements of exercise performance. The study showed that lower exercise capacity in thalassemia patients is attributed to muscular deconditioning and reduced cardiac inotropism due to iron deposition [10]. Myocardial siderosis contributes to this by reducing physical activity levels, as patients may avoid physical activity due to concerns of cardiac complications, therefore reducing overall muscle strength and endurance [10]. In this case, the patient underwent low-intensity cardiovascular exercise before participating in his first marathon run (2017). This is aligned with the findings of Piatti et al. [10], where thalassemia patients who participated in moderate/vigorous physical activities are associated with better V’O2 peak values, resulting in increased exercise tolerance. Wagner et al. [11] also found a strong positive relationship between high V’O2 peak values and other performance-related cardiopulmonary exercise testing (CPET) parameters, emphasizing the benefits of incorporating higher-intensity aerobic exercise into health promotion strategies. Exercise, particularly aerobic activities, has been shown to enhance the quality of life in thalassemia major patients [12].
3.2. Pharmacological Management for TDT Patients with Poor Cardiac Function
Following the patient’s ROSC in 2020, he was given a triple combination of iron chelation therapy, which showed improved cardiac function and lower ferritin levels compared to 2019. The American Heart Association (AHA) has summarized the iron chelators given to thalassemia patients with cardiac complications, in which it can be seen that a combination of DFO and DFP has greater improvement in MRI T2* values and LVEF compared to DFO only or DFP only [13]. A study by Berdoukas et al. [14] found that patients given DFO only experienced lowered liver iron, while patients with DFP only experienced lowered heart iron. This statement was followed by the TIF guideline, which saw the combination of DFO and DFP as the best available intensive chelation for TDT patients with cardiac iron overload and with/without overt cardiac dysfunction or heart failure [1]. Meanwhile, a combination of DFX and DFO was said to achieve faster removal of liver iron compared to only DFX monotherapy regimens, with previous studies suggesting cardiac iron may not be improved unless the liver iron is lowered [15].
Under iron overload conditions, excess iron in the blood exists as NTBI, which can be taken into tissues susceptible to iron loading. Hence, the removal of NTBI by chelators is desired; however, DFO monotherapy is only partially effective [16]. The key role in optimizing iron removal is the “shuttle” effect, which refers to the interaction between different chelators. Since DFO has limited efficacy in targeting intracellular iron, the NTBI can be chelated more efficiently by incorporating DFP as a recipient and transferring it to DFO, forming a stable complex called ferroxamine (FO), while DFP continues binding iron to be excreted again [16,17]. This process enhances the efficiency of iron chelation, making the combination of DFP and DFO more effective than monotherapy.
As seen from the case above, a triple iron chelator (DFO 50 mg/kg, DFP 75 mg/kg, and DFX 25 mg/kg) showed higher improvement compared to the dual therapy (DFP 75–100 mg/kg and DFX 37.5 mg/kg) used by the patient in the past 1 year. An RCT study for triple iron chelator was performed in Sri Lanka, showing a significantly higher cardiac MRI T2* improvement (mean change +6.72 ± 9.63 ms, p = 0.06) and significantly higher mean ferritin reduction (−1094 ± 907 ng/mL, p = 0.042) after 6 months of triple iron chelator therapy compared to patients given dual therapy (DFO and DFX) [16]. However, the primary limitations of triple combination therapy include a higher incidence of arthralgia, a known adverse effect of DFP, along with increased pill burden and limited drug availability. For instance, in Indonesia, the availability of DFO is limited, requiring patients to bear the financial burden independently. Additionally, the local versions of DFX and DFP are available in Indonesia; however, they are not as efficacious as the original versions. The urine discoloration is less pronounced compared to the original, but arthralgia is a predominant adverse effect, which may result in decreased patient compliance.
Rather than just iron overload, this case presents the patient with various comorbidities, such as reduced cardiac function and metabolic disorders. The patient has a history of cardiac resuscitation and admission to ICU with electrolyte imbalance. Fortunately, extensive monitoring and appropriate management were implemented, which focused on chelating the iron and managing the cardiac and metabolic changes in this patient. As highlighted by Woods et al. [18], managing cardiovascular health in thalassemia patients with HFpEF is a challenge as the molecular basis is still unknown. One of the known factors that may cause further impairment is insulin resistance, as it triggers metabolic changes in heart utilization of fat and glucose, which further impair vascular reactivity. Thus, the target, according to current guidelines for HFpEF, is to continue to control patients’ hypertension with angiotensin 2 blocker (ARB) and consider administering sodium–glucose cotransporter 2 (SGLT-2). Meanwhile, antifibrotic agents are still under trial, and the patient needs to be continuously monitored by a trained hematologist [18].
3.3. Non-Pharmacological Management for TDT Patients with Poor Cardiac Function
Other than appropriate iron chelation and medications needed to manage comorbidities in thalassemia patients, non-pharmacological treatments should also be focused on. In this case, although the patient has increased ferritin level and reduced heart function this past year, clinically he has a competent cardiovascular function. This might be attributed to his lifestyle, in which the patient routinely performs high-intensity interval training, such as running and marathons, but did not fully control his diet. For thalassemia patients, managing iron levels effectively involves following a diet low in iron-rich foods. A previous study showed that every 1 mg of heme iron intake increased ferritin levels by 1.334 mmol/L in men and 1.249 mmol/L in women. Likewise, intake of non-heme iron also increased ferritin levels in both men and women, albeit to a slightly lesser extent, with increases of 0.988 mmol/L and 0.972 mmol/L, respectively [19]. In this case, the patient’s diet included popular or fast foods without specific dietary restrictions regarding iron content. Hence, to mitigate a higher intake of iron absorption through diets, incorporating calcium-rich foods can be beneficial, as calcium competes with iron for absorption in the intestines. Calcium consumption can also be integrated along with vitamin D, as it complements calcium absorption, and both are essential for maintaining bone health. Studies showed that vitamin D-deficient thalassemia patients had higher serum ferritin levels [20,21]. Serum vitamin D levels (25-OHD) have also been positively correlated with measures of cardiac output, such as LVEF, indicating that higher vitamin D levels may support better cardiac function [19]. Conversely, NTproBNP, a marker of cardiac stress, is negatively correlated with 25-OHD, suggesting that lower vitamin D levels may be associated with increased cardiac stress [21]. Dietary management appears to contribute to the reduction in ferritin levels and improvement in ejection fraction in the patient following the adoption of a structured, balanced, diet low in iron, sugar, and fat.
In patients requiring life-long transfusion therapy, ensuring the safety and compatibility of each transfusion is crucial. Implementing proper hemovigilance system is key to deliver safe and effective transfusion for thalassemia patients. Blood donors should be carefully selected and tested to omit any possibilities of transmitting hematogenic diseases. The Thalassaemia International Federation recommends specific screening for hepatitis B, hepatitis C, HIV, syphilis, and, in several countries, other infectious diseases (HTLV I/II, malaria, toxoplasma, hepatitis A, hepatitis E, west nile virus, and chagas disease) [1]. Leukocyte reduction to 1 × 106/L or less leukocytes per unit is recommended to eliminate the risk of adverse reactions, with pre-storage filtration as the preferred method [1]. This method offers high filtration efficiency and provides consistent low residual of leukocytes compared to other methods such as pre-transfusion or bedside filtration. However, in countries with limited resources settings, pre-storage filtration may be difficult to access; therefore, bedside filtration is more often used. This method may not allow optimal quality control as the techniques are highly variable. The current recommended practice for blood storage is to use additive solutions for blood with shelf life of less than 14 days. Before proceeding to transfusion, compatibility testing needs to be performed to avoid alloimmunization against ABO and Rh antigens. The result of antigen typing and any reaction of previous transfusion should be completely recorded for each patient. Before transfusion, it is recommended for the patient to maintain pre-transfusion Hb level at around 9.5–10.5 g/dL. However, in cases of patients with heart disease or clinically significant extramedullary hematopoiesis, it is recommended to have a higher pre-transfusion Hb level of 11.0–12.0 g/dL [1].
Beyond adhering to iron chelation therapy and a controlled diet, maintaining physical health, such as participating in regular exercise, is pivotal to their care. Despite having multiple comorbidities, the patient engages in regular physical exercise, which helps maintain his cardiovascular function. A study on the effects of physical activity on thalassemia patients shows that patients who feel higher pain severity lead a more sedentary lifestyle, which creates a cycle that exacerbates the issue and may lead to osteoporosis [22]. This chronic pain is multifactorial and may be caused by their low hemoglobin level, iron overload, or low bone mass, and is said to be associated with a greater spine BMD Z-score. However, if patients were assigned an appropriate exercise that did not burden their bodies, such as water exercise three times a week, it showed a potentially beneficial effect and increased their quality of life [12].
A crucial aspect of patient management, particularly in individuals with chronic conditions such as thalassemia, is addressing psychosocial challenges. In this case, the patient required psychiatric evaluation and was diagnosed with severe depression. Mental health disorders, including depression and anxiety, are prevalent among individuals with thalassemia, as evidenced by a study conducted in Indonesia that reported a high incidence of such conditions in this population [23]. Several factors, including physical changes, growth delays, yellowish-bronze skin pigmentation, splenomegaly, and distinct craniofacial features, may contribute to feelings of social isolation, reduced self-esteem, and decreased motivation for behavioral change. Additionally, a study by Dimitroglou et al. demonstrated that patients with thalassemia who experience greater severity of cardiac failure tend to have a lower quality of life, particularly in aspects related to social functioning, vitality, and physical health [24]. In this case, the patient had a history of cardiac arrest and multiple comorbidities, necessitating extensive daily pharmacological management. Consequently, the development of severe depression and anxiety was not unexpected. However, psychiatric intervention significantly improved the patient’s psychological well-being, enhancing self-confidence and giving him a sense of purpose even with all the limitations he has. The patient resumed activities he previously enjoyed, such as running, participating in marathons, and advocating for thalassemia awareness through social media. This case highlights the importance of comprehensive patient monitoring and a patient-centered approach, which not only improved physical health but also contributed to significant mental health benefits.
4. Conclusions
Iron overload in β-Thalassemia leads to a range of complications, with cardiac complications being associated with the highest mortality rate. Patients with TDT who exhibit severe cardiac MRI T2* values generally have a poor prognosis, particularly when compounded by additional comorbidities such as pre-existing cardiac abnormalities and glucose dysregulation. Furthermore, many thalassemia patients have a more compromised EF, contributing to a more sedentary lifestyle and mental health problems. However, in this case, despite the presence of diabetes and a history of cardiac arrest, the patient demonstrated exceptional physical endurance. This outcome may be attributable to comprehensive monitoring, multidisciplinary collaboration (including cardiology, endocrinology, hematology, and psychiatry), and lifestyle interventions. An important aspect that should be focused on in each thalassemia major patient is their psychological aspects, as it will affect their motivation and compliance with their medications and their quality of life. Consequently, this case suggests that a combination of personalized medical interventions (such as tailored iron chelation therapy, heart function management, and glucose control), regular monitoring, dietary management, an active lifestyle, and psychological support in thalassemia patients can improve their overall health and sustain cardiovascular function, even in the presence of additional challenges. Further studies are warranted to determine the optimal exercise regimens and the use of dual iron chelation therapy (DFP + DFX) in TDT patients with compromised cardiac function to better understand their prognosis and enhance their quality of life.
Author Contributions
Conceptualization, N.B.S. and N.Y.S.; methodology N.B.S. and P.A.W.; resources, K.R.U.; writing—original draft preparation, N.B.S., K.R.U., A.F. and N.Y.S.; writing—review and editing, P.A.W., A.F., S.D.I., N.B.S. and N.Y.S.; supervision P.A.W.; project administration, N.B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study because it involved a retrospective analysis of de-identified data, ensuring no direct contact with participants and no risk to individual privacy or well-being.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
We would like to thank Marni S for the administrative support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cappellini, M.D.; Farmakis, D.; Porter, J.; Taher, A. 2021 Thalassemia International Federation Guidelines for the Management of Transfusion-dependent Thalassemia. Hemasphere 2022, 6, e372. [Google Scholar]
- Sadiq, I.Z.; Abubakar, F.S.; Usman, H.S.; Abdullahi, A.D.; Ibrahim, B.; Kastayal, B.S.; Ibrahim, M.; Hassan, H.A. Thalassemia: Pathophysiology, Diagnosis, and Advances in Treatment. Thalass. Rep. 2024, 14, 81–102. [Google Scholar] [CrossRef]
- Coates, T.D. Physiology and pathophysiology of iron in hemoglobin-associated diseases. Free. Radic. Biol. Med. 2014, 72, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Taher, A.T.; Saliba, A.N. Iron overload in thalassemia: Different organs at different rates. Hematol. Am. Soc. Hematol. Educ. Program 2017, 2017, 265–271. [Google Scholar] [CrossRef]
- Ansharullah, B.A.; Sutanto, H.; Romadhon, P.Z. Thalassemia and Iron Overload Cardiomyopathy: Pathophysiological Insights, Clinical Implications, and Management Strategies. Curr. Probl. Cardiol. 2024, 50, 102911. [Google Scholar] [CrossRef]
- Chaosuwannakit, N.; Makarawate, P.; Wanitpongpun, C. The importance of cardiac T2* magnetic resonance imaging for monitoring cardiac siderosis in thalassemia major patients. Tomography 2021, 7, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Westwood, M.A.; Anderson, L.J.; Maceira, A.M.; Shah, F.T.; Prescott, E.; Porter, J.B.; Wonke, B.; Walker, J.M.; Pennell, D.J. Normalized left ventricular volumes and function in thalassemia major patients with normal myocardial iron. J. Magn. Reason. Imaging 2007, 25, 1147–1151. [Google Scholar] [CrossRef]
- Kirk, P.; Roughton, M.; Porter, J.B.; Walker, J.M.; Tanner, M.A.; Pennell, D.J.; Patel, J.; Wu, D.; Taylor, J.; Westwood, M.A.; et al. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation 2009, 120, 1961–1968. [Google Scholar] [CrossRef]
- Pepe, A.; Meloni, A.; Rossi, G.; Midiri, M.; Missere, M.; Valeri, G.; Cuccia, L.; Sorrentino, F.; Giuseppe D’Ascola, D.; Spasiano, A.; et al. Prediction of cardiac complications for thalassemia major in the widespread cardiac magnetic resonance era: A prospective multicentre study by a multi-parametric approach. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 299–309. [Google Scholar] [CrossRef]
- Piatti, G.; Giuditta, M.; Consonni, D.; Cassinerio, E.; Cappellini, M.D. Cardiopulmonary testing in adult patients with β-thalassemia major in comparison to healthy subjects. Ann. Hematol. 2022, 101, 2445–2452. [Google Scholar] [CrossRef]
- Wagner, J.; Knaier, R.; Infanger, D.; Königstein, K.; Klenk, C.; Carrard, J.; Hanssen, H.; Hinrichs, T.; Seals, D.; Schmidt-Trucksäss, A. Novel CPET Reference Values in Healthy Adults: Associations with Physical Activity. Med. Sci. Sports Exerc. 2021, 53, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Hasanpour, D.A.; Hasani, T.; Fekri, K.; Deris, F.; Etemadifar, S. Effects of Aquatic Exercise on Dimensions of Quality of Life and Blood Indicators in Patients with Beta-Thalassemia Major. Int. J. Prev. Med. 2020, 11, 128. [Google Scholar]
- Kremastinos, D.T.; Farmakis, D.; Aessopos, A.; Hahalis, G.; Hamodraka, E.; Tsiapras, D.; Keren, A. β-thalassemia cardiomyopathy: History, present considerations, and future perspectives. Circ. Heart Fail. 2010, 3, 451–458. [Google Scholar] [CrossRef]
- Berdoukas, V.; Chouliaras, G.; Moraitis, P.; Zannikos, K.; Berdoussi, E.; Ladis, V. The efficacy of iron chelator regimes in reducing cardiac and hepatic iron in patients with thalassaemia major: A clinical observational study. J. Cardiovasc. Magn. Reson. 2009, 11, 20. [Google Scholar] [CrossRef]
- Aydinok, Y. Combination chelation therapy. Ann. N. Y. Acad Sci. 2023, 1529, 33–41. [Google Scholar] [CrossRef]
- Evans, P.; Kayyali, R.; Hider, R.C.; Eccleston, J.; Porter, J.B. Mechanisms for the shuttling of plasma non-transferrin-bound iron (NTBI) onto deferoxamine by deferiprone. Transl. Res. 2010, 156, 55–67. [Google Scholar] [CrossRef]
- Chuang, T.Y.; Li, J.P.; Weng, T.F.; Wu, K.H.; Chao, Y.H. Combined chelation with high-dose deferiprone and deferoxamine to improve survival and restore cardiac function effectively in patients with transfusion-dependent thalassemia presenting severe cardiac complications. Ann. Hematol. 2020, 99, 2289–2294. [Google Scholar] [CrossRef]
- Wood, J.C. Cardiac complications in thalassemia throughout the lifespan: Victories and challenges. Ann. N. Y. Acad. Sci. 2023, 1530, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Timmer, T.C.; de Groot, R.; Rijnhart, J.J.M.; Lakerveld, J.; Brug, J.; Perenboom, C.W.M.; Baart, A.M.; Prinsze, F.J.; Zalpuri, S.; van der Schoot, C.E.; et al. Dietary intake of heme iron is associated with ferritin and hemoglobin levels in Dutch blood donors: Results from Donor InSight. Haematologica 2020, 105, 2400–2406. [Google Scholar] [CrossRef]
- Bajoria, R.; Rekhi, E.; Almusawy, M.; Chatterjee, R. Hepatic hemosiderosis contributes to abnormal vitamin D-PTH axis in thalassemia major. J. Pediatr. Hematol. Oncol. 2019, 41, e83–e89. [Google Scholar] [CrossRef]
- Napoli, N.; Carmina, E.; Bucchieri, S.; Sferrazza, C.; Rini, G.B.; Di Fede, G. Low serum levels of 25-hydroxy vitamin D in adults affected by thalassemia major or intermedia. Bone 2006, 38, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Fung, E.B.; Goldberg, E.K.; Bambot, S.; Manzo, R.; Lal, A. Relationships among Physical Activity, Pain, and Bone Health in Youth and Adults with Thalassemia: An Observational Study. Thalass. Rep. 2022, 12, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Sari, T.T.; Rahmartani, L.D.; Wirahmadi, A.; Selene, N.B.; Iskandar, S.D.; Wahidiyat, P.A. Psychological Burden among Pediatric Thalassemia Major Patients in Indonesia: A Review. Thalass. Rep. 2024, 14, 33–43. [Google Scholar] [CrossRef]
- Dimitroglou, Y.; Anagnostopoulos, F.; Aggeli, C.; Delicou, S.; Xydaki, A.; Patsourakos, D.; Tousoulis, D. Severity of heart failure and health-related quality of life in beta-thalassemia patients: A cross-sectional study. Ann. Haematol. 2020, 99, 2037–2046. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).