Amlodipine Therapy in β-Thalassemia Patients: A Systematic Review and Meta-Analysis on Ferritin Levels and Liver MRI T2*
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Systematic Search
2.3. Search Strategy
2.4. Selection Process
2.5. Data Collection Process
2.6. Risk of Bias Assessment
2.7. Level of Evidence Assessment
2.8. Strategy for Data Synthesis
3. Results
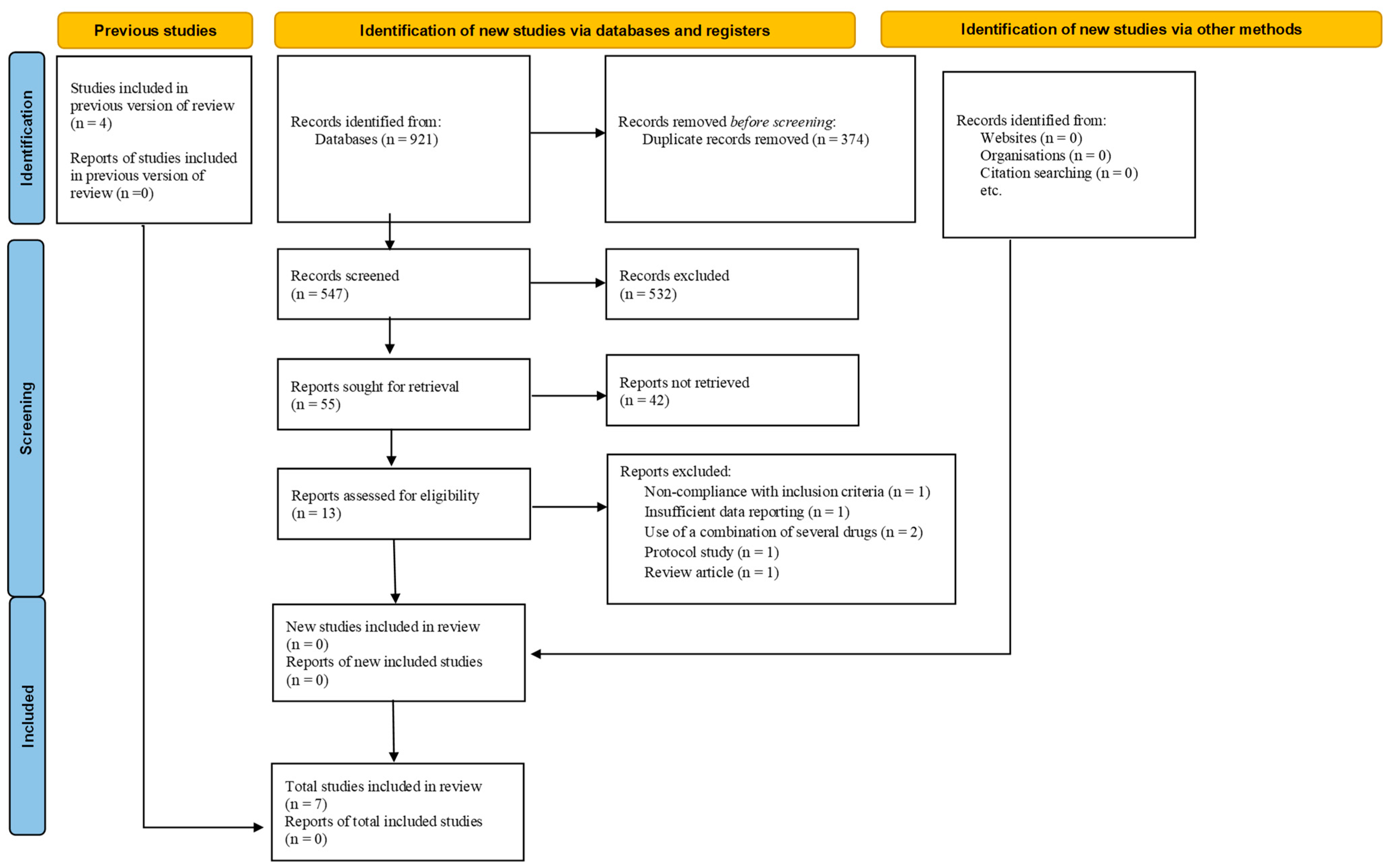
3.1. Search Findings
3.2. Studies Characteristics
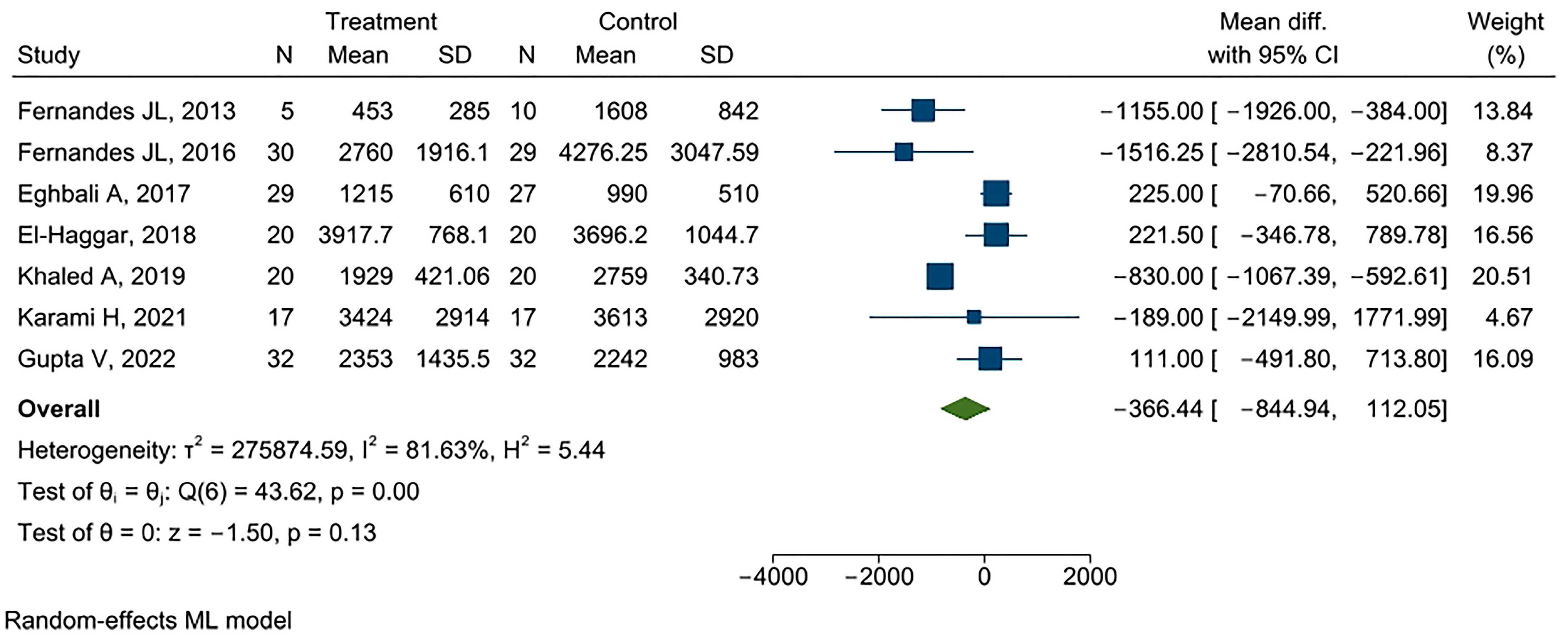
3.3. Outcomes
Serum Ferritin
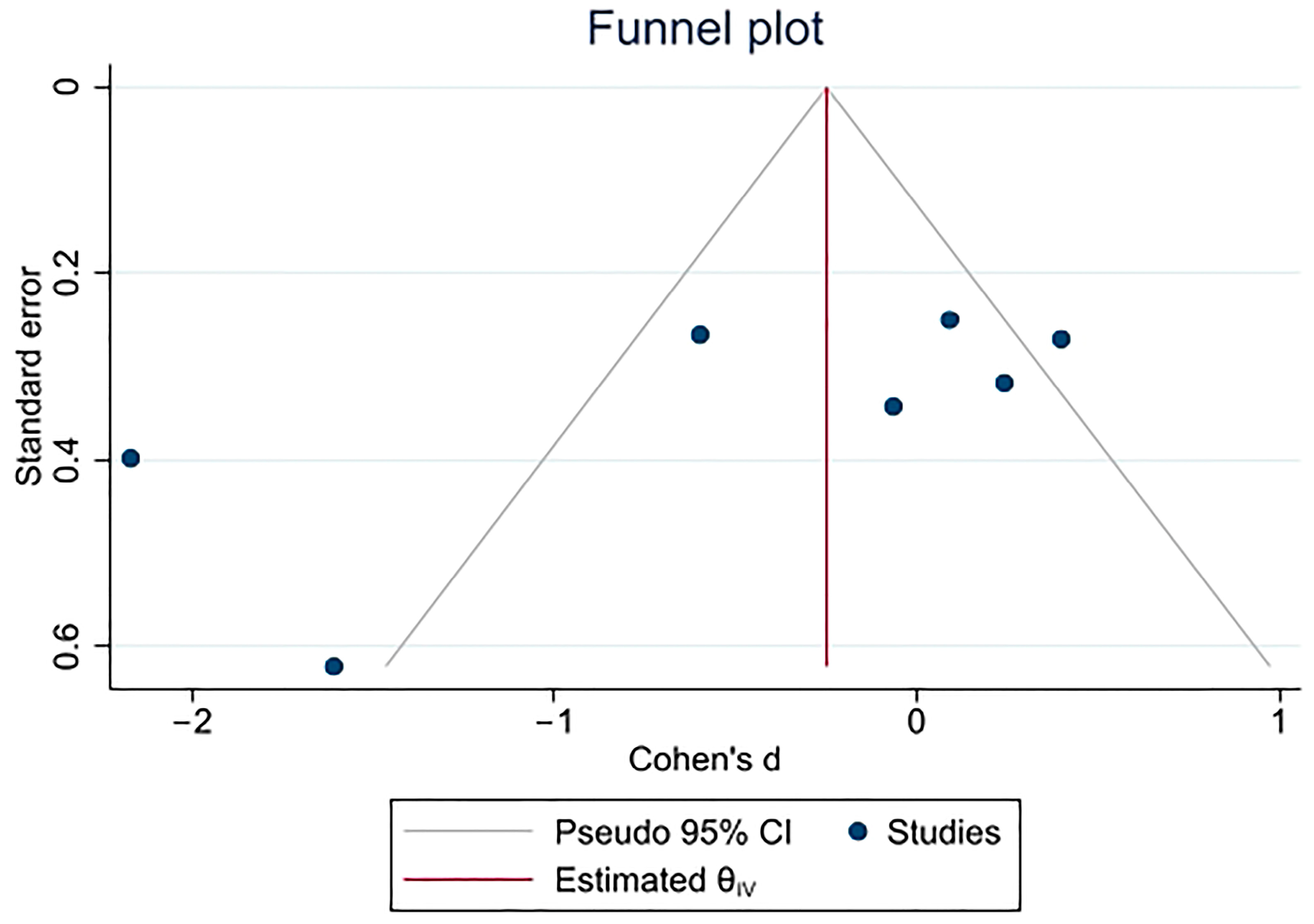
3.4. Quality and Sensitive Analyses
3.5. Liver MRI T2*
3.6. Risk of Bias Assessment
3.7. Level of Evidence Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rund, D.; Rachmilewitz, E. β-Thalassemia. N. Engl. J. Med. 2005, 353, 1135–1146. [Google Scholar] [CrossRef]
- De Sanctis, V.; Kattamis, C.; Canatan, D.; Soliman, A.T.; Elsedfy, H.; Karimi, M.; Daar, S.; Wali, Y.; Yassin, M.; Soliman, N.; et al. β-thalassemia distribution in the old world: An ancient disease seen from a historical standpoint. Mediterr. J. Hematol. Infect. Dis. 2017, 9, e2017018. [Google Scholar] [CrossRef]
- Sadaf, A.; Hasan, B.; Das, J.K.; Colan, S.; Alvi, N. Calcium channel blockers for preventing cardiomyopathy due to iron overload in people with transfusion-dependent beta thalassaemia. Cochrane Database Syst. Rev. 2018, 7, CD011626. [Google Scholar] [CrossRef]
- El-Haggar, S.M.; El-Shanshory, M.R.; El-shafey, R.A.; Dabour, M.S. Decreasing cardiac iron overload with Amlodipine and Spirulina in children with β-thalassemia. Pediatr. Hematol. Oncol. J. 2018, 3, 64–69. [Google Scholar] [CrossRef]
- Khaled, A.; Salem, H.A.; Ezzat, D.A.; Seif, H.M.; Rabee, H. A randomized controlled trial evaluating the effects of amlodipine on myocardial iron deposition in pediatric patients with thalassemia major. Drug Des. Dev. Ther. 2019, 13, 2427–2436. [Google Scholar] [CrossRef]
- Borgna-Pignatti, C.; Rugolotto, S.; De Stefano, P.; Zhao, H.; Cappellini, M.D.; Del Vecchio, G.C.; Romeo, M.A.; Forni, G.L.; Gamberini, M.R.; Ghilardi, R.; et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica 2004, 89, 1187–1193. [Google Scholar] [PubMed]
- Eghbali, A.; Kazemi, H.; Taherahmadi, H.; Ghandi, Y.; Rafiei, M.; Bagheri, B. A randomized, controlled study evaluating effects of amlodipine addition to chelators to reduce iron loading in patients with thalassemia major. Eur. J. Haematol. 2017, 99, 577–581. [Google Scholar] [CrossRef]
- Aydinok, Y.; Porter, J.B.; Piga, A.; Elalfy, M.; El-Beshlawy, A.; Kilinç, Y.; Viprakasit, V.; Yesilipek, A.; Habr, D.; Quebe-Fehling, E.; et al. Prevalence and distribution of iron overload in patients with transfusion-dependent anemias differs across geographic regions: Results from the CORDELIA study. Eur. J. Haematol. 2015, 95, 244–253. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. Introduction of higher doses of deferasirox: Better efficacy but not effective iron removal from the heart and increased risks of serious toxicities. Expert Opin. Drug Saf. 2010, 9, 633–641. [Google Scholar] [CrossRef]
- Pennell, D.J.; Berdoukas, V.; Karagiorga, M.; Ladis, V.; Piga, A.; Aessopos, A.; Gotsis, E.D.; Tanner, M.A.; Smith, G.C.; Westwood, M.A.; et al. Randomized controlled trial of deferiprone or deferoxamine in beta-thalassemia major patients with asymptomatic myocardial siderosis. Blood 2006, 107, 3738–3744. [Google Scholar] [CrossRef]
- Viprakasit, V.; Gattermann, N.; Lee, J.W.; Porter, J.B.; Taher, A.T.; Habr, D.; Martin, N.; Domokos, G.; Cappellini, M.D. Geographical variations in current clinical practice on transfusions and iron chelation therapy across various transfusion-dependent anaemias. Blood Transfus. 2013, 11, 108. [Google Scholar] [PubMed]
- Fernandes, J.L.; Sampaio, E.F.; Fertrin, K.; Coelho, O.R.; Loggetto, S.; Piga, A.; Verissimo, M.; Saad, S.T. Amlodipine reduces cardiac iron overload in patients with thalassemia major: A pilot trial. Am. J. Med. 2013, 126, 834–837. [Google Scholar] [CrossRef]
- Oudit, G.Y.; Trivieri, M.G.; Khaper, N.; Liu, P.P.; Backx, P.H. Role of L-type Ca2+ channels in iron transport and iron-overload cardiomyopathy. J. Mol. Med. 2006, 84, 349–364. [Google Scholar] [CrossRef]
- Oudit, G.Y.; Sun, H.; Trivieri, M.G.; Koch, S.E.; Dawood, F.; Ackerley, C.; Yazdanpanah, M.; Wilson, G.J.; Schwartz, A.; Liu, P.P.; et al. L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat. Med. 2003, 9, 1187–1194. [Google Scholar] [CrossRef]
- Kumfu, S.; Chattipakorn, S.; Chinda, K.; Fucharoen, S.; Chattipakorn, N. T-type calcium channel blockade improves survival and cardiovascular function in thalassemic mice. Eur. J. Haematol. 2012, 88, 535–548. [Google Scholar] [CrossRef]
- Ludwiczek, S.; Theurl, I.; Muckenthaler, M.U.; Jakab, M.; Mair, S.M.; Theurl, M.; Kiss, J.; Paulmichl, M.; Hentze, M.W.; Ritter, M.; et al. Ca2+ channel blockers reverse iron overload by a new mechanism via divalent metal transporter-1. Nat. Med. 2007, 13, 448–454. [Google Scholar] [CrossRef]
- Karami, H.; Khalilzadeh Arjmandi, H.; Salehifar, E.; Darvishi Khezri, H.; Dabirian, M.; Kosaryan, M.; Aliasgharian, A.; Akbarzadeh, R.; Aali, R.N.; Nasirzadeh, A.; et al. A Double-Blind, Controlled, Crossover Trial of Amlodipine on Iron Overload Status in Transfusion Dependent β-Thalassemia Patients. Int. J. Clin. Pract. 2021, 75, e14337. [Google Scholar] [CrossRef]
- Fernandes, J.L.; Loggetto, S.R.; Veríssimo, M.P.; Fertrin, K.Y.; Baldanzi, G.R.; Fioravante, L.A.; Tan, D.M.; Higa, T.; Mashima, D.A.; Piga, A.; et al. A randomized trial of amlodipine in addition to standard chelation therapy in patients with thalassemia major. Blood J. Am. Soc. Hematol. 2016, 128, 1555–1561. [Google Scholar] [CrossRef]
- Elfaituri, M.K.; Ghozy, S.; Ebied, A.; Morra, M.E.; Hassan, O.G.; Alhusseiny, A.; Abbas, A.S.; Sherif, N.A.; Fernandes, J.L.; Huy, N.T. Amlodipine as adjuvant therapy to current chelating agents for reducing iron overload in thalassaemia major: A systematic review, meta-analysis and simulation of future studies. Vox Sang. 2021, 116, 887–897. [Google Scholar] [CrossRef]
- Soliman, Y.; Abdelaziz, A.; Mouffokes, A.; Amer, B.E.; Goudy, Y.M.; Abdelwahab, O.A.; Badawy, M.M.; Diab, R.A.; Elsharkawy, A. Efficacy and safety of calcium channel blockers in preventing cardiac siderosis in thalassemia patients: An updated meta-analysis with trial sequential analysis. Eur. J. Haematol. 2023, 110, 414–425. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Chen, M.-P.; Cabantchik, Z.I.; Chan, S.; Chan GC-f Cheung, Y.-F. Iron overload and apoptosis of HL-1 cardiomyocytes: Effects of calcium channel blockade. PLoS ONE 2014, 9, e112915. [Google Scholar] [CrossRef]
- Chowdhury, D.; Alvi, N.; Tomredle, R.; Bijnens, B.; Hasan, B. Improvements in Regional Myocardial Function on 2D STE With Optimized Chelation in Patients With Thalassemia Major (TM). Circulation 2017, 136 (Suppl. S1), A21067-A. [Google Scholar] [CrossRef]
- Basavaiah, K.; Chandrashekar, U.; Prameela, H. Sensitive spectrophotometric determination of amlodipine and felodipine using iron (III) and ferricyanide. Il Farmaco 2003, 58, 141–148. [Google Scholar] [CrossRef]
- Motta, I.; Scaramellini, N.; Cappellini, M.D. Investigational drugs in phase I and phase II clinical trials for thalassemia. Expert Opin. Investig. Drugs 2017, 26, 793–802. [Google Scholar] [CrossRef]
- Shakoor, A.; Zahoor, M.; Sadaf, A.; Alvi, N.; Fadoo, Z.; Rizvi, A.; Quadri, F.; Tipoo, F.A.; Khurshid, M.; Sajjad, Z.; et al. Effect of L-type calcium channel blocker (amlodipine) on myocardial iron deposition in patients with thalassaemia with moderate-to-severe myocardial iron deposition: Protocol for a randomised, controlled trial. BMJ Open 2014, 4, e005360. [Google Scholar] [CrossRef]
- Manglani, M.V.; Kini, P.S. Management of ß-thalassemia–Consensus and controversies! Pediatr. Hematol. Oncol. J. 2017, 2, 94–97. [Google Scholar] [CrossRef]
- Gupta, V.; Kumar, I.; Raj, V.; Aggarwal, P.; Agrawal, V. Comparison of the effects of calcium channel blockers plus iron chelation therapy versus chelation therapy only on iron overload in children and young adults with transfusion-dependent thalassemia: A randomized double-blind placebo-controlled trial. Pediatr. Blood Cancer 2022, 69, e29564. [Google Scholar] [CrossRef]
- Alali, M.A.; Alanazi, K.M.; Alsayil, S.N.; Omari, Z.; Shaaban, A. Calcium Channel Blockers in Conjunction with Standard Iron-Chelating Agents for β-Thalassemia Major: Systematic Literature Search. Hemoglobin 2020, 44, 446–450. [Google Scholar] [CrossRef]
- Taher, A.T.; Saliba, A.N. Iron overload in thalassemia: Different organs at different rates. Hematology 2017, 2017, 265–271. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Chang, Y.; Zhang, Y.; Chu, X.; Zhang, X.; Liu, Z.; Guo, H.; Wang, N.; Gao, Y.; et al. Calcium channel blockers ameliorate iron overload-associated hepatic fibrosis by altering iron transport and stellate cell apoptosis. Toxicol. Appl. Pharmacol. 2016, 301, 50–60. [Google Scholar] [CrossRef]
- Bataller, R.; Gasull, X.; Ginès, P.; Hellemans, K.; Görbig, M.N.; Nicolás, J.M.; Sancho-Bru, P.; Heras, D.D.L.; Gual, A.; Geerts, A.; et al. In vitro and in vivo activation of rat hepatic stellate cells results in de novo expression of L-type voltage-operated calcium channels. Hepatology 2001, 33, 956–962. [Google Scholar] [CrossRef]
- Byler, R.M.; Sherman, N.A.; Wallner, J.S.; Horwitz, L.D. Hydrogen peroxide cytotoxicity in cultured cardiac myocytes is iron dependent. Am. J. Physiol. Heart Circ. Physiol. 1994, 266, H121–H127. [Google Scholar] [CrossRef]
- Winegar, B.D.; Kelly, R.; Lansman, J.B. Block of current through single calcium channels by Fe, Co, and Ni. Location of the transition metal binding site in the pore. J. Gen. Physiol. 1991, 97, 351–367. [Google Scholar] [CrossRef]
- Tsushima, R.G.; Wickenden, A.D.; Bouchard, R.A.; Oudit, G.Y.; Liu, P.P.; Backx, P.H. Modulation of iron uptake in heart by L-type Ca2+ channel modifiers: Possible implications in iron overload. Circ. Res. 1999, 84, 1302–1309. [Google Scholar] [CrossRef]
- Hershko, C.; Link, G.; Cabantchik, I. Pathophysiology of Iron Overload a. Ann. N. Y. Acad. Sci. 1998, 850, 191–201. [Google Scholar] [CrossRef]
- Sugawara, H.; Tobise, K.; Kikuchi, K. Antioxidant effects of calcium antagonists on rat myocardial membrane lipid peroxidation. Hypertens. Res. 1996, 19, 223–228. [Google Scholar] [CrossRef]
- Sevanian, A.; Shen, L.; Ursini, F. Inhibition of LDL oxidation and oxidized LDL-induced cytotoxicity by dihydropyridine calcium antagonists. Pharm. Res. 2000, 17, 999–1006. [Google Scholar] [CrossRef]
- Savigni, D.L.; Morgan, E.H. Mediation of iron uptake and release in erythroid cells by photodegradation products of nifedipine. Biochem. Pharmacol. 1996, 51, 1701–1709. [Google Scholar] [CrossRef]
- Fares, H.; DiNicolantonio, J.J.; O’Keefe, J.H.; Lavie, C.J. Amlodipine in hypertension: A first-line agent with efficacy for improving blood pressure and patient outcomes. Open Heart 2016, 3, e000473. [Google Scholar] [CrossRef]
- Abernethy, D.R.; Schwartz, J.B. Calcium-antagonist drugs. N. Engl. J. Med. 1999, 341, 1447–1457. [Google Scholar] [CrossRef]
- Dougall, H.T.; McLay, J. A comparative review of the adverse effects of calcium antagonists. Drug Safety 1996, 15, 91–106. [Google Scholar] [CrossRef]
- Masumoto, K.; Takeyasu, A.; Oizumi, K.; Kobayashi, T. Studies of novel 1, 4-dihydropyridine Ca antagonist CS-905. I. Measurement of partition coefficient (log P) by high performance liquid chromatography (HPLC). Yakugaku Zasshi J. Pharm. Soc. Jpn. 1995, 115, 213–220. [Google Scholar] [CrossRef]
- Darvishi-Khezri, H.; Khalilzadeh Arjmandi, H.; Aliasgharian, A.; Shaki, F.; Zahedi, M.; Kosaryan, M.; Karami, H.; Aali, R.N.; Salehifar, E. Amlodipine: Can act as an antioxidant in patients with transfusion-dependent β-thalassemia? A double-blind, controlled, crossover trial. J. Clin. Lab. Anal. 2022, 36, e24752. [Google Scholar] [CrossRef]
- Ackerman, Z.; Oron-Herman, M.; Rosenthal, T.; Pappo, O.; Link, G.; Sela, B.-A.; Grozovski, M. Effects of amlodipine, captopril, and bezafibrate on oxidative milieu in rats with fatty liver. Dig. Dis. Sci. 2008, 53, 777–784. [Google Scholar] [CrossRef]
| Author | Year | Iron Chelator | Red-Cell-Transfusion Dependency | Measuring Tools | Amlodipine Dosage | Duration, Month |
|---|---|---|---|---|---|---|
| Fernandes J.L. [12] | 2013 | Deferoxamine/deferasirox/deferiprone | Yes | MRI T2* | 5 mg/d | 12 |
| Shakoor A. [28] | 2014 | Deferoxamine/deferasirox | Yes | MRI T2* | <2.5 mg/day | 12 |
| Fernandes J.L. [18] | 2016 | Deferoxamine/deferasirox/deferiprone | Yes | MRI T2* | 2.5–5 mg/day | 12 |
| Eghbali A. [7] | 2017 | Deferoxamine/deferasirox/deferiprone | Yes | MRI T2* | 2.5–5 mg/day | 12 |
| El-Haggar [4] | 2018 | Spirulina/deferoxamine/deferasirox | No | MRI T2* | 5 mg/day | 3 |
| Khaled A. [5] | 2019 | deferasirox | Yes | MRI T2* | 2.5–5 mg/day | 6 |
| Karami H. [17] | 2021 | Deferoxamine/deferiprone | Yes | MRI T2* | 5 mg/day | 12 |
| Gupta V. [30] | 2022 | Deferasirox/deferasirox/deferiprone | Yes | MRI T2* | 2.5–5 mg/day | 12 |
| Study | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | Other Bias | Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Fernandes et al. (2013) [12] | Unclear | Low risk | High risk | Low risk | Low risk | Low risk | Low risk | High |
| Fernandes et al. (2016) [18] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low |
| Eghbali et al. (2017) [7] | Low risk | Low risk | Unclear | Unclear | Low risk | Low risk | Low risk | Moderate |
| El-Haggar et al. (2018) [4] | Low risk | Low risk | Unclear | Unclear | Low risk | Low risk | Low risk | Moderate |
| Khaled et al. (2019) [5] | Low risk | Low risk | Unclear | Unclear | Low risk | Low risk | Low risk | Moderate |
| Karami et al. (2021) [17] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low |
| Gupta V et al. (2022) [30] | Low risk | Low risk | Low risk | Unclear | Low risk | Low risk | Low risk | Low |
| Outcome | Quality Assessment | Number of Participants | Cohen’s d, 95% CI 6 | Quality of Evidence 7 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Risk of Bias 1 | Inconsistency 2 | Indirectness 3 | Imprecision 4 | Publication Bias 5 | Amlodipine | Comparator | |||
| Ferritin | serious, −1 | serious, −1 | not serious, 0 | serious, −1 | serious, −1 | 113 | 114 | −0.46, −1.11 to 0.19, 0 | Very low, −4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliasgharian, A.; Karami, H.; Zahedi, M.; Jahanshahi, R.; Bakhtiari-Dovvombaygi, H.; Nasirzadeh, A.; Naderisorki, M.; Kosaryan, M.; Salehifar, E.; Ghazaiean, M.; et al. Amlodipine Therapy in β-Thalassemia Patients: A Systematic Review and Meta-Analysis on Ferritin Levels and Liver MRI T2*. Thalass. Rep. 2023, 13, 241-252. https://doi.org/10.3390/thalassrep13040021
Aliasgharian A, Karami H, Zahedi M, Jahanshahi R, Bakhtiari-Dovvombaygi H, Nasirzadeh A, Naderisorki M, Kosaryan M, Salehifar E, Ghazaiean M, et al. Amlodipine Therapy in β-Thalassemia Patients: A Systematic Review and Meta-Analysis on Ferritin Levels and Liver MRI T2*. Thalassemia Reports. 2023; 13(4):241-252. https://doi.org/10.3390/thalassrep13040021
Chicago/Turabian StyleAliasgharian, Aily, Hossein Karami, Mohammad Zahedi, Reza Jahanshahi, Hossein Bakhtiari-Dovvombaygi, Amirreza Nasirzadeh, Mohammad Naderisorki, Mehrnoush Kosaryan, Ebrahim Salehifar, Mobin Ghazaiean, and et al. 2023. "Amlodipine Therapy in β-Thalassemia Patients: A Systematic Review and Meta-Analysis on Ferritin Levels and Liver MRI T2*" Thalassemia Reports 13, no. 4: 241-252. https://doi.org/10.3390/thalassrep13040021
APA StyleAliasgharian, A., Karami, H., Zahedi, M., Jahanshahi, R., Bakhtiari-Dovvombaygi, H., Nasirzadeh, A., Naderisorki, M., Kosaryan, M., Salehifar, E., Ghazaiean, M., Bitaraf, S., & Darvishi-Khezri, H. (2023). Amlodipine Therapy in β-Thalassemia Patients: A Systematic Review and Meta-Analysis on Ferritin Levels and Liver MRI T2*. Thalassemia Reports, 13(4), 241-252. https://doi.org/10.3390/thalassrep13040021