Molecular Epidemiology of HCV Infection among Multi-Transfused β-Thalassemia Patients in Eastern India: A Six-Year Observation
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Study Design
2.3. Detection of HCV RNA and Genotyping
2.4. Phylogenetic Analysis
2.5. Phylogeographic Analysis
2.6. Statistical Analysis
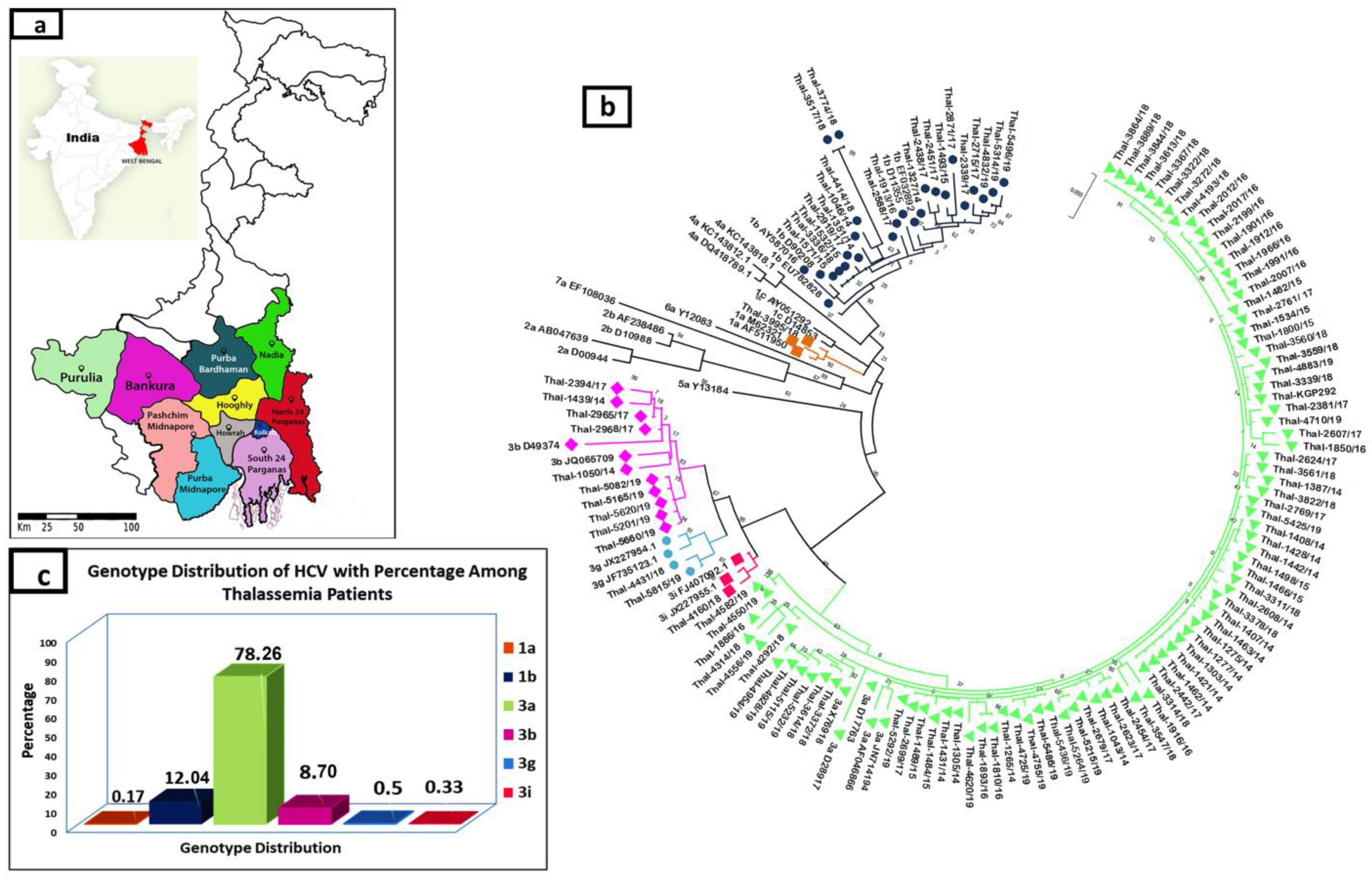
3. Results
3.1. Demographic Data and Distribution of HCV Viremia
3.2. Distribution of HCV Genotype
3.3. HCV Spontaneous Clearance
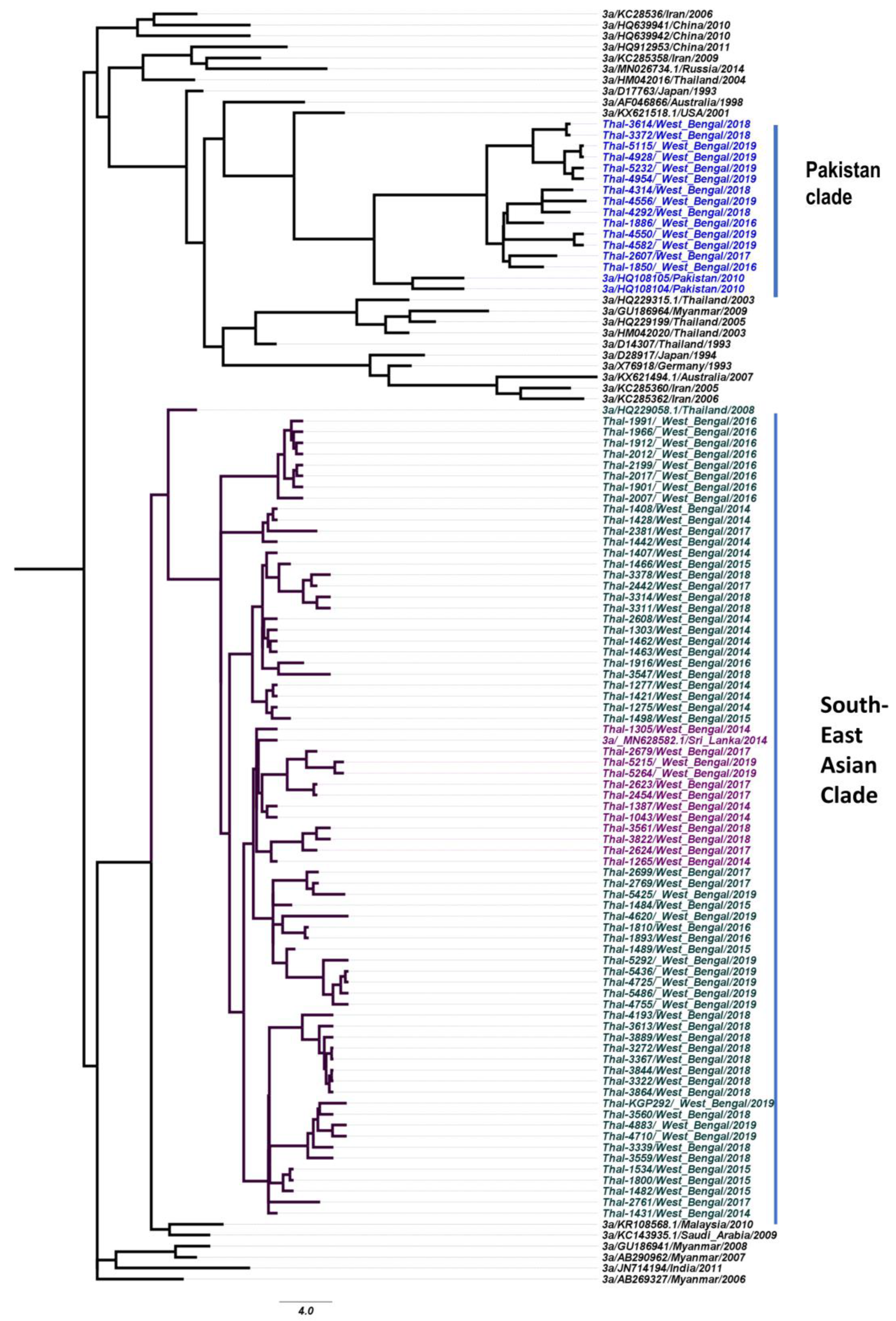
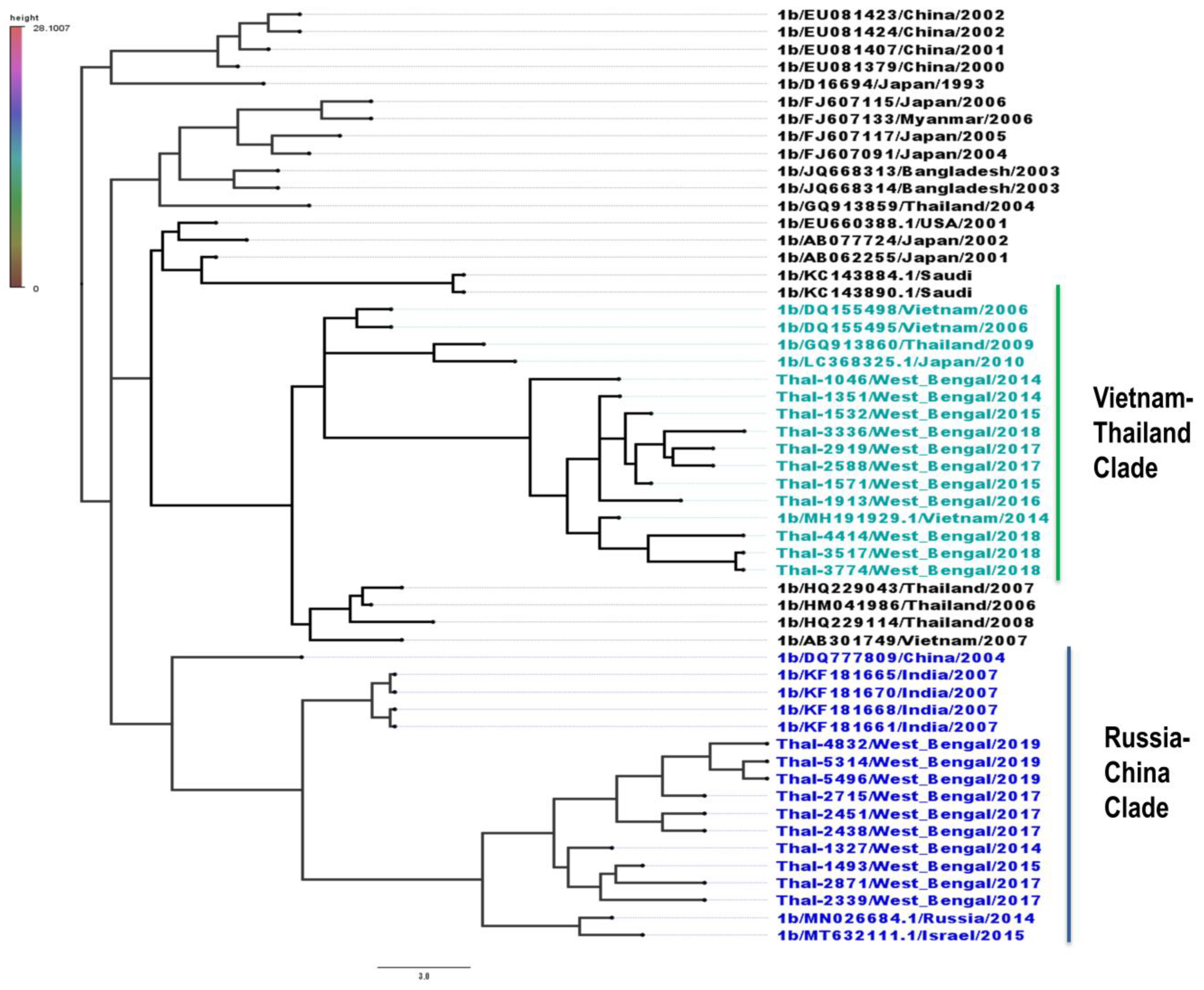
3.4. Phylogeography of HCV Isolates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeller, H.; Rabarijaona, L.; Rakoto-Andrianarivelo, M.; Boisier, P. Prevalence of hepatitis C virus infection in the general population of Madagascar. Bull. Soc. Pathol. Exot. 1997, 90, 3–5. [Google Scholar]
- Smith, D.B.; Bukh, J.; Kuiken, C.; Muerhoff, A.S.; Rice, C.M.; Stapleton, J.T.; Simmonds, P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: Updated criteria and genotype assignment web resource. Hepatology 2014, 59, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-M.; Kao, J.-T.; Tsai, Y.-F. How hepatitis C patients manage the treatment process of pegylated interferon and ribavirin therapy: A qualitative study. BMC Health Serv. Res. 2016, 16, 247. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, L.N.; Lyra, A.C. Predictive factors associated with hepatitis C antiviral therapy response. World J. Hepatol. 2015, 7, 1617–1631. [Google Scholar] [CrossRef] [PubMed]
- Diagnosis & Management of Viral Hepatitis. Available online: https://www.inasl.org.in/diagnosis-management-viral-hepatitis.pdf (accessed on 15 June 2023).
- Ghany, M.G.; Morgan, T.R.; Panel, A.H.C.G.; Marks, K.M.; Wyles, D.L.; Aronsohn, A.I.; Bhattacharya, D.; Broder, T.; Falade-Nwulia, O.O.; Feld, J.J.; et al. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases–Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology 2020, 71, 686–721. [Google Scholar] [CrossRef]
- Pozzetto, B.; Memmi, M.; Garraud, O.; Roblin, X.; Berthelot, P. Health care-associated hepatitis C virus infection. World J. Gastroenterol. 2014, 20, 17265–17278. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Galanello, R. Beta-thalassemia. Anesthesia Analg. 2010, 12, 61–76. [Google Scholar] [CrossRef]
- Vidja, P.J.; Vachhani, J.H.; Sheikh, S.S.; Santwani, P.M. Blood Transfusion Transmitted Infections in Multiple Blood Transfused Patients of Beta Thalassaemia. Indian J. Hematol. Blood Transfus. 2011, 27, 65–69. [Google Scholar] [CrossRef]
- Mohanty, D.; Colah, R.B.; Gorakshakar, A.C.; Patel, R.Z.; Master, D.C.; Mahanta, J.; Sharma, S.K.; Chaudhari, U.; Ghosh, M.; Das, S.; et al. Prevalence of β-thalassemia and other haemoglobinopathies in six cities in India: A multicentre study. J. Community Genet. 2013, 4, 33–42. [Google Scholar] [CrossRef]
- Joseph, N.; Pai, S.; Sengupta, S.; Bharadwaj, S.; Dhawan, S.; Khare, K. A clinico-epidemiological study of thalassemia cases in India. J. Nat. Sci. Biol. Med. 2018, 9, 236. [Google Scholar] [CrossRef]
- Colah, R.B.; Seth, T. Thalassemia in India. Hemoglobin 2022, 46, 20–26. [Google Scholar] [CrossRef]
- Biceroglu, S.U.; Turhan, A. Probable hepatitis C virus transmission from a seronegative blood donor via cellular blood products. Blood Transfus. 2014, 12, s69–s70. [Google Scholar] [CrossRef]
- Finianos, A.; Matar, C.F.; Taher, A. Hepatocellular Carcinoma in β-Thalassemia Patients: Review of the Literature with Molecular Insight into Liver Carcinogenesis. Int. J. Mol. Sci. 2018, 19, 4070. [Google Scholar] [CrossRef]
- Gluba-Brzózka, A.; Franczyk, B.; Rysz-Górzyńska, M.; Rokicki, R.; Koziarska-Rościszewska, M.; Rysz, J. Pathomechanisms of Immunological Disturbances in β-Thalassemia. Int. J. Mol. Sci. 2021, 22, 9677. [Google Scholar] [CrossRef]
- Petruzziello, A.; Marigliano, S.; Loquercio, G.; Cozzolino, A.; Cacciapuoti, C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J. Gastroenterol. 2016, 22, 7824–7840. [Google Scholar] [CrossRef]
- Satsangi, S.; Chawla, Y.K. Viral hepatitis: Indian scenario. Med. J. Armed Forces India 2016, 72, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Sarkar, K.; Firdaus, R.; Saha, K.; Gupta, D.; Ghosh, M.; Chowdhury, P.; Bhattacharyya, D.; Bhattacharyya, M.; Sadhukhan, P.C. Prevalence of ANTI-HCV, HBSAG, HIV among multi-transfused thalassemic individuals and their socio-economic background in Eastern India. Asian J. Pharm. Clin. Res. 2016, 9, 290–294. [Google Scholar]
- Saha, K.; Firdaus, R.; Biswas, A.; Mukherjee, A.; Sadhukhan, P. A novel nested reverse-transcriptase polymerase chain reaction method for rapid hepatitis C virus detection and genotyping. Indian J. Med. Microbiol. 2014, 32, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, R.; Biswas, A.; Saha, K.; Mukherjee, A.; Chaudhuri, S.; Chandra, A.; Konar, A.; Sadhukhan, P.C. Impact of Host IL28B rs12979860, rs8099917 in Interferon Responsiveness and Advanced Liver Disease in Chronic Genotype 3 Hepatitis C Patients. PLoS ONE 2014, 9, e99126. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.J.; Ho, S.Y.W.; Phillips, M.J.; Rambaut, A. Relaxed Phylogenetics and Dating with Confidence. PLoS Biol. 2006, 4, e88. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.-M.; Sun, W.-L. Relationship between Hepatitis C Virus Infection and Iron Overload. Chin. Med. J. 2017, 130, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Marcus, E.L.; Tur-Kaspa, R. Chronic Hepatatis C Virus Infection in Older Adults. Clin. Infect. Dis. 2013, 41, 1606–1612. [Google Scholar]
- Guy, J.; Peters, M.G. Liver disease in women: The influence of gender on epidemiology, natural history, and patient outcomes. Gastroenterol. Hepatol. 2013, 9, 633–639. [Google Scholar]
- Ruggieri, A.; Gagliardi, M.C.; Anticoli, S. Sex-Dependent Outcome of Hepatitis B and C Viruses Infections: Synergy of Sex Hormones and Immune Responses? Front. Immunol. 2018, 9, 2302. [Google Scholar] [CrossRef]
- Baden, R.; Rockstroh, J.K.; Buti, M. Natural History and Management of Hepatitis C: Does Sex Play a Role? J. Infect. Dis. 2014, 209 (Suppl. 3), S81–S85. [Google Scholar] [CrossRef]
- De Sanctis, V.; Di Maio, S. A Retrospective Long-Term Study on Age at Menarche and Menstrual Characteristics in 85 Young Women with Transfusion-Dependent β-Thalassemia (tdt) Born between 1965 and 1995. Mediterr. J. Hematol. Infect. Dis. 2021, 13, e2021040. [Google Scholar] [CrossRef]
- Shahraki, T.; Shahraki, M.; Moghaddam, E.S.; Najafi, M.; Bahari, A. Determination of Hepatitis C Genotypes and the Viral Titer Distribution in Children and Adolescents with Major Thalassemia. Iran. J. Pediatr. 2010, 20, 75–81. [Google Scholar]
- Gower, E.; Estes, C.; Blach, S.; Razavi-Shearer, K.; Razavi, H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J. Hepatol. 2014, 61, S45–S57. [Google Scholar] [CrossRef]
- De Sanctis, V. β-thalassemia distribution in the old world: A historical standpoint of an ancient disease. Mediterr. J. Hematol. Infect. Dis. 2017, 9, e2017018. [Google Scholar] [CrossRef] [PubMed]
- Bonini-Domingos, C.R. Thalassemia screening in Brazil: Results for 20 years. Rev. Bras. Hematol. Hemoter. 2004, 26, 288–289. [Google Scholar] [CrossRef]
- Paraná, R.; Vitvitski, L.; Berby, F.; Portugal, M.; Cotrim, H.P.; Cavalcante, A.; Lyra, L.; Trepo, C. HCV infection in northeastern Brazil: Unexpected high prevalence of genotype 3a and absence of African genotypes. Arq. de Gastroenterol. 2000, 37, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Christdas, J.; Sivakumar, J.; David, J.; Daniel, H.; Raghuraman, S.; Abraham, P. Genotypes of hepatitis C virus in the Indian sub-continent: A decade-long experience from a tertiary care hospital in South India. Indian J. Med. Microbiol. 2013, 31, 349–353. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, Y.; Zhang, H.; Shang, G.; Gao, J.; Jiang, J.-D.; Peng, Z. Replication priority of hepatitis C virus genotype 2a in a Chinese cohort. Acta Pharm. Sin. B 2014, 4, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Westin, J.; Nordlinder, H.; Lagging, M.; Norkrans, G.; Wejstål, R. Steatosis accelerates fibrosis development over time in hepatitis C virus genotype 3 infected patients. J. Hepatol. 2002, 37, 837–842. [Google Scholar] [CrossRef]
- Nkontchou, G.; Ziol, M.; Aout, M.; Lhabadie, M.; Baazia, Y.; Mahmoudi, A.; Roulot, D.; Ganne-Carrié, N.; Grando-Lemaire, V.; Trinchet, J.-C.; et al. HCV genotype 3 is associated with a higher hepatocellular carcinoma incidence in patients with ongoing viral C cirrhosis. J. Viral Hepat. 2011, 18, e516–e522. [Google Scholar] [CrossRef]
- Bochud, P.-Y.; Cai, T.; Overbeck, K.; Bochud, M.; Dufour, J.-F.; Müllhaupt, B.; Borovicka, J.; Heim, M.; Moradpour, D.; Cerny, A.; et al. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J. Hepatol. 2009, 51, 655–666. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.K.; Mukhopadhyay, S.; Biswas, A.B. Enduring starvation in silent Population: A study on prevalence and factors contributing to household food security in the tribal population in Bankura, West Bengal. Indian J. Public Health 2010, 54, 92–97. [Google Scholar] [CrossRef]
- Modak, B.K.; Gorai, P.; Dhan, R.; Mukherjee, A.; Dey, A. Tradition in treating taboo: Folkloric medicinal wisdom of the aboriginals of Purulia district, West Bengal, India against sexual, gynaecological and related disorders. J. Ethnopharmacol. 2015, 169, 370–386. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, C.; Yuan, J.; Lu, T.; Okamoto, H.; Murphy, D.G. Full-length genome sequences of five hepatitis C virus isolates representing subtypes 3g, 3h, 3i and 3k, and a unique genotype 3 variant. J. Gen. Virol. 2013, 94, 543–548. [Google Scholar] [CrossRef] [PubMed]
| HCV Positive (N = 598) | HCV Viremia (65.21%) | p-Value | ||
|---|---|---|---|---|
| Variables | (n = 381) Male (%) | (n = 217) Female (%) | ||
| Age group | 1 to 5 | 67.69 | 60.24 | 0.008 * |
| 6 to 10 | 75.78 | 69.31 | ||
| 11 to 15 | 62.78 | 54.62 | ||
| 16 to 20 | 62.26 | 60 | ||
| 21 to 25 | 65.22 | 64.29 | ||
| 26 to 30 | 71.43 | 57.14 | ||
| >30 | 71.43 | 57.14 | ||
| Transfusion centers | TC-1 | 63.33 | 55 | <0.0001 * |
| TC-2 | 76 | 73.33 | ||
| TC-3 | 68 | 58.82 | ||
| TC-4 | 67.16 | 60.93 | ||
| TC-5 | 67.21 | 62.16 | ||
| TC-6 | 71.42 | 64.28 | ||
| TC-7 | 64.86 | 57.44 | ||
| TC-8 | 73.91 | 73.33 | ||
| TC-9 | 67.39 | 58.06 | ||
| TC-10 | 71.15 | 60 | ||
| Locality of Residence | Rural | 69.9 | 63.3 | 0.042 * |
| Urban | 60.5 | 52.56 | ||
| Economic class | Above poverty level | 58.18 | 51.38 | 0.002 * |
| Below poverty level | 70.22 | 63.5 | ||
| HCV Positive + = 592 | HCV Genotypes | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Genotype 1b (N = 72) | Genotype 3a (N = 468) | Genotype 3b (N = 52) | |||||
| (n = 50) Male (%) | (n = 22) Female (%) | (n = 295) Male (%) | (n = 173) Female (%) | (n = 33) Male (%) | (n = 19) Female (%) | |||
| Age-group | 1 to 5 | 2.17 | 1.45 | 54.35 | 31.88 | 5.80 | 4.35 | 0.0007 * |
| 6 to 10 | 2.11 | 1.05 | 55.26 | 33.16 | 5.26 | 3.16 | ||
| 11 to 15 | 13.07 | 5.11 | 46.59 | 27.28 | 5.11 | 2.84 | ||
| 16 to 20 | 20.84 | 8.33 | 39.58 | 22.92 | 6.25 | 2.08 | ||
| 21 to 25 | 31.82 | 13.64 | 31.82 | 18.18 | 4.54 | 0.00 | ||
| 26 to 30 | 11.11 | 11.11 | 44.45 | 22.22 | 11.11 | 0.00 | ||
| >30 | 22.22 | 11.11 | 33.34 | 11.11 | 11.11 | 11.11 | ||
| Transfusion Centre | TC 1 | 10 | 3.33 | 50 | 26.67 | 6.67 | 3.33 | <0.0001 * |
| TC 2 | 6.67 | 3.33 | 50 | 30 | 6.67 | 3.33 | ||
| TC 3 | 11.11 | 3.70 | 48.15 | 25.93 | 7.41 | 3.70 | ||
| TC 4 | 8.53 | 4.27 | 49.29 | 29.38 | 4.74 | 3.79 | ||
| TC 5 | 9.38 | 3.13 | 50 | 28.13 | 6.25 | 3.13 | ||
| TC 6 | 8.33 | 4.17 | 50 | 29.17 | 4.17 | 4.17 | ||
| TC 7 | 4.05 | 1.35 | 54.05 | 32.43 | 5.41 | 2.70 | ||
| TC 8 | 11.11 | 3.70 | 48.15 | 25.93 | 7.41 | 3.70 | ||
| TC 9 | 8.51 | 4.26 | 48.94 | 29.79 | 6.38 | 2.13 | ||
| TC 10 | 10.34 | 5.17 | 48.28 | 29.31 | 5.17 | 1.72 | ||
| Locality of residence | Rural | 7.63 | 3.30 | 50.52 | 29.48 | 5.57 | 3.50 | 0.0519 |
| Urban | 12.15 | 5.61 | 46.72 | 28.03 | 5.60 | 1.89 | ||
| Economic class | Above poverty level | 7.13 | 3.05 | 50.91 | 29.74 | 5.91 | 3.26 | 0.0898 |
| Below poverty Level | 14.86 | 6.93 | 44.55 | 26.73 | 3.96 | 2.97 | ||
| Gender | Male (Total Spontaneous Clearance: Followed up: Total = 136:309:381) | Female Total Spontaneous Clearance: Followed up: Total = 114:176:217) | p-Value | ||
|---|---|---|---|---|---|
| Age Group | Spontaneous Clearance | No Clearance | Spontaneous Clearance | No Clearance | |
| 1 to 5 | 30 | 42 | 25 | 18 | <0.001 * |
| 6 to 10 | 45 | 55 | 39 | 19 | |
| 11 to 15 | 52 | 47 | 43 | 10 | |
| 16 to 20 | 6 | 19 | 5 | 10 | |
| 21 to 25 | 3 | 10 | 2 | 5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutta, S.; Biswas, A.; Bakshi, S.; Choudhury, P.; Das, R.; Nath, S.; Chowdhury, P.; Bhattacharyya, M.; Chakraborty, S.; Dutta, S.; et al. Molecular Epidemiology of HCV Infection among Multi-Transfused β-Thalassemia Patients in Eastern India: A Six-Year Observation. Thalass. Rep. 2023, 13, 165-178. https://doi.org/10.3390/thalassrep13030016
Dutta S, Biswas A, Bakshi S, Choudhury P, Das R, Nath S, Chowdhury P, Bhattacharyya M, Chakraborty S, Dutta S, et al. Molecular Epidemiology of HCV Infection among Multi-Transfused β-Thalassemia Patients in Eastern India: A Six-Year Observation. Thalassemia Reports. 2023; 13(3):165-178. https://doi.org/10.3390/thalassrep13030016
Chicago/Turabian StyleDutta, Supradip, Aritra Biswas, Sagnik Bakshi, Promisree Choudhury, Raina Das, Shreyasi Nath, Prosanto Chowdhury, Maitreyee Bhattacharyya, Sharmistha Chakraborty, Shanta Dutta, and et al. 2023. "Molecular Epidemiology of HCV Infection among Multi-Transfused β-Thalassemia Patients in Eastern India: A Six-Year Observation" Thalassemia Reports 13, no. 3: 165-178. https://doi.org/10.3390/thalassrep13030016
APA StyleDutta, S., Biswas, A., Bakshi, S., Choudhury, P., Das, R., Nath, S., Chowdhury, P., Bhattacharyya, M., Chakraborty, S., Dutta, S., & Sadhukhan, P. C. (2023). Molecular Epidemiology of HCV Infection among Multi-Transfused β-Thalassemia Patients in Eastern India: A Six-Year Observation. Thalassemia Reports, 13(3), 165-178. https://doi.org/10.3390/thalassrep13030016









