Abstract
Beta thalassemia is an inherited disorder resulting in abnormal or decreased production of hemoglobin, leading to hemolysis and chronic anemia. The long-term complications can affect multiple organ systems, namely the liver, heart, and endocrine. Myocardial iron overload is a common finding in β-thalassemia. As a result, different cardiovascular complications in the form of cardiomyopathy, pulmonary hypertension, arrhythmias, and vasculopathies can occur, and in extreme cases, sudden cardiac death. Each of these complications pertains to underlying etiologies and risk factors, which highlights the importance of early diagnosis and prevention. In this review, we will discuss different types of cardiovascular complications that can manifest in patients with β-thalassemia, in addition to the current diagnostic modalities, preventive and treatment modalities for these complications.
1. Introduction
Thalassemia is one of the most common inherited hemoglobinopathies with an autosomal recessive pattern, characterized by anemia and small-sized red blood cells [1]. The anemia is caused by a partial or complete impaired production of one of the globin chains leading to an alpha/beta-globin imbalance, ineffective erythropoiesis, and chronic hemolysis [2]. Beta thalassemia is a major form of thalassemia with a prevalence of 1.5% of the world population and is currently expanding to other regions where the disease was previously rare, mainly due to the increased migration patterns around the world [3,4]. The thalassemia spectrum is clinically divided into two main categories based on the patient’s need for blood transfusion. Patients with transfusion-dependent thalassemia (TDT) commonly present with severe anemia in early childhood, requiring a lifelong therapy of regular transfusions to survive. On the contrary, non-transfusion-dependent thalassemia (NTDT) patients usually present with mild to moderate anemia in a later stage of childhood or even in adulthood, requiring only occasional or short-course regular transfusions in certain clinical settings [5]. The classification of NTDT is not always straightforward since patients might require frequent regular transfusions in later stages of life. Thus, NTDT patients can only be placed under the category of TDT if they are dependent on transfusions for the remainder of the disease [5].
Cardiovascular disease still remains the major cause of death in both TDT and NTDT patients with a prevalence of 71% [6]. The primary reason for cardiac damage in these patients is iron overload, which is caused by frequent blood transfusions, hemolysis, increased intestinal absorption, and a lack of iron excretion mechanism in the body [6,7].
The incidence of cardiomyopathy in TDT patients due to iron accumulation ranges from 11.4% to 15.1% [7]. This is due to recurrent blood transfusions leading to iron accumulation at high levels in the body. However, in the case of NTDT, excess iron still builds up even in the absence of regular transfusions as a response to ineffective erythropoiesis and hepcidin suppression, which leads to increased iron absorption [8]. In NTDT, iron accumulation occurs more commonly in the liver rather than the heart, but the underlying pathophysiology related to NTDT can result in an increase in cardiac output, volume overload, endothelial dysfunction, inflammation, and hypercoagulability, thus leading to cardiac complications [2]. Despite the prevalence of these cardiac complications in adults, it has been also shown that cardiac iron has also been detected in one-third of pediatric β-thalassemia patients ranging between 15–18 years of age, with the majority remaining asymptomatic [9].
Throughout the past years, advances in the monitoring of iron and the accessibility of iron chelators have increased the life expectancy of patients, however comorbidities are still manifested at an advanced age in β-thalassemia patients [10,11]. Despite this, however, there are cardiac complications such as heart failure, arrhythmias, cardiomyopathies, fibrosis, and vasculopathies [11]. Thus, education and awareness about cardiac complications is beneficial [12]. In this review, we will discuss different types of cardiovascular complications that can manifest in patients with β-thalassemia, in addition to the current diagnostic modalities, preventive and treatment modalities available for these complications.
2. Pathophysiology of Cardiac Complications in β-Thalassemia
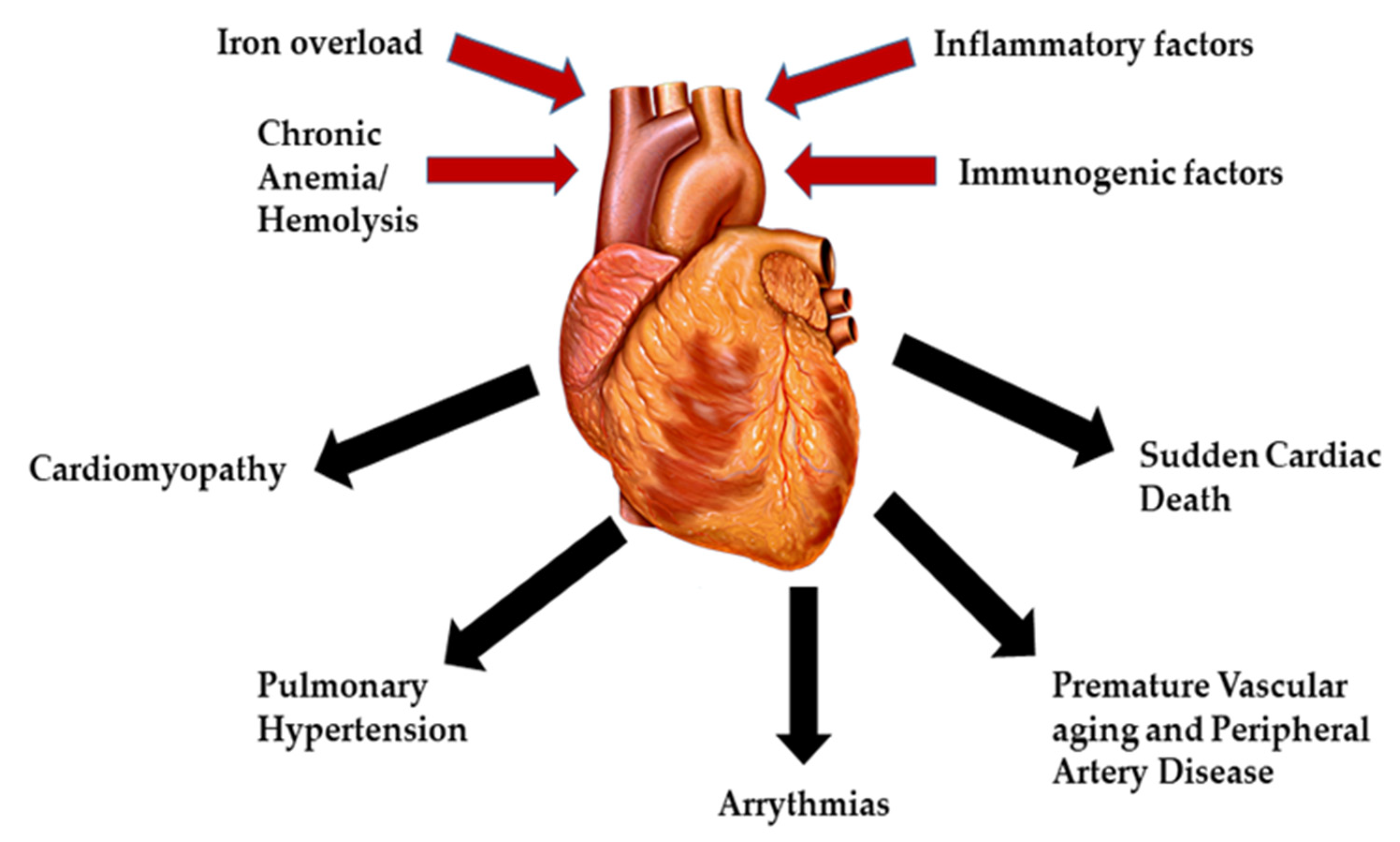
The main factor involved in the pathophysiology of cardiac dysfunction in β-thalassemia patients is iron overload, especially in TDT. Other factors that play a role include deficiencies in carnitine, thiamine, vitamin D, selenium, autoimmune diseases (hypothyroidism, hypoparathyroidism and hypogonadism) and hepatitis C (Figure 1) [13]. The etiology of iron overload is mainly attributed to repetitive blood transfusions alongside ineffective erythropoiesis, peripheral hemolysis, and increased intestinal absorption [14]. Before the introduction of iron chelation therapy for the management of β-thalassemia patients, a broad range of heart related complications was constantly being reported including pericarditis, myocarditis, high output HF, and arrhythmias. However, there has been a significant decline in heart related mortalities in β-thalassemia patients with the use of iron chelators, thus increasing survival [15]. Unfortunately, despite advances in iron chelation therapy, cardiac iron accumulation and toxicity continues to be the leading cause of mortality in thalassemia patients today [16].
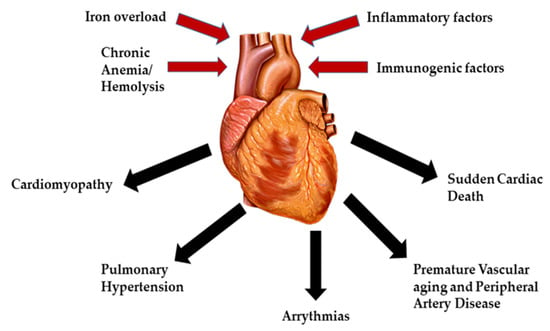
Figure 1.
Major causes and complications of cardiac diseases in β-thalassemia.
The exact pathophysiology behind these clinical manifestations is attributed to the free iron generated by the Fenton reaction and Haber-Weiss reactions. These free radical mediated pathways can damage different types of cells in the heart, such as cardiomyocytes [17]. The normal transport of iron throughout the circulation is mediated by protein transferrin, which limits its toxic effect [18]. In the case of excess iron in β-thalassemia, ferritin becomes fully saturated, which allows labile plasma iron to enter cardiomyocytes as ferrous iron (Fe2+) via voltage-dependent L-type Ca2+ channels. The three forms of iron storage in cardiomyocytes are ferritin, hemosiderin and labile iron, which is the accessible form for iron chelation and the most toxic one, responsible for the free radical reactions [14].
When compared to normal cardiomyocytes, it was shown that iron overloaded cardiomyocytes showed changes in parameters of the action potential, such as decreased overshoot and shorter duration of the action potential [15]. One of the underlying mechanisms affecting the action potential of the cardiomyocytes is the decrease in calcium currents [19]. This is due to the similarity in size and charge between ferrous iron and the calcium ions responsible for the excitation-contraction coupling and the action potential. Ferrous iron directly acts on the ryanodine-sensitive calcium channel responsible for contraction and for the reuptake of calcium in the sarcoplasmic reticulum [20]. The electrophysiological changes caused by iron overload make the heart susceptible to re-entry circuits, bradycardia, atrial fibrillation (AF), and arrhythmias, all of which can be life-threatening [21].
In the case of NTDT patients who do not require regular blood transfusions, the main pathophysiology of cardiac dysfunctions is due to chronic untreated anemia, leading to prolonged tissue hypoxia. This leads to an increase in pulmonary vascular resistance, a high output state and pulmonary hypertension. Thus, chronic hypoxia is a key factor in differentiating the clinical cardiac complications of TDT and NTDT patients [22].
2.1. Cardiomyopathy
Cardiomyopathies are groups of diseases related to structural and functional changes in the heart that can be primary (genetic and acquired causes) or secondary (from infiltrative, toxic, or inflammatory etiologies) in nature [23,24]. They are defined by the American Heart Association as a group of myocardial diseases with inappropriate ventricular hypertrophy which can lead to progressive heart failure or cardiovascular death [25].
In the case of patients with β-thalassemia, inadequate iron chelation therapy in addition to poor patient adherence and compliance is a major contributor to patients developing HF. Clinically, dyspnea and fatigue are reported by this patient population. Both, the left and right ventricles may be involved, with right sided HF symptoms usually presenting later on during the course of left HF. In terms of ventricular function, two major HF phenotypes have been described [20]. The first phenotype commonly presents as dilated cardiomyopathy associated with left ventricular dilatation and reduced ejection fraction. The second phenotype manifests as restrictive cardiomyopathy characterized by a restrictive diastolic filling pattern on echocardiography and elevated left ventricular filling pressures eventually leading to pulmonary hypertension (PH) and right sided HF. Both phenotypes may manifest clinically as congestive HF [14]. Moreover, chronic anemia, reduced oxygen tissue delivery, co-existent liver disease, and iron overload can contribute to a high output state leading to left ventricular dilatation and eccentric hypertrophy; thus, high output HF [26]. Iron starts to accumulate from early childhood in TDT patients leading to progressive HF, which manifests through the development of signs and symptoms of right-sided HF [27]. Iron accumulation in the heart can have either a homogenous, or a heterogenous pattern [28]. A study by Borgna-Pignatti et al. revealed a higher risk of HF development in cases of homogenous patterns of myocardial iron distribution [MIO] in β-thalassemia patients. This was mainly due to cardiomyocyte iron overload which eventually led to myocardial fibrosis and necrosis [28,29]. In contrast to the general population where left-sided HF is more frequent, TDT patients tend to develop right-sided HF much earlier [21]. Another factor that seems to impact the pathogenesis of cardiomyopathy in β-thalassemia is myocarditis [14,30]. β-thalassemia patients are more susceptible to developing infections because of a possible deficiency in their immune system [14,31]. In the case of myocytes, iron overload might enhance their vulnerability to developing infections [14].
In the general population, acute myocarditis was shown to be associated with an increased prevalence of dilated cardiomyopathy and a significant deterioration of ejection fraction [14]. Acute myocarditis in TDT patients caused chronic left sided HF in 27.6% of cases in an approximate period of 3.5 years [27]. Pericarditis was also reported early by Engle et al. when it coincided with fatal arrhythmias and HF in TDT patients [27]. To some degree, both pericarditis and myocarditis can usually coexist [27]. Myocardial infarction (MI) on the other hand is an uncommon cause of death in β-thalassemia patients with the first case being reported in 2004 [32]. It has been shown that patients with β-thalassemia have low total cholesterol and low LDL levels that protect them against MI [33,34]. The incidence of MI in TDT is higher than that in NTDT with a prevalence of 2.11% TDT compared to 0.71% in NTDT [35]. Multiple studies were conducted to determine the sensitivity of different serum markers for MI in β-thalassemia patients [36]. Like in the general population, Troponin T serves as a good biological marker for the early diagnosis of MI in β-thalassemia patients [36].
2.2. Pulmonary Hypertension
Pulmonary hypertension is prevalent in both TDT and NTDT patients and is a leading cause of right sided HF [11,14,37]. Pulmonary Hypertension is defined by a mean pulmonary arterial pressure higher than 20 mmHg at rest. The clinical presentation and symptomatology of this entity is mainly linked to right ventricle dysfunction and typically occurs with exercise in the early phases of the disease. The main symptom is dyspnoea upon exertion. Other common symptoms include fatigue, bendopnea, haemoptysis, fluid overload, syncope and rapid exhaustion [38]. The prevalence of PH in β-thalassemia patients was found to be between 10–78.8%, with a five-fold higher occurrence in NTDT patients compared to TDT [11,39,40]. This disease has a progressive onset and a poor prognosis with a 30% survival rate over a five-year period if left untreated [41]. In β-thalassemia patients, PH is characterized by precapillary PH and the absence of left-sided heart disease and lung disease [11,37]. Nonetheless, hypercoagulability along with other factors are thought to influence the development of PHT in patients with β-thalassemia [37]. Moreover, the most common type of PH occurs as a result of a pulmonary vascular disease, known as Chronic Thromboembolic Pulmonary Hypertension (CETPH), and is more commonly found in those patients who are splenectomized [42].
Splenectomy per se is a risk factor for developing PH by triggering platelet activation and abnormal erythrocyte aggregation, thus promoting pulmonary micro-thrombosis and red cell adhesion to the vascular endothelium [41,43]. Observational studies have determined a four–five-fold higher risk of developing PH in splenectomized NTDT patients [36]. NTDT who are splenectomized are at higher chances of developing PH due to several risk factors that include Splenectomy, naivety to iron chelation, naivety to hydroxyurea treatment, naivety to blood transfusions and history of previous thromboembolic events [44]. Other risk factors of PH include chronic hemolysis, female gender, HCV infection, iron overload (serum ferritin > 800), liver iron concentration > 5 mg Fe/g dry weight older age, platelet count ≥ 500 × 106/L, hypoxia, and anemia [Hemoglobin level < 9 g/dL] [2,11]. Therefore, accurately diagnosing the cause of PH enables accurate management and prognosis of this disease [41].
2.3. Arrhythmias
Studies conducted using animal models have demonstrated the effect of iron toxicity in inducing electrical conduction disturbances that lead to arrhythmias [15]. One of the specific indicators for iron cardiotoxicity are ventricular arrhythmias, couplets, and non-sustained ventricular tachycardia. Supraventricular ectopic beats might be present early on in cardiomyopathy, contrary to malignant arrhythmias, which are usually present in advanced stages of the disease [1]. The most frequently reported arrhythmias in TDT patients include atrial flutter and atrial re-entrant tachycardia [45]. In patients with severe iron overload, other types of arrhythmias can manifest such as ectopic atrial tachycardia and chaotic atrial rhythm [1]. A prospective study showed that 14% of patients with severe iron overload developed arrhythmias within one year of this scan [7]. It was also shown that even though NTDT patients had larger cardiac chamber volumes, the incidence of arrhythmia in these patients compared to those with TDT was much less. This is attributed to a lower level of cardiac iron overload in NTDT compared to TDT patients, emphasizing the importance of iron toxicity in such situations [7,46].
Atrial fibrillation is a common form of arrhythmia in β-thalassemia patients with a prevalence ranging from 2–33%, which is greater than the general population. Atrial fibrillation begins at a younger age in β-thalassemia with an increase in prevalence of 40% in the patients above 40 [47]. Age was found to be one of the main risk factors for AF in β-thalassemia alongside diabetes, atrial and ventricular dysfunctions and an increased level of natriuretic peptides [NT-proBNP and proANP] [47]. The clinical presentation of AF is mostly paroxysmal with symptoms, while a persistent pattern is less frequently observed in this population [47]. To be considered a symptomatic patient with atrial fibrillation, one or more of these following symptoms should be present: Dizziness, palpitations, chest pain, syncope, dyspnea and fatigue [48].
According to the concept of Coumel’s triangle, the three elements needed to produce a clinical arrhythmia are: Arrhythmogenic factors, trigger factors and autonomic nervous system dysfunction [7]. The ideal environment for developing AF would be a combination of iron deposition in myocytes and atrial dilatation [49]. Factors triggering AF could be ventricular asystole, infrequent premature atrial or ventricular contractions, and first degree atrio-ventricular blocks, which are all present in β-thalassemia patients. The third factor would be the nervous system, monitored through the HRV, which is an important parameter reflecting its function and balance. HRV is usually lower in β-thalassemia patients, especially in TDT patients, compared to normal subjects. It is used as an early sign of cardiac dysfunction, and for detecting arrhythmias, especially AF [7,47]. Even though the main etiology was found to be from iron overload, AF can still develop in β-thalassemia patients without iron deposition and in the presence of normal iron levels [11,47]. This could be attributed to previous resolved iron deposition episodes that resulted in fibrosis [11]. Atrial fibrillation can be reversed by chelation if the cause is iron overload and if heart remodeling did not occur [15]. A greater mortality rate occurs if AF occurs simultaneously with HF. AF increases mortality and morbidity by raising the risk of thromboembolic events and strokes [15]. The early detection of AF in TDT patients is extremely important in optimizing medical therapy and assessing the need to start anticoagulation therapy for stroke prevention [1].
2.4. Premature Vascular Aging and Peripheral Artery Disease
Other pathologies found in β-thalassemia patients are vascular complications and parenchymal damage of the myocardium. Iron overload, hemolysis-induced reduction in NO bioavailability, and an increase in lipid peroxidation products were found to play a role in endothelial dysfunction and arterial stiffness in β-thalassemia patients [14,33,50]. Other factors that could influence the damage of vessels include inflammatory factors associated with the immune system. These factors are altered in β-thalassemia patients due to transfusions, infectious agents, cytokine levels of stored allogeneic blood, and stromal cells of hyperplastic bone marrow [51].
High arterial stiffness was observed in both peripheral and central elastic arteries in β-thalassemia patients. Postmortem examinations have demonstrated increased fibrosis and glycosaminoglycan histologically in the aorta, iliac and pulmonary arteries. Radiological studies have also exhibited posterior tibial artery calcifications [52]. A study done by Nassef et al. compared the central and peripheral atherosclerosis in patients with β-thalassemia intermedia (β-TI). Results concluded that central vascular ischemia is more frequent than peripheral ischemia in β-TI patients. In this study, no significant correlation between Intima-media Thickness (IMT), a marker for subclinical atherosclerosis, and ferritin or cholesterol level was found in such patients [53].
The effect of iron chelation therapy on premature vascular aging and peripheral artery disease was also assessed. Despite the use of iron chelation, patients with TDT still suffered from high iron levels which led to alterations in arterial structures, damage of elastic tissue, and calcification deposits in the vascular structures. Many studies have confirmed the early changes of atherosclerosis in both TDT and NTDT patients [53]. On the one hand, the mechanism of atherosclerosis in NTDT is not fully clear in the absence of a correlation between IMT and ferritin/cholesterol. On the other hand, a study in TDT patients showed a positive correlation between carotid atherosclerosis and serum ferritin levels [54].
2.5. Sudden Cardiac Death
The high incidence of sudden cardiac death (SCD) in TDT patients can be attributed to the discrepancy between the symptoms and the severity of arrhythmias, which leads to delayed diagnosis. Before the start of iron chelation therapy, it was common for bradyarrhythmia and complete heart block to cause SCD. Nowadays, SCD is rare but might still occur in cases of severe cardiomyopathy from iron overload [1,55]. Patients with TDT are at an increased risk for SCD [56]. According to a study by Russo et al., SCD occurred in TDT patients with longer QTd and JTd compared to patients with normal electrocardiogram (ECG). In particular, patients with a QTD > 70 ms and JTd > 100 ms were found to have an increased possibility of asystole [1]. This increase in QTd might be a precursor to developing ventricular arrhythmias and sudden cardiac death in patients with comorbidities, such as diabetes mellitus, chronic HF, left ventricular hypertrophy, obesity, and patients above 55 years [25]. Other studies have shown that males are more prone to have SCD than females, which supports the hypothesis of a worse life expectancy in male patients with β-thalassemia [21].
3. Diagnosis of Cardiovascular Complications in β-Thalassemia
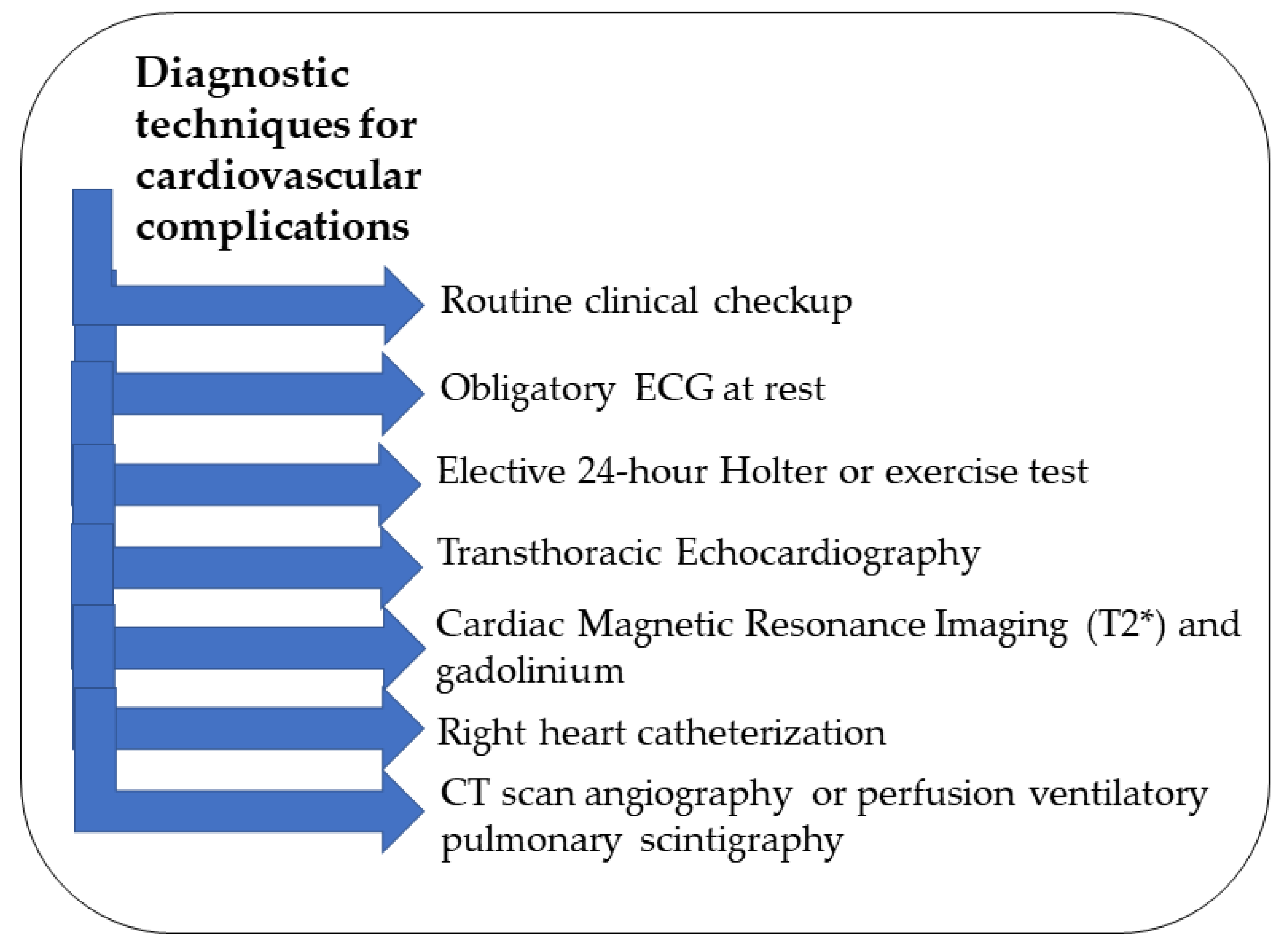
Since cardiac abnormalities can remain asymptomatic for a while, β-thalassemia patients should undergo routine clinical checkups and assessments to evaluate their overall functional capacity (Figure 2) [2,57]. An ECG at rest is obligatory for monitoring along with an elective 24 h Holter or exercise test in specific cases [2,52]. Imaging techniques that are noninvasive are the best tools for early detection of iron accumulation in the heart in all β-thalassemia patients who received multiple transfusions [58]. Transthoracic echocardiography is a noninvasive tool along with the ECG to evaluate arrhythmias in β-thalassemia patients [59]. The parameters that should be obtained for early diagnosis measure the dimensions, function, blood flow, and morphology of the heart [52]. The analysis of the ECG must include the P wave and the QT interval measurements [1]. For the echocardiography, the atrial electromechanical delay should be taken into consideration [54]. If these parameters are monitored continuously, minor changes become apparent, indicating the need for further investigations with CMR [52]. For optimal early detection of cardiac complications, it is recommended to repeat the ECG every three–four months, echocardiography once or twice per year, and a 24 h Holter ECG and cardiovascular magnetic resonance (CMR) T2 annually [2].
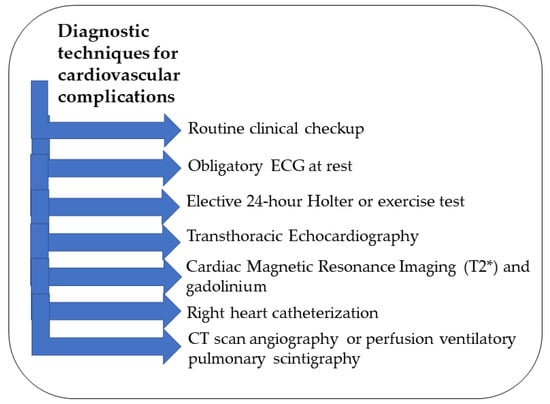
Figure 2.
Summary of the different diagnostic techniques for cardiovascular complications.
To assess the structure of the heart and myocardial iron deposition (MIC), cardiac magnetic resonance imaging (MRI) is performed by applying the T2 technique [60]. A typical Myocardial T2* value of <20 ms indicates the presence of an abnormal amount of myocardial iron, which can increase the possibility of ventricular impairment [10]. Several studies showed evidence supporting the use of T2* MRI to monitor cardiac iron overload in patients who received multiple blood transfusions. This helps in identifying all the patients who are at higher risk of cardiac mortality and who can be managed by optimization of iron chelation [61]. The fibrosis caused by previous iron deposition in the myocardium is measured with late gadolinium enhancement which was found to be safe in patients with hemoglobinopathies.
The gold standard for identifying PH is the right heart catheterization which is an invasive procedure and is recommended only for selected patients [2]. All patients must be screened routinely, especially those with NTDT, utilizing echocardiography (Table 1) and pro-BNP measurements [2]. Perfusion-ventilatory pulmonary scintigraphy or CT scan angiography should be considered for the diagnosis of CTEPH (2). The diagnosis is mainly dependent on the tricuspid valve regurgitation jet velocity (TRV) of more than 2.5–2.8 ms/s, which is linked to a pulmonary arterial systolic pressure of 30–35 mmHg on echocardiography [39]. If the patient is symptomatic with a value > 2.5 m/s, consulting a cardiologist and managing according to guidelines are recommended [11]. However, most data available based their diagnosis mainly on echocardiographic results without a confirmation from cardiac catheterization, resulting in an increased level of false positive cases in such patients [39].

Table 1.
Transthoracic Echocardiographic Findings in Pulmonary Hypertension.
4. Prevention and Management
The unique characteristics of every β-thalassemia patient make standard treatments inapplicable in all cases (Table 2) [47]. The general strategy that can be implemented is maintaining a pre-transfusion hemoglobin value of 10 g/dL and administering an effective iron chelation therapy regimen and keep a CMR T2* value > 20 ms [52]. As mentioned earlier, iron overload has a significant cardiotoxic effect. Therefore, iron chelation therapy should be initiated before iron accumulates in the heart. There are three widely known and used iron chelators: deferoxamine (DFO), deferiprone (DFP), and deferasirox (DFX). Better cardiac functions were seen in patients taking DFP in comparison to those who took DFX or DFO. DFP proved to be a better iron chelator in cases of iron-related cardiac disease since it infiltrates the cell membranes faster than DFO [15]. However, DFX was given as a monotherapy for three years and resulted in a significant reduction in cardiac iron when compared to baseline levels, confirming its efficacy in such cases [62]. However, in case of severe persistent iron overload with monotherapy, initiation of combination therapy may be introduced for better results [63]. Combination therapies that were found to be effective in reducing cardiac iron overload are DFO with DFP and DFX with DFP [64]. A recent study by Gupta et al. demonstrated the beneficial and safe use of Amlodipine, which is an L- calcium channel blocker, in combination with iron chelation therapies in improving cardiac iron overload in children and young adults with TDT [63]. Iron crosses cardiomyocytes through high-capacity L-type calcium channels, which explains the use of a calcium channel blocker as a treatment modality to prevent iron overload [65,66].

Table 2.
Summary of different cardiovascular complications and management techniques in β- thalassemia.
Different clinical studies have shown a resolution of arrhythmia after the initiation of an intensive iron chelation regimen, even after discontinuation of antiarrhythmic events [15].
There has also been an increased interest in using antioxidants or naturally occurring products in β-thalassemia for the prevention of arrhythmias and AF [15]. Many previous studies have demonstrated the effect of several antioxidants such as curcumin, silymarin, and green tea on TDT patients. As there exists no solid evidence on the effectiveness of these antioxidants in preventing arrhythmias and AF, these compounds should therefore be used with caution. When antioxidants are used in a combination with iron chelators, their effect is synergetic, and they showed benefits in decreasing iron levels in both the plasma and the heart [15]. Besides the therapies mentioned above, AF should be treated similarly to other settings, using rhythm or rate control strategies, depending on the condition of the patient. For the rhythm control, amiodarone has been used in inpatient and emergency settings because of its broad mode of action and minimal cardiac function effect [11]. It should be used with caution however in β-thalassemia patients as it can have adverse effects on the liver and thyroid of such patients [11]. The rate control can be managed with β-blockers or calcium antagonists in the presence of normal ejection fraction [EF] [7,11]. In case of diminished EF or low T2* value, digoxin can be adapted [7]. Ablation therapy can be used when rhythm control fails, and AF recurs [2]. Since AF is linked with an increased risk of stroke in β-thalassemia patients, especially in those splenectomized, a careful anticoagulation assessment and therapy are recommended [67].
The approach to anticoagulation therapy in β-thalassemia patients with AF is controversial [2]. In the general population, the assessment of AF patients is usually done depending on the CHADs-VASC score, which is a point-based system that stratifies the risk of stroke in patients with AF and determines patients requiring anticoagulation therapy [68]. It includes risk factors related to ischemic stroke, namely patient history, age, gender and concomitant disease [68]. In the case of β-thalassemia patients, it is related to other risk factors, making such approach an individualized one [2]. Due to the increased risk of thrombosis in β-thalassemia patients, anticoagulation is considered at early stages for all patients with AF and arrhythmia, except for those who experience mild and infrequent symptoms of AF [7,47]. The most commonly used drug for anticoagulation in such cases is warfarin which is considered as a standardized treatment for thromboembolic prophylaxis [47]. Being a vitamin K antagonist, warfarin necessitates frequent phlebotomies and laboratory tests for better monitoring [67]. However, when compared to vitamin K inhibitors, direct oral anticoagulants (DOACs) are more manageable and have a better safety profile. Only small studies have demonstrated the efficacy of DOACs on β-thalassemia patients, and further data will be needed to support this claim [47].
Treatment of HF involves the mandatory intensification of an appropriate iron chelation regiment. Iron overload in this case is partially or completely reversible and the overall heart functions might considerably improve with the removal of iron, provided that the patients are compliant with their therapy [2,52]. The clearance of cardiac iron might sometimes take up to three years or more in cases of extreme cardiac iron deposition [52]. Even mild diminution of ventricular functions detected through monitoring requires a prominent increase in the dose of iron chelation therapy, even in cases of asymptomatic patients [52]. Initiation of angiotensin-converting enzyme inhibitors with the possible addition of Angiotensin II receptor blockers should be considered early when the ejection fraction approaches 50% [69]. In cases of symptomatic HF, patients should be admitted and given a gentle treatment of preload fluid reduction with diuretics to avoid acute renal failure [52,69]. Treatment with bolus diuretics might be difficult in cases of low blood pressure values observed in thalassemia patients, thus furosemide drips and constant monitoring is required [2,52,69]. A continuous dose of DFO at a rate of 50 mg/kg/day should be also administered in the presence of acceptable urine output and followed by an oral dose of DFP at a dose of 75 mg/kg/day [52]. Considering that right ventricular involvement and asynchronous left ventricular contraction are frequent in end stage HF of such patients, resynchronization of contractility with a pacing is needed to reduce the symptoms of refractory systolic HF [69]. In cases of severe HF, heart transplantation should be considered since previous results proved its feasibility in such cases [52,70]. The management of PH in the case of thromboembolism etiology generally includes starting anticoagulants with Vitamin K antagonists [2]. If thromboembolism is not the cause, the therapy of PH can include sildenafil, bosentan, epoprostenol, or individualized transfusion therapy [2]. In more severe cases of β-thalassemia, chronically transfusing blood with iron chelation therapy was thought to prevent PH [43]. Patients treated intensively from infancy with transfusion and chelation did not show any sign of PH on echocardiography later in life [43]. The higher percentage of PH in NTDT compared to TDT is attributed to the protective role blood transfusion presents in this setting [37]. Even NTDT patients who were transfused blood had a lower incidence of PH compared to non-transfused patients. This is due to the improvement of anemia, hemolysis, and the hypercoagulable state [37]. A role of hydroxyurea has also been suggested in the therapy of PH in β-thalassemia, and it has a similar protective effect as transfusions [11,37].
A study conducted by Cheung et al., concluded that the arterial system in β-thalassemia patients is affected through endothelial dysfunction and an increase in stiffness, which may be leading to a reduced mechanical ability of the heart [33]. Potential treatments for β-thalassemia vasculopathies are thought to potentially target hemolysis related complications, such as erythrocyte decompartmentalization of hemoglobin and arginase. Further investigations are needed for better understanding the underlying molecular processes of the pathogenesis [7]. Lastly, since β-thalassemia patients are at an increased risk of sudden cardiac death, symptom abnormalities and ECG changes need to be thoroughly monitored and taken into consideration. Patients, particularly at risk of SCD, might be eligible for a “blind” Holter ECG for thorough monitoring [2]. As stated earlier, there is an association between pancreatic iron and cardiovascular complications [71]. Therefore, if a β-thalassemia patient was found to have pancreatic iron overload, intensification of iron chelation therapy would be recommended to prevent future effects on glucose metabolism and cardiac iron accumulation [71]. Previous studies have discussed the improvement of glucose metabolism and hence future cardiac outcomes with a combination of DFP with DFO as iron chelators [71].
5. Conclusions
In conclusion, there have been significant advancements and breakthroughs in the diagnosis and management of β-thalassemia in the past few decades in addition to a better understanding of the disease itself. This in turn has led to an increase in the life expectancy of patients. Cardiac complications, however, still remain among the primary causes of mortality and morbidity in β-thalassemia patients. Early detection through regular follow-up visits for cardiovascular monitoring in the context of multidisciplinary care with a close collaboration between the hematologist and cardiologist is a key prevention strategy. In addition, adherence and compliance to chelation therapy can prevent and improve cardiac functions and cardiac complications and reduce overall morbidity and mortality rates. Despite the constant revelations addressing this topic, many unanswered questions remain in the management and treatment of cardiac disease in β-thalassemia.
Funding
This research received no external funding.
Conflicts of Interest
N.A., M.H.H., R.B. and K.M. have nothing to disclose. A.T.T. reports receiving consultancy from Novartis, Bristol Myers Squibb/Celgene, Vifor Pharma, and Agios Pharmaceuticals, and research funding from Novartis, Bristol Myers Squibb, Vifor Pharma, and Agios Pharmaceuticals.
References
- Russo, V.; Melillo, E.; Papa, A.A.; Rago, A.; Chamberland, C.; Nigro, G. Arrhythmias and Sudden Cardiac Death in Beta-Thalassemia Major Patients: Noninvasive Diagnostic Tools and Early Markers. Cardiol. Res. Pract. 2019, 2019, 9319832. [Google Scholar] [CrossRef] [PubMed]
- Motta, I.; Mancarella, M.; Marcon, A.; Vicenzi, M.; Cappellini, M.D. Management of age-associated medical complications in patients with beta-thalassemia. Expert Rev. Hematol. 2020, 13, 85–94. [Google Scholar] [CrossRef]
- Angastiniotis, M.; Lobitz, S. Thalassemias: An Overview. Int. J. Neonatal Screen. 2019, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Taher, A.T.; Musallam, K.M.; Cappellini, M.D. β-Thalassemias. N. Engl. J. Med. 2021, 384, 727–743. [Google Scholar] [CrossRef] [PubMed]
- Musallam, K.M.; Cappellini, M.D.; Viprakasit, V.; Kattamis, A.; Rivella, S.; Taher, A.T. Revisiting the non-transfusion-dependent (NTDT) vs. transfusion-dependent (TDT) thalassemia classification 10 years later. Am. J. Hematol. 2021, 96, E54–E56. [Google Scholar] [CrossRef]
- Ali, S.; Mumtaz, S.; Shakir, H.A.; Khan, M.; Tahir, H.M.; Mumtaz, S.; Mughal, T.A.; Hassan, A.; Kazmi, S.A.R.; Sadia; et al. Current status of beta-thalassemia and its treatment strategies. Mol. Genet. Genom. Med. 2021, 9, e1788. [Google Scholar] [CrossRef]
- Barbero, U.; Fornari, F.; Guarguagli, S.; Gaglioti, C.M.; Longo, F.; Doronzo, B.; Anselmino, M.; Piga, A. Atrial fibrillation in β-thalassemia Major Patients: Diagnosis, Management and Therapeutic Options. Hemoglobin 2018, 42, 189–193. [Google Scholar] [CrossRef]
- Pinto, V.M.; Forni, G.L. Management of Iron Overload in Beta-Thalassemia Patients: Clinical Practice Update Based on Case Series. Int. J. Mol. Sci. 2020, 21, 8771. [Google Scholar] [CrossRef]
- Adramerina, A.; Printza, N.; Hatzipantelis, E.; Symeonidis, S.; Tarazi, L.; Teli, A.; Economou, M. Use of Deferasirox Film-Coated Tablets in Pediatric Patients with Transfusion Dependent Thalassemia: A Single Center Experience. Biology 2022, 11, 247. [Google Scholar] [CrossRef]
- Aydinok, Y.; Porter, J.B.; Piga, A.; Elalfy, M.; El-Beshlawy, A.; Kilinc, Y.; Viprakasit, V.; Yesilipek, A.; Habr, D.; Quebe-Fehling, E.; et al. Prevalence and distribution of iron overload in patients with transfusion-dependent anemias differs across geographic regions: Results from the CORDELIA study. Eur. J. Haematol. 2015, 95, 244–253. [Google Scholar] [CrossRef]
- Taher, A.T.; Cappellini, M.D. How I manage medical complications of β-thalassemia in adults. Blood 2018, 132, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Koohi, F.; Kazemi, T.; Miri-Moghaddam, E. Cardiac complications and iron overload in beta thalassemia major patients—A systematic review and meta-analysis. Ann. Hematol. 2019, 98, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Adjimani, J.P.; Asare, P. Antioxidant and free radical scavenging activity of iron chelators. Toxicol. Rep. 2015, 2, 721–728. [Google Scholar] [CrossRef]
- Kremastinos, D.T.; Farmakis, D.; Aessopos, A.; Hahalis, G.; Hamodraka, E.; Tsiapras, D.; Keren, A. Beta-thalassemia cardiomyopathy: History, present considerations, and future perspectives. Circ. Heart Fail. 2010, 3, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Nomani, H.; Bayat, G.; Sahebkar, A.; Fazelifar, A.F.; Vakilian, F.; Jomezade, V.; Johnston, T.P.; Mohammadpour, A.H. Atrial fibrillation in β-thalassemia patients with a focus on the role of iron-overload and oxidative stress: A review. J. Cell. Physiol. 2019, 234, 12249–12266. [Google Scholar] [CrossRef]
- Advani, N.; Advani, N.; Andriastuti, M. The corrected QT interval prolongation in adolescents with cardiac iron overload β-thalassemia major. Turk. J. Pediatr. 2020, 62, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Hershko, C.; Link, G.; Cabantchik, I. Pathophysiology of iron overload. Ann. N. Y. Acad. Sci. 1998, 850, 191–201. [Google Scholar] [CrossRef]
- Wood, J.C. Cardiac iron across different transfusion-dependent diseases. Blood Rev. 2008, 22, S14–S21. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.A.; Sellan, M.; Simpson, J.A.; Izaddoustdar, F.; Cifelli, C.; Panama, B.K.; Davis, M.; Zhao, D.; Markhani, M.; Murphy, G.G.; et al. Iron overload decreases CaV1.3-dependent L-type Ca2+ currents leading to bradycardia, altered electrical conduction, and atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2011, 4, 733–742. [Google Scholar] [CrossRef]
- Wood, J.C.; Enriquez, C.; Ghugre, N.; Otto-Duessel, M.; Aguilar, M.; Nelson, M.D.; Moats, R.; Coates, T.D. Physiology and pathophysiology of iron cardiomyopathy in thalassemia. Ann. N. Y. Acad. Sci. 2005, 1054, 386–395. [Google Scholar] [CrossRef]
- Russo, V.; Rago, A.; Pannone, B.; Papa, A.A.; Di Meo, F.; Mayer, M.C.; Spasiano, A.; Russo, M.G.; Golino, P.; Calabro, R.; et al. Dispersion of repolarization and beta-thalassemia major: The prognostic role of QT and JT dispersion for identifying the high-risk patients for sudden death. Eur. J. Haematol. 2011, 86, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Hahalis, G.; Manolis, A.S.; Gerasimidou, I.; Alexopoulos, D.; Sitafidis, G.; Kourakli, A.; Körfer, R.; Koerner, M.M.; Vagenakis, A.G.; Zoumbos, N.C. Right ventricular diastolic function in β-thalassemia major: Echocardiographic and clinical correlates. Am. Heart J. 2001, 141, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Ciarambino, T.; Menna, G.; Sansone, G.; Giordano, M. Cardiomyopathies: An Overview. Int. J. Mol. Sci. 2021, 22, 7722. [Google Scholar] [CrossRef] [PubMed]
- Wexler, R.K.; Elton, T.; Pleister, A.; Feldman, D. Cardiomyopathy: An overview. Am. Fam. Physician 2009, 79, 778–784. [Google Scholar]
- Debonnaire, P.; Katsanos, S.; Joyce, E.; Van Den Brink, O.V.W.; Atsma, D.E.; Schalij, M.J.; Bax, J.J.; Delgado, V.; Marsan, N.A. QRS Fragmentation and QTc Duration Relate to Malignant Ventricular Tachyarrhythmias and Sudden Cardiac Death in Patients with Hypertrophic Cardiomyopathy. J. Cardiovasc. Electrophysiol. 2015, 26, 547–555. [Google Scholar] [CrossRef]
- Farmakis, D.; Triposkiadis, F.; Lekakis, J.; Parissis, J. Heart failure in haemoglobinopathies: Pathophysiology, clinical phenotypes, and management. Eur. J. Heart Fail. 2017, 19, 479–489. [Google Scholar] [CrossRef]
- Kremastinos, D.T. Heart failure in β-thalassemia. Congest. Heart Fail. 2001, 7, 312–314. [Google Scholar] [CrossRef]
- Borgna-Pignatti, C.; Meloni, A.; Guerrini, G.; Gulino, L.; Filosa, A.; Ruffo, G.B.; Casini, T.; Chiodi, E.; Lombardi, M.; Pepe, A. Myocardial iron overload in thalassaemia major. How early to check? Br. J. Haematol. 2014, 164, 579–585. [Google Scholar] [CrossRef]
- Meloni, A.; Gulino, L.; Rossi, G.; Pitrolo, L.; De Marchi, D.; Vallone, A.; Resta, M.; Positano, V.; Lombardi, M.; Pepe, A. Prognostic CMR parameters for heart failure and arrhythmias in large cohort of well treated thalssemia major patients. Eur. Heart J. 2013, 34, 1509. [Google Scholar] [CrossRef]
- Du, Z.D.; Roguin, N.; Milgram, E.; Saab, K.; Koren, A. Pulmonary hypertension in patients with thalassemia major. Am. Heart J. 1997, 134, 532–537. [Google Scholar] [CrossRef]
- Jabbar, D.A.; Davison, G.; Muslin, A.J. Getting the iron out: Preventing and treating heart failure in transfusion-dependent thalassemia. Cleve. Clin. J. Med. 2007, 74, 807–810, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, Z.G.; Rund, D. Myocardial infarction in a patient with beta-thalassemia major: First report. Am. J. Hematol. 2004, 75, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.F.; Chan, G.C.; Ha, S.Y. Arterial stiffness and endothelial function in patients with beta-thalassemia major. Circulation 2002, 106, 2561–2566. [Google Scholar] [CrossRef]
- Maioli, M.; Vigna, G.B.; Tonolo, G.; Brizzi, P.; Ciccarese, M.; Donegà, P.; Maioli, M.; Fellin, R. Plasma lipoprotein composition, apolipoprotein(a) concentration and isoforms in β-thalassemia. Atherosclerosis 1997, 131, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Rhee, T.-M.; Jeon, K.; Cho, Y.; Lee, S.-W.; Han, K.-D.; Seong, M.-W.; Park, S.-S.; Lee, Y.K. Epidemiologic Trends of Thalassemia, 2006–2018: A Nationwide Population-Based Study. J. Clin. Med. 2022, 11, 2289. [Google Scholar]
- Helmi, N.; Choudhry, H.; Qari, M.; Kumosani, T.A.; Al-Malki, A.L.; Moselhy, S.S.; Kumosani, A.T. Association of serum asymmetric dimethyl-arginine and troponin I levels as a risk of myocardial infarction in thalassemia. Afr. Health Sci. 2018, 18, 720–726. [Google Scholar] [CrossRef]
- Derchi, G.; Galanello, R.; Bina, P.; Cappellini, M.D.; Piga, A.; Lai, M.E.; Quarta, A.; Casu, G.; Perrotta, S.; Pinto, V.; et al. Prevalence and risk factors for pulmonary arterial hypertension in a large group of β-thalassemia patients using right heart catheterization: A Webthal study. Circulation 2014, 129, 338–345. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Taher, A.; Vichinsky, E.; Musallam, K.; Cappellini, M.D.; Viprakasit, V. Guidelines for the Management of Non Transfusion Dependent Thalassaemia (NTDT); Weatherall, D., Ed.; Thalassaemia International Federation: Nicosia, Cyprus, 2013. [Google Scholar]
- Musallam, K.M.; Taher, A.T.; Rachmilewitz, E.A. β-thalassemia intermedia: A clinical perspective. Cold Spring Harb. Perspect. Med. 2012, 2, a013482. [Google Scholar] [CrossRef]
- Mokhtar, G.M.; Adly, A.A.; El Alfy, M.S.; Tawfik, L.M.; Khairy, A.T. N-terminal natriuretic peptide and ventilation-perfusion lung scan in sickle cell disease and thalassemia patients with pulmonary hypertension. Hemoglobin 2010, 34, 78–94. [Google Scholar] [CrossRef]
- Taher, A.T.; Cappellini, M.D.; Bou-Fakhredin, R.; Coriu, D.; Musallam, K.M. Hypercoagulability and Vascular Disease. Hematol. Oncol. Clin. North Am. 2018, 32, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Fraidenburg, D.R.; Machado, R.F. Pulmonary hypertension associated with thalassemia syndromes. Ann. N. Y. Acad. Sci. 2016, 1368, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, J.; Tarhini, A.; Bou-Fakhredin, R.; Saliba, A.N.; Cappellini, M.D.; Taher, A.T. Non-Transfusion-Dependent Thalassemia: An Update on Complications and Management. Int. J. Mol. Sci. 2018, 19, 182. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Rago, A.; Pannone, B.; Mayer, M.C.; Spasiano, A.; Calabro, R.; Russo, M.G.; Gerardo, N.; Papa, A.A. Atrial Fibrillation and Beta Thalassemia Major: The Predictive Role of the 12-lead Electrocardiogram Analysis. Indian Pacing Electrophysiol. J. 2014, 14, 121–132. [Google Scholar] [CrossRef]
- Pennell, D.J.; Udelson, J.E.; Arai, A.E.; Bozkurt, B.; Cohen, A.R.; Galanello, R.; Hoffman, T.M.; Kiernan, M.S.; Lerakis, S.; Piga, A.; et al. Cardiovascular function and treatment in β-thalassemia major: A consensus statement from the American Heart Association. Circulation 2013, 128, 281–308. [Google Scholar] [CrossRef]
- Malagù, M.; Marchini, F.; Fiorio, A.; Sirugo, P.; Clò, S.; Mari, E.; Gamberini, M.R.; Rapezzi, C.; Bertini, M. Atrial Fibrillation in β-Thalassemia: Overview of Mechanism, Significance and Clinical Management. Biology 2022, 11, 148. [Google Scholar] [CrossRef]
- Guerra, F.; Brambatti, M.; Nieuwlaat, R.; Marcucci, M.; Dudink, E.; Crijns, H.; Matassini, M.V.; Capucci, A. Symptomatic atrial fibrillation and risk of cardiovascular events: Data from the Euro Heart Survey. Europace 2017, 19, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.; Mohamed, S.; Ako, E.; Chatterjee, R.; Bajoria, R.; Porter, J.; Walker, J. The prevalence and risk factors for atrial fibrillation in beta-thalassemia major: A cross-sectional study in a UK specialist cardio-haematology clinic. Eur. Heart J. 2015, 36, 916. [Google Scholar]
- Stoyanova, E.; Trudel, M.; Felfly, H.; Lemsaddek, W.; Garcia, D.; Cloutier, G. Vascular endothelial dysfunction in beta-thalassemia occurs despite increased eNOS expression and preserved vascular smooth muscle cell reactivity to NO. PLoS ONE 2012, 7, e38089. [Google Scholar] [CrossRef]
- Hahalis, G.; Kremastinos, D.T.; Terzis, G.; Kalogeropoulos, A.P.; Chrysanthopoulou, A.; Karakantza, M.; Kourakli, A.; Adamopoulos, S.; Tselepis, A.D.; Grapsas, N.; et al. Global vasomotor dysfunction and accelerated vascular aging in β-thalassemia major. Atherosclerosis 2008, 198, 448–457. [Google Scholar] [CrossRef]
- Cappellini, M.D.; Cohen, A.; Porter, J.; Taher, A.; Viprakasit, V. (Eds.) Guidelines for the Management of Transfusion Dependent Thalassaemia (TDT); Thalassaemia International Federation: Nicosia, Cyprus, 2021. [Google Scholar]
- Nassef, S.; El Shenoufy, M.; Rawi, R.; El Demerdash, D.; Hassan, M.; Mustafa, H.; Mattar, M.; El Husseiny, N. Assessment of Atherosclerosis in Peripheral and Central Circulation in Adult β Thalassemia Intermedia Patients by Color Doppler Ultrasound: Egyptian Experience. J. Vasc. Res. 2020, 57, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Adly, A.A.; El-Sherif, N.H.; Ismail, E.A.; El-Zaher, Y.A.; Farouk, A.; El-Refaey, A.M.; Wahba, M.S. Vascular dysfunction in patients with young β-thalassemia: Relation to cardiovascular complications and subclinical atherosclerosis. Clin. Appl. Thromb. Hemost. 2015, 21, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.; Zhang, L.; Kim, C.; Uy-Evanado, A.; Teodorescu, C.; Reinier, K.; Zheng, Z.J.; Gunson, K.; Jui, J.; Chugh, S.S. QRS fragmentation and sudden cardiac death in the obese and overweight. J. Am. Heart Assoc. 2015, 4, e001654. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, S. Abnormal QT Dispersion Predicts Unexpected Sudden Death in Young Patients with Thalassemia Major. Ann. Noninvasive Electrocardiol. 1999, 4, 295–300. [Google Scholar] [CrossRef]
- Cogliandro, T.; Derchi, G.; Mancuso, L.; Mayer, M.C.; Pannone, B.; Pepe, A.; Pili, M.; Bina, P.; Cianciulli, P.; De Sanctis, V.; et al. Guideline recommendations for heart complications in thalassemia major. J. Cardiovasc. Med. 2008, 9, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Najimi, M.; Ghandi, Y.; Mehrabi, S.; Eghbali, A.; Habibi, D. Correlation between Myocardial Iron Overload Detected by CMRT2* and Left Ventricular Function Assessed by Tissue Doppler Imaging in Patients with Thalassemia Major. J. Cardiovasc. Echogr. 2022, 32, 17–22. [Google Scholar]
- Ramazzotti, A.; Pepe, A.; Positano, V.; Scattini, B.; Santarelli, M.F.; Landini, L.; De Marchi, D.; Keilberg, P.; Derchi, G.; Formisano, F.; et al. Standardized T2* map of a normal human heart to correct T2* segmental artefacts; myocardial iron overload and fibrosis in thalassemia intermedia versus thalassemia major patients and electrocardiogram changes in thalassemia major patients. Hemoglobin 2008, 32, 97–107. [Google Scholar] [CrossRef]
- Anderson, L.J.; Holden, S.; Davis, B.; Prescott, E.; Charrier, C.C.; Bunce, N.H.; Firmin, D.N.; Wonke, B.; Porter, J.; Walker, J.M.; et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur. Heart J. 2001, 22, 2171–2179. [Google Scholar] [CrossRef]
- Chaosuwannakit, N.; Makarawate, P.; Wanitpongpun, C. The Importance of Cardiac T2* Magnetic Resonance Imaging for Monitoring Cardiac Siderosis in Thalassemia Major Patients. Tomography 2021, 7, 130–138. [Google Scholar] [CrossRef]
- Pennell, D.J.; Porter, J.B.; Cappellini, M.D.; Chan, L.L.; El-Beshlawy, A.; Aydinok, Y.; Ibrahim, H.; Li, C.K.; Viprakasit, V.; Elalfy, M.S.; et al. Deferasirox for up to 3 years leads to continued improvement of myocardial T2* in patients with β-thalassemia major. Haematologica 2012, 97, 842–848. [Google Scholar] [CrossRef]
- Gupta, V.; Kumar, I.; Raj, V.; Aggarwal, P.; Agrawal, V. Comparison of the effects of calcium channel blockers plus iron chelation therapy versus chelation therapy only on iron overload in children and young adults with transfusion-dependent thalassemia: A randomized double-blind placebo-controlled trial. Pediatr. Blood Cancer 2022, 69, e29564. [Google Scholar] [CrossRef] [PubMed]
- Zargari, A.; Wu, S.; Greenway, A.; Cheng, K.; Kaplan, Z. Effects of dual chelation therapy with deferasirox and deferoxamine in patients with beta thalassaemia major. Vox Sang. 2022, 117, 733–737. [Google Scholar] [CrossRef]
- Elfaituri, M.K.; Ghozy, S.; Ebied, A.; Morra, M.E.; Hassan, O.G.; Alhusseiny, A.; Abbas, A.S.; Sherif, N.A.; Fernandes, J.L.; Huy, N.T. Amlodipine as adjuvant therapy to current chelating agents for reducing iron overload in thalassaemia major: A systematic review, meta-analysis and simulation of future studies. Vox Sang. 2021, 116, 887–897. [Google Scholar] [CrossRef]
- Oudit, G.Y.; Trivieri, M.G.; Khaper, N.; Liu, P.P.; Backx, P.H. Role of L-type Ca2+ channels in iron transport and iron-overload cardiomyopathy. J. Mol. Med. 2006, 84, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Bahrani, S.; Teimouri-Jervekani, Z.; Sadeghi, M. Thrombotic Events and Anticoagulants in Beta-thalassemia Patients with Focus on Anticoagulants for Atrial Fibrillation: A Brief Review. Curr. Probl. Cardiol. 2022, 47, 100912. [Google Scholar] [CrossRef]
- Gažová, A.; Leddy, J.J.; Rexová, M.; Hlivák, P.; Hatala, R.; Kyselovič, J. Predictive value of CHA2DS2-VASc scores regarding the risk of stroke and all-cause mortality in patients with atrial fibrillation (CONSORT compliant). Medicine 2019, 98, e16560. [Google Scholar] [CrossRef]
- Hahalis, G.; Alexopoulos, D.; Kremastinos, D.T.; Zoumbos, N.C. Heart failure in beta-thalassemia syndromes: A decade of progress. Am. J. Med. 2005, 118, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Koerner, M.M.; Tenderich, G.; Minami, K.; zu Knyphausen, E.; Mannebach, H.; Kleesiek, K.; Meyer, H.; Koerfer, R. Heart transplantation for end-stage heart failure caused by iron overload. Br. J. Haematol. 1997, 97, 293–296. [Google Scholar] [CrossRef]
- Pepe, A.; Pistoia, L.; Gamberini, M.R.; Cuccia, L.; Peluso, A.; Messina, G.; Spasiano, A.; Allò, M.; Bisconte, M.G.; Putti, M.C.; et al. The Close Link of Pancreatic Iron With Glucose Metabolism and With Cardiac Complications in Thalassemia Major: A Large, Multicenter Observational Study. Diabetes Care 2020, 43, 2830–2839. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).