A Concept for MRI-Based Cholesteatoma Detection in Cochlear Implant Recipients
Abstract
1. Introduction
2. Method
3. Results
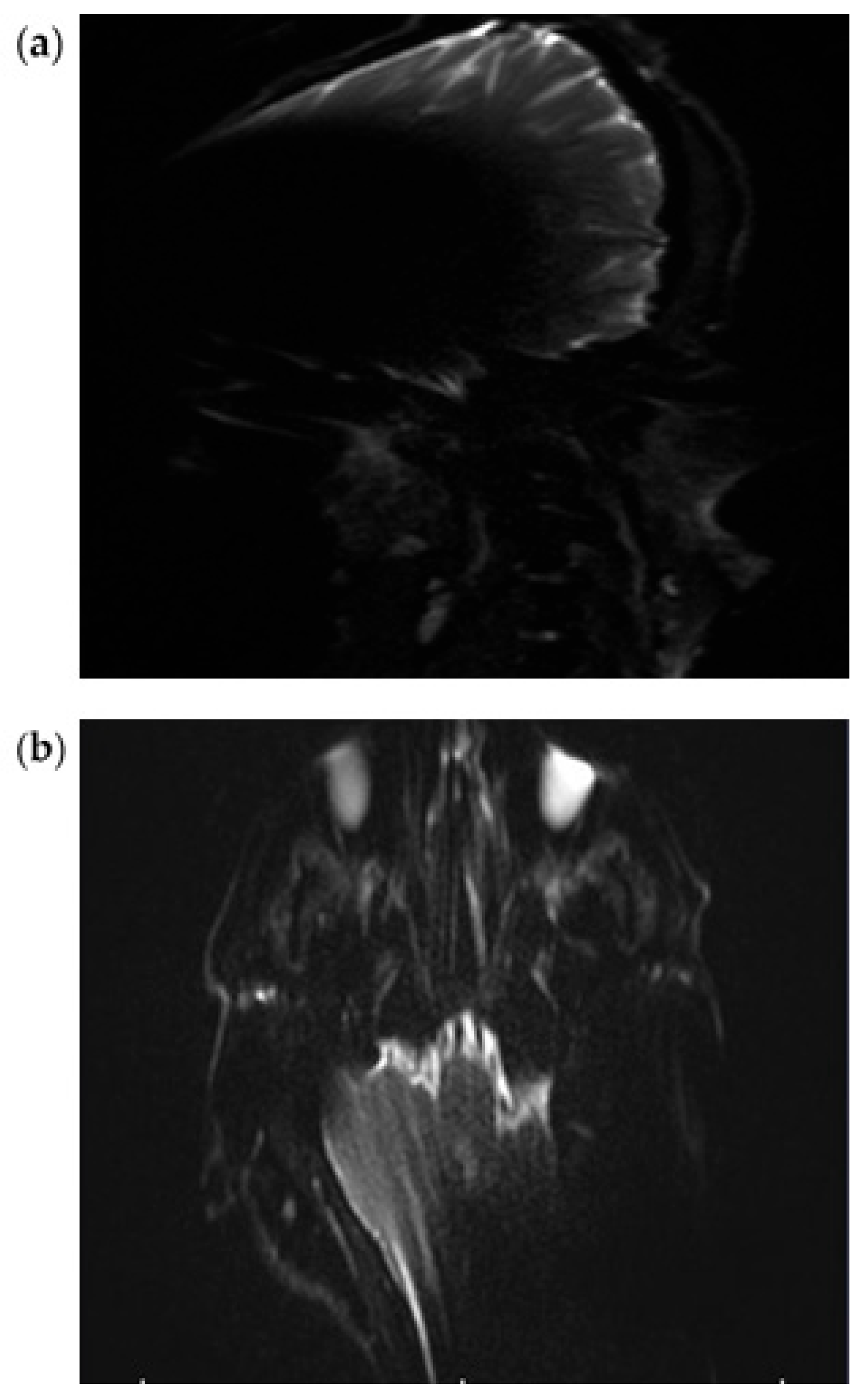
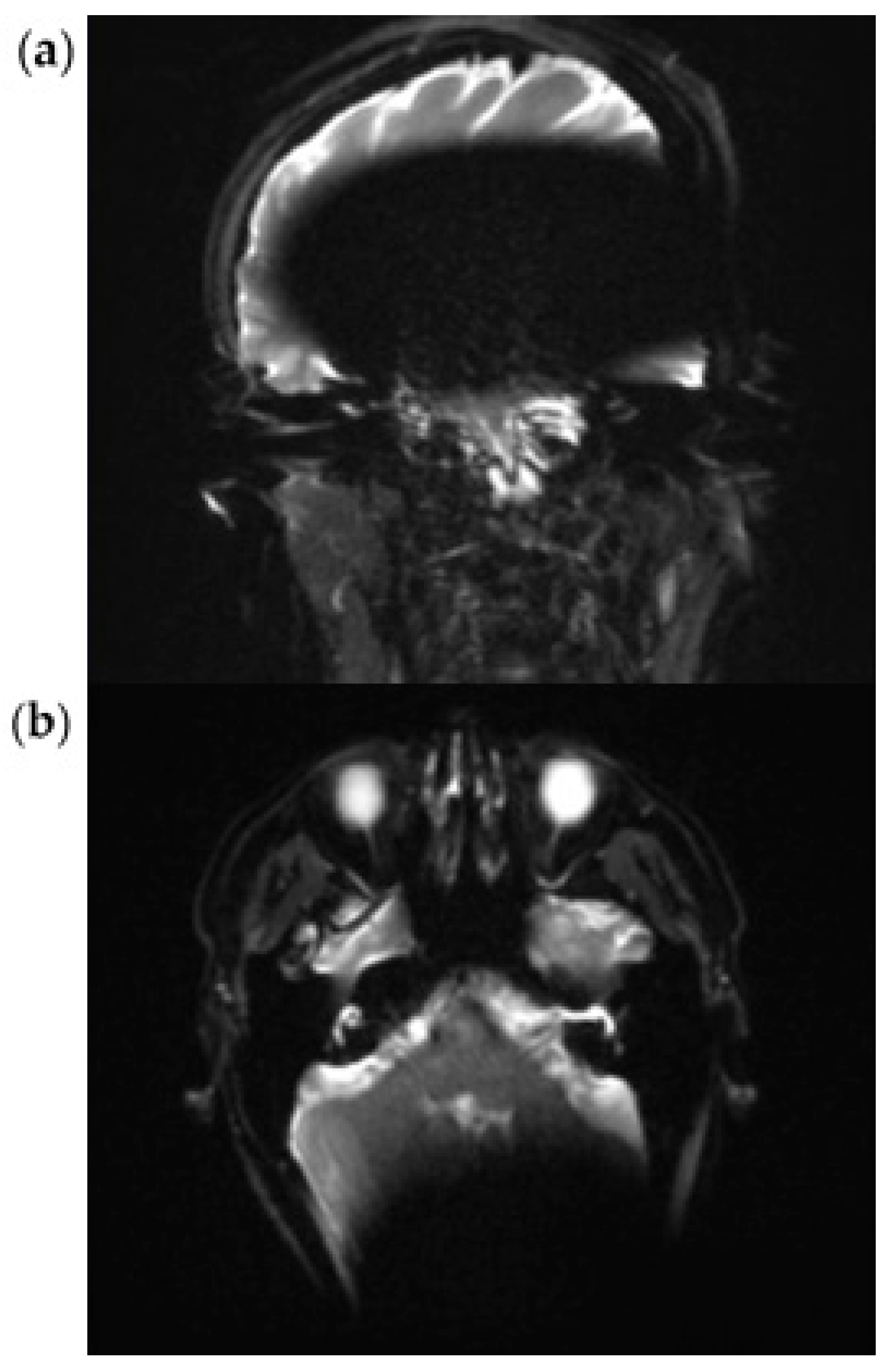
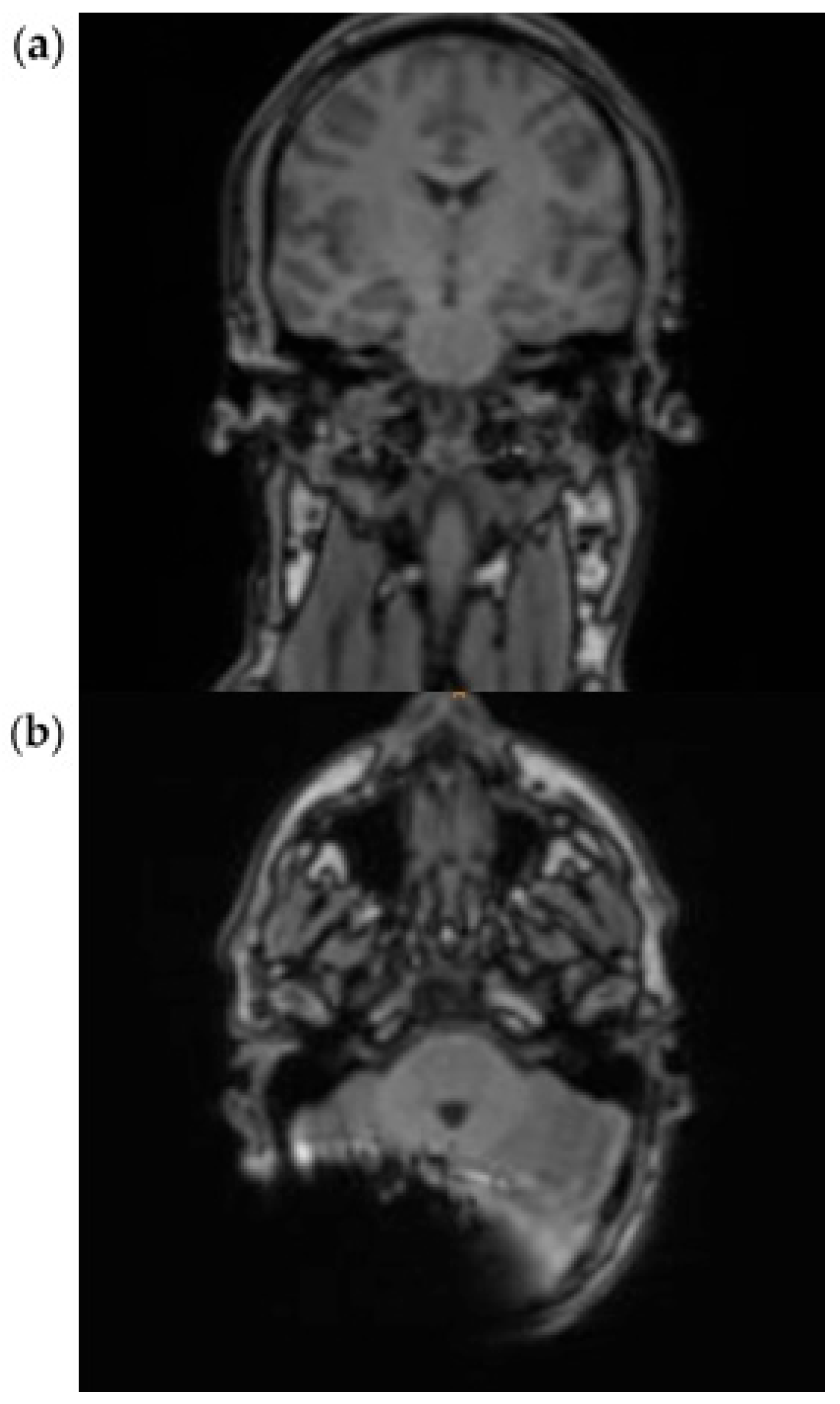
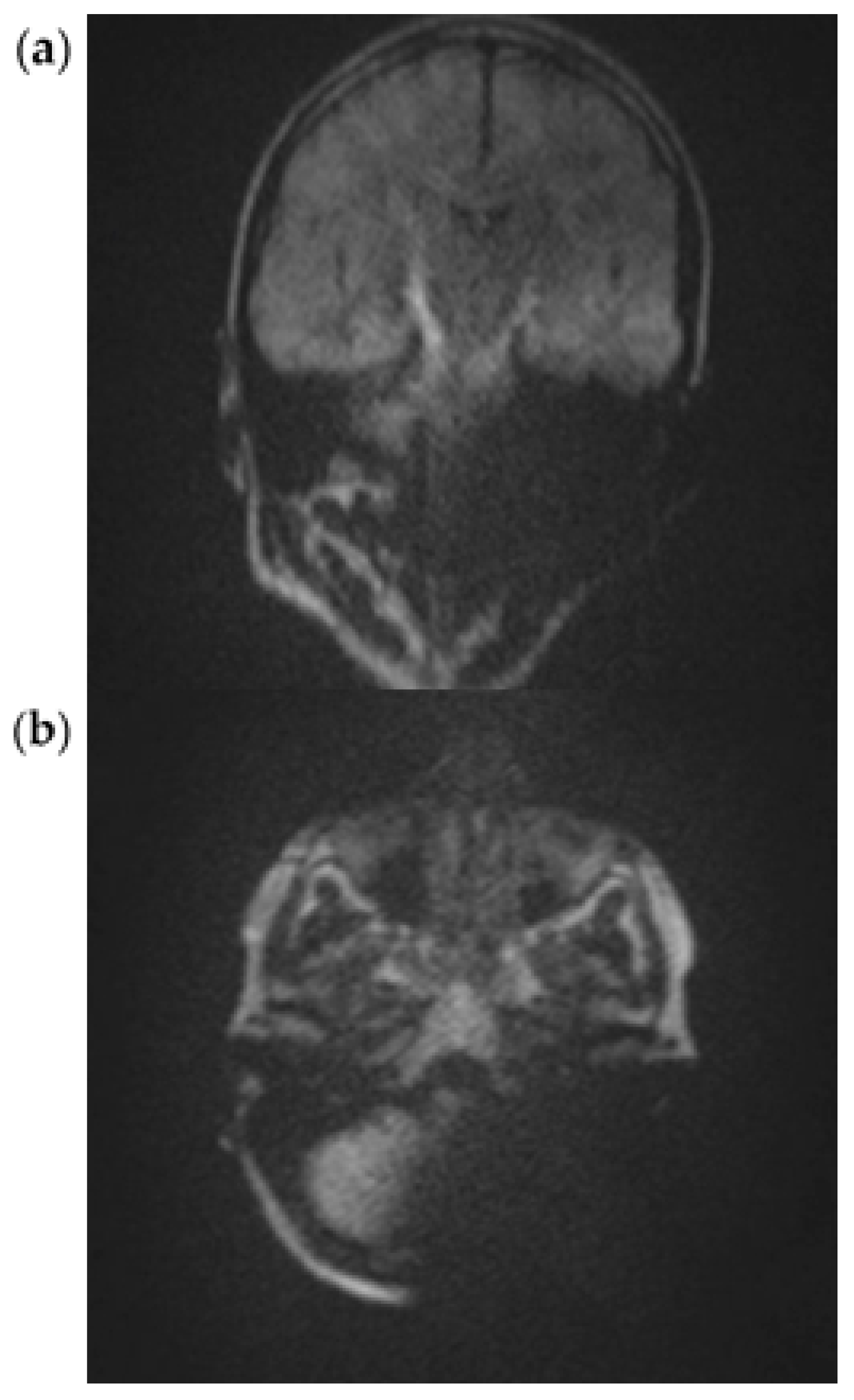
3.1. EP2D-Diff-Sequence
3.2. RESOLVE Sequence
3.3. HASTE Sequence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van de Heyning, P.; Mertens, G.; Topsakal, V.; de Brito, R.; Wimmer, W.; Caversaccio, M.D.; Dazert, S.; Volkenstein, S.; Zernotti, M.; Parnes, L.S.; et al. Two-phase survey on the frequency of use and safety of MRI for hearing implant recipients. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 4225–4233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Todt, I.; Rademacher, G.; Mittmann, P.; Wagner, J.; Mutze, S.; Ernst, A. MRI Artifacts and Cochlear Implant Positioning at 3 T In Vivo. Otol. Neurotol. 2015, 36, 972–976. [Google Scholar] [CrossRef]
- Todt, I.; Tittel, A.; Ernst, A.; Mittmann, P.; Mutze, S. Pain Free 3 T MRI Scans in Cochlear Implantees. Otol. Neurotol. 2017, 38, e401–e404. [Google Scholar] [CrossRef]
- Ay, N.; Gehl, H.B.; Sudhoff, H.; Todt, I. Effect of head position on cochlear implant MRI artifact. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 2763–2767. [Google Scholar] [CrossRef] [PubMed]
- Sudhoff, H.; Gehl, H.B.; Scholtz, L.U.; Todt, I. MRI observation after intralabyrinthine and vestibular schwannoma resection and cochlear implantation. Front. Neurol. 2020, 11, 759. [Google Scholar] [CrossRef] [PubMed]
- Sudhoff, H.; Scholtz, L.U.; Gehl, H.B.; Todt, I. Quality Control after Intracochlear Intralabyrinthine Schwannoma Resection and Cochlear Implantation. Brain Sci. 2021, 11, 1221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Edmonson, H.A.; Carlson, M.L.; Patton, A.C.; Watson, R.E. MR imaging and cochlear implants with retained internal magnets: Reducing artifacts near highly inhomogeneous magnetic fields. RadioGraphics 2018, 38, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Canzi, P.; Carlotto, E.; Simoncelli, A.; Lafe, E.; Scribante, A.; Minervini, D.; Nardo, M.; Malpede, S.; Chiapparini, L.; Benazzo, M. The usefulness of the O-MAR algorithm in MRI skull base assessment to manage cochlear implant-related artifacts. Acta Otorhinolaryngol. Ital. 2023, 43, 273–282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Winchester, A.; Cottrell, J.; Kay-Rivest, E.; Friedmann, D.; McMenomey, S.; Thomas Roland, J., Jr.; Bruno, M.; Hagiwara, M.; Moonis, G.; Jethanamest, D. Image Quality Improvement in MRI of Cochlear Implants and Auditory Brainstem Implants After Metal Artifact Reduction Techniques. Otol. Neurotol. 2025, 46, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Tran Ba Huy, P.; Lévy, C.; Bensimon, J.L.; Cophignon, J. Cinq cas de kyste épidermoïde (ou cholestéatome primitif) de la pyramide pétreuse.Aspects clinique, radiologique, pathogénique et thérapeutique. Intérêt de la RMN dans la diagnostic et la surveillance post-operatoire [5 cases of epidermoid cyst (or primary cholesteatoma) of the petrous pyramid. Clinical, radiologic, pathogenic and therapeutic aspects. Value of nuclear magnetic resonance in the diagnosis and postoperative monitoring]. Ann. Otolaryngol. Chir. Cervicofac. 1986, 103, 363–371. [Google Scholar] [PubMed]
- Fitzek, C.; Mewes, T.; Fitzek, S.; Mentzel, H.J.; Hunsche, S.; Stoeter, P. Diffusion–weighted MRI of cholesteatomas of the petrous bone. J. Magn. Reson. Imaging 2002, 15, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Jindal, M.; Riskalla, A.; Jiang, D.; Connor, S.; O’Connor, A.F. A systematic review of diffusion-weighted magnetic resonance imaging in the assessment of postoperative cholesteatoma. Otol. Neurotol. 2011, 32, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- De Foer, B.; Vercruysse, J.P.; Pilet, B.; Michiels, J.; Vertriest, R.; Pouillon, M.; Somers, T.; Casselman, J.W.; Offeciers, E. Single-shot, turbo spin-echo, diffusion-weighted imaging versus spin-echo-planar, diffusion-weighted imaging in the detection of acquired middle ear cholesteatoma. Am. J. Neuroradiol. 2006, 27, 1480–1482. [Google Scholar] [PubMed] [PubMed Central]
- Nassiri, A.M.; Messina, S.A.; Benson, J.C.; Lane, J.I.; McGee, K.P.; Trzasko, J.D.; Carlson, M.L. Magnetic Resonance Imaging Artifact Associated with Transcutaneous Bone Conduction Implants: Cholesteatoma and Vestibular Schwannoma Surveillance. Otolaryngol, Head Neck Surg. 2024, 170, 187–194. [Google Scholar] [CrossRef]
- Bozer, A.; Adıbelli, Z.H.; Yener, Y.; Dalgıç, A. Diagnostic performance of multishot echo-planar imaging (RESOLVE) and non-echo-planar imaging (HASTE) diffusion-weighted imaging in cholesteatoma with an emphasis on signal intensity ratio measurement. Diagn. Interv. Radiol. 2024, 30, 370–377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rambo, J.H. Primary closure of the radical mastoidectomy wound; a technique to eliminate postoperative care. Laryngoscope 1958, 68, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Bendet, E.; Cerenko, D.; Linder, T.E.; Fisch, U. Cochlear implantation after subtotal petrosectomies. Eur. Arch. Oto-Rhino-Laryngol. 1998, 255, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Canzi, P.; Berrettini, S.; Albera, A.; Barbara, M.; Bruschini, L.; Canale, A.; Carlotto, E.; Covelli, E.; Cuda, D.; Dispenza, F.; et al. Current trends on subtotal petrosectomy with cochlear implantation in recalcitrant chronic middle ear disorders. Acta Otorhinolaryngol. Ital. 2023, 43 (Suppl. 1), S67–S75. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L.; Damam, S.K.; Yilala, M.H.; Fancello, G.; Ferraro, M.; Caruso, A.; Sanna, M. Subtotal petrosectomy in cochlear implantation: Gruppo Otologico experience after 348 cases. Eur. Arch. Oto-Rhino-Laryngol. 2025, 282, 5091–5099. [Google Scholar] [CrossRef] [PubMed]
- Arístegui, I.; Aranguez, G.; Casqueiro, J.C.; Gutiérrez-Triguero, M.; Pozo, A.; Arístegui, M. Subtotal Petrosectomy (SP) in Cochlear Implantation (CI): A Report of 92 Cases. Audiol. Res. 2022, 12, 113–125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gray, F.; Ray, J.; McFerran, J. Further experience with fat graft obliteration of mastoid cavities for cochlear. J. Laryngol. Otol. 1999, 113, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Reddy, P.D.; Isaac, M.J.; Nguyen, S.A.; McRackan, T.R.; Meyer, T.A. Subtotal Petrosectomy and Cochlear Implantation: A Systematic Review and Meta-analysis. Arch. Otolaryngol. Neck Surg. 2020, 147, 1–12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Macielak, R.J.; Kull, A.J.; Carlson, M.L.; Patel, N.S. Disease recidivism after subtotal petrosectomy and ear canal closure. Am. J. Otolaryngol. 2023, 44, 103743. [Google Scholar] [CrossRef] [PubMed]
- Eichler, T.; Ibrahim, A.; Riemann, C.; Scholtz, L.U.; Gehl, H.B.; Goon, P.; Sudhoff, H.; Todt, I. Prospective Evaluation of 3 T MRI Effect on Residual Hearing Function of Cochlea Implantees. Brain Sci. 2022, 12, 1406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| 1.5 T | 3 T | |||
|---|---|---|---|---|
| MAGNETOM Altea, Siemens Healthineers, Erlangen, Germany | 1.5 T | MAGNETOM Skyra, Siemens Healthineers, Erlangen, Germany | 3 T | 3 T |
| Transmit Coil | Body | Transmit Coil | Body | Body |
| Receive Coil | HeadNeck_20_TCS | Receive Coil | HeadNeck_20 | HeadNeck_20 |
| Sequence Type | HASTE DIFFUSION | Sequence Type | EPI DIFFUSION | RESOLVE DIFFUSION |
| Protocol Name | t2_haste_diff_p2 | Protocol Name | ep2d_diff_4scan_trace_p2 | resolve_4scan_trace_p2_192 |
| voxel size | 1.2 × 1.6 × 3 mm | 3voxel size | 1.2 × 1.2 × 3 mm3 | 1.2 × 1.2 × 3 mm3 |
| FOV | 230 × 230 mm | 2FOV | 230 × 230 mm2 | 230 × 230 mm2 |
| TR | 2000 ms | TR | 10,400 ms | 7280 ms |
| TE | 107 ms | TE | 85 ms | 65.18 ms |
| flip angle | 150° | flip angle | 90° | 180° |
| Slice Thickness | 3 mm | Slice Thickness | 3 mm | 3 mm |
| Spacing Between Slices | 3.3 mm | Spacing Between Slices | 3.9 mm | 3.9 mm |
| Acquisition Matrix | 192 × 144 | Acquisition Matrix | 192 × 192 | 192 × 192 |
| Number of Averages | 12 | Number of Averages (b0) | 2 | 1 |
| TA | 07:17 min | Number of Averages (b1000) | 3 | 1 |
| TA | 03:19 min | 04:45 min | ||
| Structure | EPI | RESOLVE | HASTE |
|---|---|---|---|
| Ipsilateral Mastoid | Not assessable | assessable | assessable |
| Contralateral Mastoid | Not assessable | assessable | assessable |
| Ipsilateral IAC | Not assessable | mainly assessable | assessable |
| Contralateral IAC | Not assessable | assessable | assessable |
| Ipsilateral EAC | Not assessable | mainly assessable | assessable |
| Contralateral EAC | Not assessable | assessable | assessable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woltersdorf, L.; Kim, R.; Rempen, A.; Pfeiffer, C.; Scholtz, L.-U.; Schimmack, C.; Eickenjäger, D.; Steinbach, R.; Todt, I. A Concept for MRI-Based Cholesteatoma Detection in Cochlear Implant Recipients. Audiol. Res. 2025, 15, 162. https://doi.org/10.3390/audiolres15060162
Woltersdorf L, Kim R, Rempen A, Pfeiffer C, Scholtz L-U, Schimmack C, Eickenjäger D, Steinbach R, Todt I. A Concept for MRI-Based Cholesteatoma Detection in Cochlear Implant Recipients. Audiology Research. 2025; 15(6):162. https://doi.org/10.3390/audiolres15060162
Chicago/Turabian StyleWoltersdorf, Lukas, Rayoung Kim, Alexander Rempen, Christoph Pfeiffer, Lars-Uwe Scholtz, Christiane Schimmack, Daniela Eickenjäger, Rüdiger Steinbach, and Ingo Todt. 2025. "A Concept for MRI-Based Cholesteatoma Detection in Cochlear Implant Recipients" Audiology Research 15, no. 6: 162. https://doi.org/10.3390/audiolres15060162
APA StyleWoltersdorf, L., Kim, R., Rempen, A., Pfeiffer, C., Scholtz, L.-U., Schimmack, C., Eickenjäger, D., Steinbach, R., & Todt, I. (2025). A Concept for MRI-Based Cholesteatoma Detection in Cochlear Implant Recipients. Audiology Research, 15(6), 162. https://doi.org/10.3390/audiolres15060162







