Brainstem Stroke and Dysphagia Treatment: A Narrative Review on the Role of Neuromodulation, Skill-Based Swallowing Training and Transient Receptor Potential Agonists
Abstract
1. Introduction
2. Physiology and Pathophysiology of Dysphagia After Brainstem Stroke
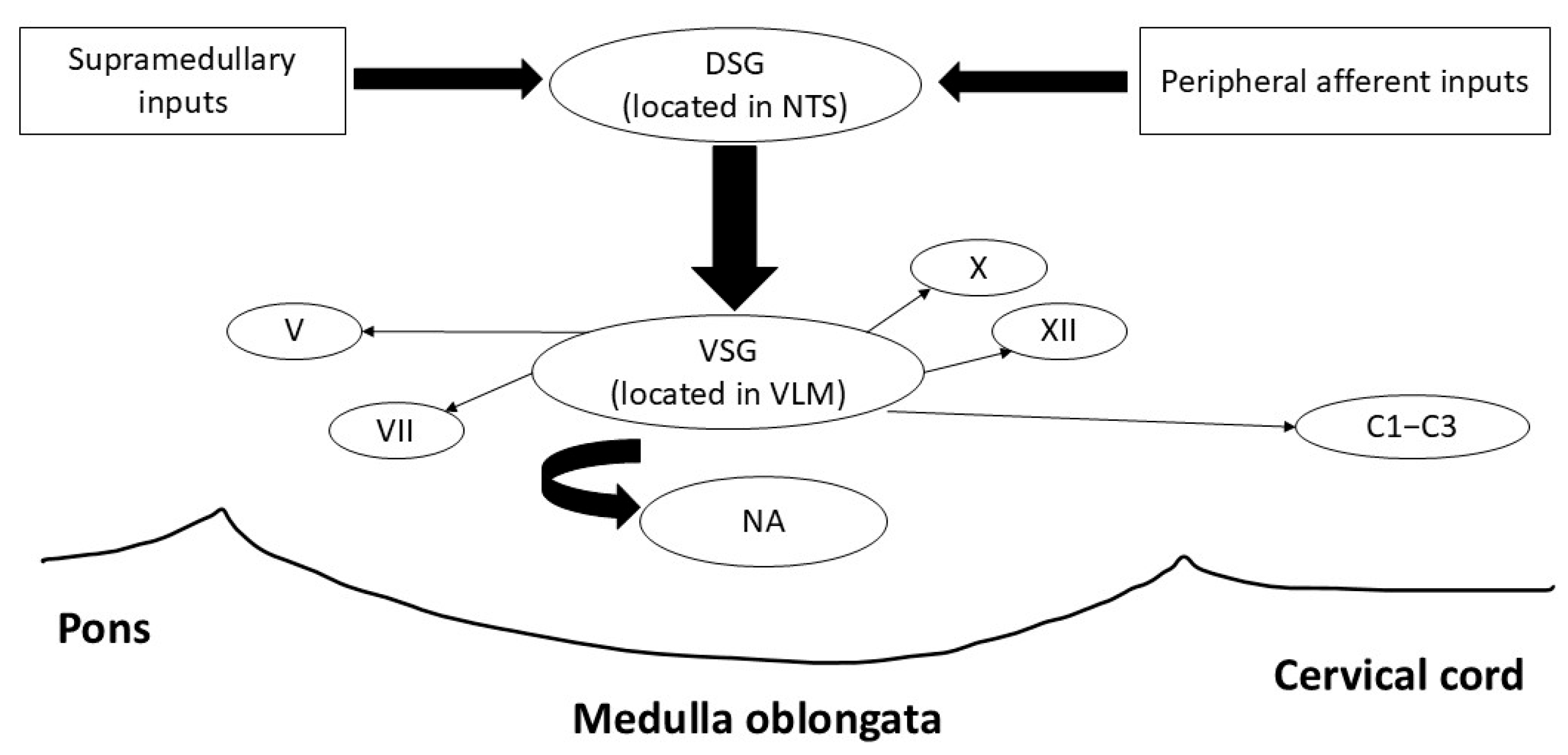
2.1. Swallowing and Brainstem Anatomy
2.2. Pathophysiology of Dysphagia After Brainstem Stroke
3. Neuromodulation for Dysphagia After Brainstem Stroke
3.1. Pharyngeal Electrical Stimulation (PES)
3.2. Repetitive Transcranial Magnetic Stimulation (rTMS)
3.3. Transcranial Direct Current Stimulation (tDCS)
4. Skill-Based Swallowing Training
5. Transient Receptors Potential (TRP) Agonists
Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AusTOMs | Australian Therapy Outcome Measures |
| CRB | Cerebellum |
| CGRP | Calcitonin gene-related peptide |
| CPD | Cricopharyngeal muscle dysfunction |
| CPG | Central pattern generator |
| CT | Computerized topography |
| DG | Dysphagic grade |
| DOSS | Dysphagia Outcome and Severity Scale |
| DSG | Dorsal swallowing group |
| EC | European Commission |
| FDA | Food and Drug Administration |
| FDS | Functional Dysphagia Scale |
| FEES | Fibreoptic endoscopic evaluation of swallowing |
| fMRI | Functional magnetic resonance imaging |
| FOIS | Functional Oral Intake Scale |
| LMS | Lateral medullary syndrome |
| LVC | Laryngeal vestibule closure |
| MEP | Motor evoked potential |
| MRI | Magnetic resonance imaging |
| NA | Nucleus ambiguus |
| NIBS | Non-invasive brain stimulation |
| NMDA | N-methyl-D-aspartate |
| NTS | Nucleus tractus solitarius |
| PAS | Penetration Aspiration Scale |
| PES | Pharyngeal electrical stimulation |
| PSD | Post-stroke dysphagia |
| RCT | randomized controlled trial |
| RMT | Resting motor threshold |
| RTMS | Repetitive transcranial magnetic stimulation |
| SMA | supplementary motor area |
| SMD | standardized mean difference |
| sEMG | Surface electromyography |
| TENS | Transcutaneous electrical nerve stimulation |
| TDCS | Transcranial direct current stimulation |
| TRP | Transient receptors potential |
| TRPA1 | Transient receptor potential vanilloid 1 |
| TRPM8 | Transient receptor potential ankyrin 1 |
| TRPV1 | Transient receptor potential melastatin 8 |
| UESO | Upper esophageal sphincter opening |
| VSG | Ventral swallowing group |
References
- Sasegbon, A.; Cheng, I.; Hamdy, S. The neurorehabilitation of post-stroke dysphagia: Physiology and pathophysiology. J. Physiol. 2024, 603, 617–634. [Google Scholar] [CrossRef]
- Steuer, I.; Guertin, P.A. Central pattern generators in the brainstem and spinal cord: An overview of basic principles, similarities and differences. Rev. Neurosci. 2019, 30, 107–164. [Google Scholar] [CrossRef]
- Gowda, S.N.; Munakomi, S.; De Jesus, O. Brainstem Stroke. In StatPearls; StatPearls Publishing: Orlando, FL, USA, 2024. [Google Scholar]
- González-Fernández, M.; Ottenstein, L.; Atanelov, L.; Christian, A.B. Dysphagia after stroke: An overview. Curr. Phys. Med. Rehabil. Rep. 2013, 1, 187–196. [Google Scholar] [CrossRef]
- Forstenpointner, J.; Maallo, A.M.S.; Elman, I.; Holmes, S.; Freeman, R.; Baron, R.; Borsook, D. The solitary nucleus connectivity to key autonomic regions in humans. Eur. J. Neurosci. 2022, 56, 3938–3966. [Google Scholar] [CrossRef] [PubMed]
- Cheng, I.; Takahashi, K.; Miller, A.J.; Hamdy, S. Cerebral control of swallowing: An update on neurobehavioral evidence. J. Neurol. Sci. 2022, 442, 120434. [Google Scholar] [CrossRef] [PubMed]
- Jean, A. Brain stem control of swallowing: Neuronal network and cellular mechanisms. Physiol. Rev. 2001, 81, 929–969. [Google Scholar] [CrossRef]
- Martin-Harris, B.; Brodsky, M.B.; Price, C.C.; Michel, Y.; Walters, B. Temporal coordination of pharyngeal and laryngeal dynamics with breathing during swallowing: Single liquid swallows. J. Appl. Physiol. 2003, 94, 1735–1743. [Google Scholar] [CrossRef]
- Martin-Harris, B.; Brodsky, M.B.; Michel, Y.; Ford, C.L.; Walters, B.; Heffner, J. Breathing and swallowing dynamics across the adult lifespan. Arch. Otolaryngol. Head Neck Surg. 2005, 131, 762–770. [Google Scholar] [CrossRef]
- Bautista, T.G.; Sun, Q.-J.; Pilowsky, P.M. The generation of pharyngeal phase of swallow and its coordination with breathing: Interaction between the swallow and respiratory central pattern generators. Prog. Brain Res. 2014, 212, 253–275. [Google Scholar] [PubMed]
- Qiao, J.; Wu, Z.-m.; Ye, Q.-p.; Dai, M.; Dai, Y.; He, Z.-t.; Dou, Z.-l. Characteristics of dysphagia among different lesion sites of stroke: A retrospective study. Front. Neurosci. 2022, 16, 944688. [Google Scholar] [CrossRef]
- Flowers, H.L.; Skoretz, S.A.; Streiner, D.L.; Silver, F.L.; Martino, R. MRI-based neuroanatomical predictors of dysphagia after acute ischemic stroke: A systematic review and meta-analysis. Cerebrovasc. Dis. 2011, 32, 1–10. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, L.; Xing, X.; Zhu, L.; Wu, H.; Xu, S.; Wan, P.; Ding, R. Brain activation site of laryngeal elevation during swallowing: An fMRI Study. Dysphagia 2023, 38, 268–277. [Google Scholar] [CrossRef]
- Im Moon, H.; Pyun, S.B.; Kwon, H.K. Correlation between location of brain lesion and cognitive function and findings of videofluoroscopic swallowing study. Ann. Rehabil. Med. 2012, 36, 347–355. [Google Scholar] [CrossRef]
- Konak, H.E.; Alemdaroğlu, E.; Altaş, E.U. The relationship between dysphagia and the localisation of brain lesion in stroke: Is the involvement of the pons and medulla important? Somatosens. Mot. Res. 2024, 41, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.A.; Bakir, A.; Sidow, N.O.; Ali, A.M.; Osman, M.F.; Ahmed, A.; Hassan, M.S. Lateral medullary syndrome: Uncommon form of brainstem stroke. Ann. Med. Surg. 2023, 85, 589–591. [Google Scholar] [CrossRef]
- Jang, S.H.; Kim, M.S. Dysphagia in lateral medullary syndrome: A narrative review. Dysphagia 2021, 36, 329–338. [Google Scholar] [CrossRef]
- Chang, M.C.; Kwak, S.G.; Chun, M.H. Dysphagia in patients with isolated pontine infarction. Neural Regen. Res. 2018, 13, 2156–2159. [Google Scholar] [CrossRef]
- Yang, Q.-l.; Chen, Y.; Wang, X.-j.; Qiu, H.-y.; Chen, M.-t.; Zhou, X.-h.; Jian, C.-y.; Zhao, S.-f. Correlation between lesion location and dysphagia characteristics in post-stroke patients. J. Stroke Cerebrovasc. Dis. 2024, 33, 107682. [Google Scholar] [CrossRef]
- Martino, R.; Foley, N.; Bhogal, S.; Diamant, N.; Speechley, M.; Teasell, R. Dysphagia after stroke: Incidence, diagnosis, and pulmonary complications. Stroke 2005, 36, 2756–2763. [Google Scholar] [CrossRef] [PubMed]
- Wilmskoetter, J.; Daniels, S.K.; Miller, A.J. Cortical and subcortical control of swallowing—Can we use information from lesion locations to improve diagnosis and treatment for patients with stroke? Am. J. Speech-Lang. Pathol. 2020, 29, 1030–1043. [Google Scholar] [CrossRef] [PubMed]
- Lankapothu, P.B.R.; Kumar, S.; Dasi, S.C.; Bhaskaran, S.; Bathena, A.K.; Shrinidhi, B.; kumar Bathena, A. Heart-Shaped Infarct on MRI and Its Implications in Bilateral Medullary Syndrome. Cureus 2024, 16, e70361. [Google Scholar] [CrossRef]
- Nikolaidou, F.; Krasnikova, E.; Vamvaka, E.; Potolidis, E. From locked-in syndrome to recovery: Thrombolysis success in bilateral pontine infarction with ‘heart appearance’ sign. BMJ Case Rep. CP 2024, 17, e262763. [Google Scholar] [CrossRef]
- Kumral, E.; Bayülkem, G.; Evyapan, D. Clinical spectrum of pontine infarction: Clinical-MRI correlations. J. Neurol. 2002, 249, 1659–1670. [Google Scholar] [CrossRef]
- Ertekin, C.; Aydogdu, I.; Tarlaci, S.; Turman, A.B.; Kiylioglu, N. Mechanisms of dysphagia in suprabulbar palsy with lacunar infarct. Stroke 2000, 31, 1370–1376. [Google Scholar] [CrossRef]
- Ekberg, O.; Hamdy, S.; Woisard, V.; Wuttge-Hannig, A.; Ortega, P. Social and psychological burden of dysphagia: Its impact on diagnosis and treatment. Dysphagia 2002, 17, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Labeit, B.; Kremer, A.; Muhle, P.; Claus, I.; Warnecke, T.; Dziewas, R.; Suntrup-Krueger, S. Costs of post-stroke dysphagia during acute hospitalization from a health-insurance perspective. Eur. Stroke J. 2023, 8, 361–369. [Google Scholar] [CrossRef]
- Hamdy, S.; Rothwell, J.C.; Aziz, Q.; Singh, K.D.; Thompson, D.G. Long-term reorganization of human motor cortex driven by short-term sensory stimulation. Nat. Neurosci. 1998, 1, 64–68. [Google Scholar] [CrossRef]
- Jefferson, S.; Mistry, S.; Michou, E.; Singh, S.; Rothwell, J.C.; Hamdy, S. Reversal of a virtual lesion in human pharyngeal motor cortex by high frequency contralesional brain stimulation. Gastroenterology 2009, 137, 841–849.e1. [Google Scholar] [CrossRef]
- Jefferson, S.; Mistry, S.; Singh, S.; Rothwell, J.; Hamdy, S. Characterizing the application of transcranial direct current stimulation in human pharyngeal motor cortex. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G1035–G1040. [Google Scholar] [CrossRef] [PubMed]
- Mistry, S.; Verin, E.; Singh, S.; Jefferson, S.; Rothwell, J.C.; Thompson, D.G.; Hamdy, S. Unilateral suppression of pharyngeal motor cortex to repetitive transcranial magnetic stimulation reveals functional asymmetry in the hemispheric projections to human swallowing. J. Physiol. 2007, 585, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.; Rothwell, J.; Power, M.; Hobson, A.; Thompson, D.; Hamdy, S. Differential changes in human pharyngoesophageal motor excitability induced by swallowing, pharyngeal stimulation, and anesthesia. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G137–G144. [Google Scholar] [CrossRef]
- Fraser, C.; Power, M.; Hamdy, S.; Rothwell, J.; Hobday, D.; Hollander, I.; Tyrell, P.; Hobson, A.; Williams, S.; Thompson, D. Driving plasticity in human adult motor cortex is associated with improved motor function after brain injury. Neuron 2002, 34, 831–840. [Google Scholar] [CrossRef]
- Jayasekeran, V.; Singh, S.; Tyrrell, P.; Michou, E.; Jefferson, S.; Mistry, S.; Gamble, E.; Rothwell, J.; Thompson, D.; Hamdy, S. Adjunctive functional pharyngeal electrical stimulation reverses swallowing disability after brain lesions. Gastroenterology 2010, 138, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Suntrup, S.; Teismann, I.; Wollbrink, A.; Winkels, M.; Warnecke, T.; Pantev, C.; Dziewas, R. Pharyngeal electrical stimulation can modulate swallowing in cortical processing and behavior—Magnetoencephalographic evidence. Neuroimage 2015, 104, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Suntrup-Krueger, S.; Bittner, S.; Recker, S.; Meuth, S.G.; Warnecke, T.; Suttrup, I.; Marian, T.; Dziewas, R. Electrical pharyngeal stimulation increases substance P level in saliva. Neurogastroenterol. Motil. 2016, 28, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Muhle, P.; Suntrup-Krueger, S.; Bittner, S.; Ruck, T.; Claus, I.; Marian, T.; Schröder, J.B.; Minnerup, J.; Warnecke, T.; Meuth, S.G.; et al. Increase of Substance P Concentration in Saliva after Pharyngeal Electrical Stimulation in Severely Dysphagic Stroke Patients—An Indicator of Decannulation Success? Neurosignals 2017, 25, 74–87. [Google Scholar] [CrossRef]
- Cheng, I.; Sasegbon, A.; Hamdy, S. Effects of Neurostimulation on Poststroke Dysphagia: A Synthesis of Current Evidence From Randomized Controlled Trials. Neuromodulation 2021, 24, 1388–1401. [Google Scholar] [CrossRef]
- Cabib, C.; Nascimento, W.; Rofes, L.; Arreola, V.; Tomsen, N.; Mundet, L.; Palomeras, E.; Michou, E.; Clave, P.; Ortega, O. Short-term neurophysiological effects of sensory pathway neurorehabilitation strategies on chronic poststroke oropharyngeal dysphagia. Neurogastroenterol. Motil. 2020, 32, e13887. [Google Scholar] [CrossRef]
- Dziewas, R.; Stellato, R.; van der Tweel, I.; Walther, E.; Werner, C.J.; Braun, T.; Citerio, G.; Jandl, M.; Friedrichs, M.; Notzel, K.; et al. Pharyngeal electrical stimulation for early decannulation in tracheotomised patients with neurogenic dysphagia after stroke (PHAST-TRAC): A prospective, single-blinded, randomised trial. Lancet Neurol. 2018, 17, 849–859. [Google Scholar] [CrossRef]
- Michou, E.; Mistry, S.; Jefferson, S.; Tyrrell, P.; Hamdy, S. Characterizing the mechanisms of central and peripheral forms of neurostimulation in chronic dysphagic stroke patients. Brain Stimul. 2014, 7, 66–73. [Google Scholar] [CrossRef]
- Suntrup, S.; Marian, T.; Schroder, J.B.; Suttrup, I.; Muhle, P.; Oelenberg, S.; Hamacher, C.; Minnerup, J.; Warnecke, T.; Dziewas, R. Electrical pharyngeal stimulation for dysphagia treatment in tracheotomized stroke patients: A randomized controlled trial. Intensive Care Med. 2015, 41, 1629–1637. [Google Scholar] [CrossRef]
- Suntrup-Krueger, S.; Labeit, B.; Marian, T.; Schröder, J.; Claus, I.; Ahring, S.; Warnecke, T.; Dziewas, R.; Muhle, P. Pharyngeal electrical stimulation for postextubation dysphagia in acute stroke: A randomized controlled pilot trial. Crit. Care 2023, 27, 383. [Google Scholar] [CrossRef]
- Suntrup-Krueger, S.; Labeit, B.; von Itter, J.; Jung, A.; Claus, I.; Ahring, S.; Warnecke, T.; Dziewas, R.; Muhle, P. Treating postextubation dysphagia after stroke with pharyngeal electrical stimulation–insights from a randomized controlled pilot trial. Neurotherapeutics 2025, 22, e00613. [Google Scholar] [CrossRef]
- Cheng, I.; Bath, P.M.; Hamdy, S.; Muhle, P.; Mistry, S.; Dziewas, R.; Suntrup-Krueger, S. Predictors of pharyngeal electrical stimulation treatment success in tracheotomised stroke patients with dysphagia: Secondary analysis from PHADER cohort study. Neurotherapeutics 2024, 21, e00433. [Google Scholar] [CrossRef]
- Bath, P.M.; Woodhouse, L.J.; Suntrup-Krueger, S.; Likar, R.; Koestenberger, M.; Warusevitane, A.; Herzog, J.; Schuttler, M.; Ragab, S.; Everton, L.; et al. Pharyngeal electrical stimulation for neurogenic dysphagia following stroke, traumatic brain injury or other causes: Main results from the PHADER cohort study. eClinicalMedicine 2020, 28, 100608. [Google Scholar] [CrossRef]
- Florea, C.; Bräumann, C.; Mussger, C.; Leis, S.; Hauer, L.; Sellner, J.; Golaszewski, S.M. Therapy of Dysphagia by Prolonged Pharyngeal Electrical Stimulation (Phagenyx) in a Patient with Brainstem Infarction. Brain Sci. 2020, 10, 256. [Google Scholar] [CrossRef]
- Sasegbon, A.; Hamdy, S. The Role of the Cerebellum in Swallowing. Dysphagia 2023, 38, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Dong, L.; Cong, X.; Luo, H.; Li, W.; Meng, P.; Wang, Q. Comparative efficacy of non-invasive neurostimulation therapies for poststroke dysphagia: A systematic review and meta-analysis. Neurophysiol. Clin. 2021, 51, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.-h.; Pan, X.; Wang, Y.; Bai, G.; Han, C.; Wang, Q.; Meng, P. High-Frequency Cerebellar rTMS Improves the Swallowing Function of Patients with Dysphagia after Brainstem Stroke. Neural Plast. 2022, 2022, 6259693. [Google Scholar] [CrossRef]
- Rosenbek, J.C.; Robbins, J.A.; Roecker, E.B.; Coyle, J.L.; Wood, J.L. A penetration-aspiration scale. Dysphagia 1996, 11, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Han, T.R.; Paik, N.J.; Park, J.W. The Functional Dysphagia Scale Using Videofluoroscopic Swallowing Study in Stroke Patients. J. Korean Acad. Rehabil. Med. 1999, 23, 1118–1126. [Google Scholar]
- Sasegbon, A.; Smith, C.J.; Bath, P.; Rothwell, J.; Hamdy, S. The effects of unilateral and bilateral cerebellar rTMS on human pharyngeal motor cortical activity and swallowing behavior. Exp. Brain Res. 2020, 238, 1719–1733. [Google Scholar] [CrossRef] [PubMed]
- Khedr, E.M.; Abo-Elfetoh, N. Therapeutic role of rTMS on recovery of dysphagia in patients with lateral medullary syndrome and brainstem infarction. J. Neurol. Neurosurg. Psychiatry 2010, 81, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Verin, E.; Leroi, A.M.; Marie, J.P. Restoration of normal swallowing function in Wallenberg syndrome by repetitive transcranial magnetic stimulation and surgery. Ann. Phys. Rehabil. Med. 2016, 59, 343–345. [Google Scholar] [CrossRef]
- Lin, W.-S.; Chou, C.-L.; Chang, M.-H.; Chung, Y.-M.; Lin, F.-G.; Tsai, P.-Y. Vagus nerve magnetic modulation facilitates dysphagia recovery in patients with stroke involving the brainstem-A proof of concept study. Brain Stimul. 2018, 11, 264–270. [Google Scholar] [CrossRef]
- Dai, M.; Qiao, J.; Shi, Z.; Wei, X.; Chen, H.; Shen, L.; Wen, H.; Dou, Z. Effect of cerebellar transcranial magnetic stimulation with double-cone coil on dysphagia after subacute infratentorial stroke: A randomized, single-blinded, controlled trial. Brain Stimul. 2023, 16, 1012–1020. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, B.; Ambler, G.; Chen, Q.; Huang, H.; Lin, H.; Fang, S.; Liu, N.; Du, H. Repetitive transcranial magnetic stimulation strategies for post-stroke dysphagia: A systematic review and network meta-analysis. Arch. Phys. Med. Rehabil. 2024. [Google Scholar] [CrossRef]
- Shigematsu, T.; Fujishima, I.; Ohno, K. Transcranial direct current stimulation improves swallowing function in stroke patients. Neurorehabilit. Neural Repair 2013, 27, 363–369. [Google Scholar] [CrossRef]
- Suntrup-Krueger, S.; Ringmaier, C.; Muhle, P.; Wollbrink, A.; Kemmling, A.; Hanning, U.; Claus, I.; Warnecke, T.; Teismann, I.; Pantev, C.; et al. Randomized trial of transcranial direct current stimulation for poststroke dysphagia. Ann. Neurol. 2018, 83, 328–340. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Chen, J.M.; Lin, Z.K.; Ni, G.X. Transcranial direct current stimulation improves the swallowing function in patients with cricopharyngeal muscle dysfunction following a brainstem stroke. Neurol. Sci. 2020, 41, 569–574. [Google Scholar] [CrossRef]
- Farpour, S.; Asadi-Shekaari, M.; Borhani Haghighi, A.; Farpour, H.R. Improving swallowing function and ability in post stroke dysphagia: A randomized clinical trial. Dysphagia 2023, 38, 330–339. [Google Scholar] [CrossRef]
- Mao, H.; Lyu, Y.; Li, Y.; Gan, L.; Ni, J.; Liu, L.; Xiao, Z. Clinical study on swallowing function of brainstem stroke by tDCS. Neurol. Sci. 2021, 43, 477–484. [Google Scholar] [CrossRef]
- Crary, M.A.; Mann, G.D.; Groher, M.E. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch. Phys. Med. Rehabil. 2005, 86, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, K.H.; Purdy, M.; Falk, J.; Gallo, L. The dysphagia outcome and severity scale. Dysphagia 1999, 14, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Priori, A. Brain polarization in humans: A reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clin. Neurophysiol. 2003, 114, 589–595. [Google Scholar] [CrossRef]
- Liebetanz, D.; Nitsche, M.A.; Tergau, F.; Paulus, W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain 2002, 125, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef]
- Radman, T.; Ramos, R.L.; Brumberg, J.C.; Bikson, M. Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul. 2009, 2, 215–228.e3. [Google Scholar] [CrossRef]
- Suntrup, S.; Teismann, I.; Wollbrink, A.; Winkels, M.; Warnecke, T.; Floel, A.; Pantev, C.; Dziewas, R. Magnetoencephalographic evidence for the modulation of cortical swallowing processing by transcranial direct current stimulation. Neuroimage 2013, 83, 346–354. [Google Scholar] [CrossRef]
- Zhao, S.; Dou, Z.; Wei, X.; Li, J.; Dai, M.; Wang, Y.; Yang, Q.; He, H. Task-concurrent anodal tDCS modulates bilateral plasticity in the human suprahyoid motor cortex. Front. Hum. Neurosci. 2015, 9, 370. [Google Scholar] [CrossRef]
- Doeltgen, S.H.; Rigney, L.; Cock, C.; Omari, T. Effects of cortical anodal transcranial direct current stimulation on swallowing biomechanics. Neurogastroenterol. Motil. 2018, 30, e13434. [Google Scholar] [CrossRef]
- Vasant, D.H.; Mistry, S.; Michou, E.; Jefferson, S.; Rothwell, J.C.; Hamdy, S. Transcranial direct current stimulation reverses neurophysiological and behavioural effects of focal inhibition of human pharyngeal motor cortex on swallowing. J. Physiol. 2014, 592, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Han, Y.; Park, G.-Y.; Lee, S.; Park, H.-Y.; Im, S. Role of Catechol-O-methyltransferase Val158Met Polymorphism on Transcranial Direct Current Stimulation in Swallowing. J. Pers. Med. 2022, 12, 488. [Google Scholar] [CrossRef]
- He, K.; Wu, L.; Huang, Y.; Chen, Q.; Qiu, B.; Liang, K.; Ma, R. Efficacy and safety of transcranial direct current stimulation on post-stroke dysphagia: A systematic review and meta-analysis. J. Clin. Med. 2022, 11, 2297. [Google Scholar] [CrossRef]
- Speyer, R.; Sutt, A.L.; Bergström, L.; Hamdy, S.; Pommée, T.; Balaguer, M.; Kaale, A.; Cordier, R. Neurostimulation in People with Oropharyngeal Dysphagia: A Systematic Review and Meta-Analysis of Randomised Controlled Trials-Part II: Brain Neurostimulation. J. Clin. Med. 2022, 11, 993. [Google Scholar] [CrossRef]
- Marchina, S.; Pisegna, J.M.; Massaro, J.M.; Langmore, S.E.; McVey, C.; Wang, J.; Kumar, S. Transcranial direct current stimulation for post-stroke dysphagia: A systematic review and meta-analysis of randomized controlled trials. J. Neurol. 2021, 268, 293–304. [Google Scholar] [CrossRef]
- Zhao, N.; Sun, W.; Xiao, Z.; Fan, C.; Zeng, B.; Xu, K.; Liao, M.; Lu, W. Effects of transcranial direct current stimulation on poststroke dysphagia: A systematic review and meta-analysis of randomized controlled trials. Arch. Phys. Med. Rehabil. 2022, 103, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gu, L. Noninvasive Brain Stimulation for Poststroke Dysphagia: A Meta-Analysis for Randomized Controlled Trials. Eur. Neurol. 2022, 85, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Raginis-Zborowska, A.; Cheng, I.; Pendleton, N.; Payton, A.; Ollier, W.; Michou, E.; Hamdy, S. Genetic influences on the variability of response to repetitive transcranial magnetic stimulation in human pharyngeal motor cortex. Neurogastroenterol. Motil. 2019, 31, e13612. [Google Scholar] [CrossRef]
- Cheng, I.; Scarlett, H.; Zhang, M.; Hamdy, S. Preconditioning human pharyngeal motor cortex enhances directional metaplasticity induced by repetitive transcranial magnetic stimulation. J. Physiol. 2020, 598, 5213–5230. [Google Scholar] [CrossRef]
- Antal, A.; Alekseichuk, I.; Bikson, M.; Brockmoller, J.; Brunoni, A.R.; Chen, R.; Cohen, L.G.; Dowthwaite, G.; Ellrich, J.; Floel, A.; et al. Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clin. Neurophysiol. 2017, 128, 1774–1809. [Google Scholar] [CrossRef] [PubMed]
- Dziewas, R.; Michou, E.; Trapl-Grundschober, M.; Lal, A.; Arsava, E.M.; Bath, P.M.; Clave, P.; Glahn, J.; Hamdy, S.; Pownall, S.; et al. European Stroke Organisation and European Society for Swallowing Disorders guideline for the diagnosis and treatment of post-stroke dysphagia. Eur. Stroke J. 2021, 6, LXXXIX-CXV. [Google Scholar] [CrossRef]
- Rossi, S.; Antal, A.; Bestmann, S.; Bikson, M.; Brewer, C.; Brockmöller, J.; Carpenter, L.L.; Cincotta, M.; Chen, R.; Daskalakis, J.D. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert guidelines. Clin. Neurophysiol. 2020, 1, 269–306. [Google Scholar] [CrossRef]
- Jean, A.; Car, A. Inputs to the swallowing medullary neurons from the peripheral afferent fibers and the swallowing cortical area. Brain Res. 1979, 178, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Jean, A. Control of the central swallowing program by inputs from the peripheral receptors. A review. J. Auton. Nerv. Syst. 1984, 10, 225–233. [Google Scholar] [CrossRef]
- Miller, A.J. Characteristics of the swallowing reflex induced by peripheral nerve and brain stem stimulation. Exp. Neurol. 1972, 34, 210–222. [Google Scholar] [CrossRef]
- Miller, A.J. Deglutition. Physiol. Rev. 1982, 62, 129–184. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.J. Neurophysiological basis of swallowing. Dysphagia 1986, 1, 91–100. [Google Scholar] [CrossRef]
- Bahia, M.M.; Lowell, S.Y. A Systematic Review of the Physiological Effects of the Effortful Swallow Maneuver in Adults with Normal and Disordered Swallowing. Am. J. Speech Lang. Pathol. 2020, 29, 1655–1673. [Google Scholar] [CrossRef]
- Crary, M.A.; Carnaby, G.D.; Groher, M.E.; Helseth, E. Functional benefits of dysphagia therapy using adjunctive sEMG biofeedback. Dysphagia 2004, 19, 160–164. [Google Scholar] [CrossRef]
- Shaker, R.; Kern, M.; Bardan, E.; Taylor, A.; Stewart, E.T.; Hoffmann, R.G.; Arndorfer, R.C.; Hofmann, C.; Bonnevier, J. Augmentation of deglutitive upper esophageal sphincter opening in the elderly by exercise. Am. J. Physiol. Gastrointest. Liver Physiol. 1997, 272, G1518–G1522. [Google Scholar] [CrossRef]
- Kim, J.; Sapienza, C.M. Implications of expiratory muscle strength training for rehabilitation of the elderly: Tutorial. J. Rehabil. Res. Dev. 2005, 42, 211–224. [Google Scholar] [CrossRef]
- Fujiu, M.; Logemann, J.A. Effect of a tongue-holding maneuver on posterior pharyngeal wall movement during deglutition. Am. J. Speech Lang. Pathol. 1996, 5, 23–30. [Google Scholar] [CrossRef]
- Martin, R.E.; Sessle, B.J. The role of the cerebral cortex in swallowing. Dysphagia 1993, 8, 195–202. [Google Scholar] [CrossRef]
- Ertekin, C. Voluntary versus spontaneous swallowing in man. Dysphagia 2011, 26, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Mosier, K.; Bereznaya, I. Parallel cortical networks for volitional control of swallowing in humans. Exp. Brain Res. 2001, 140, 280–289. [Google Scholar] [CrossRef]
- Michou, E.; Hamdy, S. Cortical input in control of swallowing. Curr. Opin. Otolaryngol. Head Neck Surg. 2009, 17, 166–171. [Google Scholar] [CrossRef]
- Huckabee, M.-L.; Deecke, L.; Cannito, M.P.; Gould, H.J.; Mayr, W. Cortical control mechanisms in volitional swallowing: The Bereitschaftspotential. Brain Topogr. 2003, 16, 3–17. [Google Scholar] [CrossRef]
- McDonnell, M.N.; Hillier, S.L.; Miles, T.S.; Thompson, P.D.; Ridding, M.C. Influence of combined afferent stimulation and task-specific training following stroke: A pilot randomized controlled trial. Neurorehabilit. Neural Repair 2007, 21, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Bryant, M. Biofeedback in the treatment of a selected dysphagic patient. Dysphagia 1991, 6, 140–144. [Google Scholar] [CrossRef]
- Huckabee, M.L.; Cannito, M.P. Outcomes of swallowing rehabilitation in chronic brainstem dysphagia: A retrospective evaluation. Dysphagia 1999, 14, 93–109. [Google Scholar] [CrossRef]
- Bogaardt, H.; Grolman, W.; Fokkens, W. The use of biofeedback in the treatment of chronic dysphagia in stroke patients. Folia Phoniatr. Logop. 2009, 61, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Benfield, J.K.; Everton, L.F.; Bath, P.M.; England, T.J. Does therapy with biofeedback improve swallowing in adults with dysphagia? A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2019, 100, 551–561. [Google Scholar] [CrossRef]
- Battel, I.; Calvo, I.; Walshe, M. Interventions involving biofeedback to improve swallowing in people with parkinson disease and dysphagia: A systematic review. Arch. Phys. Med. Rehabil. 2021, 102, 314–322. [Google Scholar] [CrossRef]
- Larsen, G. Rehabilitation for Dysphagia Paralytica. J. Speech Hear. Disord. 1972, 37, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Kleim, J.A.; Jones, T.A. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. J. Speech Lang. Hear. Res. 2008, 51, S225–S239. [Google Scholar] [CrossRef]
- Robbins, J.; Butler, S.G.; Daniels, S.K.; Gross, R.D.; Langmore, S.; Lazarus, C.L.; Martin-Harris, B.; McCabe, D.; Musson, N.; Rosenbek, J. Swallowing and dysphagia rehabilitation: Translating principles of neural plasticity into clinically oriented evidence. J. Speech Lang. Hear. Res. 2008, 51, S276–S300. [Google Scholar] [CrossRef]
- Jing, Y.H.; Lin, T.; Li, W.Q.; Wu, C.; Li, X.; Ding, Q.; Wu, M.F.; Xu, G.Q.; Lan, Y. Comparison of activation patterns in mirror neurons and the swallowing network during action observation and execution: A task-based fMRI study. Front. Neurosci. 2020, 14, 867. [Google Scholar] [CrossRef] [PubMed]
- Kober, S.E.; Grössinger, D.; Wood, G. Effects of motor imagery and visual neurofeedback on activation in the swallowing network: A real-time fMRI study. Dysphagia 2019, 34, 879–895. [Google Scholar] [CrossRef]
- Szynkiewicz, S.H.; Nobriga, C.V.; O’Donoghue, C.R.; Becerra, B.J.; LaForge, G. Motor imagery practice and increased tongue strength: A case series feasibility report. J. Speech Lang. Hear. Res. 2019, 62, 1676–1684. [Google Scholar] [CrossRef]
- Athukorala, R.P.; Jones, R.D.; Sella, O.; Huckabee, M.-L. Skill training for swallowing rehabilitation in patients with Parkinson’s disease. Arch. Phys. Med. Rehabil. 2014, 95, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- McHorney, C.A.; Robbins, J.; Lomax, K.; Rosenbek, J.C.; Chignell, K.; Kramer, A.E.; Earl Bricker, D. The SWAL–QOL and SWAL–CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia 2002, 17, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.A.; Wiles, C.M. Clinical measurement of swallowing in health and in neurogenic dysphagia. QJM 1996, 89, 109–116. [Google Scholar] [CrossRef]
- Perry, S.E.; Sevitz, J.S.; Curtis, J.A.; Kuo, S.H.; Troche, M.S. Skill training resulted in improved swallowing in a person with multiple system atrophy: An endoscopy study. Mov. Disord. Clin. Pract. 2018, 5, 451. [Google Scholar] [CrossRef]
- Battel, I.; Walshe, M. An intensive neurorehabilitation programme with sEMG biofeedback to improve swallowing in idiopathic Parkinson’s disease (IPD): A feasibility study. Int. J. Lang. Commun. Disord. 2023, 58, 813–825. [Google Scholar] [CrossRef]
- Nordio, S.; Arcara, G.; Berta, G.; Dellai, A.; Brisotto, C.; Koch, I.; Cazzador, D.; Aspidistria, M.; Ventura, L.; Turolla, A. Biofeedback as an adjunctive treatment for post-stroke dysphagia: A pilot-randomized controlled trial. Dysphagia 2022, 37, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Benfield, J.K.; Hedstrom, A.; Everton, L.F.; Bath, P.M.; England, T.J. Randomized controlled feasibility trial of swallow strength and skill training with surface electromyographic biofeedback in acute stroke patients with dysphagia. J. Oral Rehabil. 2023, 50, 440–451. [Google Scholar] [CrossRef]
- Hou, M.; Zhao, Y.; Zhao, L.; Yuan, X.; Liu, Z.; Li, H. Efficacy of game training combined with surface electromyography biofeedback on post-stroke dysphagia. Geriatr. Nurs. 2024, 55, 255–262. [Google Scholar] [CrossRef]
- Huckabee, M.L.; Lamvik, K.; Jones, R. Pharyngeal mis-sequencing in dysphagia: Characteristics, rehabilitative response, and etiological speculation. J. Neurol. Sci. 2014, 343, 153–158. [Google Scholar] [CrossRef]
- Huckabee, M.-L.; Lamvik-Gozdzikowska, K. Reconsidering Rehabilitation for Neurogenic Dysphagia: Strengthening Skill in Swallowing. Curr. Phys. Med. Rehabil. Rep. 2018, 6, 186–191. [Google Scholar] [CrossRef]
- Zimmerman, E.; Carnaby, G.D.; Lazarus, C.L.; Malandraki, G.A. Motor Learning, Neuroplasticity, and Strength and Skill Training: Moving From Compensation to Retraining in Behavioral Management of Dysphagia. Am. J. Speech-Lang. Pathol. 2020, 29, 1065–1077. [Google Scholar] [CrossRef] [PubMed]
- Huckabee, M.-L.; Flynn, R.; Mills, M. Expanding Rehabilitation Options for Dysphagia: Skill-Based Swallowing Training. Dysphagia 2023, 38, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.J. The neurobiology of swallowing and dysphagia. Dev. Disabil. Res. Rev. 2008, 14, 77–86. [Google Scholar] [CrossRef]
- Capra, N.F. Mechanisms of oral sensation. Dysphagia 1995, 10, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Berdugo, D.; Rofes, L.; Farre, R.; Casamitjana, J.F.; Enrique, A.; Chamizo, J.; Padron, A.; Navarro, X.; Clave, P. Localization and expression of TRPV1 and TRPA1 in the human oropharynx and larynx. Neurogastroenterol. Motil. 2016, 28, 91–100. [Google Scholar] [CrossRef]
- Clapham, D.E. Signal transduction. Hot and cold TRP ion channels. Science 2002, 295, 2228–2229. [Google Scholar] [CrossRef]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef]
- Namer, B.; Seifert, F.; Handwerker, H.O.; Maihofner, C. TRPA1 and TRPM8 activation in humans: Effects of cinnamaldehyde and menthol. Neuroreport 2005, 16, 955–959. [Google Scholar] [CrossRef]
- Tominaga, M.; Caterina, M.J. Thermosensation and pain. J. Neurobiol. 2004, 61, 3–12. [Google Scholar] [CrossRef]
- Cheng, I.; Sasegbon, A.; Hamdy, S. Effects of pharmacological agents for neurogenic oropharyngeal dysphagia: A systematic review and meta-analysis. Neurogastroenterol. Motil. 2021, 34, e14220. [Google Scholar] [CrossRef] [PubMed]
- Kittipanya-Ngam, P.; Benjapornlert, P.; Rattanakanokchai, S.; Wattanapan, P. Effect of TRP-stimulating compounds to reduce swallowing response time in the elderly: A systematic review. Dysphagia 2021, 36, 614–622. [Google Scholar] [CrossRef]
- van Oosterhout, W.; Schoonman, G.; Garrelds, I.; Danser, A.; Chan, K.; Terwindt, G.; Ferrari, M.; MaassenVanDenBrink, A. A human capsaicin model to quantitatively assess salivary CGRP secretion. Cephalalgia 2015, 35, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Suntrup-Krueger, S.; Muhle, P.; Kampe, I.; Egidi, P.; Ruck, T.; Lenze, F.; Jungheim, M.; Gminski, R.; Labeit, B.; Claus, I.; et al. Effect of Capsaicinoids on Neurophysiological, Biochemical, and Mechanical Parameters of Swallowing Function. Neurotherapeutics 2021, 18, 1360–1370. [Google Scholar] [CrossRef]
- Tomsen, N.; Ortega, O.; Nascimento, W.; Carrión, S.; Clavé, P. Oropharyngeal Dysphagia in Older People is Associated with Reduced Pharyngeal Sensitivity and Low Substance P and CGRP Concentration in Saliva. Dysphagia 2022, 37, 48–57. [Google Scholar] [CrossRef]
- Niimi, M.; Hashimoto, G.; Hara, T.; Yamada, N.; Abo, M.; Fujigasaki, H.; Ide, T. Relationship Between Frequency of Spontaneous Swallowing and Salivary Substance P Level in Patients with Acute Stroke. Dysphagia 2018, 33, 414–418. [Google Scholar] [CrossRef]
- Carnaby, G.; Sia, I.; Crary, M. Associations Between Spontaneous Swallowing Frequency at Admission, Dysphagia, and Stroke-Related Outcomes in Acute Care. Arch. Phys. Med. Rehabil. 2019, 100, 1283–1288. [Google Scholar] [CrossRef]
- Tomsen, N.; Ortega, O.; Rofes, L.; Arreola, V.; Martin, A.; Mundet, L.; Clave, P. Acute and subacute effects of oropharyngeal sensory stimulation with TRPV1 agonists in older patients with oropharyngeal dysphagia: A biomechanical and neurophysiological randomized pilot study. Ther. Adv. Gastroenterol. 2019, 12, 1756284819842043. [Google Scholar] [CrossRef]
- Rofes, L.; Arreola, V.; Martin, A.; Clavé, P. Effect of oral piperine on the swallow response of patients with oropharyngeal dysphagia. J. Gastroenterol. 2014, 49, 1517–1523. [Google Scholar] [CrossRef]
- Rofes, L.; Arreola, V.; Martin, A.; Clavé, P. Natural capsaicinoids improve swallow response in older patients with oropharyngeal dysphagia. Gut 2013, 62, 1280. [Google Scholar] [CrossRef] [PubMed]
- Tomsen, N.; Alvarez-Berdugo, D.; Rofes, L.; Ortega, O.; Arreola, V.; Nascimento, W.; Martin, A.; Cabib, C.; Bolivar-Prados, M.; Mundet, L. A randomized clinical trial on the acute therapeutic effect of TRPA1 and TRPM8 agonists in patients with oropharyngeal dysphagia. Neurogastroenterol. Motil. 2020, 32, e13821. [Google Scholar] [CrossRef] [PubMed]
- Tomsen, N.; Ortega, O.; Alvarez-Berdugo, D.; Rofes, L.; Clavé, P. A Comparative Study on the Effect of Acute Pharyngeal Stimulation with TRP Agonists on the Biomechanics and Neurophysiology of Swallow Response in Patients with Oropharyngeal Dysphagia. Int. J. Mol. Sci. 2022, 23, 10773. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, T.; Ebihara, S.; Maruyama, M.; Kobayashi, M.; Itou, A.; Arai, H.; Sasaki, H. A randomized trial of olfactory stimulation using black pepper oil in older people with swallowing dysfunction. J. Am. Geriatr. Soc. 2006, 54, 1401–1406. [Google Scholar] [CrossRef]
- Nascimento, W.; Tomsen, N.; Acedo, S.; Campos-Alcantara, C.; Cabib, C.; Alvarez-Larruy, M.; Clavé, P. Effect of Aging, Gender and Sensory Stimulation of TRPV1 Receptors with Capsaicin on Spontaneous Swallowing Frequency in Patients with Oropharyngeal Dysphagia: A Proof-of-Concept Study. Diagnostics 2021, 11, 461. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, L.; Fang, Q.; Shen, M.; Zhang, L.; Liu, X. Effects of capsaicin on swallowing function in stroke patients with dysphagia: A randomized controlled trial. J. Stroke Cerebrovasc. Dis. 2019, 28, 1744–1751. [Google Scholar] [CrossRef]
- Belafsky, P.C.; Mouadeb, D.A.; Rees, C.J.; Pryor, J.C.; Postma, G.N.; Allen, J.; Leonard, R.J. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann. Otol. Rhinol. Laryngol. 2008, 117, 919–924. [Google Scholar] [CrossRef] [PubMed]
| Structure | Location | Role | Function | Symptoms After Lesion |
|---|---|---|---|---|
| Nucleus Tractus Solitarius (NTS) | Medulla | Sensory Centre |
|
|
| Nucleus Ambiguus (NA) | Medulla | Motor Centre |
|
|
| Central pattern generator (CPG) | Medulla | Coordination Centre |
|
|
| Cranial Nerve Nuclei | Pons, Medulla | Swallowing Regulator |
|
|
| Study | Design | Population | Stimulation Parameters | Outcomes | Limitations |
|---|---|---|---|---|---|
| rTMS | |||||
| Khedr & Abo-Elfetoh, 2010 [54] | RCT | 22 patients with brainstem infarction and LMS | 3 Hz, bilateral hemisphere, 10 min/day, 5 days | Improved swallowing severity (DG score) | No blinding; limited sample |
| Verin et al., 2016 [55] | Case series | 2 patients with chronic aphagia post-LMS | 1 Hz, bilateral motor cortex, 20% above threshold, 5 × 5 days + TENS + surgery | Full restoration of oral intake | Small sample; multimodal approach limits causal inference |
| Lin et al., 2018 [56] | Proof-of-concept | 28 patients with brainstem stroke with dysphagia | Vagal magnetic modulation, 600 pulses/day, 10 days | Significant recovery in AusTOMs swallowing domain | Non-TMS coil; vagus targeting—limited generalizability |
| Dong et al., 2022 [50] | RCT | 34 patients with medullary/pontine stroke | 10 Hz, 250 pulses, 80% RMT, bilateral/unilateral cerebellum vs. sham, 2 weeks | Improved PAS & FDS scores; increased MEP amplitudes | No direct correlation between MEP gain & clinical improvement |
| Dai et al., 2023 [57] | RCT (single-blinded) | 42 subacute infratentorial stroke patients | 10 Hz, 5 × 50 stimuli, 90% RMT, bilateral/unilateral cerebellum vs. sham, 10 days | Significant FOIS, PAS, DOSS improvements; Bilateral > unilateral | No significant MEP differences; cerebellar lesion variability may affect results |
| Wu et al., 2024 [58] | Network meta-analysis | 760 PSD patients (including brainstem stroke) | Multiple protocols including HF/ipsi-CRB, HF/bi-CRB | HF/bi-CRB, HF/ipsi-CRB improved swallowing (PAS, FDS) | Protocol heterogeneity; brainstem subgroup effects not isolated |
| tDCS | |||||
| Shigematsu et al., 2013 [59] | RCT | 20 stroke patients (7 with brainstem stroke) | 1 mA, 20 min, 10 days; Ipsilesional pharyngeal motor cortex | Improved DOSS | Unclear which side was targeted for brainstem stroke patients |
| Suntrup-Krueger et al., 2018 [60] | RCT | 59 stroke patients (14 with brainstem stroke) | 1 mA, 20 min, 4 days; Swallowing (pharyngeal) motor cortex; Right hemisphere for brainstem stroke | Improved FEDSS; associated with increase in activation of contralesional swallowing neural network | Brainstem subgroup effects not isolated |
| Wang et al., 2020 [61] | RCT | 28 patients with brainstem stroke and CPD | 1 mA, 20 min, 20 days; Bilateral oesophageal motor cortex | Improved FDS and FOIS; Improved PESO scores | Unclear methodology: one anodal electrode for bilateral stimulation; Sequence of hemispheric stimulation unclear |
| Farpour et al., 2022 [62] | RCT | 44 stroke patients (2 with brainstem stroke, both received active tDCS) | 2 mA, 20 min, 5 days; Supramarginal gyrus; Right hemisphere for brainstem stroke | Improved MASA and FOIS | No patients with brainstem stroke in the sham group |
| Mao et al., 2022 [63] | RCT | 40 patients with brainstem stroke | 1.6 mA, 20 min, 54 days; Unlesioned swallowing sensory motor cortex | Improved DOSS and FDS; Improved nutritional indexes | Unclear which hemisphere was targeted for brainstem stroke patients |
| Study | Design | Population | Skill Training Protocol | Biofeedback | Outcomes |
|---|---|---|---|---|---|
| Athukorala et al. [113] | Observational | 10 patients with PD | Skill training targeted at improving strength and timing of swallowing movements. 10 sessions over 2 weeks | sEMG activity of submental muscles with sEMG activity displayed on a computer monitor | Improved functional swallowing measures, sEMG activity, and swallowing-related quality of life |
| Battel & Walshe, 2023 [117] | Observational | 10 patients with PD | Skill training targeted at coordinating swallowing and increasing submental muscle activity. 5 days a week for 4 weeks | sEMG activity of submental muscles with visualization of sEMG activity through a computer game | Improved oral intake methods and in pharyngeal residue from saliva and solids. |
| Benfield et al., 2023 [119] | RCT on feasibility | 27 patients with acute (≤4 weeks) * post-stroke dysphagia | Experimental group: CDT + sEMG-BF training; Skill training targeted at improving strength and timing of swallowing movements. Control: CDT 10 sessions over 2 weeks | sEMG activity of submental muscles with sEMG activity displayed on a computer monitor | The treatment protocol is feasible with compliance rate of 80%. |
| Hou et al., 2024 [120] | RCT | 90 patients with acute (≤2 weeks) post-stroke dysphagia (25 with brainstem stroke) |
| Group 1: sEMG activity of submental muscles Group 2: sEMG activity of submental muscles with visualization of sEMG activity through a computer game | Improved functional swallowing measures, sEMG activity, swallowing timing and tongue pressure in both experimental groups. Game training combined with biofeedback showed the greatest improvement among the three groups. |
| Huckabee et al., 2014 [121] | Observational | 16 patients with infratentorial stroke or brain tumour resection, and all with atypical pharyngeal pressure generation | Skill training targeted at increasing the temporal separation between the upper and lower pharyngeal pressure waveforms when swallowing. Twice daily for a minimum of one week. | Manometric measurement of the pharynx with visualization of pharyngeal pressure displayed the manometric system | 12 patients returned to normal oral diet, with resolution of nasal redirection, aspiration, and pharyngeal residue. |
| Nordio et al., 2022 [118] | RCT | 16 patients with post-stroke (>6 weeks) dysphagia (12 with brainstem stroke) | Experimental group: sEMG-BF rehabilitation; Skill training targeted at performing effortful swallow, supraglottic swallow and Masako maneuver. Control: Behavioural training without sEMG-BF. All treatments were delivered for 1 h per day for 5 days | sEMG activity of submental muscles with sEMG activity displayed on a computer monitor | sEMG-BF improved pharyngeal clearance and swallowing safety compared to control. |
| Perry et al., 2018 [116] | Case study | 1 patient with multiple system atrophy | Skill training targeted at improving strength and timing of swallowing movements. 6 sessions over 6 weeks + daily home practice | sEMG activity of submental muscles with sEMG activity displayed on a computer monitor | Improved accuracy in swallowing movements; reduced premature spillage and aspiration and post-swallow residue; subjective improvement in swallowing symptoms |
| Study | Design | Population | Treatment Protocol | Outcomes |
|---|---|---|---|---|
| Ebihara et al., 2006 [144] | RCT | 67 patients with * post-stroke dysphagia | Nasal inhalation of black pepper oil (concentration unspecified) vs. lavender oil vs. distilled water. 1 min before each meal for 30 days | Improved latent time of swallowing reflex, increased serum substance P level, increased number of involuntary swallowing movements during nasal inhalation of black pepper oil. |
| Nascimento et al., 2021 [145] | Observational | 141 healthy volunteers and 17 patients with * post-stroke dysphagia | 10 μM oral capsaicin | Capsaicin increased spontaneous swallowing frequency when comparing to basal condition. |
| Rofes et al., 2013 [141] | Observational | 33 patients with neurogenic dysphagia | 150 μM capsaicinoid (oral) | Treatment with capsaicinoids reduced penetration and pharyngeal residue, shortened the time of laryngeal vestibule closure, upper esophageal sphincter opening, and maximal hyoid and laryngeal displacement |
| Rofes et al., 2014 [140] | RCT with active control | 40 elderly with dysphagia associated with ageing, non-progressive neurological disease or neurodegenerative disease | 150 μM piperine (oral) vs. 1 mM piperine (oral) | Improved swallowing safety and reduced laryngeal vestibule closure time. Greater effects observed at higher concentration. |
| Tomsen et al., 2019 [139] | RCT | 14 elderly with dysphagia associated with ageing |
| The 10-day treatment regimen induced cortical changes that were correlated with reduced laryngeal vestibule closure time and aspiration and penetration in older patients with dysphagia. |
| Tomsen et al., 2022 [143] | Retrospective | 329 patients with dysphagia | Oral capsaicin (TRPV1, 150 μM/10 μM), piperine (TRPA1/V1, 1 mM/150 μM), menthol (TRPM8, 1 mM/10 mM), cinnamaldehyde-zinc (TRPA1, 100 ppm–70 mM), citral (TRPA1, 250 ppm) and citral-isopulegol (TRPA1-TRPM8, 250–200 ppm) | Capsaicin 150 μM or piperine 1 mM significantly improved swallowing safety and time of laryngeal vestibule closure and bolus velocity. |
| Wang et al., 2019 [146] | RCT | 60 patients with post-stroke dysphagia (12 with brainstem or cerebellar stroke) | 150 μM/L capsaicin (oral) (thermal tactile stimulation + nectar bolus). 3 times per day for 21 days | Improved swallowing function as assessed by Eating Assessment Tool [147] and Standardized Swallowing Assessment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, I.; Li, W.-Q.; Hamdy, S.; Michou, E.; Huckabee, M.-L.; Tomsen, N.; Clavé, P.; Dziewas, R. Brainstem Stroke and Dysphagia Treatment: A Narrative Review on the Role of Neuromodulation, Skill-Based Swallowing Training and Transient Receptor Potential Agonists. Audiol. Res. 2025, 15, 156. https://doi.org/10.3390/audiolres15060156
Cheng I, Li W-Q, Hamdy S, Michou E, Huckabee M-L, Tomsen N, Clavé P, Dziewas R. Brainstem Stroke and Dysphagia Treatment: A Narrative Review on the Role of Neuromodulation, Skill-Based Swallowing Training and Transient Receptor Potential Agonists. Audiology Research. 2025; 15(6):156. https://doi.org/10.3390/audiolres15060156
Chicago/Turabian StyleCheng, Ivy, Wan-Qi Li, Shaheen Hamdy, Emilia Michou, Maggie-Lee Huckabee, Noemí Tomsen, Pere Clavé, and Rainer Dziewas. 2025. "Brainstem Stroke and Dysphagia Treatment: A Narrative Review on the Role of Neuromodulation, Skill-Based Swallowing Training and Transient Receptor Potential Agonists" Audiology Research 15, no. 6: 156. https://doi.org/10.3390/audiolres15060156
APA StyleCheng, I., Li, W.-Q., Hamdy, S., Michou, E., Huckabee, M.-L., Tomsen, N., Clavé, P., & Dziewas, R. (2025). Brainstem Stroke and Dysphagia Treatment: A Narrative Review on the Role of Neuromodulation, Skill-Based Swallowing Training and Transient Receptor Potential Agonists. Audiology Research, 15(6), 156. https://doi.org/10.3390/audiolres15060156







