Oculomotor Abnormalities and Nystagmus in Brainstem Disease: A Mini Review
Abstract
1. Introduction
2. Symptoms and Signs in Brainstem Lesions
- -
- rotatory vertigo;
- -
- postural instability or unsteadiness;
- -
- postural crises;
- -
- unclear or blurred vision.
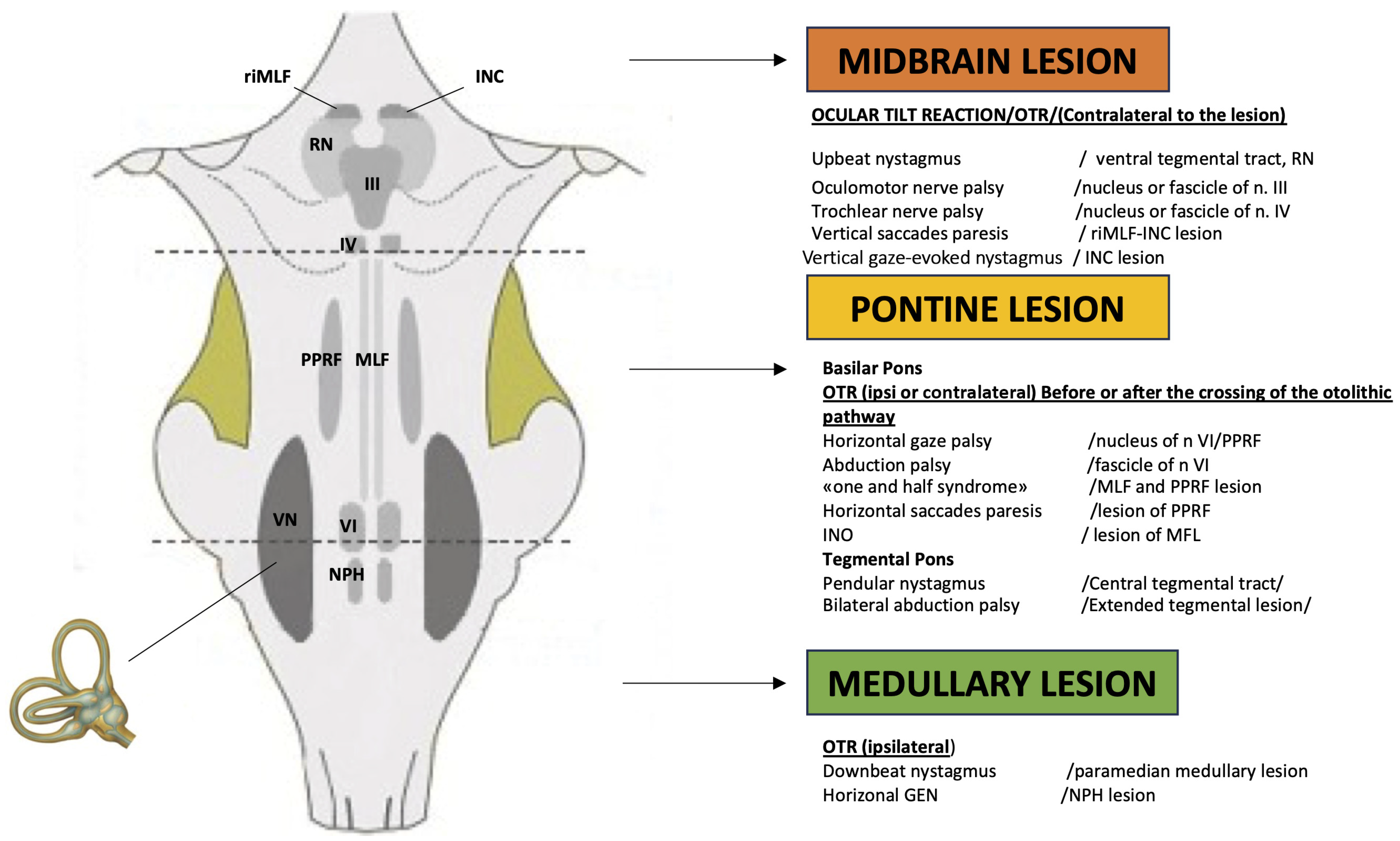
2.1. Abnormal Eye Movements in Medullary Lesions
2.2. Abnormal Eye Movements in Pontine Lesions
2.3. Abnormal Eye Movements in Midbrain Lesions
3. Vascular Disorders of the Brainstem
4. Neuro-Otological Signs Associated with Brainstem Involvement
4.1. Central Positional Nystagmus
- The CPN may have any trajectory, but pure downbeat and apogeotropic bidirectional horizontal forms are far more common than upbeat, torsional, or mixed forms.
- Nystagmus that occurs during or shortly after a change in position, with little or no latency, suggests a central cause.
- Failure to fatigue/persistence of nystagmus, especially after repeated supine roll tests, suggests a central cause.
- Intense positional nystagmus with little to no vertiginous sensation may also suggest a central cause.
- Poor or no response to repeated repositioning maneuvers.
- Apogeotropic bidirectional horizontal nystagmus. More commonly associated with cerebellar disease [56,64], this type of CPN shows no latency and no associated vertigo, lasts as long as the position is maintained, and is reproduced by returning the patient to the same position. A brainstem lesion could induce an apogeotropic CPN because of damage to the connection from the nodulus and uvula (and sometimes tonsil) to the vestibular nuclei [18,58,59,60,61,62,63,64] (Figure 4).
- Positional downbeating nystagmus (PDN). In the past, the presence of PDN during the head-hanging position and/or in Dix–Hallpike was considered a sign of central vestibular involvement; in the present time, PDN is more frequently associated with an apogeotropic variant of posterior canal BPPV [65] or anterior canal BPPV [66]. Two patterns of PDN can be recognized: paroxysmal, with poor or no latency, duration less than 1 min, and occasionally with an upbeating nystagmus when the patient returns to the sitting position; and persistent, sometimes preceded by a paroxysmal component [67]. The pathophysiology of PDN during a brainstem lesion is similar to that described for the apogeotropic horizontal positional nystagmus. Recently a case of paroxysmal CPN mimicking posterior canal BPPV due to a pontine infarction was described [68]. Finally, upbeating nystagmus and central bidirectional geotropic nystagmus of central origin are much rarer.
4.2. Head-Shaking Nystagmus (HSN)
Smooth Pursuit and Saccades Abnormalities in Brainstem Lesions
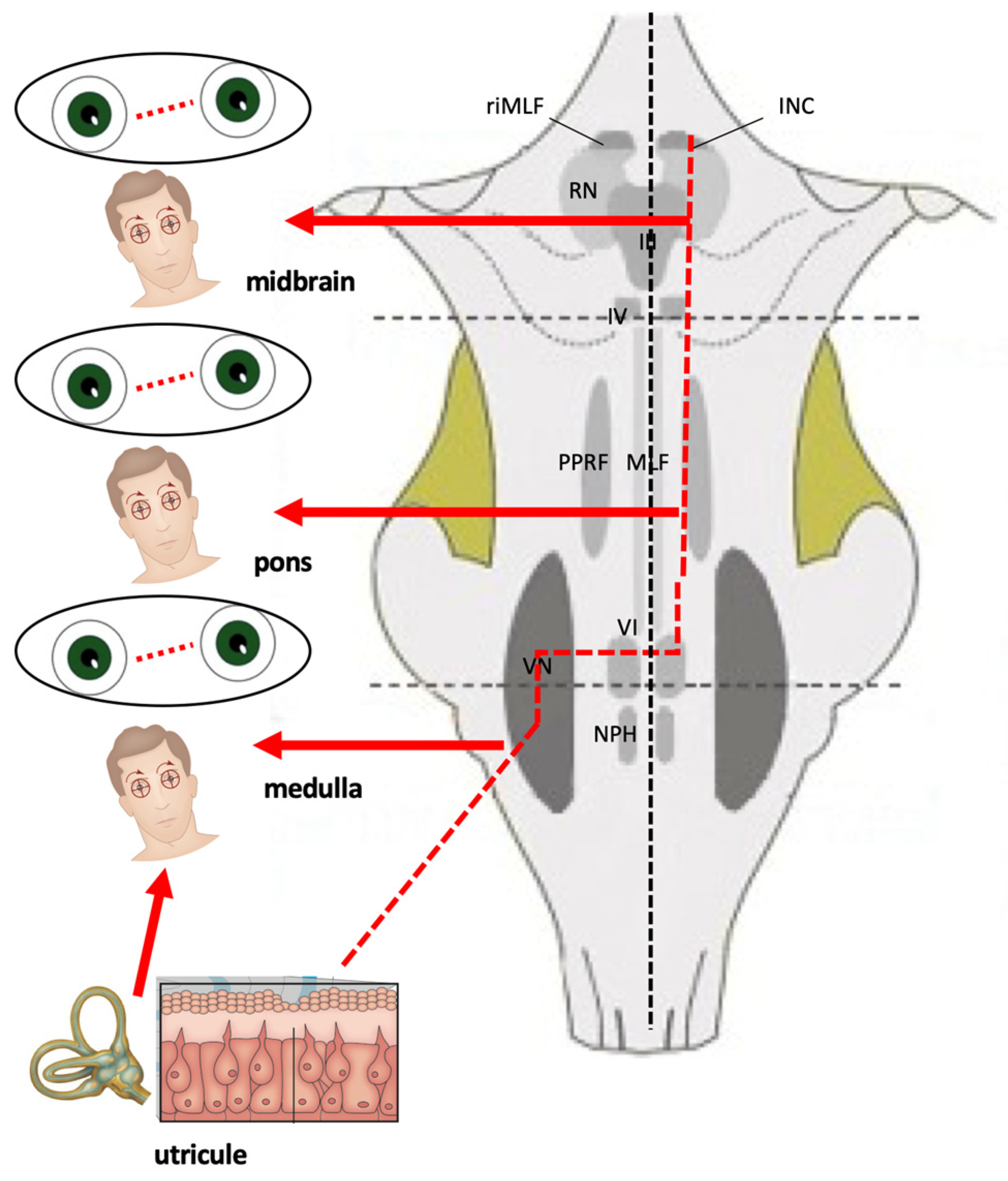
4.3. Ocular Tilt Reaction (OTR)
- Skew deviation is a vertical misalignment of the eyes due to unilateral impairment of the otolith–ocular reflex. Hypotropia of the eye (on the side of the lesion if the damage affects the peripheral receptor and/or the pathways before their crossing, contralaterally in case of deficit after the commissure).
- Ocular torsion (in the case of the right labyrinth, counterclockwise torsion from the viewer’s point of view in the case of a pre-decussation lesion, clockwise in the case of a post-decussation lesion).
- Head tilt (to the side of the lesion if the damage affects the peripheral receptor and/or the pathways before their crossing, contralaterally in case of deficit after the commissure).
4.4. Spontaneous Acquired Nystagmus in Brainstem Lesion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VOR | Vestibulo-ocular reflex |
| NPH | Nucleus propositus Hypoglossi |
| NR | Nucleus of Roller |
| OTR | Ocular tilt reaction |
| PPRF | Paramedian pontine reticular formation |
| GEN | Gaze-evoked nystagmus |
| HSN | Head-shaking nystagmus |
| SP | Smooth pursuit |
| MLF | Medial longitudinal fascicle |
| INO | Internuclear ophthalmoplegia |
| SD | Skew deviation |
| riMLF | rostral interstitial nucleus of the medial longitudinal fasciculus |
| PICA | Posterior inferior cerebellar artery |
| AICA | Anterior inferior cerebellar artery |
| ICP | Inferior cerebellar peduncle |
| MRI | Magnetic resonance imaging |
| HIT | Head impulse test |
| CNP | Central positional nystagmus |
| PDN | Positional downbeating nystagmus |
| BPPV | Benign paroxysmal positional vertigo |
| DBN | Downbeat nystagmus |
| UBN | Upbeat nystagmus |
| TN | Torsional nystagmus |
References
- Geser, R.; Straumann, D. Referral and final diagnoses of patients assessed in an academic vertigo center. Front. Neurol. 2012, 3, 169. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Si, L.; Zhang, W.; Wang, Y.; Li, K.; Yang, X. Etiologic distribution of dizziness/vertigo in a neurological outpatient clinic according to the criteria of the international classification of vestibular disorders: A single-center study. J. Neurol. 2024, 271, 2446–2457. [Google Scholar] [CrossRef] [PubMed]
- Brandt, T. (Ed.) Central vestibular disorders. In Vertigo: Its Multisensory Syndromes; Springer: London, UK, 2000; pp. 146–167. [Google Scholar]
- Leigh, R.J.; Zee, D.S. The Neurology of Eye Movements, 5th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Strupp, M.; Hüfner, K.; Sandmann, R.; Zwergal, A.; Dieterich, M.; Jahn, K.; Brandt, T. Central oculomotor disturbances and nystagmus: A window into the brainstem and cerebellum. Dtsch. Arztebl. Int. 2011, 108, 197–204. [Google Scholar] [PubMed]
- Molnár, A.; Maihoub, S.; Tamás, L.; Szirmai, Á. Comparison between caloric and video-head impulse tests in Ménière’s disease and vestibular neuritis. Int. J. Audiol. 2023, 62, 393–399. [Google Scholar] [CrossRef]
- Molnár, A.; Jassoy, B.D.; Maihoub, S.; Mavrogeni, P.; Tamás, L.; Szirmai, Á. Long-term follow-up of patients with vestibular neuritis by caloric testing and directional preponderance calculation. Eur. Arch. Oto-Rhino-Laryngol. 2023, 280, 1695–1701. [Google Scholar] [CrossRef]
- Maihoub, S.; Molnár, A.; Tamás, L.; Szirmai, Á. The diagnosis of central vestibular disorders based on the complementary examination of the vestibulospinal reflex. J. Otol. 2022, 17, 1–4. [Google Scholar] [CrossRef]
- Dieterich, M. Central vestibular disorders. J. Neurol. 2007, 254, 559–568. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.J.; Kim, J.S. Ocular motor dysfunction due to brainstem disorders. J. Neuroophthalmol. 2018, 13, 393–412. [Google Scholar] [CrossRef]
- Brandt, T.; Dieterich, M. The dizzy patient: Don’t forget disorders of the central vestibular system. Nat. Rev. Neurol. 2017, 13, 352–362. [Google Scholar] [CrossRef]
- Kattah, J.C.; Saber Tehrani, A.S.; Roeber, S.; Gujrati, M.; Bach, S.E.; Newman Toker, D.E.; Blitz, A.M.; Horn, A.K.E. Transient Vestibulopathy in Wallenberg’s Syndrome: Pathologic Analysis. Front. Neurol. 2017, 8, 191. [Google Scholar] [CrossRef]
- Choi, W.Y.; Gold, D.R. Ocular Motor and Vestibular Disorders in Brainstem Disease. J. Clin. Neurophysiol. 2019, 36, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, M.; Brandt, T. Wallenberg’s syndrome: Lateropulsion, cyclorotation, and subjective visual vertical in thirty-six patients. Ann. Neurol. 1992, 31, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Moon, S.Y.; Kim, K.Y.; Kim, H.C.; Park, S.H.; Yoon, B.W.; Roh, J.K. Ocular contrapulsion in rostral medial medullary infarction. Neurology 2004, 63, 1325–1327. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Zee, D.S.; Du Lac, S.; Kim, H.J.; Kim, J.S. Nucleus prepositus hypoglossi lesions produce a unique ocular motor syndrome. Neurology 2016, 87, 2026–2033. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, H.J.; Kim, J.S. Recent advances in head impulse test findings in central vestibular disorders. Neurology 2018, 90, 602–612. [Google Scholar] [CrossRef]
- Choi, J.H.; Seo, J.D.; Choi, Y.R.; Kim, M.J.; Kim, H.J.; Kim, J.S.; Choi, K.D. Inferior cerebellar peduncular lesion causes a distinct vestibular syndrome. Eur. J. Neurol. 2015, 22, 1062–1067. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, S.H.; Kim, H.J.; Kim, J.S. Isolated central vestibular syndrome. Ann. N. Y. Acad. Sci. 2015, 1343, 80–89. [Google Scholar] [CrossRef]
- Pierrot Deseilligny, C.; Milea, D. Vertical nystagmus: Clinical facts and hypotheses. Brain 2005, 128, 1237–1246. [Google Scholar] [CrossRef]
- Nakamagoe, K.; Shimizu, K.; Koganezawa, T.; Tamaoka, A. Downbeat nystagmus due to a paramedian medullary lesion. J. Clin. Neurosci. 2012, 19, 1597–1599. [Google Scholar] [CrossRef]
- Marcelli, V.; Giannoni, B.; Volpe, G.; Faralli, M.; Marcelli, E.; Cavaliere, M.; Fetoni, A.R.; Pettorossi, V.E. Upbeat nystagmus: A clinical and pathophysiological review. Front. Neurol. 2025, 16, 1601434. [Google Scholar] [CrossRef]
- Geiner, S.; Horn, A.K.; Wadia, N.H.; Sakai, H.; Buttner-Ennever, J.A. The neuroanatomical basis of slow saccades in spinocerebellar ataxia type 2 (Wadia-subtype). Prog. Brain Res. 2008, 171, 575–581. [Google Scholar] [PubMed]
- Nishida, T.; Tychsen, L.; Corbett, J.J. Resolution of saccadic palsy after treatment of brain-stem metastasis. Arch. Neurol. 1986, 43, 1196–1197. [Google Scholar] [CrossRef]
- Bhidayasiri, R.; Plant, G.T.; Leigh, R.J. A hypothetical scheme for the brainstem control of vertical gaze. Neurology 2000, 54, 1985–1993. [Google Scholar] [CrossRef]
- Frohman, E.M.; Frohman, T.C.; Zee, D.S.; McColl, R.; Galetta, S. The neuro-ophthalmology of multiple sclerosis. Lancet Neurol. 2005, 4, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Zwergal, A.; Cnyrim, C.; Arbusow, V.; Glaser, M.; Fesl, G.; Brandt, T.; Strupp, M. Unilateral INO is associated with ocular tilt reaction in pontomesencephalic lesions: INO plus. Neurology 2008, 71, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.U.; Kim, H.J.; Kim, J.S. Evolution of symmetric upbeat into dissociated torsional-upbeat nystagmus in internuclear ophthalmoplegia. Clin. Neurol. Neurosurg. 2013, 115, 1882–1884. [Google Scholar] [CrossRef]
- Prasad, S.; Galetta, S.L. Eye movement abnormalities in multiple sclerosis. Neurol. Clin. 2010, 28, 641–655. [Google Scholar] [CrossRef]
- Kim, J.S. Internuclear ophthalmoplegia as an isolated or predominant symptom of brainstem infarction. Neurology 2004, 62, 1491–1496. [Google Scholar] [CrossRef]
- de Seze, J.; Lucas, C.; Leclerc, X.; Sahli, A.; Vermersch, P.; Leys, D. One-and-a-half syndrome in pontine infarcts: MRI correlates. Neuroradiology 1999, 41, 666–669. [Google Scholar] [CrossRef]
- Bronstein, A.M.; Rudge, P.; Gresty, M.A.; Du Boulay, G.; Morris, J. Abnormalities of horizontal gaze. Clinical, oculographic and magnetic resonance imaging findings. II. Gaze palsy and internuclear ophthalmoplegia. J. Neurol. Neurosurg. Psychiatry 1990, 53, 200–207. [Google Scholar] [CrossRef]
- Xue, F.; Zhang, L.; Zhang, L.; Ying, Z.; Sha, O.; Ding, Y. One-and-a-half syndrome with its spectrum disorders. Quant. Imaging Med. Surg. 2017, 7, 691. [Google Scholar] [CrossRef]
- Takahashi, M.; Sugiuchi, Y.; Shinoda, Y. Brainstem neural circuits triggering vertical saccades and fixation. J. Neurosci. 2024, 44, e1657232023. [Google Scholar] [CrossRef]
- Strupp, M.; Kremmyda, O.; Adamczyk, C.; Böttcher, N.; Muth, C.; Yip, C.W.; Bremova, T. Central ocular motor disorders, including gaze palsy and nystagmus. J. Neurol. 2014, 261, 542–558. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Buttner-Ennever, J.A.; Straumann, D.; Hepp, K.; Hess, B.J.; Henn, V. Deficits in torsional and vertical rapid eye movements and shift of listing’s plane after uni- and bilateral lesions of the rostral interstitial nucleus of the medial longitudinal fasciculus. Exp. Brain Res. 1995, 106, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Helmchen, C.; Rambold, H.; Fuhry, L.; Büttner, U. Deficits in vertical and torsional eye movements after uni- and bilateral muscimol inactivation of the interstitial nucleus of Cajal of the alert monkey. Exp. Brain Res. 1998, 119, 436–452. [Google Scholar] [CrossRef] [PubMed]
- Helmchen, C.; Rambold, H.; Kempermann, U.; Buttner-Ennever, J.A.; Buttner, U. Localizing value of torsional nystagmus in small midbrain lesions. Neurology 2002, 59, 1956–1964. [Google Scholar] [CrossRef]
- Merwick, A.; Werring, D. Posterior circulation ischaemic stroke. Bmj 2014, 348, g3175. [Google Scholar] [CrossRef]
- Kerber, K.A.; Meurer, W.J.; Brown, D.L.; Burke, J.F.; Hofer, T.P.; Tsodikov, A.; Hoeffner, E.G.; Fendrick, A.M.; Adelman, E.E.; Morgenstern, L.B. Stroke risk stratification in acute dizziness presentations: A prospective imaging-based study. Neurology 2015, 85, 1869–1878. [Google Scholar] [CrossRef]
- Tarnutzer, A.A.; Berkowitz, A.L.; Robinson, K.A.; Hsieh, Y.H.; Newman-Toker, D.E. Does my dizzy patient have a stroke? A systematic review of bedside diagnosis in acute vestibular syndrome. CMAJ 2011, 183, E571–E592. [Google Scholar] [CrossRef]
- Edlow, J.A.; Newman-Toker, D.E.; Savitz, S.I. Diagnosis and initial management of cerebellar infarction. Lancet Neurol. 2008, 7, 951–964. [Google Scholar] [CrossRef]
- Lee, H.; Sohn, S.I.; Cho, Y.W.; Lee, S.R.; Ahn, B.H.; Park, B.R.; Baloh, R.W. Cerebellar infarction presenting isolated vertigo: Frequency and vascular topographical patterns. Neurology 2006, 67, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Saber Tehrani, A.S.; Kattah, J.C.; Kerber, K.A.; Gold, D.R.; Zee, D.S.; Urrutia, V.C.; Newman-Toker, D.E. Diagnosing Stroke in Acute Dizziness and Vertigo: Pitfalls and Pearls. Stroke 2018, 49, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Vanni, S.; Vannucchi, P.; Pecci, R.; Pepe, G.; Paciaroni, M.; Pavellini, A.; Ronchetti, M.; Pelagatti, L.; Bartolucci, M.; Konze, A.; et al. Consensus paper on the management of acute isolated vertigo in the emergency department. Intern. Emerg. Med. 2024, 19, 1181–1202. [Google Scholar] [CrossRef]
- Cnyrim, C.D.; Newman-Toker, D.; Karch, C.; Brandt, T.; Strupp, M. Bedside differentiation of vestibular neuritis from central “vestibular pseudoneuritis”. J. Neurol. Neurosurg. Psychiatry 2008, 79, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Kattah, J.C.; Talkad, A.V.; Wang, D.Z.; Hsieh, Y.H.; Newman-Toker, D.E. HINTS to diagnose stroke in the acute vestibular syndrome: Three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke 2009, 40, 3504–3510. [Google Scholar] [CrossRef]
- Edlow, J.A. Distinguishing Peripheral from Central Causes of Dizziness and Vertigo without using HINTS or STANDING. J. Emerg. Med. 2024, 67, e622–e633. [Google Scholar] [CrossRef]
- Lawhn-Heath, C.; Buckle, C.; Christoforidis, G.; Straus, C. Utility of head CT in the evaluation of vertigo/dizziness in the emergency department. Emerg. Radiol. 2013, 20, 45–49. [Google Scholar]
- Edlow, J.A.; Carpenter, C.; Akhter, M.; Khoujah, D.; Marcolini, E.; Meurer, W.J.; Morrill, D.; Naples, J.G.; Ohle, R.; Omron, R.; et al. Guidelines for reasonable and appropriate care in the emergency department 3 (GRACE-3): Acute dizziness and vertigo in the emergency department. Acad. Emerg. Med. 2023, 30, 442–486. [Google Scholar] [CrossRef]
- Carmona, S.; Martinez, C.; Zalazar, G.; Moro, M.; Batuecas-Caletrio, A.; Luis, L.; Gordon, C. The Diagnostic Accuracy of Truncal Ataxia and HINTS as Cardinal Signs for Acute Vestibular Syndrome. Front. Neurol. 2016, 7, 125. [Google Scholar] [CrossRef]
- Tarnutzer, A.A.; Edlow, J.A. Bedside Testing in Acute Vestibular Syndrome—Evaluating HINTS Plus and Beyond—A Critical Review. Audiol. Res. 2023, 13, 670–685. [Google Scholar]
- Baloh, R.W.; Yee, R.D.; Honrubia, V. Eye movements in patients with Wallenberg’s syndrome. Ann. N. Y. Acad. Sci. 1981, 374, 600–613. [Google Scholar] [CrossRef]
- Kumral, E.; Bayulkem, G.; Evyapan, D. Clinical spectrum of pontine infarction: Clinical—MRI correlations. J. Neurol. 2002, 249, 1659–1670. [Google Scholar] [CrossRef]
- Frohman, E.M.; Frohman, T.C.; Fleckenstein, J.; Racke, M.K.; Hawker, K.; Kramer, P.D. Ocular contrapulsion in multiple sclerosis: Clinical features and pathophysiological mechanisms. J. Neurol. Neurosurg. Psychiatry 2001, 70, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Lemos, J.; Strupp, M. Central positional nystagmus: An update. J. Neurol. 2021, 269, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lee, S.H.; Kim, H.J.; Kim, J.S. Less talked variants of benign paroxysmal positional vertigo. J. Neurol. Sci. 2022, 442, 120440. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Kim, J.H.; Kim, H.J.; Glasauer, S.; Kim, J.S. Central paroxysmal positional nystagmus: Characteristics and possible mechanisms. Neurology 2015, 84, 2238–2246. [Google Scholar] [CrossRef]
- De Schutter, E.; Adham, Z.O.; Kattah, J.C. Central positional vertigo: A clinical-imaging study. Prog. Brain Res. 2019, 249, 345–360. [Google Scholar]
- Walker, M.F.; Tian, J.; Shan, X.; Tamargo, R.J.; Ying, H.; Zee, D.S. The cerebellar nodulus/uvula integrates otolith signals for the translational vestibulo-ocular reflex. PLoS ONE 2010, 5, e13981. [Google Scholar] [CrossRef]
- Macdonald, N.K.; Kaski, D.; Saman, Y.; Sulaiman, A.A.-S.; Anwer, A.; Bamiou, D.E. Central Positional Nystagmus: A Systematic Literature Review. Front. Neurol. 2017, 8, 141. [Google Scholar] [CrossRef]
- Lee, S.U.; Kim, H.J.; Lee, E.S.; Choi, J.H.; Choi, J.Y.; Kim, J.S. Central positional nystagmus in inferior cerebellar peduncle lesions: A case series. J. Neurol. 2021, 268, 2851–2857. [Google Scholar] [CrossRef]
- Buttner, U.; Helmchen, C.; Brandt, T. Diagnostic Criteria for Central Versus Peripheral Positioning Nystagmus and Vertigo: A Review. Acta Otolaryngol. 1999, 119, 1–5. [Google Scholar]
- Imai, T.; Horii, A.; Takeda, N.; Higashi-Shingai, K.; Inohara, H. A case of apogeotropic nystagmus with brainstem lesion: An implication for mechanism of central apogeotropic nystagmus. Auris Nasus Larynx 2010, 37, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Califano, L.; Mazzone, S.; Salafia, F.; Melillo, M.G.; Manna, G. Less common forms of posterior canal benign paroxysmal positional vertigo. Acta Otorhinolaryngol. Ital. 2021, 41, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Casani, A.P.; Cerchiai, N.; Dallan, I.; Sellari-Franceschini, S. Anterior canal lithiasis: Diagnosis and treatment. Otolaryngol. Head Neck Surg. 2011, 144, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Yacovino, D.A.; Cherchi, M. Clinical spectrum of positional downbeat nystagmus: A diagnostic approach. J. Neurol. 2025, 272, 163. [Google Scholar] [CrossRef]
- Kwon, E.; Jeong, H.S.; Jeong, S.H.; Kim, H.J.; Kim, J.S. Central paroxysmal positional nystagmus mimicking posterior canal benign paroxysmal positional vertigo in pontine infarction: A case report and literature review. J. Neurol. 2024, 271, 3672–3677. [Google Scholar] [CrossRef]
- Hain, T.C.; Spindler, J. Head-shaking nystagmus. In The Vestibulo-Ocular Reflex and Vertigo; Sharpe, J.A., Barber, H.O., Eds.; Raven Press: New York, NY, USA, 1993; pp. 217–228. [Google Scholar]
- Kim, H.A.; Lee, H.; Sohn, S.I.; Kim, J.S.; Baloh, R.W. Perverted head shaking nystagmus in focal pontine infarction. J. Neurol. Sci. 2011, 301, 93–95. [Google Scholar] [CrossRef]
- Minagar, A.; Sheremata, W.A.; Tusa, R.J. Perverted head-shaking nystagmus: A possible mechanism. Neurology 2001, 57, 887–889. [Google Scholar] [CrossRef]
- Choi, K.D.; Oh, S.Y.; Park, S.H.; Kim, J.H.; Koo, J.W.; Kim, J.S. Head-shaking nystagmus in lateral medullary infarction: Patterns and possible mechanisms. Neurology 2007, 68, 1337–1344. [Google Scholar] [CrossRef]
- Yang, T.H.; Lee, J.H.; Oh, S.Y.; Kang, J.J.; Kim, J.S.; Dieterich, M. Clinical implications of head-shaking nystagmus in central and peripheral vestibular disorders: Is perverted head-shaking nystagmus specific for central vestibular pathology? Eur. J. Neurol. 2020, 27, 1296–1303. [Google Scholar] [CrossRef]
- Choi, J.Y.; Jung, I.; Jung, J.M.; Kwon, D.Y.; Park, M.H.; Kim, H.J.; Kim, J.S. Characteristics and mechanism of perverted head-shaking nystagmus in central lesions: Video-oculography analysis. Clin. Neurophysiol. 2016, 127, 2973–2978. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.B.; Boo, S.H.; Ban, J.H. Nystagmus-based approach to vertebrobasilar stroke presenting as vertigo without initial neurologic signs. Eur. Neurol. 2013, 70, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.L.; Sharpe, J.A.; Morrow, M.J. Paresis of contralateral smooth pursuit and normal vestibular smooth eye movements after unilateral brainstem lesions. Ann. Neurol. 1992, 31, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Fracica, E.; Hale, D.; Gold, D.R. Diagnosing and localizing the acute vestibular syndrome–beyond the HINTS exam. J. Neurol. Sci. 2022, 442, 120451. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, J.M.; Kim, J.S. Update on the medial longitudinal fasciculus syndrome. Neurol. Sci. 2022, 43, 3533–3540. [Google Scholar] [CrossRef]
- Bianchi, F.; Vidailhet, M.; Gaymard, B. Ipsilateral Saccade Hypometria and Contralateral Saccadic Pursuit in a Focal Brainstem Lesion: A Rare Oculomotor Pattern. Cerebellum 2018, 17, 485–488. [Google Scholar] [CrossRef]
- Brandt, T.; Dieterich, M. Vestibular syndromes in the roll plane: Topographic diagnosis from brainstem to cortex. Ann. Neurol. 1994, 36, 337–347. [Google Scholar] [CrossRef]
- Brodsky, M.C.; Donahue, S.P.; Vaphiades, M.; Brandt, T. Skew deviation revisited. Surv. Ophthalmol. 2006, 51, 105–128. [Google Scholar] [CrossRef]
- Halmagyi, G.; Brandt, T.; Dieterich, M.; Curthoys, I.; Stark, R.; Hoyt, W. Tonic contraversive ocular tilt reaction due to unilateral meso-diencephalic lesion. Neurology 1990, 40, 1503–1509. [Google Scholar] [CrossRef]
- Hedges, T.R.D.; Hoyt, W.F. Ocular tilt reaction due to an upper brainstem lesion: Paroxysmal skew deviation, torsion, and oscillation of the eyes with head tilt. Ann. Neurol. 1982, 11, 537–540. [Google Scholar] [CrossRef]
- Rodriguez, A.R.; Egan, R.A.; Barton, J.J. Pearls & Oysters: Paroxysmal ocular tilt reaction. Neurology 2009, 72, e67–e68. [Google Scholar] [CrossRef] [PubMed]
- Kattah, J.C. Update on HINTS Plus, With Discussion of Pitfalls and Pearls. J. Neurol. Phys. Ther. 2019, 43, S42–S45. [Google Scholar] [CrossRef] [PubMed]
- Korda, A.; Zamaro, E.; Wagner, F.; Morrison, M.; Caversaccio, M.D.; Sauter, T.C.; Schneider, E.; Mantokoudis, G. Acute vestibular syndrome: Is skew deviation a central sign? J. Neurol. 2022, 269, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Gufoni, M. Uphill/downhill nystagmus. Acta Otorhinolaryngol. Ital. 2017, 37, 513–518. [Google Scholar] [CrossRef]
- Wagner, J.N.; Glaser, M.; Brandt, T.; Strupp, M. Downbeat nystagmus: Aetiology and comorbidity in 117 patients. J. Neurol. Neurosurg. Psychiatry 2008, 79, 672–677. [Google Scholar] [CrossRef]
- Marcelli, V.; Giannoni, B.; Volpe, G.; Faralli, M.; Fetoni, A.R.; Pettorossi, V.E. Downbeat nystagmus: A clinical and pathophysiological review. Front. Neurol. 2024, 15, 1394859. [Google Scholar] [CrossRef]
- Himi, T.; Kataura, A.; Tokuda, S.; Sumi, Y.; Kamiyama, K.; Shitamichi, M. Downbeat nystagmus with compression of the medulla oblongata by the dolichoectatic vertebral arteries. Am. J. Otol. 1995, 16, 377–381. [Google Scholar]
- Rosengart, A.; Hedges, T.R., 3rd; Teal, P.A.; DeWitt, L.D.; Wu, J.K.; Wolpert, S.; Caplan, L.R. Intermittent downbeat nystagmus due to vertebral artery compression. Neurology 1993, 43, 216–218. [Google Scholar] [CrossRef]
- Kim, J.S.; Yoon, B.; Choi, K.D.; Oh, S.Y.; Park, S.H.; Kim, B.K. Upbeat nystagmus: Clinic-anatomical correlations in 15 patients. J. Clin. Neurol. 2006, 2, 58–65. [Google Scholar] [CrossRef]
- Choi, K.D.; Jung, D.S.; Park, K.P.; Jo, J.W.; Kim, J.S. Bowtie and upbeat nystagmus evolving into hemi-seesaw nystagmus in medial medullary infarction: Possible anatomic mechanisms. Neurology 2004, 62, 663–665. [Google Scholar] [CrossRef]
- Hirose, G.; Ogasawara, T.; Shirakawa, T.; Kawada, J.; Kataoka, S.; Yoshioka, A.; Halmagyi, G.M. Primary position upbeat nystagmus due to unilateral medial medullary infarction. Ann. Neurol. 1998, 43, 403–406. [Google Scholar] [CrossRef]
- Gilman, N.; Baloh, R.W.; Tomiyasu, U. Primary position upbeat nystagmus. A clinicopathologic study. Neurology 1977, 27, 294–298. [Google Scholar] [CrossRef]
- Lopez, L.; Bronstein, A.M.; Gresty, M.A.; Rudge, P.; du Boulay, E.P. Torsional nystagmus. A neuro-otological and MRI study of thirty-five cases. Brain 1992, 115, 1107–1124. [Google Scholar] [CrossRef] [PubMed]
- Morrow, M.J.; Sharpe, J.A. Torsional nystagmus in the lateral medullary syndrome. Ann. Neurol. 1988, 24, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Cannon, S.C.; Robinson, D.A. Loss of the neural integrator of the oculomotor system from brain stem lesions in monkey. J. Neurophysiol. 1987, 57, 1383–1409. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.D.; Kayan, A.; Leech, J. Rebound nystagmus. Brain 1973, 96, 507–526. [Google Scholar] [CrossRef]
- Khan, S.R.; Lueck, C.J. Hemi-seesaw nystagmus in lateral medullary syndrome. Neurology 2013, 80, 1261–1262. [Google Scholar] [CrossRef]
- Halmagy, G.; Aw, S.; Dehaene, I.; Curthoys, I.; Todd, M. Jerk-waveform see-saw nystagmus due to unilateral meso-diencephalic lesion. Brain 1994, 117, 789–803. [Google Scholar] [CrossRef]
- Tsutsumi, T.; Ikeda, T.; Kikuchi, S. Periodic alternating nystagmus caused by a medullary lesion in acute disseminated encephalomyelitis. Otol. Neurotol. 2014, 35, 861–865. [Google Scholar] [CrossRef]
- Averbuch-Heller, L.; Zivotofsky, A.Z.; Das, V.E.; DiScenna, A.O.; Leigh, R.J. Investigations of the pathogenesis of acquired pendular nystagmus. Brain 1995, 118, 369–378. [Google Scholar] [CrossRef]
- Gresty, M.A.; Ell, J.J.; Findley, L.J. Acquired pendular nystagmus: Its characteristics, localizing value and pathophysiology. J. Neurol. Neurosurg. Psychiatry 1982, 45, 431–439. [Google Scholar] [CrossRef]
- Kang, S.; Shaikh, A.G. Acquired pendular nystagmus. J. Neurol. Sci. 2017, 375, 8–17. [Google Scholar] [CrossRef]
- Rambold, H.; Kömpf, D.; Helmchen, C. Convergence retraction nystagmus: A disorder of vergence? Ann. Neurol. 2001, 50, 677–681. [Google Scholar] [CrossRef]
- Schon, F.; Hodgson, T.L.; Mort, D.; Kennard, C. Ocular flutter associated with a localized lesion in the paramedian pontine reticular formation. Ann. Neurol. 2001, 50, 413–416. [Google Scholar] [CrossRef]
- Chang, T.P.; Gold, D.R.; Otero-Millan, J.; Huang, B.R.; Zee, D.S. Pendular oscillation and ocular bobbing after pontine hemorrhage. Cerebellum 2021, 20, 734–743. [Google Scholar] [CrossRef]


| Type of Examination | Search for |
|---|---|
| Head posture | Head tilt |
| Eye movements Position of the eyes Straight ahead, look to the right, left, upward, and downward, cover test | Primary misalignment, Spontaneous nystagmus Gaze function End-point nystagmus |
| Smooth pursuit | Saccadic, |
| Reduction in gain | |
| Saccades | Latency, velocity, accuracy |
| VOR functionality Clinical head impulse test | Presence of corrective saccades |
| Visual fixation suppression of the VOR | No suppression of VOR (mainly occur in cerebellar diseases) |
| Symptoms and Signs | Structures Involved |
|---|---|
| Vertigo, nystagmus | Vestibular nuclei |
| Tinnitus, hearing loss | Auditory nerve, cochlear nuclei |
| Gait and limb ataxia | Ventral spinocerebellar tract, middle cerebellar peduncle |
| Dysphagia, dysarthria | Vagal nuclei and nerve |
| Facial hemianesthesia | Fifth nerve and nucleus |
| Facial paralysis | Seventh nerve |
| Crossed hemisensory loss | Spinothalamic tract |
| Horner’s syndrome (ptosis, miosis, facial anhidrosis) | Descending sympathetic tract |
| Lesion/Syndrome | Primary Structure(s) Involved | Key Oculomotor and Nystagmus Characteristics |
|---|---|---|
| Wallenberg Syndrome | Lateral medulla (PICA territory) | Nystagmus: Spontaneous horizontal–torsional (fast phase beating away from the lesion). Saccades: Hypermetric (ipsilesional), hypometric (controlesional). Associated sign: ocular tilt reaction ipsilesional. |
| Medial Medullary Infarction | Nucleus propositus hypoglossi (NPH) | Nystagmus: Ipsilateral horizontal (sometimes upbeating); gaze-evoked nystagmus (GEN) more intense when looking toward the affected side. Smooth pursuit: Central pattern of head-shaking nystagmus (HSN). |
| Pons Lesions (Horizontal Gaze) | PPRF (paramedian pontine reticular formation) | Saccades: Isolated horizontal saccadic palsy. Smooth pursuit: Severely impaired or absent. |
| Internuclear Ophthalmoplegia | Medial longitudinal fasciculus (MLF) | Unilateral: Impaired adduction (ipsilateral eye) and abducting nystagmus (contralateral eye). Bilateral: Bilateral adduction latency/impairment and bilateral abducting nystagmus. Convergence is typically spared. |
| “One and a Half” Syndrome | PPRF + ipsilateral MLF | Horizontal movements: Loss of all horizontal movements, except for abduction in the eye contralateral to the lesion. |
| Midbrain Lesions (Vertical Gaze) | riMLF and interstitial nucleus of Cajal | Saccades: Isolated vertical saccadic palsy. Nystagmus: Possible isolated vertical GEN. |
| Central Positional Nystagmus | Vestibulo-cerebellar pathways (e.g., nodule/uvula) | Latency/Fatigue: Absent/minimal latency and non-fatigable (persistent). Direction: Often pure downbeat or apogeotropic bidirectional horizontal. |
| Isolated Vestibular Nuclei Infarction | Vestibular nuclei | Nystagmus: Spontaneous torsional–horizontal, beating away from the side of the lesion; direction-changing GEN. HIT: May be positive (an atypical finding for a central lesion). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casani, A.P.; Gufoni, M.; Ducci, N.; Asprella Libonati, G.; Chiarella, G. Oculomotor Abnormalities and Nystagmus in Brainstem Disease: A Mini Review. Audiol. Res. 2025, 15, 150. https://doi.org/10.3390/audiolres15060150
Casani AP, Gufoni M, Ducci N, Asprella Libonati G, Chiarella G. Oculomotor Abnormalities and Nystagmus in Brainstem Disease: A Mini Review. Audiology Research. 2025; 15(6):150. https://doi.org/10.3390/audiolres15060150
Chicago/Turabian StyleCasani, Augusto Pietro, Mauro Gufoni, Nicola Ducci, Giacinto Asprella Libonati, and Giuseppe Chiarella. 2025. "Oculomotor Abnormalities and Nystagmus in Brainstem Disease: A Mini Review" Audiology Research 15, no. 6: 150. https://doi.org/10.3390/audiolres15060150
APA StyleCasani, A. P., Gufoni, M., Ducci, N., Asprella Libonati, G., & Chiarella, G. (2025). Oculomotor Abnormalities and Nystagmus in Brainstem Disease: A Mini Review. Audiology Research, 15(6), 150. https://doi.org/10.3390/audiolres15060150









