The Presence of Serotonin in the Vestibular System: Supporting the Use of SSRIs/SNRIs in the Treatment of Vestibular Disorders—A Narrative Review
Abstract
1. Introduction
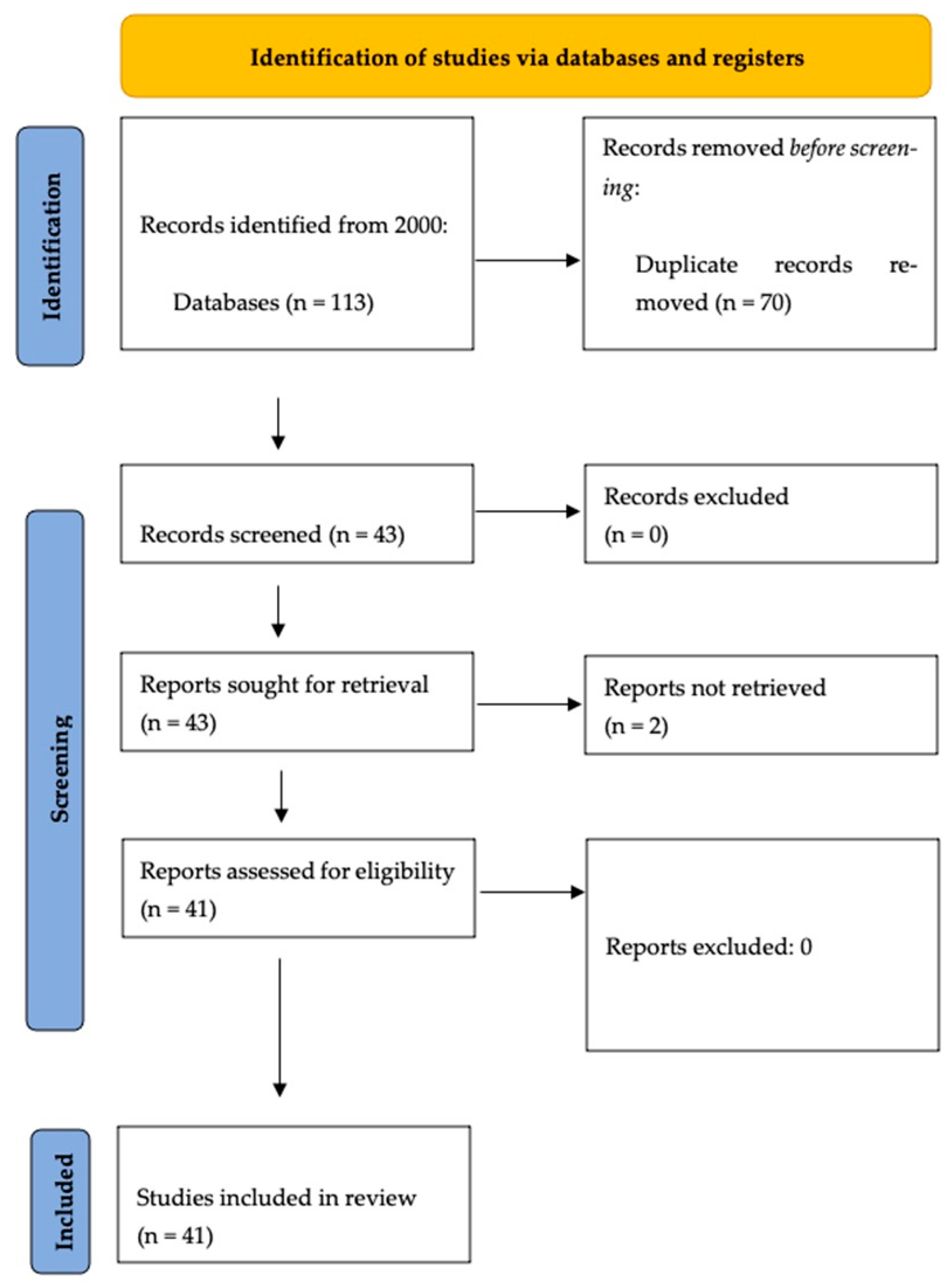
2. Materials and Methods
3. Results
3.1. SSRIs in Persistent Postural Perceptual Dizziness and Chronic Subjective Dizziness
3.2. SSRIs in Other Vestibular Disorders
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- Kroeze, W.K.; Kristiansen, K.; Roth, B.L. Molecular biology of serotonin receptors: Structure and function at the molecular level. Curr. Top. Med. Chem. 2002, 2, 507–528. [Google Scholar] [CrossRef] [PubMed]
- Airan, R.D.; Meltzer, L.A.; Roy, M.; Gong, Y.; Chen, H.; Deisseroth, K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science 2007, 317, 819–823. [Google Scholar] [CrossRef]
- Canli, T.; Lesch, K.P. Long story short: The serotonin transporter in emotion regulation and social cognition. Nat. Neurosci. 2007, 10, 1103–1109. [Google Scholar] [CrossRef]
- Kaumann, A.J.; Levy, F.O. 5-hydroxytryptamine receptors in the human cardiovascular system. Pharmacol. Ther. 2006, 111, 674–706. [Google Scholar] [CrossRef]
- Hamel, E. Serotonin and migraine: Biology and clinical implications. Cephalalgia 2007, 27, 1293–1300. [Google Scholar] [CrossRef]
- Obermann, M.; Strupp, M. Current treatment options in vestibular migraine. Front. Neurol. 2014, 5, 257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neumann, J.; Hofmann, B.; Dhein, S.; Gergs, U. Cardiac Roles of Serotonin (5-HT) and 5-HT-Receptors in Health and Disease. Int. J. Mol. Sci. 2023, 24, 4765. [Google Scholar] [CrossRef]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging Roles for Serotonin in Regulating Metabolism: New Implications for an Ancient Molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef]
- Scotton, W.J.; Hill, L.J.; Williams, A.C.; Barnes, N.M. Serotonin Syndrome: Pathophysiology, Clinical Features, Management, and Potential Future Directions. Int. J. Tryptophan Res. 2019, 12, 1178646919873925. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koo, J.W.; Balaban, C.D. Serotonin-induced plasma extravasation in the murine inner ear: Possible mechanism of migraine-associated inner ear dysfunction. Cephalalgia 2006, 26, 1310–1319. [Google Scholar] [CrossRef]
- Ahn, S.K.; Balaban, C.D. Distribution of 5-HT1B and 5-HT1D receptors in the inner ear. Brain Res. 2010, 1346, 92–101. [Google Scholar] [CrossRef]
- Ma, F.R.; Liu, J.X.; Li, X.P.; Mao, J.-J.; Zhang, Q.-D.; Jia, H.-B.; Mao, L.-Q.; Zhao, R. Effects of caloric vestibular stimulation on serotoninergic system in the media vestibular nuclei of guinea pigs. Chin. Med. J. 2007, 120, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Halberstadt, A.L.; Balaban, C.D. Organization of projections from the raphe nuclei to the vestibular nuclei in rats. Neuroscience 2003, 120, 573–594. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.K.; Khalmuratova, R.; Jeon, S.Y.; Kim, J.-P.; Park, J.-J.; Hur, D.-G.; Kim, D.-W.; Balaban, C.D. Colocalization of 5-HT1F receptor and glutamate in neurons of the vestibular nuclei in rats. NeuroReport 2009, 20, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Heesbeen, E.J.; van Kampen, D.; Vendouw, P.M.; van Lissa, C.; Bijlsma, E.Y.; Groenink, L. The effect of SSRIs on unconditioned anxiety: A systematic review and meta-analysis of animal studies. Psychopharmacology 2024, 241, 1731–1755. [Google Scholar] [CrossRef] [PubMed]
- Teggi, R.; Caldirola, D.; Colombo, B.; Perna, G.; Comi, G.; Bellodi, L.; Bussi, M. Dizziness, migrainous vertigo and psychiatric disorders. J. Laryngol. Otol. 2010, 124, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Balaban, C.D.; Thayer, J.F. Neurological bases for balance anxiety links. J. Anxiety Disord. 2001, 15, 53–79. [Google Scholar] [CrossRef]
- Waterston, J.; Chen, L.; Mahony, K.; Gencarelli, J.; Stuart, G. Persistent Postural-Perceptual Dizziness: Precipitating Conditions, Co-Morbidities and Treatment with Cognitive Behavioral Therapy. Front. Neurol. 2021, 12, 795516. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Edinoff, A.N.; Akuly, H.A.; Hanna, T.A.; Ochoa, C.O.; Patti, S.J.; Ghaffar, Y.A.; Kaye, A.D.; Viswanath, O.; Urits, I.; Boyer, A.G.; et al. Selective Serotonin Reuptake Inhibitors and Adverse Effects: A Narrative Review. Neurol. Int. 2021, 13, 387–401. [Google Scholar] [CrossRef]
- Perna, G.; Alpini, D.; Caldirola, D.; Raponi, G.; Cesarani, A.; Bellodi, L. Serotonergic modulation of the balance system in panic disorder: An open study. Depress. Anxiety 2003, 17, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Staab, J.P.; Eckhardt-Henn, A.; Horii, A.; Jacob, R.; Strupp, M.; Brandt, T.; Bronstein, A. Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): Consensus document of the committee for the Classification of Vestibular Disorders of the Bárány Society. J. Vestib. Res. 2017, 27, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Staab, J.P. Persistent Postural-Perceptual Dizziness. Semin. Neurol. 2020, 40, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.E.; Harrington-Benton, N.A.; Judd, O.; Kaski, D.; Maarsingh, O.R.; MacKeith, S.; Ray, J.; A Van Vugt, V.; Burton, M.J. Pharmacological interventions for persistent postural-perceptual dizziness (PPPD). Cochrane Database Syst. Rev. 2023, 3, CD015188. [Google Scholar] [CrossRef]
- Tang, B.; Jiang, W.; Zhang, C.; Tan, H.; Luo, M.; He, Y.; Yu, X. Effect of public square dancing combined with serotonin reuptake inhibitors on persistent postural-perceptual dizziness (PPPD) in middle-aged and older women. J. Vestib. Res. 2024, 34, 63–72. [Google Scholar] [CrossRef]
- Popkirov, S.; Staab, J.P.; Stone, J. Persistent postural-perceptual dizziness (PPPD): A common, characteristic and treatable causeof chronic dizziness. Pract. Neurol. 2018, 18, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Fleischman, K.M.; Kawai, K.; Corcoran, M.; Brodsky, J.R. Persistent Postural-Perceptual Dizziness in Children and Adolescents. Otol. Neurotol. 2021, 42, e1093–e1100. [Google Scholar] [CrossRef]
- Staab, J.P.; Ruckenstein, M.J.; Amsterdam, J.D. A prospective trial of sertraline for chronic subjective dizziness. Laryngoscope 2004, 114, 1637–1641. [Google Scholar] [CrossRef]
- Horii, A.; Mitani, K.; Kitahara, T. Paroxetine, a selective serotonin reuptake inhibitor, reduces depressive symptoms and subjective handicaps in patients with dizziness. Otol. Neurotol. 2004, 25, 536–543. [Google Scholar] [CrossRef]
- Horii, A.; Imai, T.; Kitahara, T.; Uno, A.; Morita, Y.; Takahashi, K.; Inohara, H. Psychiatric comorbidities and use of milnacipran in patients with chronic dizziness. J. Vestib. Res. 2016, 26, 335–340. [Google Scholar] [CrossRef]
- Banzi, R.; Cusi, C.; Randazzo, C.; Sterzi, R.; Tedesco, D.; Moja, L.; Cochrane Pain, Palliative and Supportive Care Group. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of migraine in adults. Cochrane Database Syst. Rev. 2015, 4, CD002919. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salviz, M.; Yuce, T.; Acar, H.; Karatas, A.; Acikalin, R.M. Propranolol and venlafaxine for vestibular migraine prophylaxis: A randomized controlled trial. Laryngoscope 2016, 126, 169–174. [Google Scholar] [CrossRef]
- Liu, F.; Ma, T.; Che, X.; Wang, Q.; Yu, S. The Efficacy of Venlafaxine, Flunarizine, and Valproic Acid in the Prophylaxis of Vestibular Migraine. Front. Neurol. 2017, 8, 524. [Google Scholar] [CrossRef] [PubMed]
- Kıroğlu, O.; Sürmelioğlu, Ö.; Kıroğlu, M. Effects of Selective Serotonine Re-Uptake Inhibitors on Meniere’s Disease. J. Int. Adv. Otol. 2017, 13, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Goto, F.; Tsutsumi, T.; Ogawa, K. Successful treatment of relapsed Ménière’s disease using selective serotonin reuptake inhibitors: A report of three cases. Exp. Ther. Med. 2014, 7, 488–490. [Google Scholar] [CrossRef] [PubMed]
- Nagliya, D.; Daryanani, S. Mal de Debarquement Syndrome: A Case Presentation of a Vestibular Enigma. Cureus 2024, 16, e65787. [Google Scholar] [CrossRef] [PubMed]
- Thorlund, K.; Druyts, E.; Wu, P.; Balijepalli, C.; Keohane, D.; Mills, E. Comparative efficacy and safety of selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors in older adults: A network meta-analysis. J. Am. Geriatr. Soc. 2015, 63, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Brandt, T.; Dieterich, M. “Excess anxiety” and “less anxiety”: Both depend on vestibular function. Curr. Opin. Neurol. 2020, 33, 136–141. [Google Scholar] [CrossRef]
- Hilber, P. The role of the cerebellar and vestibular networks in anxiety disorders and depression: The internal modelhypothesis. Cerebellum 2022, 21, 791–800. [Google Scholar] [CrossRef]
- Balaban, C.D. Neurotransmitters in the vestibular system. Handb. Clin. Neurol. 2016, 137, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Teggi, R.; Colombo, B.; Trimarchi, M.; Bianco, M.; Manfredi, A.; Bussi, M.; Corti, A. Altered chromogranin a circulating levels in Meniere’s disease. Dis. Markers 2015, 2015, 643420. [Google Scholar] [CrossRef]
- Schatzberg, A.F.; Haddad, P.; Kaplan, E.M.; Lejoyeux, M.; Rosenbaum, J.F.; Young, A.H.; Zajecka, J. Possible biological mechanisms of the serotonin reuptake inhibitor discontinuation syndrome. J. Clin. Psychiatry 1997, 58 (Suppl. S7), 23–27. [Google Scholar] [PubMed]
- Smith, P.F.; Darlington, C.L. A possible explanation for dizziness following SSRI discontinuation. Acta Oto-Laryngol. 2010, 130, 981–983. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teggi, R.; Caldirola, D.; Neri, G.; Cangiano, I.; Viola, P.; Chiarella, G. The Presence of Serotonin in the Vestibular System: Supporting the Use of SSRIs/SNRIs in the Treatment of Vestibular Disorders—A Narrative Review. Audiol. Res. 2025, 15, 148. https://doi.org/10.3390/audiolres15060148
Teggi R, Caldirola D, Neri G, Cangiano I, Viola P, Chiarella G. The Presence of Serotonin in the Vestibular System: Supporting the Use of SSRIs/SNRIs in the Treatment of Vestibular Disorders—A Narrative Review. Audiology Research. 2025; 15(6):148. https://doi.org/10.3390/audiolres15060148
Chicago/Turabian StyleTeggi, Roberto, Daniela Caldirola, Giampiero Neri, Iacopo Cangiano, Pasquale Viola, and Giuseppe Chiarella. 2025. "The Presence of Serotonin in the Vestibular System: Supporting the Use of SSRIs/SNRIs in the Treatment of Vestibular Disorders—A Narrative Review" Audiology Research 15, no. 6: 148. https://doi.org/10.3390/audiolres15060148
APA StyleTeggi, R., Caldirola, D., Neri, G., Cangiano, I., Viola, P., & Chiarella, G. (2025). The Presence of Serotonin in the Vestibular System: Supporting the Use of SSRIs/SNRIs in the Treatment of Vestibular Disorders—A Narrative Review. Audiology Research, 15(6), 148. https://doi.org/10.3390/audiolres15060148









