Use of the FLEX 28 Dexamethasone-Eluting Cochlear Implant Electrode in Electric–Acoustic Stimulation: A Case Report
Abstract
1. Introduction
2. Materials and Methods
2.1. Case
2.2. Control Subjects
2.3. Clinical Evaluation
3. Results
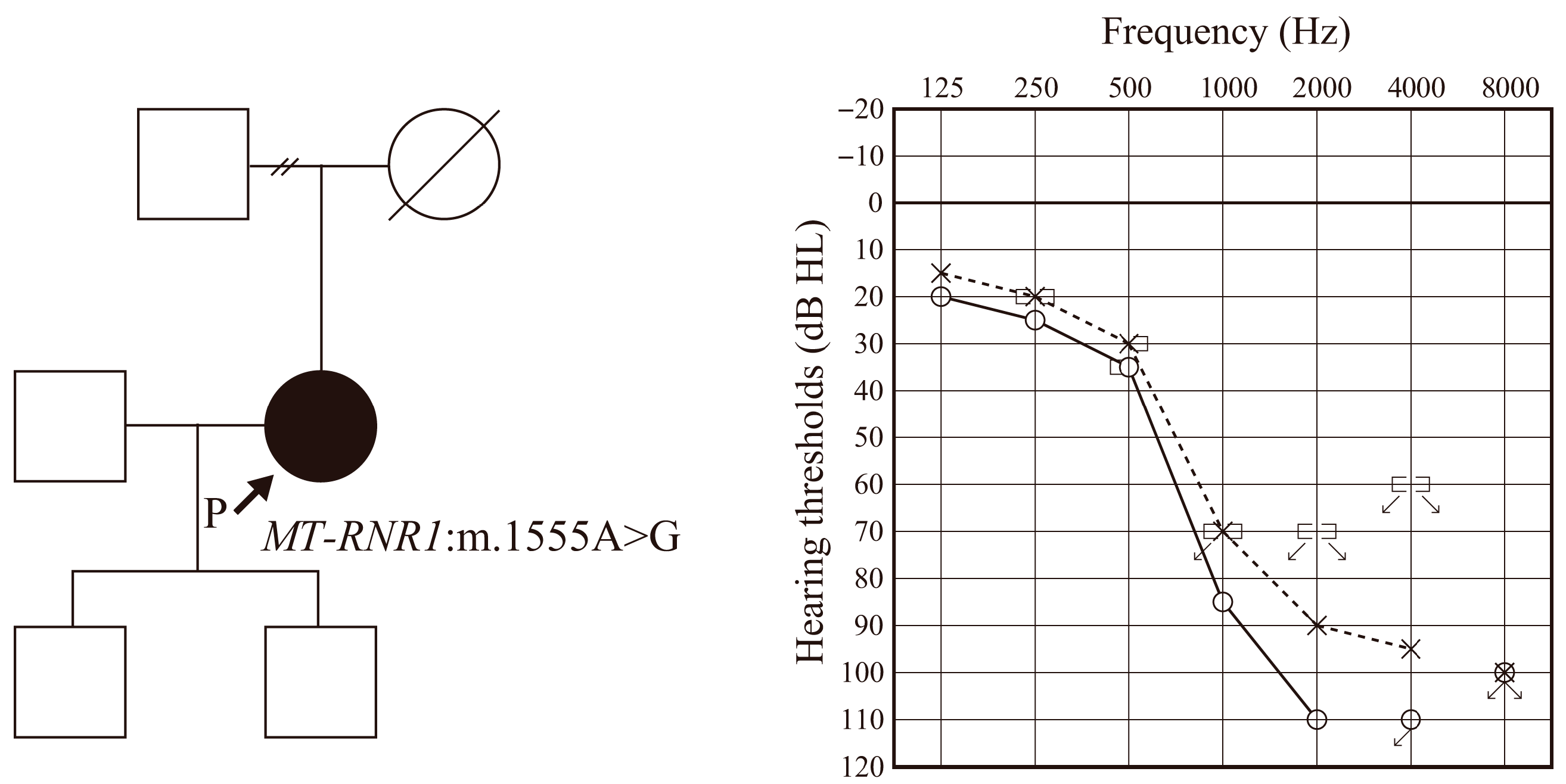
3.1. Clinical Details of the Patient
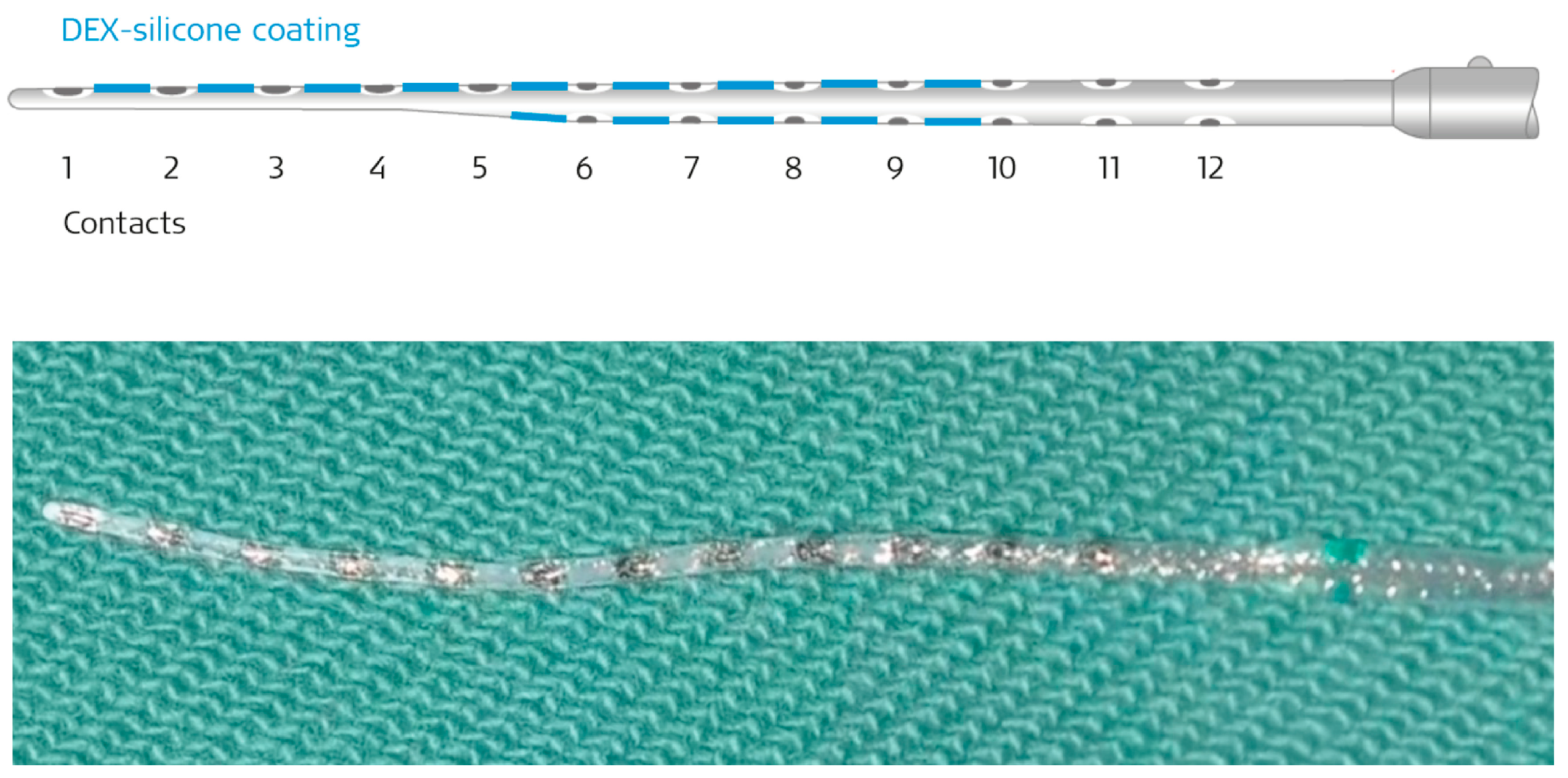
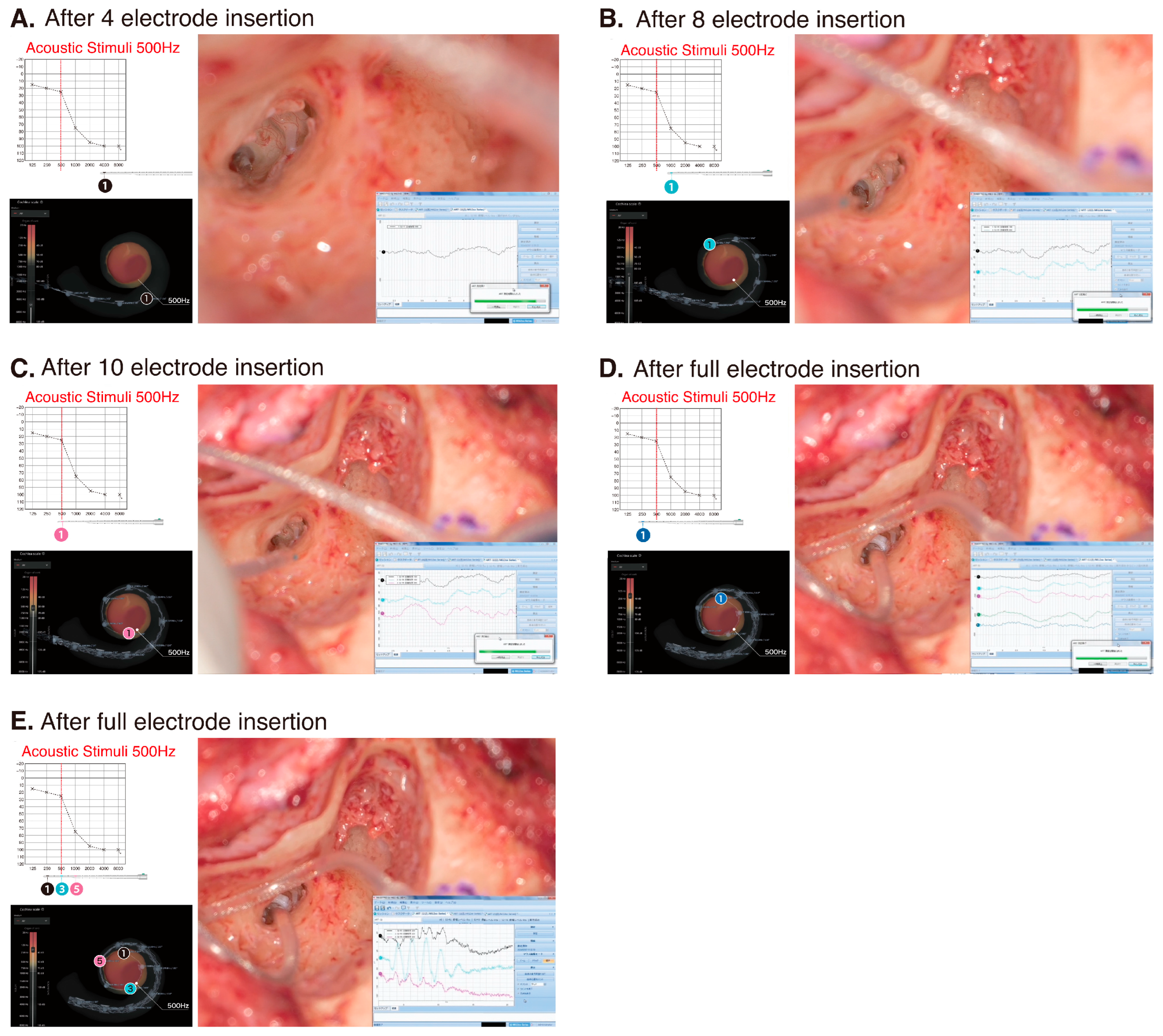
3.2. Surgery and Implantation
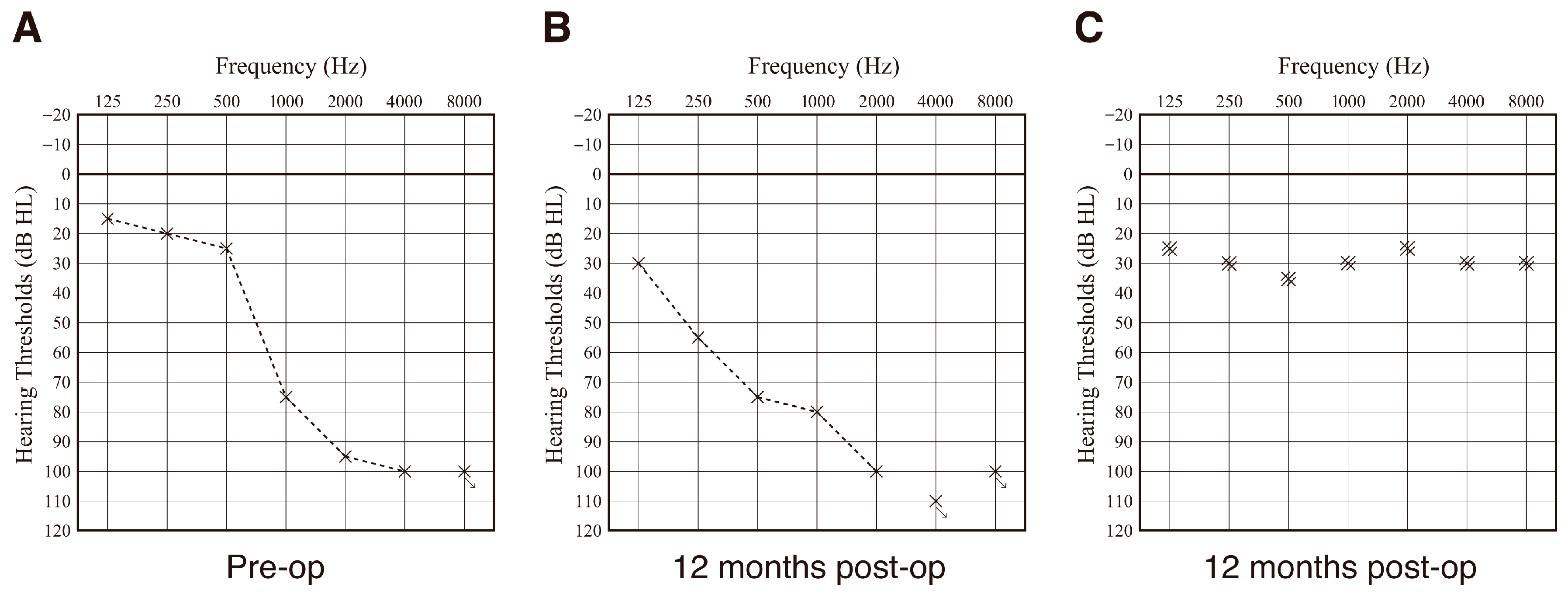
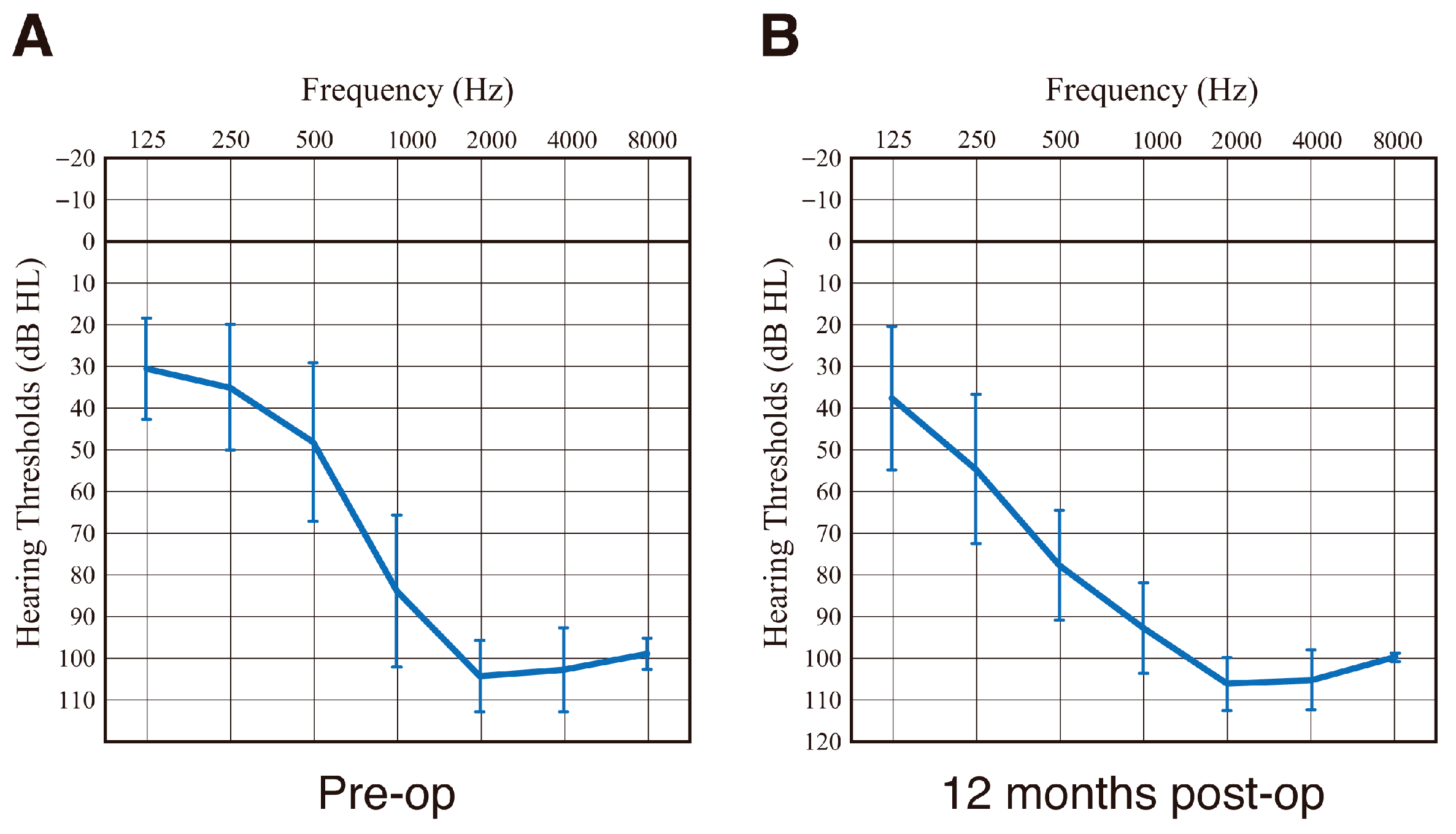
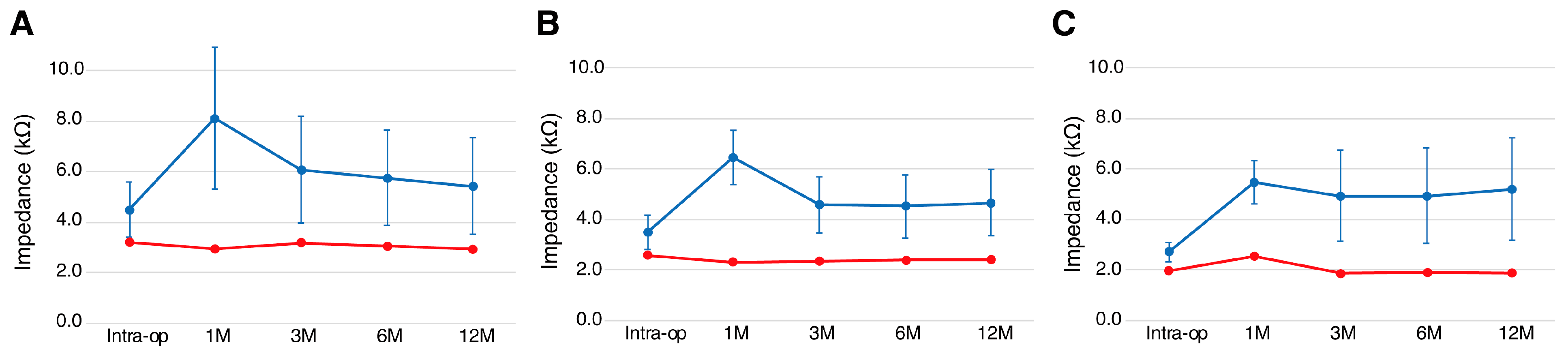
3.3. Post-Operative Hearing Outcome and Impedance Change
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Von Ilberg, C.A.; Baumann, U.; Kiefer, J.; Tillein, J.; Adunka, O.F. Electric-acoustic stimulation of the auditory system: A review of the first decade. Audiol. Neurotol. 2011, 16 (Suppl. S2), 1–30. [Google Scholar] [CrossRef] [PubMed]
- Gstoettner, W.; Helbig, S.; Settevendemie, C.; Baumann, U.; Wagenblast, J.; Arnoldner, C. A new electrode for residual hearing preservation in cochlear implantation: First clinical results. Acta Oto-Laryngol. 2009, 129, 372–379. [Google Scholar]
- Prentiss, S.; Sykes, K.; Staecker, H. Partial deafness cochlear implantation at the University of Kansas: Techniques and outcomes. J. Am. Acad. Audiol. 2010, 21, 197–203. [Google Scholar] [CrossRef]
- Helbig, S.; Van de Heyning, P.; Kiefer, J.; Baumann, U.; Kleine-Punte, A.; Brockmeier, H.; Anderson, I.; Gstoettner, W. Combined electric acoustic stimulation with the PULSARCI(100) implant system using the FLEX(EAS) electrode array. Acta Oto-Laryngol. 2011, 131, 585–595. [Google Scholar] [CrossRef]
- Tamir, S.; Ferrary, E.; Borel, S.; Sterkers, O.; Bozorg Grayeli, A. Hearing preservation after cochlear implantation using deeply inserted flex atraumatic electrode arrays. Audiol. Neurotol. 2012, 17, 331–337. [Google Scholar] [CrossRef]
- Adunka, O.F.; Dillon, M.T.; Adunka, M.C.; King, E.R.; Pillsbury, H.C.; Buchman, C.A. Hearing preservation and speech perception outcomes with electric-acoustic stimulation after 12 months of listening experience. Laryngoscope 2013, 123, 2509–2515. [Google Scholar] [CrossRef]
- Usami, S.; Moteki, H.; Tsukada, K.; Miyagawa, M.; Nishio, S.Y.; Takumi, Y.; Iwasaki, S.; Kumakawa, K.; Naito, Y.; Takahashi, H.; et al. Hearing preservation and clinical outcome of 32 consecutive electric acoustic stimulation (EAS) surgeries. Acta OtoLaryngol. 2014, 134, 717–727. [Google Scholar]
- Usami, S.; Moteki, H.; Suzuki, N.; Fukuoka, H.; Miyagawa, M.; Nishio, S.Y.; Takumi, Y.; Iwasaki, S.; Jolly, C. Achievement of hearing preservation in the presence of an electrode covering the residual hearing region. Acta Oto-Laryngol. 2011, 131, 405–412. [Google Scholar] [CrossRef]
- Carlson, M.L.; Driscoll, C.L.; Gifford, R.H.; Service, G.J.; Tombers, N.M.; Hughes-Borst, B.J.; Neff, B.A.; Beatty, C.W. Implications of minimizing trauma during conventional cochlear implantation. Otol. Neurotol. 2011, 32, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Cosetti, M.K.; Friedmann, D.R.; Zhu, B.Z.; Heman-Ackah, S.E.; Fang, Y.; Keller, R.G.; Shapiro, W.H.; Roland, J.T., Jr.; Waltzman, S.B. The effects of residual hearing in traditional cochlear implant candidates after implantation with a conventional electrode. Otol. Neurotol. 2013, 34, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Rajan, G.P.; Kontorinis, G.; Kuthubutheen, J. The effects of insertion speed on inner ear function during cochlear implantation: A comparison study. Audiol. Neurotol. 2013, 18, 17–22. [Google Scholar] [CrossRef]
- Hochmair, I.; Hochmair, E.; Nopp, P.; Waller, M.; Jolly, C. Deep electrode insertion and sound coding in cochlear implants. Hear. Res. 2015, 322, 14–23. [Google Scholar] [CrossRef]
- Mirsalehi, M.; Mohebbi, S.; Ghajarzadeh, M.; Lenarz, T.; Majdani, O. Impact of the round window membrane accessibility on hearing preservation in adult cochlear implantation. Eur. Arch. Otorhinolaryngol. 2017, 274, 3049–3056. [Google Scholar] [CrossRef] [PubMed]
- Wanna, G.B.; O’Connell, B.P.; Francis, D.O.; Gifford, R.H.; Hunter, J.B.; Holder, J.T.; Bennett, M.L.; Rivas, A.; Labadie, R.F.; Haynes, D.S. Predictive factors for short- and long-term hearing preservation in cochlear implantation with conventional-length electrodes. Laryngoscope 2018, 128, 482–489. [Google Scholar] [CrossRef]
- Helbig, S.; Adel, Y.; Leinung, M.; Stöver, T.; Baumann, U.; Weissgerber, T. Hearing Preservation Outcomes After Cochlear Implantation Depending on the Angle of Insertion: Indication for Electric or Electric-Acoustic Stimulation. Otol. Neurotol. 2018, 39, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Gotamco, G.L.; Sun, C.H.; Chou, Y.F.; Hsu, C.J.; Wu, H.P. Effect of Round Window Opening Size on Residual Hearing Preservation in Cochlear Implantation. Otolaryngol. Head Neck Surg. 2020, 163, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, H.; Moteki, H.; Nishio, S.Y.; Miyajima, H.; Miyagawa, M.; Usami, S.I. Genetic testing has the potential to impact hearing preservation following cochlear implantation. Acta Otolaryngol. 2020, 140, 438–444. [Google Scholar] [CrossRef]
- Lee, G.; Lee, S.; Chung, J.H.; Choi, J.W. Preimplant Hearing Threshold: An Important Predictor of Hearing Preservation in Cochlear Implantation With Lateral Wall Electrodes. Otol. Neurotol. 2021, 42, e145–e152. [Google Scholar] [CrossRef]
- Hollis, E.S.; Canfarotta, M.W.; Dillon, M.T.; Rooth, M.A.; Bucker, A.L.; Dillon, S.A.; Young, A.; Quinones, K.; Pillsbury, H.C.; Dedmon, M.M.; et al. Initial Hearing Preservation Is Correlated With Cochlear Duct Length in Fully-inserted Long Flexible Lateral Wall Arrays. Otol. Neurotol. 2021, 42, 1149–1155. [Google Scholar] [CrossRef]
- Sladen, M.; Nichani, J.; Kluk-de Kort, K.; Saeed, H.; Bruce, I.A. Outcomes of attempted hearing preservation after cochlear implantation (HPCI): A prognostic factor (PF) systematic review of the literature. Cochlear Implants Int. 2025, 26, 12–29. [Google Scholar] [CrossRef]
- Rajan, G.P.; Kuthubutheen, J.; Hedne, N.; Krishnaswamy, J. The role of preoperative, intratympanic glucocorticoids for hearing preservation in cochlear implantation: A prospective clinical study. Laryngoscope 2012, 122, 190–195. [Google Scholar] [CrossRef]
- Cho, H.S.; Lee, K.Y.; Choi, H.; Jang, J.H.; Lee, S.H. Dexamethasone Is One of the Factors Minimizing the Inner Ear Damage from Electrode Insertion in Cochlear Implantation. Audiol. Neurotol. 2016, 21, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Kuthubutheen, J.; Joglekar, S.; Smith, L.; Friesen, L.; Smilsky, K.; Millman, T.; Ng, A.; Shipp, D.; Coates, H.; Arnoldner, C.; et al. The Role of Preoperative Steroids for Hearing Preservation Cochlear Implantation: Results of a Randomized Controlled Trial. Audiol. Neurotol. 2017, 22, 292–302. [Google Scholar] [CrossRef]
- Braun, S.; Ye, Q.; Radeloff, A.; Kiefer, J.; Gstoettner, W.; Tillein, J. Protection of inner ear function after cochlear implantation: Compound action potential measurements after local application of glucocorticoids in the guinea pig cochlea. ORL J. Otorhinolaryngol. Relat. Spec. 2011, 73, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Prenzler, N.K.; Salcher, R.; Timm, M.; Gaertner, L.; Lenarz, T.; Warnecke, A. Intracochlear administration of steroids with a catheter during human cochlear implantation: A safety and feasibility study. Drug Deliv. Transl. Res. 2018, 8, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Prenzler, N.K.; Salcher, R.; Lenarz, T.; Gaertner, L.; Warnecke, A. Dose-Dependent Transient Decrease of Impedances by Deep Intracochlear Injection of Triamcinolone with a Cochlear Catheter Prior to Cochlear Implantation-1 Year Data. Front. Neurol. 2020, 11, 258. [Google Scholar] [CrossRef]
- Prenzler, N.K.; Salcher, R.; Lenarz, T.; Gaertner, L.; Lesinski-Schiedat, A.; Warnecke, A. Deep intracochlear injection of triamcinolone-acetonide with an inner ear catheter in patients with residual hearing. Front. Neurosci. 2023, 17, 1202429. [Google Scholar] [CrossRef] [PubMed]
- Farahmand Ghavi, F.; Mirzadeh, H.; Imani, M.; Jolly, C.; Farhadi, M. Corticosteroid-releasing cochlear implant: A novel hybrid of biomaterial and drug delivery system. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94, 388–398. [Google Scholar] [CrossRef]
- Liu, Y.; Jolly, C.; Braun, S.; Stark, T.; Scherer, E.; Plontke, S.K.; Kiefer, J. In vitro and in vivo pharmacokinetic study of a dexamethasone-releasing silicone for cochlear implants. Eur. Arch. Otorhinolaryngol. 2016, 273, 1745–1753. [Google Scholar] [CrossRef]
- Bas, E.; Bohorquez, J.; Goncalves, S.; Perez, E.; Dinh, C.T.; Garnham, C.; Hessler, R.; Eshraghi, A.A.; Van De Water, T.R. Electrode array-eluted dexamethasone protects against electrode insertion trauma induced hearing and hair cell losses, damage to neural elements, increases in impedance and fibrosis: A dose response study. Hear Res. 2016, 337, 12–24. [Google Scholar] [CrossRef]
- Liebau, A.; Schilp, S.; Mugridge, K.; Schön, I.; Kather, M.; Kammerer, B.; Tillein, J.; Braun, S.; Plontke, S.K. Long-term in vivo release profile of dexamethasone-loaded silicone rods implanted into the cochlea of guinea pigs. Front. Neurol. 2019, 10, 1377. [Google Scholar] [CrossRef]
- Vivero, R.J.; Joseph, D.E.; Angeli, S.; He, J.; Chen, S.; Eshraghi, A.A.; Balkany, T.J.; Van de Water, T.R. Dexamethasone base conserves hearing from electrode trauma-induced hearing loss. Laryngoscope 2008, 118, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- Niedermeier, K.; Braun, S.; Fauser, C.; Straubinger, R.K.; Stark, T. Pneumococcal meningitis post cochlear implantation: Development of an animal model in the guinea pig. Hear Res. 2012, 289, 108–115. [Google Scholar] [CrossRef]
- Takumi, Y.; Nishio, S.Y.; Mugridge, K.; Oguchi, T.; Hashimoto, S.; Suzuki, N.; Iwasaki, S.; Jolly, C.; Usami, S. Gene expression pattern after insertion of dexamethasone-eluting electrode into the guinea pig cochlea. PLoS ONE. 2014, 9, e110238. [Google Scholar] [CrossRef]
- Wilk, M.; Hessler, R.; Mugridge, K.; Jolly, C.; Fehr, M.; Lenarz, T.; Scheper, V. Impedance Changes and Fibrous Tissue Growth after Cochlear Implantation Are Correlated and Can Be Reduced Using a Dexamethasone Eluting Electrode. PLoS ONE. 2016, 11, e0147552. [Google Scholar] [CrossRef] [PubMed]
- Honeder, C.; Zhu, C.; Gausterer, J.C.; Schöpper, H.; Ahmadi, N.; Saidov, N.; Nieratschker, M.; Gabor, F.; Arnoldner, C. Sustained-Release Triamcinolone Acetonide Hydrogels Reduce Hearing Threshold Shifts in a Model for Cochlear Implantation with Hearing Preservation. Audiol. Neurotol. 2019, 24, 237–244. [Google Scholar] [CrossRef]
- Manrique-Huarte, R.; Zulueta-Santos, C.; Calavia, D.; Linera-Alperi, M.Á.; Gallego, M.A.; Jolly, C.; Manrique, M. Cochlear Implantation With a Dexamethasone Eluting Electrode Array: Functional and Anatomical Changes in Non-Human Primates. Otol. Neurotol. 2020, 41, e812–e822. [Google Scholar] [CrossRef] [PubMed]
- Prenzler, N.; Salcher, R.; Büchner, A.; Warnecke, A.; Kley, D.; Batsoulis, C.; Vormelcher, S.; Mitterberger-Vogt, M.; Morettini, S.; Schilp, S.; et al. Cochlear implantation with a dexamethasone-eluting electrode array: First-in-human safety and performance results. Hear Res. 2025, 461, 109255. [Google Scholar] [CrossRef]
- Usami, S.I.; Nishio, S.Y. The genetic etiology of hearing loss in Japan revealed by the social health insurance-based genetic testing of 10K patients. Hum. Genet. 2022, 141, 665–681. [Google Scholar] [CrossRef]
- Nishio, S.Y.; Tono, T.; Iwaki, T.; Moteki, H.; Suzuki, K.; Tsushima, Y.; Kashio, A.; Akamatsu, Y.; Sato, H.; Yaegashi, K.; et al. Development and validation of an iPad-based Japanese language monosyllable speech perception test (iCI2004 monosyllable). Acta Otolaryngol. 2021, 141, 267–272. [Google Scholar] [CrossRef]
- Skarzynski, H.; van de Heyning, P.; Agrawal, S.; Arauz, S.L.; Atlas, M.; Baumgartner, W.; Caversaccio, M.; de Bodt, M.; Gavilan, J.; Godey, B.; et al. Towards a consensus on a hearing preservation classification system. Acta Otolaryngol. Suppl. 2013, 133, 3–13. [Google Scholar] [CrossRef]
- O’Leary, S.J.; Choi, J.; Brady, K.; Matthews, S.; Ozdowska, K.B.; Payne, M.; McLean, T.; Rousset, A.; Lo, J.; Creber, N.; et al. Systemic methylprednisolone for hearing preservation during cochlear implant surgery: A double blinded placebo-controlled trial. Hear Res. 2021, 404, 108224. [Google Scholar] [CrossRef]
- Plontke, S.K.; Götze, G.; Rahne, T.; Liebau, A. Intracochlear drug delivery in combination with cochlear implants: Current aspects. HNO 2017, 65 (Suppl. S1), 19–28. [Google Scholar] [CrossRef] [PubMed]
- Briggs, R.; O’Leary, S.; Birman, C.; Plant, K.; English, R.; Dawson, P.; Risi, F.; Gavrilis, J.; Needham, K.; Cowan, R. Comparison of electrode impedance measures between a dexamethasone-eluting and standard Cochlear™ Contour Advance® electrode in adult cochlear implant recipients. Hear Res. 2020, 390, 107924. [Google Scholar] [CrossRef]
- Usami, S.; Abe, S.; Kasai, M.; Shinkawa, H.; Moeller, B.; Kenyon, J.B.; Kimberling, W.J. Genetic and clinical features of sensorineural hearing loss associated with the 1555 mitochondrial mutation. Laryngoscope 1997, 107, 483–490. [Google Scholar] [CrossRef]
- Lehtonen, M.S.; Uimonen, S.; Hassinen, I.E.; Majamaa, K. Frequency of mitochondrial DNA point mutations among patients with familial sensorineural hearing impairment. Eur. J. Hum. Genet. 2000, 8, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Chiu, Y.H.; Chen, P.J.; Hsu, C.J. Prevalence and clinical features of the mitochondrial m.1555A>G mutation in Taiwanese patients with idiopathic sensorineural hearing loss and association of haplogroup F with low penetrance in three families. Ear Hear. 2007, 28, 332–342. [Google Scholar] [CrossRef]
- Guo, Y.F.; Liu, X.W.; Xu, B.C.; Zhu, Y.M.; Wang, Y.L.; Zhao, F.F.; Wang, D.Y.; Zhao, Y.L.; Ji, Y.B.; Wang, Q.J. Analysis of a large-scale screening of mitochondrial DNA m.1555A>G mutation in 2417 deaf-mute students in northwest of China. Genet. Test. Mol. Biomark. 2010, 14, 527–531. [Google Scholar] [CrossRef]
- Subathra, M.; Ramesh, A.; Selvakumari, M.; Karthikeyen, N.P.; Srisailapathy, C.R. Genetic Epidemiology of Mitochondrial Pathogenic Variants Causing Nonsyndromic Hearing Loss in a Large Cohort of South Indian Hearing Impaired Individuals. Ann. Hum. Genet. 2016, 80, 257–273. [Google Scholar] [CrossRef]
- Kaheel, H.; Breß, A.; Hassan, M.A.; Shah, A.A.; Amin, M.; Bakhit, Y.H.Y.; Kniper, M. Frequency of mitochondrial m.1555A > G mutation in Syrian patients with non-syndromic hearing impairment. BMC Ear Nose Throat Disord. 2018, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Borisova, T.V.; Cherdonova, A.M.; Pshennikova, V.G.; Teryutin, F.M.; Morozov, I.V.; Bondar, A.A.; Baturina, O.A.; Kabilov, M.R.; Romanov, G.P.; Solovyev, A.V.; et al. High prevalence of m.1555A > G in patients with hearing loss in the Baikal Lake region of Russia as a result of founder effect. Sci. Rep. 2024, 14, 15342. [Google Scholar] [CrossRef]
- Gallo-Terán, J.; Salomón-Felechosa, C.; González-Aguado, R.; Onecha, E.; Fontalba, A.; Del Castillo, I.; Morales-Angulo, C. Sensorineural Hearing Loss in Patients With the m.1555A > G Mutation in the MTRNR1 Gene. Laryngoscope 2025, 135, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.X. Mitochondrial 12S rRNA mutations associated with aminoglycoside ototoxicity. Mitochondrion 2011, 11, 237–245. [Google Scholar] [CrossRef]
- Usami, S.; Miyagawa, M.; Nishio, S.Y.; Moteki, H.; Takumi, Y.; Suzuki, M.; Kitano, Y.; Iwasaki, S. Patients with CDH23 mutations and the 1555A > G mitochondrial mutation are good candidates for electric acoustic stimulation (EAS). Acta Otolaryngol. 2012, 132, 377–384. [Google Scholar] [CrossRef]
- Usami, S.; Miyagawa, M.; Suzuki, N.; Moteki, H.; Nishio, S.; Takumi, Y.; Iwasaki, S. Genetic background of candidates for EAS (Electric-Acoustic Stimulation). Audiol. Med. 2010, 8, 28–32. [Google Scholar] [CrossRef]
- Oghalai, J.S.; Tonini, R.; Rasmus, J.; Emery, C.; Manolidis, S.; Vrabec, J.T.; Haymond, J. Intra-operative monitoring of cochlear function during cochlear implantation. Cochlear Implant. Int. 2009, 10, 1–18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koka, K.; Riggs, W.J.; Dwyer, R.; Holder, J.T.; Noble, J.H.; Dawant, B.M.; Ortmann, A.; Valenzuela, C.V.; Mattingly, J.K.; Harris, M.M.; et al. Intra-Cochlear Electrocochleography During Cochlear Implant Electrode Insertion Is Predictive of Final Scalar Location. Otol. Neurotol. 2018, 39, e654–e659. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usami, S.-i.; Takumi, Y.; Yoshimura, H.; Nishio, S.-y. Use of the FLEX 28 Dexamethasone-Eluting Cochlear Implant Electrode in Electric–Acoustic Stimulation: A Case Report. Audiol. Res. 2025, 15, 112. https://doi.org/10.3390/audiolres15050112
Usami S-i, Takumi Y, Yoshimura H, Nishio S-y. Use of the FLEX 28 Dexamethasone-Eluting Cochlear Implant Electrode in Electric–Acoustic Stimulation: A Case Report. Audiology Research. 2025; 15(5):112. https://doi.org/10.3390/audiolres15050112
Chicago/Turabian StyleUsami, Shin-ichi, Yutaka Takumi, Hidekane Yoshimura, and Shin-ya Nishio. 2025. "Use of the FLEX 28 Dexamethasone-Eluting Cochlear Implant Electrode in Electric–Acoustic Stimulation: A Case Report" Audiology Research 15, no. 5: 112. https://doi.org/10.3390/audiolres15050112
APA StyleUsami, S.-i., Takumi, Y., Yoshimura, H., & Nishio, S.-y. (2025). Use of the FLEX 28 Dexamethasone-Eluting Cochlear Implant Electrode in Electric–Acoustic Stimulation: A Case Report. Audiology Research, 15(5), 112. https://doi.org/10.3390/audiolres15050112





