Vestibular Atelectasis: A Narrative Review and Our Experience
Abstract
1. Introduction
2. Materials and Methods
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Air-conducted |
| AR | Asymmetry ratio |
| ASC | Anterior semicircular canal |
| BCT | Bithermal caloric test |
| BLB | Blood–labyrinth barrier |
| BVP | Bilateral vestibulopathy |
| CSF | Cerebrospinal fluid |
| cVEMPs | Cervical vestibular-evoked myogenic potentials |
| EH | Endolymphatic hydrops |
| FLAIR | Fluid attenuated inversion recovery |
| HRCT | High-resolution computed tomography |
| ILS | Intralabyrinthine schwannoma |
| MD | Meniere’s disease |
| MRI | Magnetic resonance imaging |
| LSC | Lateral semicircular canal |
| oVEMPs | Ocular vestibular-evoked myogenic potentials |
| PLF | Perilymphatic fistula |
| PSC | Posterior semicircular canal |
| SC | Semicircular canal |
| SNHL | Sensorineural hearing loss |
| SVINT | Skull vibration-induced nystagmus test |
| UVL | Unilateral vestibular loss |
| VA | vestibular atelectasis |
| VEMPs | Vestibular-evoked myogenic potentials |
| vHIT | Video head impulse test |
| VN | Vestibular neuritis |
| VOR | Vestibulo-ocular reflex |
References
- Merchant, S.N.; Schuknecht, H.F. Vestibular atelectasis. Ann. Otol. Rhinol. Laryngol. 1988, 97 Pt 1, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, A.; Ward, B.K.; Schubert, M.C.; Kheradmand, A.; Zee, D.S.; Mantokoudis, G.; Carey, J.P. Patients with vestibular loss, Tullio phenomenon, and pressure-induced nystagmus: Vestibular atelectasis? Otol. Neurotol. 2014, 35, 866–872. [Google Scholar] [CrossRef]
- Finn, S.; Dietzek, M.; Karvouniari, P.; Klingner, C.M.; Neumann, R.; Guntinas-Lichius, O.; Witte, O.W.; Axer, H. Bilateral vestibulopathy with positive Tullio phenomenon. Laryngoscope 2018, 128, 1223–1225. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Kumarasamy, G.; Salim, R. Bilateral Vestibular Atelectasis with Tullio’s Phenomenon. Indian J. Otolaryngol. Head Neck Surg. 2019, 71 (Suppl. 2), 1599–1602. [Google Scholar] [CrossRef]
- Maslovara, S.; Butkovic-Soldo, S.; Pajic-Matic, I.; Sestak, A. Vestibular atelectasis: Decoding pressure and sound-induced nystagmus with bilateral vestibulopathy. Laryngoscope 2019, 129, 1685–1688. [Google Scholar] [CrossRef] [PubMed]
- Marc, M.; Hautefort, C.; Guichard, J.-P.; Herman, P.; Houdart, E.; Toupet, M.; Eliezer, M. Clinical characteristics in unilateral vestibular atelectasis. J. Neurol. 2021, 268, 689–700. [Google Scholar] [CrossRef]
- Eliezer, M.; Attyé, A.; Guichard, J.; Vitaux, H.; Guillonnet, A.; Toupet, M.; Herman, P.; Kania, R.; Houdart, E.; Hautefort, C. Vestibular atelectasis: Myth or reality? Laryngoscope 2019, 129, 1689–1695. [Google Scholar] [CrossRef]
- Eliezer, M.; Toupet, M.; Vitaux, H.; Guichard, J.-P.; Kania, R.; Houdart, E.; Hautefort, C. MRI Evidence of Vestibular Atelectasis in Bilateral Vestibulopathy and Tullio Phenomenon. Otol. Neurotol. 2019, 40, e944–e946. [Google Scholar] [CrossRef]
- Naganawa, S.; Yamazaki, M.; Kawai, H.; Bokura, K.; Sone, M.; Nakashima, T. Visualization of endolymphatic hydrops in Ménière’s disease after intravenous administration of single-dose gadodiamide at 1.5T. Magn. Reson. Med. Sci. 2013, 12, 137–139. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gürkov, R.; Lutsenko, V.; Babkina, T.; Valchyshyn, S.; Situkho, M. Clinical high-resolution imaging and grading of endolymphatic hydrops in Hydropic Ear Disease at 1.5 T using the two-slice grading for vestibular endolymphatic hydrops in less than 10 min. Eur. Arch. Otorhinolaryngol. 2022, 279, 751–757. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Duan, M. Current status on researches of Meniere’s disease: A review. Acta Otolaryngol. 2020, 140, 808–812. [Google Scholar] [CrossRef]
- Sobhy, O.A.; Elmoazen, D.M.; Abd-Elbaky, F.A. Towards a new staging of Ménière’s disease: A vestibular approach. Acta Otorhinolaryngol. Ital. 2019, 39, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Lermoyez, M. Le vertige qui fait entendre (Angiospasme labyrinthique). La Presse Médicale 1919, 27, 1–3. [Google Scholar]
- Tumarkin, A. The Otolithic Catastrophe: A New Syndrome. Br. Med. J. 1936, 2, 175–177. [Google Scholar] [CrossRef]
- Choudhury, B.; Carlson, M.L.; Jethanamest, D. Intralabyrinthine Schwannomas: Disease Presentation, Tumor Management, and Hearing Rehabilitation. J. Neurol. Surg. B Skull Base 2019, 80, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.J.; Shelton, C.; Salzman, K.L.; Davidson, H.C.; Harnsberger, H.R. Intralabyrinthine schwannomas: Diagnosis, management, and a new classification system. Otol. Neurotol. 2004, 25, 160–167. [Google Scholar] [CrossRef]
- Nadol, J.B., Jr.; Schuknecht, H.F. Pathology of peripheral vestibular disorders in the elderly. Am. J. Otolaryngol. 1990, 11, 213–227. [Google Scholar] [CrossRef]
- Khetarpal, U. Investigations into the cause of vertigo in sudden sensorineural hearing loss. Otolaryngol. Head Neck Surg. 1991, 105, 360–371. [Google Scholar] [CrossRef]
- Nadol, J.B., Jr. Vestibular neuritis. Otolaryngol. Head Neck Surg. 1995, 112, 162–172. [Google Scholar] [CrossRef]
- Viana, L.M.; Salviz, M.; Rauch, S.D.; Nadol, J.B. Otopathology in idiopathic Dandy’s syndrome. Otol. Neurotol. 2013, 34, 1099–1103. [Google Scholar] [CrossRef]
- Young, Y.-H.; Huang, T.-W.; Cheng, P.-W. Assessing the stage of Meniere’s disease using vestibular evoked myogenic potentials. Arch. Otolaryngol. Head. Neck Surg. 2003, 129, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Hara, M.; Young, Y.-H.; Okuno, T. Inner ear morphology of experimental perilymphatic fistula. Am. J. Otol. 1992, 13, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Young, Y.-H.; Nomura, Y.; Hara, M. Caloric irregularity in experimentally induced perilymphatic fistula. Eur. Arch. Otorhinolaryngol. 1992, 249, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Young, Y.-H.; Hara, M. Vestibular pathophysiologic changes in experimental perilymphatic fistula. Ann. Otol. Rhinol. Laryngol. 1992, 101, 612–616. [Google Scholar] [CrossRef]
- Nomura, Y.; Hara, M. Experimental perilymphatic fistula. Am. J. Otolaryngol. 1986, 7, 267–275. [Google Scholar] [CrossRef]
- Nomura, Y.; Okuno, T.; Hara, M.; Young, Y.-H. “Floating” labyrinth. Pathophysiology and treatment of perilymph fistula. Acta Otolaryngol. 1992, 112, 186–191. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Hultcrantz, M.; Jin, Z.; Ulfendahl, M.; Suzuki, M. Vestibular morphology in the German Waltzing guinea pig. J. Otolaryngol. Head Neck Surg. 2010, 39, 115–121. [Google Scholar]
- Batuecas-Caletrío, A.; Martínez-Carranza, R.; Nuñez, G.M.G.; Nava, M.J.F.; Gómez, H.S.; Ruiz, S.S.; Guillén, V.P.; Pérez-Fernández, N. Skull vibration-induced nystagmus in vestibular neuritis. Acta Otolaryngol. 2020, 140, 995–1000. [Google Scholar] [CrossRef]
- Díaz, M.P.G.; Torres-García, L.; Zamora, E.G.; Jiménez, A.B.C.; Guillén, V.P. Is Skull Vibration-Induced Nystagmus Useful in Vestibular Neuritis Follow Up? Audiol. Res. 2022, 12, 126–131. [Google Scholar] [CrossRef]
- Peng, H.; Wang, L.; Song, H.; Gao, B.; Yang, Y.; Lyu, F. Clinical Characteristics of persistent geotropic horizontal direction-changing positional nystagmus: Experience in 189 participants. J. Vestib. Res. 2023, 33, 203–211. [Google Scholar] [CrossRef]
- Lee, D.-H.; Kim, T.H.; Jang, M.; Kim, C.-H. The Light Cupula Phenomenon: A Scoping Review. Brain Sci. 2023, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Fluur, E.; Siegborn, J. Interaction between the utricles and the horizontal semicircular canals. I. Unilateral selective sectioning of the horizontal ampullar nerve followed by tilting around the longitudinal axis. Acta Otolaryngol. 1973, 75, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-H.; Choi, J.M.; Jung, H.V.; Park, H.J.; Shin, J.E. Sudden sensorineural hearing loss with simultaneous positional vertigo showing persistent geotropic direction-changing positional nystagmus. Otol. Neurotol. 2014, 35, 1626–1632. [Google Scholar] [CrossRef]
- Kim, C.-H.; Yang, Y.S.; Im, D.; Shin, J.E. Nystagmus in patients with unilateral acute otitis media complicated by serous labyrinthitis. Acta Otolaryngol. 2016, 136, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Shim, D.B.; Ko, K.M.; Song, M.H.; Song, C.E. A case of labyrinthine fistula by cholesteatoma mimicking lateral canal benign paroxysmal positional vertigo. Korean J. Audiol. 2014, 18, 153–157. [Google Scholar] [CrossRef][Green Version]
- Choi, J.W.; Han, K.; Nahm, H.; Shin, J.E.; Kim, C.-H. Direction-changing positional nystagmus in acute otitis media complicated by serous labyrinthitis: New insights into positional nystagmus. Otol. Neurotol. 2019, 40, e393–e398. [Google Scholar] [CrossRef]
- Castellucci, A.; Botti, C.; Bettini, M.; Fernandez, I.J.; Malara, P.; Martellucci, S.; Crocetta, F.M.; Fornaciari, M.; Lusetti, F.; Renna, L.; et al. Case Report: Could Hennebert’s Sign Be Evoked Despite Global Vestibular Impairment on Video Head Impulse Test? Considerations Upon Pathomechanisms Underlying Pressure-Induced Nystagmus due to Labyrinthine Fistula. Front. Neurol. 2021, 12, 634782. [Google Scholar] [CrossRef]
- Kim, C.-H.; Pham, N.C. Density difference between perilymph and endolymph: A new hypothesis for light cupula phenomenon. Med. Hypotheses 2019, 123, 55–59. [Google Scholar] [CrossRef]
- Ostrowski, V.B.; Byskosh, A.; Hain, T.C. Tullio phenomenon with dehiscence of the superior semicircular canal. Otol. Neurotol. 2001, 22, 61–65. [Google Scholar] [CrossRef]
- Kaski, D.; Davies, R.; Luxon, L.; Bronstein, A.M.; Rudge, P. The Tullio phenomenon: A neurologically neglected presentation. J. Neurol. 2012, 259, 4–21. [Google Scholar] [CrossRef]
- Nadol, J.B. Positive “fistula sign” with an intact tympanic membrane. Clinical report of three cases and histopathological description of vestibulofibrosis as the probable cause. Arch. Otolaryngol. 1974, 100, 273–278. [Google Scholar] [CrossRef]
- Pillsbury, H.C.; Postma, D.S. The Tullio phenomenon, fistula test, and Hennebert’s sign: Clinical significance. Otolaryngol. Clin. N. Am. 1983, 16, 205–207. [Google Scholar] [CrossRef]
- Acierno, M.D.N.; Trobe, J.D.; Shepard, N.T.; Cornblath, W.T.; Disher, M.J. Two types of oscillopsia in a patient with idiopathic vestibulopathy. J. Neuroophthalmol. 1997, 17, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.M.; Fernandez, C. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. I. Resting discharge and response to constant angular accelerations. J. Neurophysiol. 1971, 34, 635–660. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.G.; Chacron, M.J.; Taylor, M.C.; Cullen, K.E. Neural variability, detection thresholds, and information transmission in the vestibular system. J. Neurosci. 2007, 27, 771–781. [Google Scholar] [CrossRef]
- Rabbitt, R.D. Semicircular canal biomechanics in health and disease. J. Neurophysiol. 2019, 121, 732–755. [Google Scholar] [CrossRef] [PubMed]
- Crane, B.T. Why no Unilateral Vestibular Atelectasis? Otol. Neurotol. 2016, 37, 115. [Google Scholar] [CrossRef]
- Heidenreich, K.D.; Pitts, C.M.; Angster, K.; Zajac, T.; Havard, S.; Melendez, T.L.; Kileny, P.R. Electrocochleography Results in Patients With Bilateral Vestibular Paresis and Sound- or Pressure-Induced Horizontal Nystagmus. Otol. Neurotol. 2018, 39, e274–e279. [Google Scholar] [CrossRef]
- Attyé, A.; Eliezer, M.; Boudiaf, N.; Tropres, I.; Chechin, D.; Schmerber, S.; Dumas, G.; Krainik, A. MRI of endolymphatic hydrops in patients with Meniere’s disease: A case-controlled study with a simplified classification based on saccular morphology. Eur. Radiol. 2017, 27, 3138–3146. [Google Scholar] [CrossRef]
- Attyé, A.; Eliezer, M.; Galloux, A.; Pietras, J.; Tropres, I.; Schmerber, S.; Dumas, G.; Krainik, A. Endolymphatic hydrops imaging: Differential diagnosis in patients with Meniere disease symptoms. Diagn. Interv. Imaging. 2017, 98, 699–706. [Google Scholar] [CrossRef]
- Attyé, A.; Eliezer, M.; Medici, M.; Tropres, I.; Dumas, G.; Krainik, A.; Schmerber, S. In vivo imaging of saccular hydrops in humans reflects sensorineural hearing loss rather than Meniere’s disease symptoms. Eur. Radiol. 2018, 28, 2916–2922. [Google Scholar] [CrossRef] [PubMed]
- Eliezer, M.; Gillibert, A.; Tropres, I.; Krainik, A.; Attyé, A. Influence of inversion time on endolymphatic hydrops evaluation in 3D-FLAIR imaging. J. Neuroradiol. 2017, 44, 339–343. [Google Scholar] [CrossRef]
- Eliezer, M.; Hautefort, C.; Van Nechel, C.; Duquesne, U.; Guichard, J.-P.; Herman, P.; Kania, R.; Houdart, E.; Attyé, A.; Toupet, M. Electrophysiological and inner ear MRI findings in patients with bilateral vestibulopathy. Eur. Arch. Otorhinolaryngol. 2020, 277, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Okayasu, T.; Kamakura, T.; Kitahara, T.; Nadol, J.B., Jr. Temporal Bone Histopathology of Undiagnosed Dizziness in the Elderly. Audiol. Neurootol. 2023, 28, 94–105. [Google Scholar] [CrossRef]
- Castellucci, A.; Piras, G.; Del Vecchio, V.; Ferri, G.G.; Ghidini, A.; Brandolini, C. Which Inner Ear Disorders Lie Behind a Selective Posterior Semicircular Canal Hypofunction on Video Head Impulse Test? Otol. Neurotol. 2021, 42, 573–584. [Google Scholar] [CrossRef]
- Tarnutzer, A.A.; Bockisch, C.J.; Buffone, E.; Weber, K.P. Association of posterior semicircular canal hypofunction on video-head-impulse testing with other vestibulo-cochlear deficits. Clin. Neurophysiol. 2017, 128, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Zulueta-Santos, C.; Lujan, B.; Manrique-Huarte, R.; Perez-Fernandez, N. The vestibulo-ocular reflex assessment in patients with Ménière’s disease: Examining all semicircular canals. Acta Otolaryngol. 2014, 134, 1128–1133. [Google Scholar] [CrossRef]
- Fukushima, M.; Oya, R.; Nozaki, K.; Eguchi, H.; Akahani, S.; Inohara, H.; Takeda, N. Vertical head impulse and caloric are complementary but react opposite to Meniere’s disease hydrops. Laryngoscope 2019, 129, 1660–1666. [Google Scholar] [CrossRef]
- Ito, Y.; Seo, T.; Sasano, Y.; Mochizuki, F.; Koizuka, I. Perilymphatic fistula with characteristic findings of the inner ear by contrast-enhanced magnetic resonance imaging: A case report. Front. Neurol. 2023, 14, 1276991. [Google Scholar] [CrossRef]
- Taylor, R.L.; McGarvie, L.A.; Reid, N.; Young, A.S.; Halmagyi, G.M.; Welgampola, M.S. Vestibular neuritis affects both superior and inferior vestibular nerves. Neurology 2016, 87, 1704–1712. [Google Scholar] [CrossRef]
- Büki, B.; Hanschek, M.; Jünger, H. Vestibular neuritis: Involvement and long-term recovery of individual semicircular canals. Auris Nasus Larynx 2017, 44, 288–293. [Google Scholar] [CrossRef]
- Navari, E.; Casani, A.P. Lesion Patterns and Possible Implications for Recovery in Acute Unilateral Vestibulopathy. Otol. Neurotol. 2020, 41, e250–e255. [Google Scholar] [CrossRef] [PubMed]
- Aw, S.T.; Halmagyi, G.M.; Haslwanter, T.; Curthoys, I.S.; Yavor, R.A.; Todd, M.J. Three-dimensional vector analysis of the human vestibuloocular reflex in response to high-acceleration head rotations. II responses in subjects with unilateral vestibular loss and selective semicircular canal occlusion. J. Neurophysiol. 1996, 76, 4021–4030. [Google Scholar] [CrossRef] [PubMed]
- Palla, A.; Straumann, D. Recovery of the high-acceleration vestibulo-ocular reflex after vestibular neuritis. J. Assoc. Res. Otolaryngol. 2004, 5, 427–435. [Google Scholar] [CrossRef]
- Agrawal, Y.; Van de Berg, R.; Wuyts, F.; Walther, L.; Magnusson, M.; Oh, E.; Sharpe, M.; Strupp, M. Presbyvestibulopathy: Diagnostic criteria Consensus document of the classification committee of the Barany Society. J. Vestib. Res. 2019, 29, 161–170. [Google Scholar] [CrossRef]
- Lerchundi, F.; Laffue, A.H.; Olivier, M.; Gualtieri, F.J. Bilateral posterior semicircular canal dysfunction: A new finding with video head impulse test. J. Neurol. 2020, 267, 2347–2352. [Google Scholar] [CrossRef]
- Fancello, V.; Hatzopoulos, S.; Santopietro, G.; Fancello, G.; Palma, S.; Skarżyński, P.H.; Bianchini, C.; Ciorba, A. Vertigo in the Elderly: A Systematic Literature Review. J. Clin. Med. 2023, 12, 2182. [Google Scholar] [CrossRef] [PubMed]
- Hegemann, S.C.A.; Wenzel, A. Diagnosis and Treatment of Vestibular Neuritis/Neuronitis or Peripheral Vestibulopathy (PVP)? Open Questions and Possible Answers. Otol. Neurotol. 2017, 38, 626–631. [Google Scholar] [CrossRef]
- Van De Berg, R.; Guinand, N.; Ranieri, M.; Cavuscens, S.; Khoa Nguyen, T.A.; Guyot, J.-P.; Lucieer, F.; Starkov, D.; Kingma, H.; van Hoof, M.; et al. The Vestibular Implant Input Interacts with Residual Natural Function. Front. Neurol. 2017, 8, 644. [Google Scholar] [CrossRef]

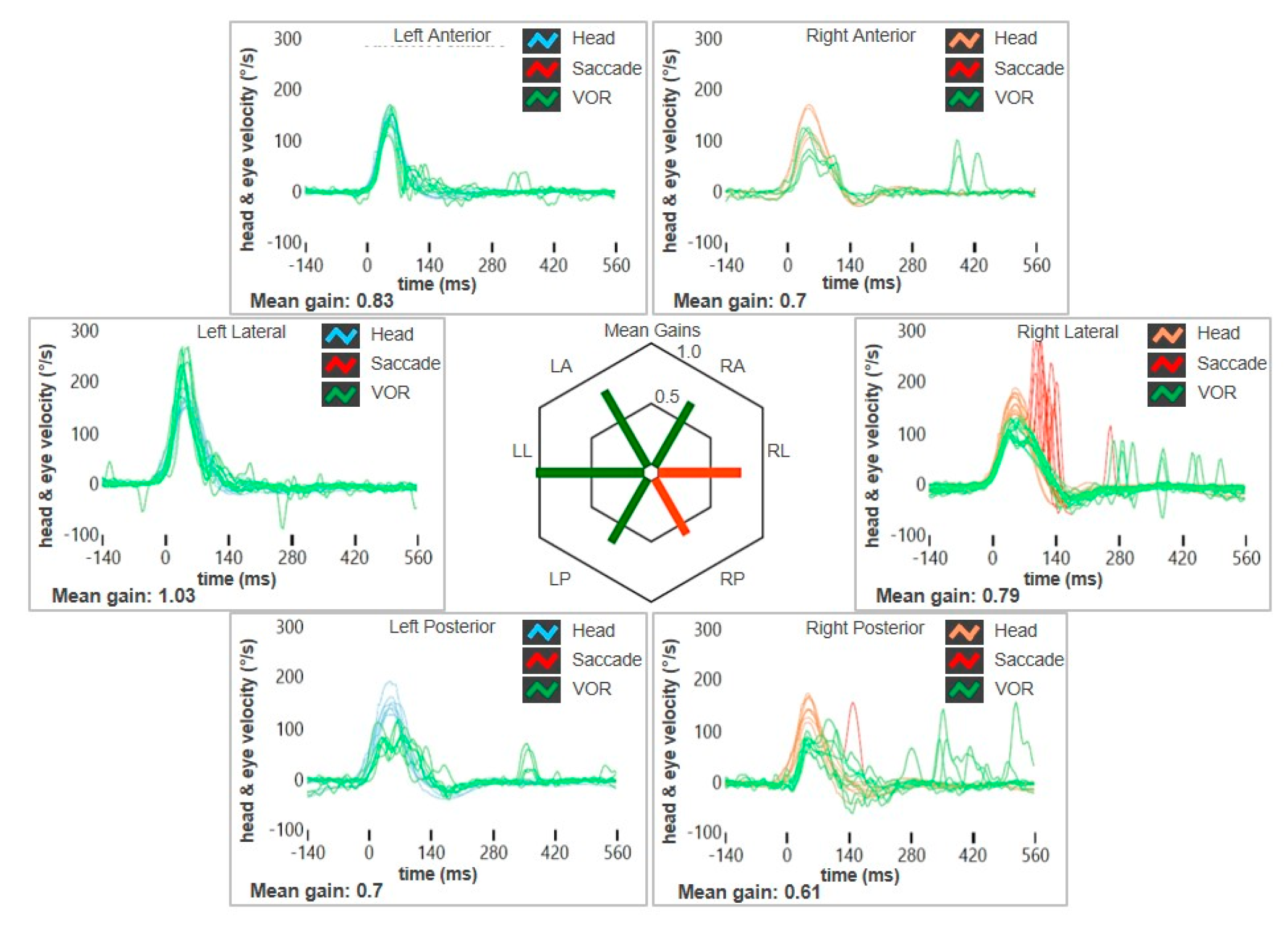
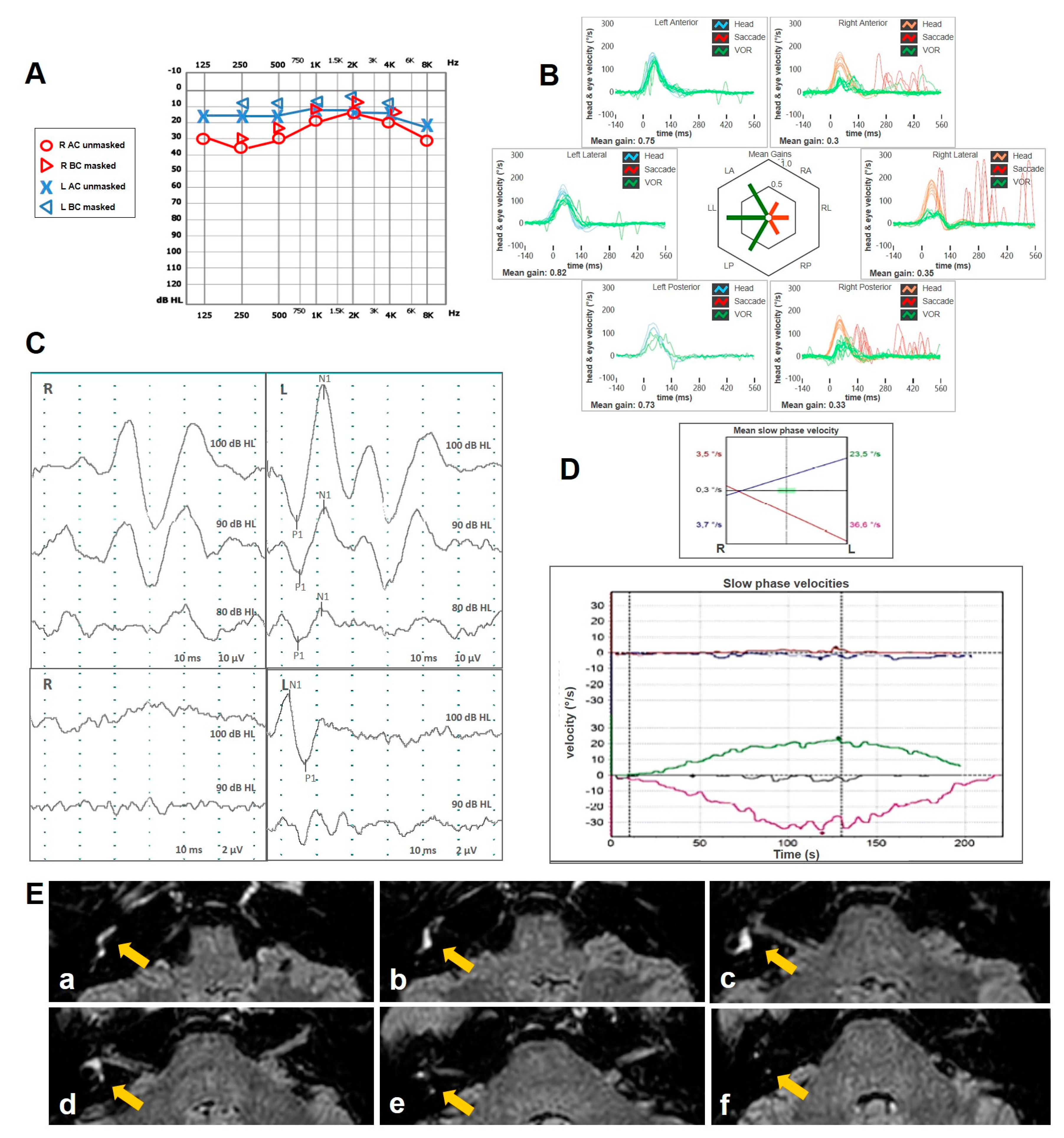
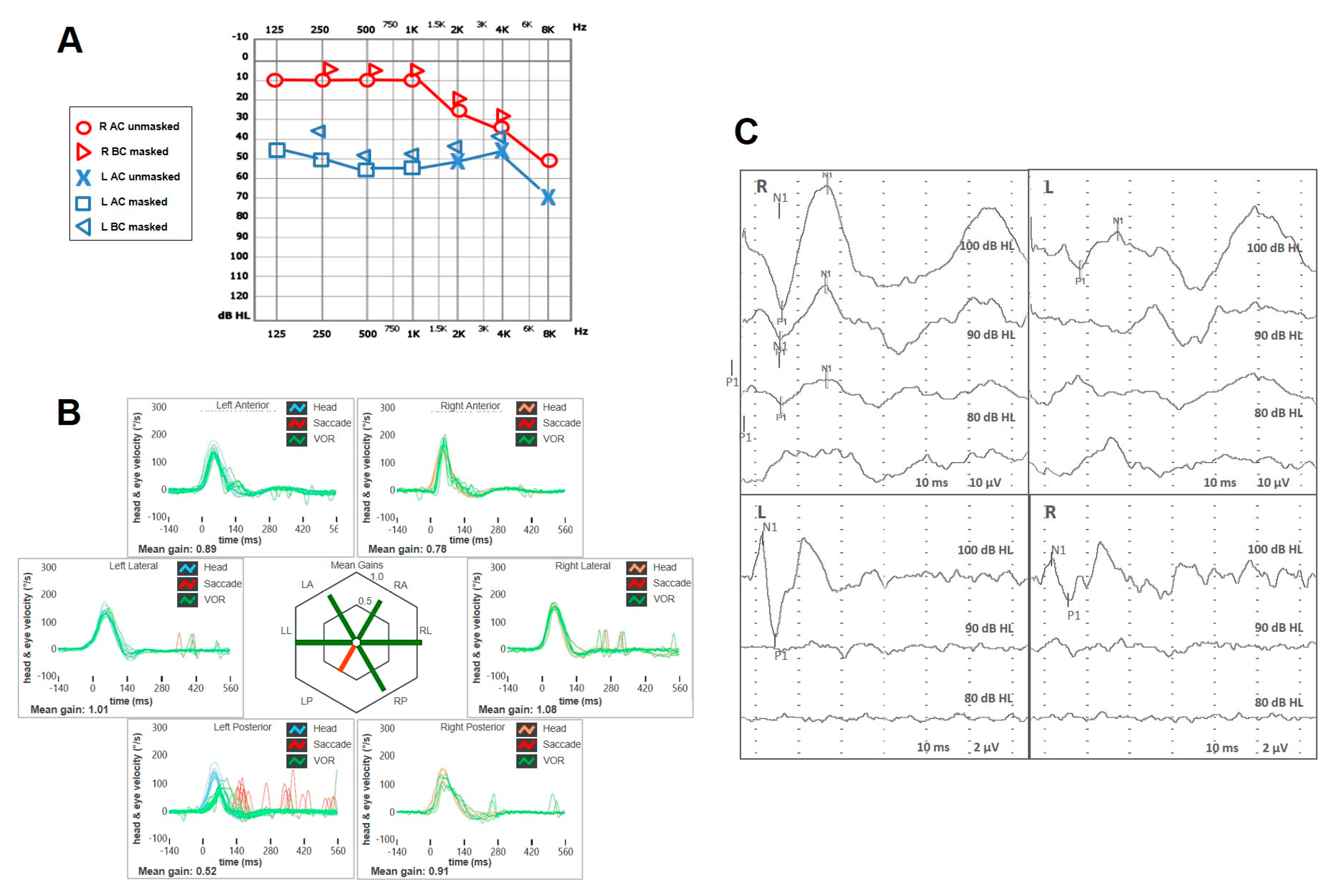
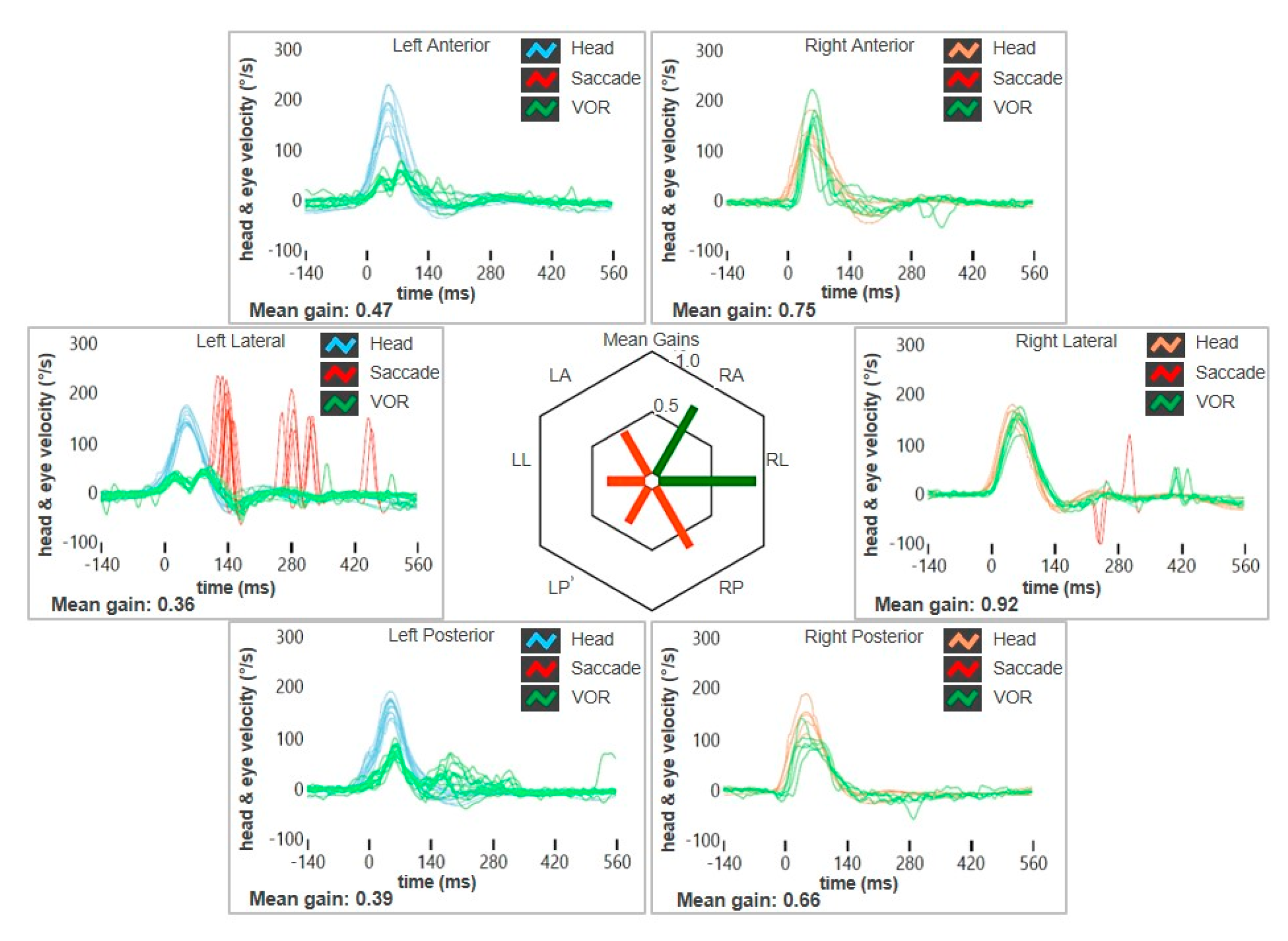

| Authors | Year | Type of Article | Field of the Study | N° of Cases with VA | Site of VA | Brief Description | Vestibular Assessment |
|---|---|---|---|---|---|---|---|
| Merchant and Schuknecht [1] | 1988 | Original Article | Histopathological | 11/426 | Unilateral degeneration of the superior labyrinth (SCs + utricle) | First histopathological evidence of a new clinical entity. Distinction between primary and secondary variants and possible pathophysiological explanations. | BCT: different responses (either normal or irregular responses) |
| Nadol and Schuknecht [17] | 1990 | Original Article | Histopathological | 1/14 | Unilateral degeneration of the superior labyrinth (SCs + utricle) | Classification of the causes of vertigo in the elderly illustrated through clinical and histopathological cases. | / |
| Kehetarpal [18] | 1991 | Original Article | Histopathological | 2 | Unilateral degeneration of the inferior labyrinth (cochlea + saccule) | First description of cochleo-saccular atelectasis in patient with idiopathic sudden SNHL. | / |
| Nomura et al. [22] | 1992 | Original Article | Animal model | / | / | An experimentally induced PLF in guinea pigs causes a collapse of the membranous labyrinth. | Paretic spontaneous nystagmus and positional nystagmus with the lesioned ear undermost. BCT: different responses (either areflexia, normal responses or irregular responses). |
| Nomura et al. [26] | 1992 | Case Report | Clinical | / | / | Two patients with PLF. | Positional nystagmus with the lesioned ear undermost BCT: different responses (either normal or irregular responses). |
| Young et al. [24] | 1992 | Original Article | Animal model | / | Partial collapse (floating labyrinth) or total collapse (VA) of the membranous labyrinth | The term ‘floating labyrinth’ corresponds to partial VA. | Paretic spontaneous nystagmus and positional nystagmus with the lesioned ear undermost. |
| Young et al. [23] | 1992 | Original Article | Animal model | 6/46 | Partial collapse of the membranous labyrinth (floating labyrinth) | Floating labyrinth as an intermediate stage VA and a normal membranous labyrinth. | BCT: different responses (either areflexia, normal responses or irregular responses). |
| Nadol [19] | 1995 | Original Article | Histopathological | 2 | / | VA can be included in the differential diagnosis with VN. | / |
| Kawaguchi et al. [27] | 2010 | Original Article | Animal model | / | / | “Waltzing” guinea pig: a new model for studying VA. Labyrinthine collapse resulting in severe degeneration in the dark cell area. | / |
| Viana et al. [20] | 2013 | Original Article | Histopathological | 1 | Bilateral degeneration of all the SCs with saccule and utricle preservation | Idiopathic Dandy Syndrome. Reduction in neurons in Scarpa’s and Spiral’s ganglions. | / |
| Wenzel et al. [2] | 2014 | Original Article | Clinical | 4 | Bilateral SCs and utricular collapse associated with sound/pressure-induced vertigo and normal hearing | First description of sound/pressure-induced vertigo in VA. Pathophysiological explanation: dissociation between low- and high-frequency responses. | BCT: bilateral areflexia vHIT: bilateral VOR reduction for all the SCs |
| Finn et al. [3] | 2018 | Case Report | Clinical | 1 | BVP. | Sound/pressure-induced nystagmus, no hearing loss. BCT: bilateral areflexia. vHIT: bilateral VOR reduction for all the SCs cVEMPs: normal, oVEMPs: bilateral reduction. | |
| Heidenreich et al. [48] | 2018 | Original Article | Clinical | 3 | BVP with sound/pressure-induced vertigo | EcochG application in patients with BVP. | Sound/pressure-induced nystagmu. BCT: bilateral hypofunction. Rotatory chair: decreased gain vHIT: bilateral VOR reduction for all the SCs cVEMPs: normal. |
| Maslovara et al. [5] | 2018 | Case Report | Clinical | 1 | Bilateral VA | Sound/pressure-induced nystagmus. BCT: bilateral areflexia. cVEMPs: normal, oVEMPs: bilateral reduction. | |
| Roy et al. [4] | 2019 | Case Report | Clinical | 1 | Bilateral VA | Sound-induced nystagmus. | |
| Eliezer et al. [7] | 2019 | Original Article | Radiological | 4/200 | Unilateral VA with collapse of the superior labyrinth (SCs + utricle) | First description in vivo of VA with 3T -MRI and radiological definition of partial and total labyrinthine collapse. | BCT: unilateral areflexia. vHIT: VOR gain. reduction for LSC and PSC. cVEMPs: normal. oVEMPs: absent. |
| Eliezer et al. [8] | 2019 | Case Report | Clinical | 1 | Bilateral VA with collapse of the superior labyrinth (SCs + utricle) | First description in vivo of bilateral labyrinthine collapse. VA as a cause of BVP. | BCT: bilateral areflexia. vHIT: bilateral VOR reduction for all the SCs. oVEMPs: bilaterally absent. |
| Eliezer et al. [53] | 2020 | Original Article | Clinical | 21/42 | Bilateral VA with collapse of the superior labyrinth (SCs + utricle) | Electrophysiological and MRI findings in patient with BVP. | vHIT: bilateral VOR reduction for all the SCs (ASC spared in four cases, LSC spared in one case). cVEMPs: impaired (5/21). oVEMPa: impaired (19/21). |
| Marc et al. [6] | 2020 | Original Article | Clinical | 22 | Unilateral VA | Description of various clinical presentation of unilateral VA. | Sound-induced vertigo (2/22). BCT: hypo/areflexia. vHIT: VOR reduction for one or more SCs. cVEMPs: abnormal in 54% of cases. oVEMP:s abnormal in 82% of cases. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tozzi, A.; Castellucci, A.; Martellucci, S.; Malara, P.; Eliezer, M.; Ferrulli, G.; Ruberto, R.R.; Brizzi, P.; Armato, E.; Marchetti, A.; et al. Vestibular Atelectasis: A Narrative Review and Our Experience. Audiol. Res. 2025, 15, 61. https://doi.org/10.3390/audiolres15030061
Tozzi A, Castellucci A, Martellucci S, Malara P, Eliezer M, Ferrulli G, Ruberto RR, Brizzi P, Armato E, Marchetti A, et al. Vestibular Atelectasis: A Narrative Review and Our Experience. Audiology Research. 2025; 15(3):61. https://doi.org/10.3390/audiolres15030061
Chicago/Turabian StyleTozzi, Andrea, Andrea Castellucci, Salvatore Martellucci, Pasquale Malara, Michael Eliezer, Giuseppe Ferrulli, Rosanna Rita Ruberto, Pasquale Brizzi, Enrico Armato, Alessio Marchetti, and et al. 2025. "Vestibular Atelectasis: A Narrative Review and Our Experience" Audiology Research 15, no. 3: 61. https://doi.org/10.3390/audiolres15030061
APA StyleTozzi, A., Castellucci, A., Martellucci, S., Malara, P., Eliezer, M., Ferrulli, G., Ruberto, R. R., Brizzi, P., Armato, E., Marchetti, A., Marchioni, D., Ghidini, A., & Moratti, C. (2025). Vestibular Atelectasis: A Narrative Review and Our Experience. Audiology Research, 15(3), 61. https://doi.org/10.3390/audiolres15030061









