Visual Fixation of Skull-Vibration-Induced Nystagmus in Patients with Peripheral Vestibulopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
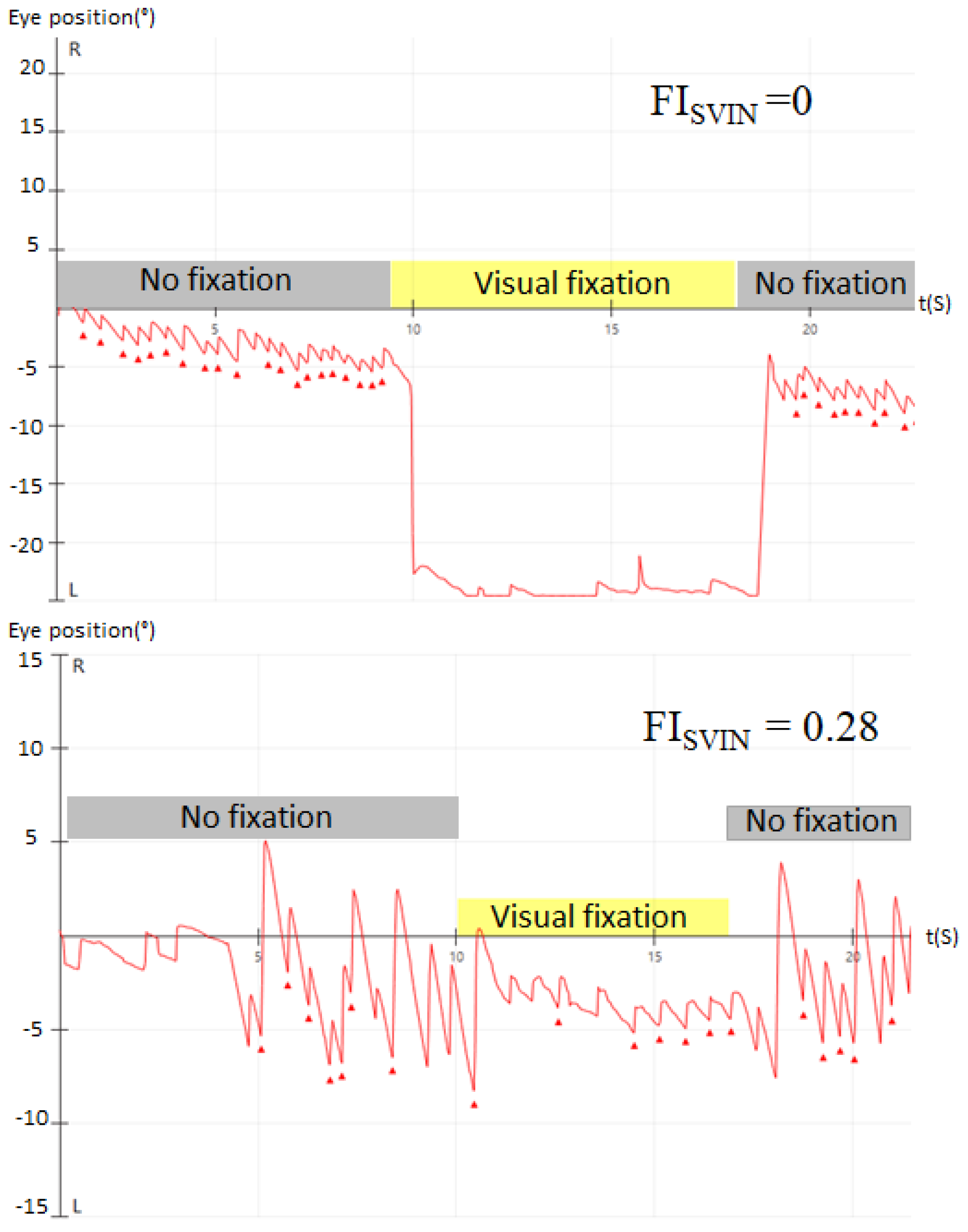
2.2. Nystagmus Evaluation
2.3. Video Head-Impulse Test (vHIT)
2.4. Statistics
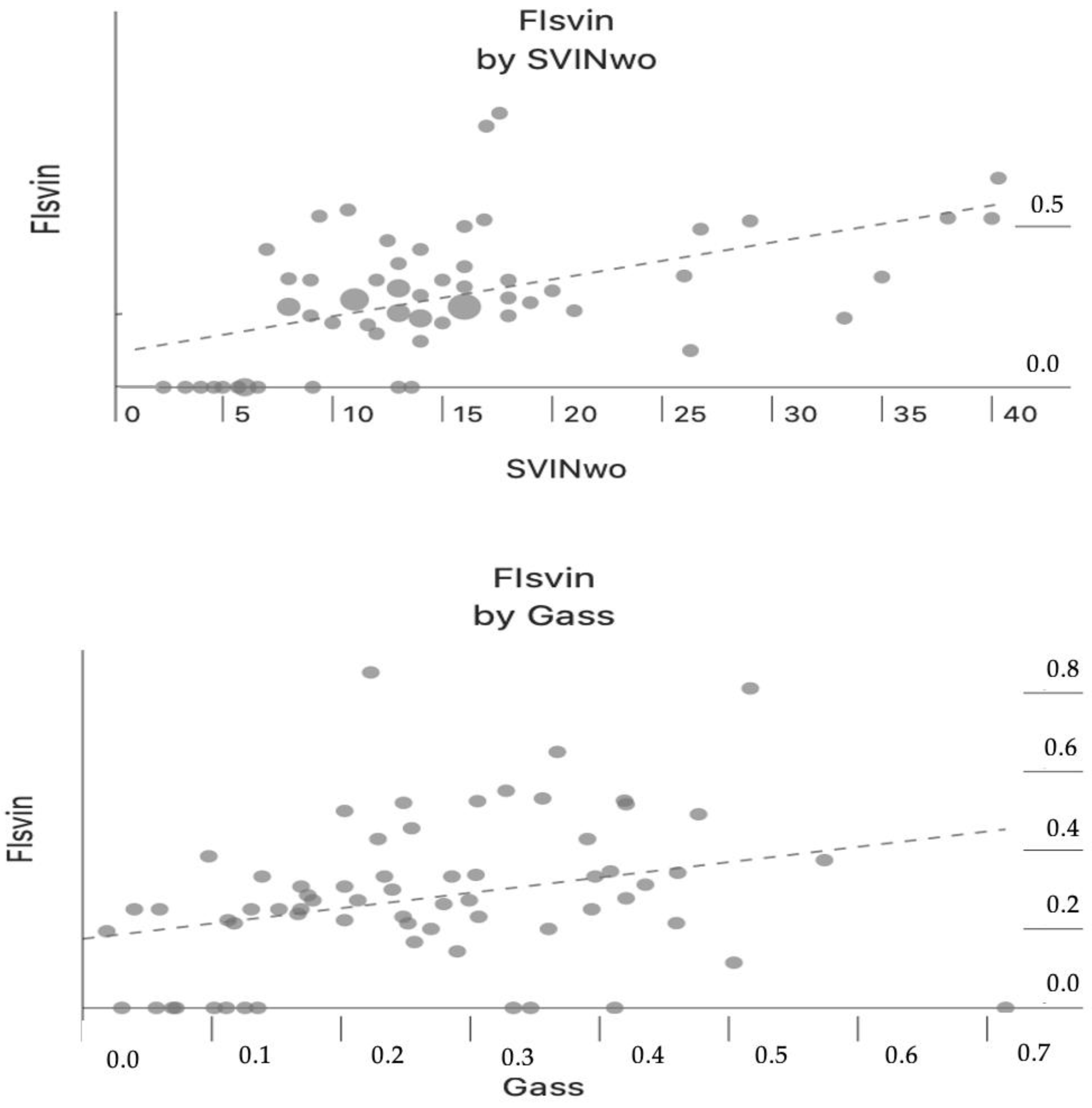
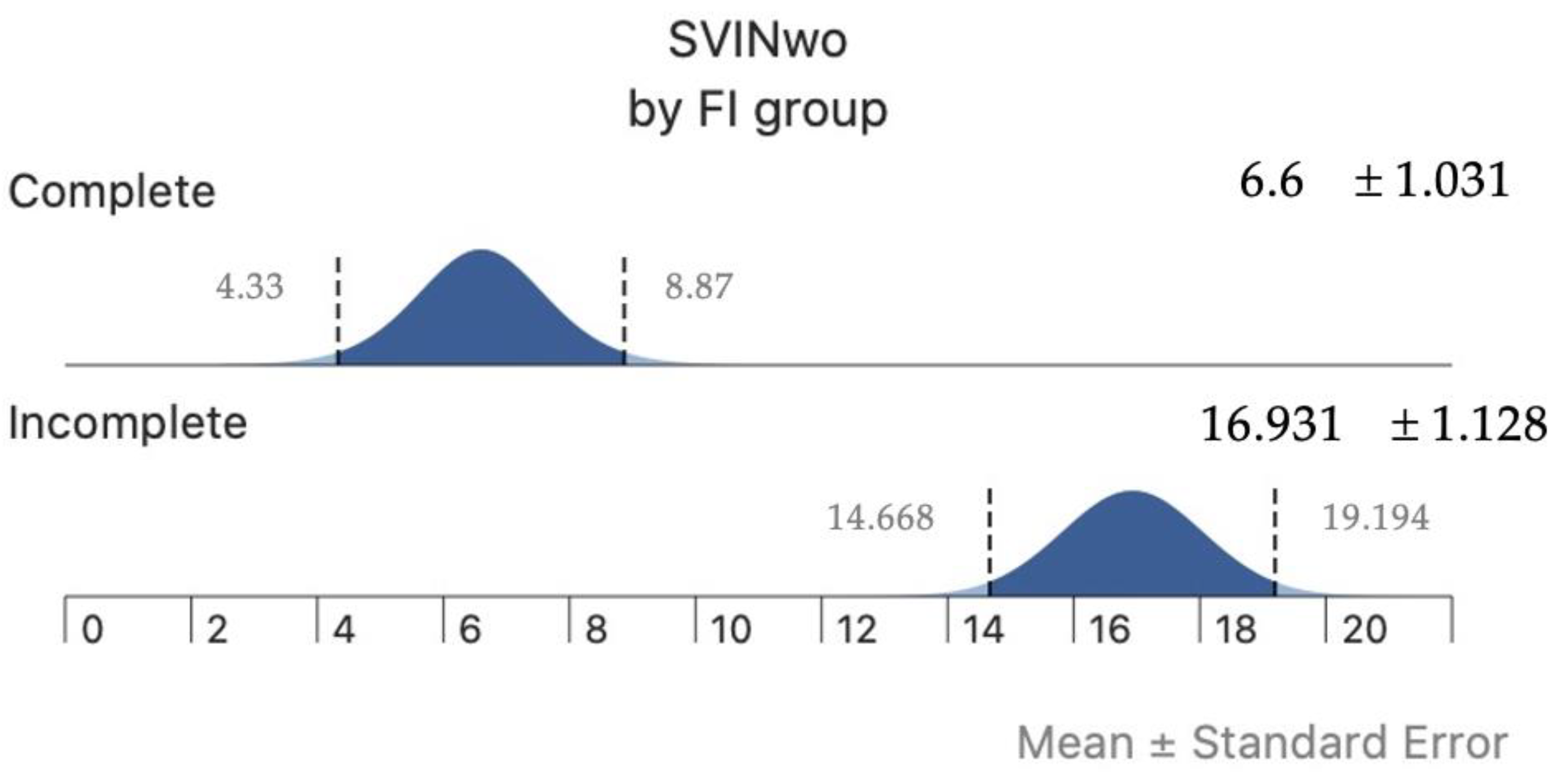
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lücke, K. Eine Methode zur Provokation eines pathologischen Nystagmus durch Vibrationsreize von 100 Hz [A vibratory stimulus of 100 Hz for provoking pathological nystagmus (author’s transl)]. Z. Laryngol. Rhinol. Otol. Grenzgeb. 1973, 52, 716–720. (In German) [Google Scholar] [PubMed]
- Ulmer, E.; Chays, A.; Brémond, G. Nystagmus induit par des vibrations: Physiopathogénie et intérêt en clinique. Ann. D’otolaryngol. Chir. Cervico-Faciale 2004, 121, 95–103. [Google Scholar] [CrossRef]
- Dumas, G.; Curthoys, I.S.; Lion, A.; Perrin, P.; Schmerber, S. The Skull Vibration-Induced Nystagmus Test of Vestibular Function—A Review. Front. Neurol. 2017, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Batuecas-Caletrío, A.; Martínez-Carranza, R.; García Nuñez, G.M.; Fernández Nava, M.J.; Sánchez Gómez, H.; Santacruz Ruiz, S.; Pérez Guillén, V.; Pérez-Fernández, N. Skull vibration-induced nystagmus in vestibular neuritis. Acta Oto-Laryngol. 2020, 140, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Perez, N. Vibration induced nystagmus in normal subjects and in patients with dizziness. A videonystagmography study. Rev. Laryngol.-Otol.-Rhinol. 2003, 124, 85–90. [Google Scholar]
- Zamora, E.G.; Araújo, P.E.; Guillén, V.P.; Gamarra, M.F.V.; Ferrer, V.F.; Rauch, M.C.; Garrigues, H.P. Parameters of skull vibration-induced nystagmus in normal subjects. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 1955–1961. [Google Scholar] [CrossRef] [PubMed]
- Teggi, R.; Familiari, M.; Gatti, O.; Bussi, M. Vertigo without cochlear symptoms: Vestibular migraine or Menière disease? Neurol. Sci. 2021, 42, 5071–5076. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Shin, J.E.; Lim, Y.C.; Shin, H.A. Clinical significance of vibration-induced nystagmus. Audiol. Neurotol. 2008, 13, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Curthoys, I.S. The Neural Basis of Skull Vibration Induced Nystagmus (SVIN). Audiol. Res. 2021, 11, 557–566. [Google Scholar] [CrossRef]
- Jareonsettasin, P.; Otero-Millan, J.; Ward, B.K.; Roberts, D.C.; Schubert, M.C.; Zee, D.S. Multiple Time Courses of Vestibular Set-Point Adaptation Revealed by Sustained Magnetic Field Stimulation of the Labyrinth. Curr. Biol. 2016, 26, 1359–1366. [Google Scholar] [CrossRef]
- Dumas, G.; Perrin, P.; Ouedraogo, E.; Schmerber, S. How to perform the skull vibration induced nystagmus test (SVINT). Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2016, 133, 343–348. [Google Scholar] [CrossRef]
- Mantokoudis, G.; Wyss, T.; Zamaro, E.; Korda, A.; Wagner, F.; Sauter, T.C.; Kerkeni, H.; Kalla, R.; Morrison, M.; Caversaccio, M.D. Stroke Prediction Based on the Spontaneous Nystagmus Suppression Test in Dizzy Patients: A Diagnostic Accuracy Study. Neurology 2021, 97, e42–e51. [Google Scholar] [CrossRef]
- Li, M.; Leng, Y.; Liu, B. Clinical Implication of Caloric and Video Head Impulse Tests for Patients with Enlarged Vestibular Aqueduct Presenting with Vertigo. Front. Neurol. 2021, 12, 717035. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Díaz, M.P.G.; Torres-García, L.; Zamora, E.G.; Belén Castilla Jiménez, A.; Pérez Guillén, V. Is Skull Vibration-Induced Nystagmus Useful in Vestibular Neuritis Follow Up? Audiol. Res. 2022, 12, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Martin-Sanz, E.; Esteban-Sánchez, J.; González-Márquez, R.; Larrán-Jiménez, A.; Cuesta, Á.; Batuecas-Caletrio, Á. Vibration-induced nystagmus and head impulse test screening for vestibular schwannoma. Acta Oto-Laryngol. 2021, 141, 340–347. [Google Scholar] [CrossRef]
- Zhang, Y.; Soper, J.; Lohse, C.M.; Eggers, S.D.Z.; Kaufman, K.R.; McCaslin, D.L. Agreement between the Skull Vibration-Induced Nystagmus Test and Semicircular Canal and Otolith Asymmetry. J. Am. Acad. Audiol. 2021, 32, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Eggers, S.D.Z.; Bisdorff, A.; von Brevern, M.; Zee, D.S.; Kim, J.S.; Perez-Fernandez, N.; Welgampola, M.S.; Della Santina, C.C.; Newman-Toker, D.E. Classification of vestibular signs and examination techniques: Nystagmus and nystagmus-like movements. J. Vestib. Res. 2019, 29, 57–87. [Google Scholar] [CrossRef]
- Matiño-Soler, E.; Esteller-More, E.; Martin-Sanchez, J.C.; Martinez-Sanchez, J.M.; Perez-Fernandez, N. Normative data on angular vestibulo-ocular responses in the yaw axis measured using the video head impulse test. Otol. Neurotol. 2015, 36, 466–471. [Google Scholar] [CrossRef]
- Sinno, S.; Schmerber, S.; Perrin, P.; Dumas, G. Fifty Years of Development of the Skull Vibration-Induced Nystagmus Test. Audiol. Res. 2022, 12, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, C.; Kawahara, T.; Yagi, M.; Murofushi, T. Association between vestibular dysfunction and findings of horizontal head-shaking and vibration-induced nystagmus. J. Vestib. Res. 2020, 30, 319–327. [Google Scholar] [CrossRef]
- Korda, A.; Zee, D.S.; Wyss, T.; Zamaro, E.; Caversaccio, M.D.; Wagner, F.; Kalla, R.; Mantokoudis, G. Impaired fixation suppression of horizontal vestibular nystagmus during smooth pursuit: Pathophysiology and clinical implications. Eur. J. Neurol. 2021, 28, 2614–2621. [Google Scholar] [CrossRef] [PubMed]
- Hirvonen, T.P.; Juhola, M.; Aalto, H. Suppression of spontaneous nystagmus during different visual fixation conditions. Eur. Arch. Otorhinolaryngol. 2012, 269, 1759–1762. [Google Scholar] [CrossRef] [PubMed]
- Özel, H.E.; Karakuzu, A.T.; Temir, H.; Alpay, M.; Özdoğan, F.; Genç, S. Effect of ocular fixation on positional nystagmus in BPPV patients. Int. J. Audiol. 2023, 62, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Huh, Y.E.; Kim, J.S. Bedside evaluation of dizzy patients. J. Clin. Neurol. 2013, 9, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Yi, H.A.; Lee, H. Failure of Fixation Suppression of Spontaneous Nystagmus in Cerebellar Infarction: Frequency, Pattern, and a Possible Structure. Cerebellum 2016, 15, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Murai, N.; Funabiki, K.; Naito, Y.; Ito, J.; Fukuyama, H. Validity and limitation of manual rotational test to detect impaired visual-vestibular interaction due to cerebellar disorders. Auris Nasus Larynx 2005, 32, 23–28. [Google Scholar] [CrossRef]
- Alonso, S.M.; Ayerve, N.A.; Roca, C.M.; Touma, G.C.; Dios, J.C.D.P.; Gómez, H.S.; Ruíz, S.S.C.; Caletrío, Á.B. Use of skull vibration-induced nystagmus in the follow-up of patients with Ménière Disease treated with intratympanic gentamicin. Clin. Exp. Otorhinolaryngol. 2023, 16, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Dumas, G.; Michel, J.; Lavieille, J.P.; Ouedraogo, E. Intérêt sémiologique et essai d’optimisation du stimulus au cours du test vibratoire: Résultats d’une analyse 3D du nystagmus. Ann. D’oto-Laryngol. Chir. Cervico-Faciale 2000, 117, 299–312. [Google Scholar]
- Kim, H.J.; Kim, H.J.; Lee, J.H.; Kim, J.S. Pearls & Oysters: Vibration-Induced Downbeat Nystagmus: A New Cerebellar Sign Observed in Paraneoplastic Syndrome. Neurology 2023, 100, 43–46. [Google Scholar] [PubMed]
- Dumas, G.; Tan, H.; Dumas, L.; Perrin, P.; Lion, A.; Schmerber, S. Skull vibration induced nystagmus in patients with superior semicircular canal dehiscence. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2019, 136, 263–272. [Google Scholar] [CrossRef]
- Karlberg, M.; Aw, S.T.; Black, R.A.; Todd, M.J.; MacDougall, H.G.; Halmagyi, G.M. Vibration-induced ocular torsion and nystagmus after unilateral vestibular deafferentation. Brain 2003, 126, 956–964. [Google Scholar] [CrossRef] [PubMed]
- McKenna, G.J.; Peng, G.C.; Zee, D.S. Neck muscle vibration alters visually perceived roll in normals. J. Assoc. Res. Otolaryngol. 2004, 5, 25–31. [Google Scholar] [PubMed]

| Variable | n | (%) |
|---|---|---|
| Sex | ||
| Female | 65 | 52.5 |
| Male | 59 | 47.5 |
| Diagnosis | ||
| Ménière’s disease | 51 | 41.1 |
| Sub-acute vestibular neuritis | 26 | 21.0 |
| BPPV | 17 | 13.7 |
| Recurrent non-positional vertigo | 16 | 12.9 |
| Post-surgical vestibular schwannoma | 14 | 11.3 |
| Affected side | ||
| Right | 59 | 47.6 |
| Left | 46 | 37.1 |
| NA | 19 | 15.3 |
| Spontaneous nystagmus | ||
| Yes | 44 | 35.5 |
| No | 80 | 64.5 |
| Diagnosis | Gasym | aSVINwo | aSVINw | FISVIN |
|---|---|---|---|---|
| Ménière’s disease | ||||
| Untreated | 0.1 ± 0.1 | 9.7 ± 6 | 3 ± 3.6 | 0.29 ± 0.2 |
| Post-gentamycin | 0.13 ± 0.9 | 12 ± 7 | 3.2 ± 4 | 0.21 ± 0.14 |
| Sub-acute vestibular neuritis | 0.36 ± 0.15 | 15 ± 9 | 5.3 ± 6 | 0.3 ± 0.2 |
| Post-surgical vestibular schwannoma | 0.28 ± 0.14 | 16 ± 9.5 | 6 ± 5.5 | 0.3 ± 0.17 |
| Recurrent vertigo | 0.08 ± 0.09 | 5.3 ± 3.5 | 2 ± 2.7 | 0.29 ± 0.49 |
| Positional (BPPV) | 0.03 ± 0.02 | 7.8 ± 8 | 2.2 ± 3.3 | 0.25 ± 0.37 |
| Non-positional | 0.03 ± 0.02 | 7.8 ± 8 | 2.2 ± 3.3 | 0.25 ± 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, M.; Monopoli-Roca, C.; Álvarez de Linera-Alperi, M.; Menéndez Fernández-Miranda, P.; Molina, B.; Batuecas-Caletrío, A.; Pérez-Fernández, N. Visual Fixation of Skull-Vibration-Induced Nystagmus in Patients with Peripheral Vestibulopathy. Audiol. Res. 2024, 14, 562-571. https://doi.org/10.3390/audiolres14040047
Blanco M, Monopoli-Roca C, Álvarez de Linera-Alperi M, Menéndez Fernández-Miranda P, Molina B, Batuecas-Caletrío A, Pérez-Fernández N. Visual Fixation of Skull-Vibration-Induced Nystagmus in Patients with Peripheral Vestibulopathy. Audiology Research. 2024; 14(4):562-571. https://doi.org/10.3390/audiolres14040047
Chicago/Turabian StyleBlanco, Melissa, Chiara Monopoli-Roca, Marta Álvarez de Linera-Alperi, Pablo Menéndez Fernández-Miranda, Bárbara Molina, Angel Batuecas-Caletrío, and Nicolás Pérez-Fernández. 2024. "Visual Fixation of Skull-Vibration-Induced Nystagmus in Patients with Peripheral Vestibulopathy" Audiology Research 14, no. 4: 562-571. https://doi.org/10.3390/audiolres14040047
APA StyleBlanco, M., Monopoli-Roca, C., Álvarez de Linera-Alperi, M., Menéndez Fernández-Miranda, P., Molina, B., Batuecas-Caletrío, A., & Pérez-Fernández, N. (2024). Visual Fixation of Skull-Vibration-Induced Nystagmus in Patients with Peripheral Vestibulopathy. Audiology Research, 14(4), 562-571. https://doi.org/10.3390/audiolres14040047







