Comparison of Tinnitus Handicap Inventory and Tinnitus Functional Index as Treatment Outcomes
Abstract
1. Introduction
2. Materials and Methods
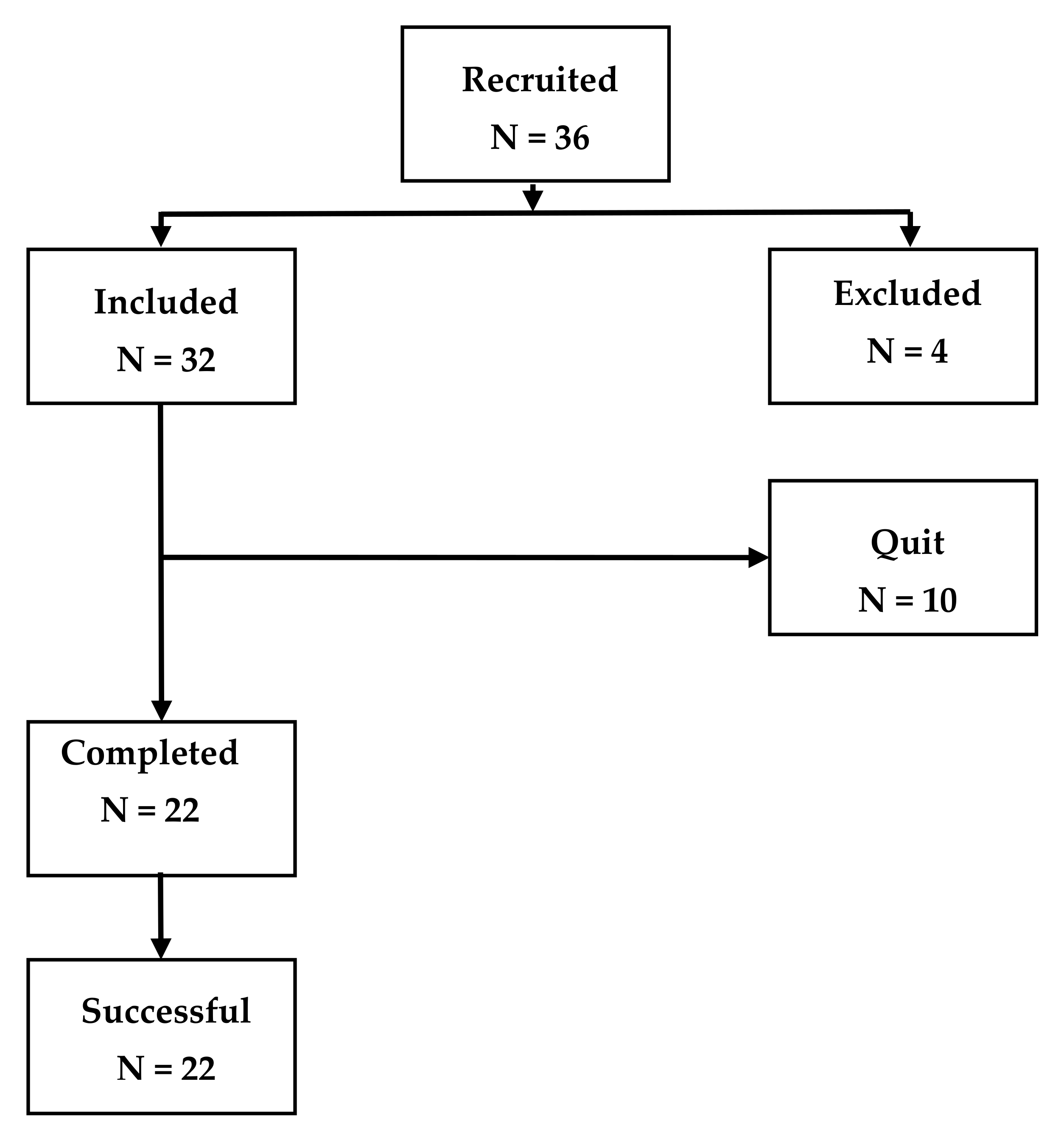
2.1. Subjects
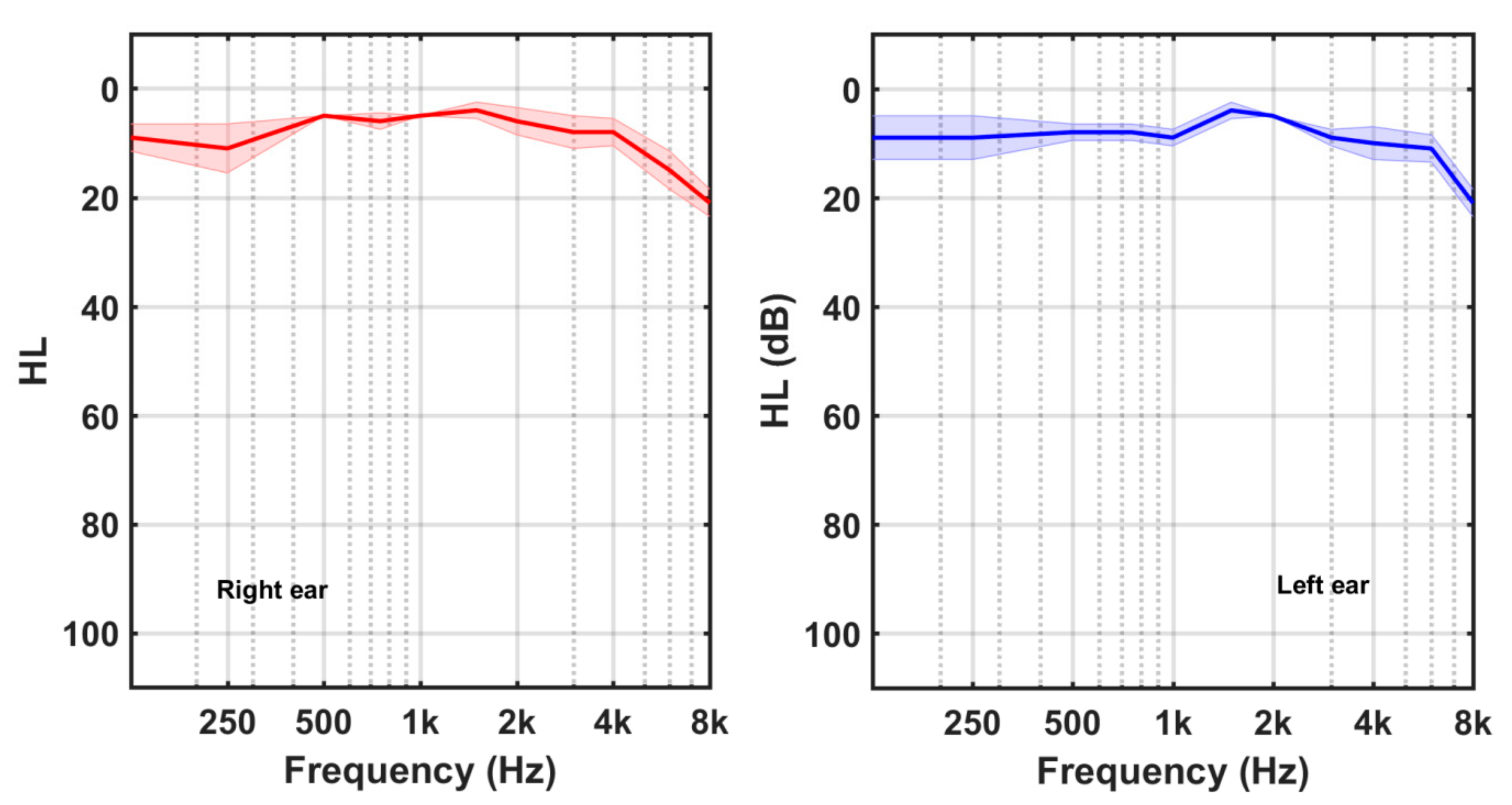
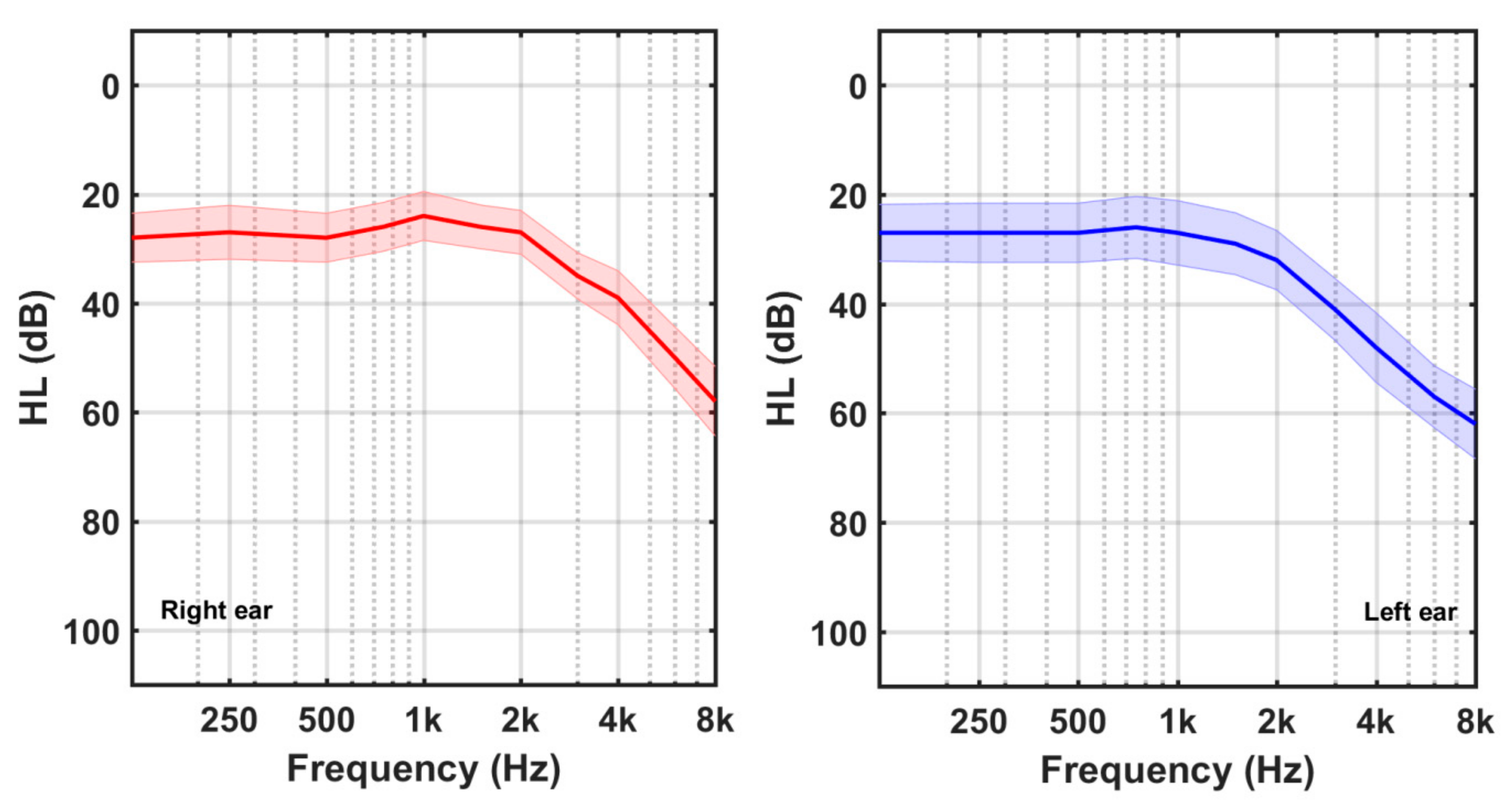
2.2. Audiometric Measurements
2.3. Tinnitus Assessment
2.4. Tinnitus Treatment
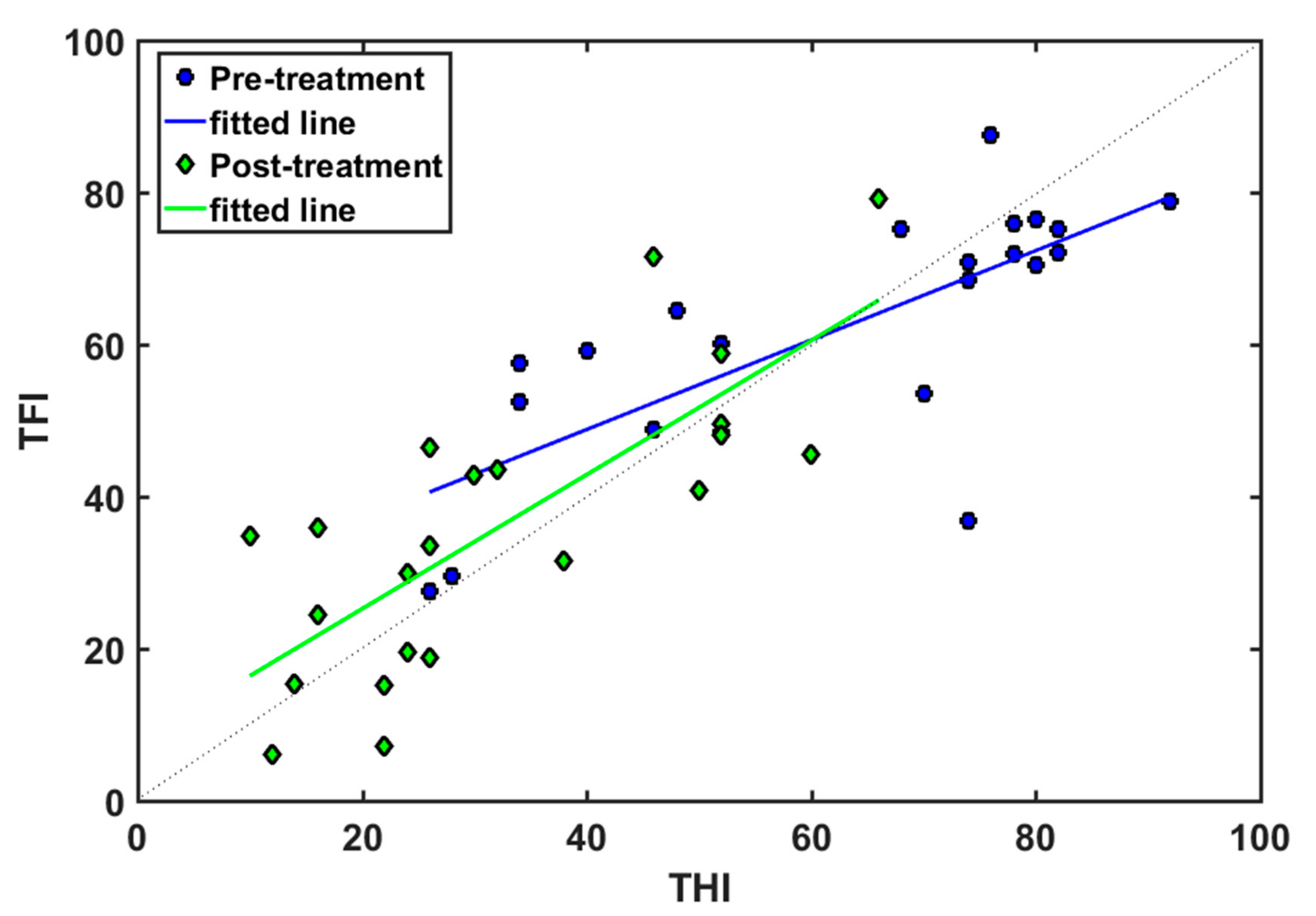
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eggermont, J.J.; Tass, P.A. Maladaptive neural synchrony in tinnitus: Origin and restoration. Front. Neurol. 2015, 6, 29. [Google Scholar] [CrossRef]
- Hall, D.A.; Haider, H.; Szczepek, A.J.; Lau, P.; Rabau, S.; Jones-Diette, J.; Londero, A.; Edvall, N.K.; Cederroth, C.R.; Mielczarek, M.; et al. Systematic Review of Outcome Domains and Instruments Used in Clinical Trials of Tinnitus Treatments in Adults. Trials 2016, 17, 270. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.A.; Fackrell, K.; Li, A.B.; Thavayogan, R.; Smith, S.; Kennedy, V.; Tinoco, C.; Rodrigues, E.D.; Campelo, P.; Martins, T.D.; et al. A Narrative Synthesis of Research Evidence for Tinnitus-Related Complaints as Reported by Patients and Their Significant Others. Health Qual. Life Outcomes 2018, 16, 61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Auditory Cortex Stimulation to Suppress Tinnitus: Mechanisms and Strategies. Hear. Res. 2013, 295, 38–57. [Google Scholar] [CrossRef] [PubMed]
- Van de Heyning, P.; Meeus, O.; Blaivie, C.; Vermeire, K.; Boudewyns, A.; De Ridder, D. Tinnitus: A Multidisciplinary Clinical Approach. B-ENT 2007, 3 (Suppl. 7), 3–10. [Google Scholar]
- Pienkowski, M. Rationale and efficacy of sound therapies for tinnitus and hyperacusis. Neuroscience 2019, 407, 120–134. [Google Scholar] [CrossRef]
- Salvi, R.; Lobarinas, E.; Sun, W. Pharmacological treatments for tinnitus: New and old. Drugs Future 2009, 34, 381–400. [Google Scholar] [CrossRef]
- Cima, R.F.; Maes, I.H.; Joore, M.A.; Scheyen, D.J.; El Refaie, A.; Baguley, D.M.; Anteunis, L.J.; van Breukelen, G.J.; Vlaeyen, J.W. Specialised treatment based on cognitive behaviour therapy versus usual care for tinnitus: A randomised controlled trial. Lancet 2012, 379, 1951–1959. [Google Scholar] [CrossRef]
- McFerran, D.J.; Stockdale, D.; Holme, R.; Large, C.H.; Baguley, D.M. Why is there no cure for tinnitus? Front. Neurosci. 2019, 13, 802. [Google Scholar] [CrossRef]
- Cobo, P. Tinnitus: Mechanisms, measures and sound treatments. Loquens 2015, 2, e024. [Google Scholar] [CrossRef]
- Cobo, P.; Cuesta, M.; De la Colina, C. Customised enriched acoustic environment for sound therapy of tinnitus. Acta Acust. 2021, 5, 34. [Google Scholar] [CrossRef]
- Cuesta, M.; Cobo, P. Broadband sound equalized by the hearing loss curves as an improved stimulus for tinnitus retraining therapy—A pilot, non-controlled observational study. J. Int. Adv. Otol. 2020, 16, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, M.; Garzón, C.; Cobo, P. Efficacy of Sound Therapy for Tinnitus Using an Enriched Acoustic Environment with Hearing-Loss Matched Broadband Noise. Brain Sci. 2022, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Langguth, B.; Searchfield, G.D.; Biesinger, E.; Greimel, K.V. History and Questionnaires. In Textbook of Tinnitus; Chapter 47; Moller, A., Langguth, B., de Rider, D., Kleinjung, T., Eds.; Springer: New York, NY, USA, 2011. [Google Scholar]
- Newman, C.W.; Jacobson, G.P.; Spitzer, J.B. Development of the Tinnitus Handicap Inventory. Arch. Otolaryngol. Head Neck Surg. 1996, 122, 143–148. [Google Scholar] [CrossRef]
- Newman, C.W.; Sandridge, S.A.; Jacobson, G.P. Psychometric adequacy of the Tinnitus Handicap Inventory (THI) for evaluating treatment outcome. J. Am. Acad. Audiol. 1998, 9, 153–160. [Google Scholar]
- Meikle, M.B.; Henry, J.A.; Griest, S.E.; Stewart, B.J.; Abrams, H.B.; McArdle, R.; Myers, P.J.; Newman, C.W.; Sandridge, S.; Turk, D.C.; et al. The Tinnitus Functional Index: Development of a New Clinical Measure for Chronic, Intrusive Tinnitus. Ear Hear. 2012, 33, 153–176. [Google Scholar] [CrossRef]
- Henry, J.A.; Griest, S.; Thielman, E.; McMillan, G.; Kaelin, C.; Carlson, K.F. Tinnitus Functional Index: Development, Validation, Outcomes Research, and Clinical Application. Hear. Res. 2016, 334, 58–64. [Google Scholar] [CrossRef]
- Fackrel, K.; Hoare, D. Questionnaires to Measure Tinnitus Severity. ENT Audiol. News 2014, 22, 718–723. [Google Scholar]
- Baguley, D.M.; Andersson, G. Factor Analysis of the Tinnitus Handicap Inventory. Am. J. Audiol. 2003, 12, 31–34. [Google Scholar] [CrossRef]
- McCombe, A.; Baguley, D.; Coles, R.; McKeyna, L.; McKinney, C.; Windle-Taylor, P. Guidelines for the grading of tinnitus severity: The results of a working group commissioned by the British Association of Otolaryngologists, Head and Neck Surgeons, 1999. Clin. Otolaryngol. Allied Sci. 2001, 26, 388–393. [Google Scholar] [CrossRef]
- Fackrel, K.; Hall, D.; Barry, J.G.; Hoare, D. Psychometric properties of the Tinnitus Functional Index (TFI): Assessment in a UK research volunteer population. Hear. Res. 2016, 335, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Langguth, B.; Goodey, R.; Azevedo, A.; Bjorne, A.; Cacace, A.; Crocetti, A.; Del Bo, L.; De Ridder, D.; Diges, I.; Elbert, T.; et al. Consensus for tinnitus patient assessment and treatment outcome measurement: Tinnitus Research Initiative meeting, Regensburg, July 2006. Prog. Brain Res. 2007, 166, 525–536. [Google Scholar] [PubMed]
- Dehghan, M.; Fatahi, F.; Rouhbakhsh, N.; Mahdavi, M.E.; Abdollahi, F.Z.; Jalaie, S. The relationship between tinnitus functional index and tinnitus handicap inventory scores in patients with chronic tinnitus. Audit. Vestib. Res. 2020, 29, 140–146. [Google Scholar]
- Cuesta, M.; Cobo, P. Audiometric Characteristics and Tinnitus Features in a Cohort of 170 Spanish Patients. Audiol. Res. 2021, 11, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Herráiz, C.; Hernández Calvín, F.J.; Plaza, G.; Tapia, M.C.; De los Santos, G. Evaluación de la incapacidad en los pacientes con acúfenos (Evaluation of handicap in tinnitus patients). Acta Otorrinolaringológica Española 2001, 52, 142–145. [Google Scholar] [CrossRef]
- Jastreboff, P.J. 25 years of tinnitus retraining therapy. HNO 2015, 63, 307–311. [Google Scholar] [CrossRef]
- Fackrell, K.; Hall, D.A.; Barry, J.; Hoare, D.J. Integrating Distribution-Based and Anchor-Based Techniques to Identify Minimal Important Change for the Tinnitus Functional Index (TFI) Questionnaire. Brain Sci. 2022, 12, 726. [Google Scholar] [CrossRef]
- Cobo, P. Preliminary results of the enriched acoustic environment as personalized sound-therapy for tinnitus. Auditio 2021, 5, 1–9. [Google Scholar]
- Schaette, R.; Kempter, R. Development of tinnitus-related neuronal hyperactivity through homeostatic plasticity after hearing loss: A computational model. Eur. J. Neurosci. 2006, 23, 3124–3138. [Google Scholar] [CrossRef]
- Landgrebe, M.; Langguth, B.; Zeman, F.; Koller, M. Methodology of Clinical Trials for Tinnitus. In Textbook of Tinnitus; Chapter 22; Moller, A., Langguth, B., de Rider, D., Kleinjung, T., Eds.; Springer: New York, NY, USA, 2011. [Google Scholar]
- Lindblad, A.C.; Rosenhall, U.; Oloffson, A.; Hagerman, B. Tinnitus and other auditory problems—Occupational noise exposure below risk limits may cause inner ear dysfunction. PLoS ONE 2014, 9, e97377. [Google Scholar] [CrossRef]
- Weisz, N.; Hartmann, T.; Dohrmann, K.; Schlee, W.; Noreña, A. High-frequency tinnitus without hearing loss does not mean absence of deafferentation. Hear. Res. 2006, 222, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.T.; Bruce, I.C.; Roberts, L.E. Evidence that hidden hearing loss underlies amplitude modulation encoding deficits in individuals with and without tinnitus. Hear. Res. 2017, 344, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Liu, Z.; Liu, Q.; Peng, Y.; Wu, H.; Lin, Y.; Zhao, X.; Sun, W. Missed hearing loss in tinnitus patients with normal audiograms. Hear. Res. 2019, 384, 107826. [Google Scholar] [CrossRef] [PubMed]


| Duration (Months) | Sound | Location | Possible Aetiology |
|---|---|---|---|
| Mean = 83 SD = 108 | Hissing (9) | Bilateral (10) | Emotional disorders (9) |
| Ringing (8) | Left ear (8) | Hearing loss (7) | |
| Tonal (5) | Right ear (4) | Conductive troubles (7) | |
| Mean = 5680 Hz | Teeth extraction (2) | ||
| SD = 2810 Hz | Noise trauma (1) |
| THI [21] | TFI [18] | ||
|---|---|---|---|
| Category | THI Range | TFI Range | Category |
| Slight | 0–16 | 0–17 | Not a problem |
| Mild | 18–37 | 18–31 | Small problem |
| Moderate | 38–56 | 32–53 | Moderate problem |
| Severe | 58–76 | 57–72 | Big problem |
| Catastrophic | 78–100 | 73–100 | Very big problem |
| r | m | b | Regression Line | |
|---|---|---|---|---|
| Pre-EAE | 0.74 | 0.59 | 25 | TFI = 0.59 THI + 25 |
| Post-EAE | 0.78 | 0.88 | 7.6 | TFI = 0.88 THI + 7.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, M.; Cuesta, M.; Sanz, R.; Cobo, P. Comparison of Tinnitus Handicap Inventory and Tinnitus Functional Index as Treatment Outcomes. Audiol. Res. 2023, 13, 23-31. https://doi.org/10.3390/audiolres13010003
Fernández M, Cuesta M, Sanz R, Cobo P. Comparison of Tinnitus Handicap Inventory and Tinnitus Functional Index as Treatment Outcomes. Audiology Research. 2023; 13(1):23-31. https://doi.org/10.3390/audiolres13010003
Chicago/Turabian StyleFernández, Marta, María Cuesta, Ricardo Sanz, and Pedro Cobo. 2023. "Comparison of Tinnitus Handicap Inventory and Tinnitus Functional Index as Treatment Outcomes" Audiology Research 13, no. 1: 23-31. https://doi.org/10.3390/audiolres13010003
APA StyleFernández, M., Cuesta, M., Sanz, R., & Cobo, P. (2023). Comparison of Tinnitus Handicap Inventory and Tinnitus Functional Index as Treatment Outcomes. Audiology Research, 13(1), 23-31. https://doi.org/10.3390/audiolres13010003








