Fluctuations of Otoacoustic Emissions and Medial Olivocochlear Reflexes: Tracking One Subject over a Year
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Description
2.2. Procedures
2.3. Data Analysis
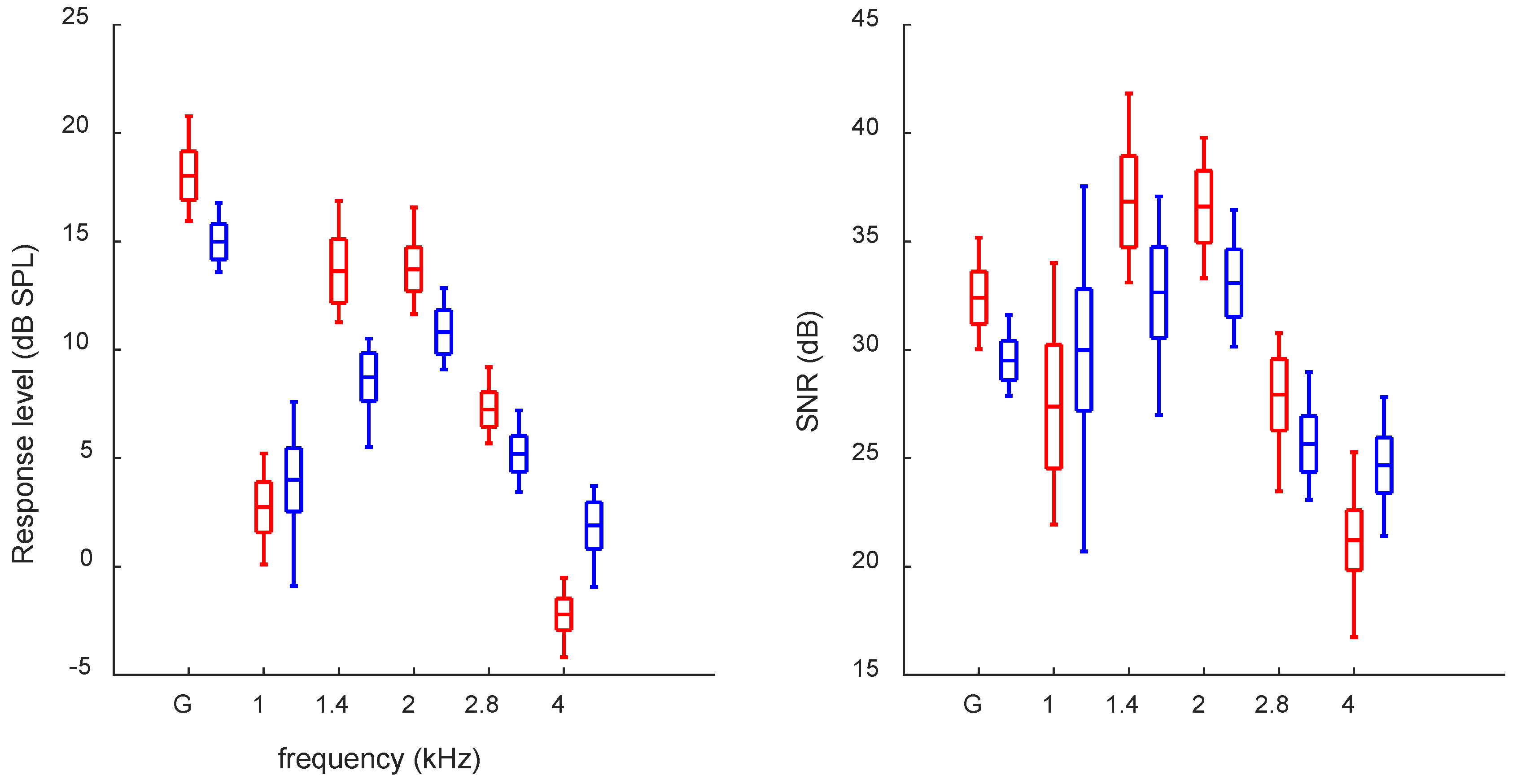
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kemp, D.T.; Ryan, S.; Bray, P. A guide to the effective use of otoacoustic emissions. Ear Hear. 1990, 11, 93–105. [Google Scholar] [CrossRef]
- Lonsbury-Martin, B.L.; Whitehead, M.L.; Martin, G.K. Clinical applications of otoacoustic emissions. J. Speech Hear. Res. 1991, 34, 964–981. [Google Scholar] [CrossRef]
- Antonelli, A.; Grandori, F. Long term stability, influence of the head position and modelling considerations for evoked otoacoustic emissions. Scand. Audiol. Suppl. 1986, 25, 97–108. [Google Scholar] [PubMed]
- Bell, A. Circadian and menstrual rhythms in frequency variations of spontaneous otoacoustic emissions from human ears. Hear. Res. 1992, 58, 91–100. [Google Scholar] [CrossRef]
- Burns, E.M. Long-term stability of spontaneous otoacoustic emissions. J. Acoust. Soc. Am. 2009, 125, 3166–3176. [Google Scholar] [CrossRef] [PubMed]
- Burns, E.M. Even-longer-term stability of spontaneous otoacoustic emissions. J. Acoust. Soc. Am. 2017, 142, 1828. [Google Scholar] [CrossRef]
- Keppler, H.; Dhooge, I.; Maes, L.; D’haenens, W.; Bockstael, A.; Philips, B.; Swinnen, F.; Vinck, B. Transient-evoked and distortion product otoacoustic emissions: A short-term test-retest reliability study. Int. J. Audiol. 2010, 49, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.H.; McPherson, B. Test-retest reliability of tone-burst-evoked otoacoustic emissions. Acta Otolaryngol. 2000, 120, 825–834. [Google Scholar] [CrossRef]
- Lapsley Miller, J.A.; Marshall, L.; Heller, L.M. A longitudinal study of changes in evoked otoacoustic emissions and pure-tone thresholds as measured in a hearing conservation program. Int. J. Audiol. 2004, 43, 307–322. [Google Scholar] [CrossRef]
- Hatzopoulos, S.; Grzanka, A.; Martini, A.; Konopka, W. New clinical insights for transiently evoked otoacoustic emission protocols. Med. Sci. Monit. 2009, 15, CR403–CR408. [Google Scholar]
- Lopez-Poveda, E.A. Olivocochlear efferents in animals and humans: From anatomy to clinical relevance. Front. Neurol. 2018, 26, 9–197. [Google Scholar] [CrossRef] [PubMed]
- Lalaki, P.; Hatzopoulos, S.; Lorito, G.; Kochanek, K.; Sliwa, L.; Skarzynski, H. A connection between the efferent auditory system and noise-induced tinnitus generation. Reduced contralateral suppression of TEOAEs in patients with noise-induced tinnitus. Med. Sci. Monit. 2011, 17, MT56–MT62. [Google Scholar] [CrossRef]
- Sliwinska-Kowalska, M.; Kotylo, P. Occupational exposure to noise decreases otoacoustic emission efferent suppression. Int. J. Audiol. 2002, 41, 113–119. [Google Scholar]
- Namyslowski, G.; Morawski, K.; Kossowska, I.; Lisowska, G.; Koehler, B.; Jarosz-Chobot, P. Contralateral suppression of TEOAE in diabetic children. Effects of 1.0 kHz and 2.0 kHz pure tone stimulation--preliminary study. Scand. Audiol. Suppl. 2001, 52, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Muchnik, C.; Ari-Even Roth, D.; Othman-Jebara, R.; Putter-Katz, H.; Shabtai, E.L.; Hildesheimer, M. Reduced medial olivocochlear bundle system function in children with auditory processing disorders. Audiol. Neurootol. 2004, 9, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.S.; Mertes, I.B.; Lewis, J.D.; Weissbeck, D.K. Medial olivocochlear-induced transient-evoked otoacoustic emission amplitude shifts in individual subjects. J. Assoc. Res. Otolaryngol. 2013, 14, 829–842. [Google Scholar] [CrossRef]
- Jedrzejczak, W.W.; Pilka, E.; Kochanek, K.; Skarzynski, H. Does the presence of spontaneous components affect the reliability of contralateral suppression of evoked otoacoustic emissions? Ear Hear. 2021, 42, 990–1005. [Google Scholar] [CrossRef]
- Killan, E.; Brooke, R.; Farrell, A.; Merrett, J. Clinically relevant long-term reliability of contralateral suppression of click-evoked otoacoustic emissions. J. Hear. Sci. 2017, 7, 2736. [Google Scholar] [CrossRef]
- Mishra, S.K.; Lutman, M.E. Repeatability of click-evoked otoacoustic emission-based medial olivocochlear efferent assay. Ear Hear. 2013, 34, 789–798. [Google Scholar] [CrossRef]
- Mertes, I.B.; Goodman, S.S. Within- and across-subject variability of repeated measurements of medial olivocochlear-induced changes in transient-evoked otoacoustic emissions. Ear Hear. 2016, 37, e72–e84. [Google Scholar] [CrossRef]
- Abdala, C. A longitudinal study of distortion product otoacoustic emission ipsilateral suppression and input/output characteristics in human neonates. J. Acoust. Soc. Am. 2003, 114 Pt 1, 3239–3250. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, C.; Kei, J.; McPherson, B. Transient evoked otoacoustic emissions in 6-year-old school children: A normative study. Scand. Audiol. 2000, 29, 103–110. [Google Scholar] [CrossRef] [PubMed]
- McFadden, D.; Plattsmier, H.S. Aspirin abolishes spontaneous oto-acoustic emissions. J. Acoust. Soc. Am. 1984, 76, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Pawlaczyk-Łuszczyńska, M.; Zamojska-Daniszewska, M.; Dudarewicz, A.; Zaborowski, K. Pure-Tone Hearing Thresholds and Otoacoustic Emissions in Students of Music Academies. Int. J. Environ. Res. Public Health 2021, 18, 1313. [Google Scholar] [CrossRef]
- Jedrzejczak, W.W.; Kochanek, K.; Skarzynski, H. Otoacoustic emissions from ears with spontaneous activity behave differently to those without: Stronger responses to tone bursts as well as to clicks. PLoS ONE 2018, 16, e0192930. [Google Scholar] [CrossRef]
- Lewis, J.D. Synchronized spontaneous otoacoustic emissions provide a signal-to-noise ratio advantage in medial-olivocochlear reflex assays. J. Assoc. Res. Otolaryngol. 2018, 19, 53–65. [Google Scholar] [CrossRef]
- Backus, B.C.; Guinan, J.J., Jr. Measurement of the distribution of medial olivocochlear acoustic reflex strengths across normal-hearing individuals via otoacoustic emissions. J. Assoc. Res. Otolaryngol. 2007, 8, 484–496. [Google Scholar] [CrossRef]
- Lewis, J.D. The effect of otoacoustic emission stimulus level on the strength and detectability of the medial olivocochlear reflex. Ear Hear. 2019, 40, 1391–1403. [Google Scholar] [CrossRef]
- Jedrzejczak, W.W.; Milner, R.; Olszewski, L.; Skarzynski, H. Heightened visual attention does not affect inner ear function as measured by otoacoustic emissions. PeerJ 2017, 5, e4199. [Google Scholar] [CrossRef][Green Version]
- Jedrzejczak, W.W.; Milner, R.; Ganc, M.; Pilka, E.; Skarzynski, H. No change in medial olivocochlear efferent activity during an auditory or visual task: Dual evidence from otoacoustic emissions and event-related potentials. Brain Sci. 2020, 10, 894. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate—Practical and powerful approach to multiple testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Jedrzejczak, W.W.; Blinowska, K.J.; Konopka, W.; Grzanka, A.; Durka, P.J. Identification of otoacoustic emissions components by means of adaptive approximations. J. Acoust. Soc. Am. 2004, 115 Pt 1, 2148–2158. [Google Scholar] [CrossRef] [PubMed]
- Seifert, E.; Brand, K.; van de Flierdt, K.; Hahn, M.; Riebandt, M.; Lamprecht-Dinnesen, A. The influence of hypothermia on outer hair cells of the cochlea and its efferents. Br. J. Audiol. 2001, 35, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Zhang, M.; Nuttall, A.L.; Miller, J.M. Heart beat modulation of spontaneous otoacoustic emissions in guinea pig. Acta Otolaryngol. 1995, 115, 725–731. [Google Scholar] [CrossRef]
- Robinette, M.S. Clinical observations with evoked otoacoustic emissions at Mayo Clinic. J. Am. Acad. Audiol. 2003, 14, 213–224. [Google Scholar] [CrossRef]
- Stuart, A.; Kerls, A.N. Does Contralateral Inhibition of Transient Evoked Otoacoustic Emissions Suggest Sex or Ear Laterality. Effects? Am. J. Audiol. 2018, 27, 272–282. [Google Scholar] [CrossRef]
- Lisowska, G.; Namyslowski, G.; Orecka, B.; Misiolek, M. Influence of aging on medial olivocochlear system function. Clin. Interv. Aging 2014, 9, 901–914. [Google Scholar] [CrossRef]
- Mishra, S.K.; Dinger, Z. Influence of medial olivocochlear efferents on the sharpness of cochlear tuning estimates in children. J. Acoust. Soc. Am. 2016, 140, 1060. [Google Scholar] [CrossRef]


| Ear | Frequency (kHz) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.125 | 0.25 | 0.5 | 0.75 | 1 | 1.5 | 2 | 3 | 4 | 6 | 8 | |
| R | 0 | −5 | −5 | 0 | −5 | 5 | 0 | −10 | −5 | −5 | 0 |
| L | 0 | −5 | −5 | −5 | −5 | −5 | 5 | 0 | −10 | 0 | 5 |
| Week | Month | First/Last Month | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (STD) | Min | Max | Mean (STD) | Min | Max | Mean (STD) | Min | Max | ||
| Resp. level | R | 0.95 (0.73) | 0.05 | 2.67 | 1.10 (0.85) | 0.04 | 3.33 | 0.57 (0.45) | 0.06 | 1.15 |
| L | 0.66 (0.52) | 0.02 | 2.31 | 0.87 (0.75) | 0.02 | 3.04 | 0.48 (0.35) | 0.12 | 0.94 | |
| SNR | R | 1.33 (1.01) | 0.04 | 4.18 | 1.43 (1.25) | 0.00 | 5.05 | 1.16 (0.65) | 0.55 | 2.08 |
| L | 1.03 (0.75) | 0.01 | 2.67 | 0.96 (0.74) | 0.05 | 2.53 | 1.35 (0.69) | 0.61 | 2.00 | |
| Latency | R | 0.13 (0.11) | 0.00 | 0.52 | 0.16 (0.12) | 0.00 | 0.48 | 0.17 (0.11) | 0.08 | 0.32 |
| L | 0.12 (0.12) | 0.00 | 0.36 | 0.13 (0.12) | 0.00 | 0.48 | 0.27 (0.11) | 0.16 | 0.40 | |
| MOCRMD | R | 0.15 (0.11) | 0.0002 | 0.52 | 0.15 (0.11) | 0.005 | 0.40 | 0.27 (0.05) | 0.23 | 0.34 |
| L | 0.14 (0.11) | 0.002 | 0.40 | 0.16 (0.11) | 0.01 | 0.42 | 0.14 (0.07) | 0.08 | 0.24 | |
| MOCRMP | R | 1.00 (0.85) | 0.02 | 3.71 | 1.17 (0.83) | 0.08 | 3.37 | 1.63 (0.55) | 0.98 | 2.17 |
| L | 1.06 (0.82) | 0.07 | 3.71 | 1.31 (1.03) | 0.01 | 3.63 | 1.14 (0.22) | 0.86 | 1.38 | |
| MOCRT | R | 2.06 (1.25) | 0.31 | 5.62 | 2.01 (1.63) | 0.07 | 7.18 | 2.11 (1.58) | 1.13 | 4.46 |
| L | 2.96 (2.12) | 0.11 | 9.02 | 3.32 (2.31) | 0.04 | 8.12 | 4.06 (3.93) | 1.08 | 9.36 | |
| r | p | |
|---|---|---|
| Response level R vs. L | 0.72 | <0.001 |
| SNR R vs. L | 0.30 | 0.04 |
| Latency R vs. L | 0.53 | <0.001 |
| MOCRMD R vs. L | 0.35 | 0.01 |
| MOCRMP R vs. L | 0.56 | <0.001 |
| MOCRT R vs. L | −0.07 | 0.62 |
| MOCR-induced latency change R vs. L | 0.06 | 0.68 |
| r | p | ||
|---|---|---|---|
| Resp. level vs. MOCRMD | R | −0.21 | 0.16 |
| L | −0.45 | <0.001 | |
| Resp. level vs. MOCRMP | R | −0.66 | <0.001 |
| L | −0.72 | <0.001 | |
| Resp. level vs. MOCRT | R | −0.43 | <0.001 |
| L | 0.02 | 0.90 |
| r | p | ||
|---|---|---|---|
| Latency vs. MOCRMD | R | −0.41 | <0.001 |
| L | −0.46 | <0.001 | |
| Latency vs. MOCRMP | R | −0.74 | <0.001 |
| L | −0.62 | <0.001 | |
| Latency vs. MOCRT | R | −0.45 | <0.001 |
| L | 0.22 | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastucha, M.; Jedrzejczak, W.W. Fluctuations of Otoacoustic Emissions and Medial Olivocochlear Reflexes: Tracking One Subject over a Year. Audiol. Res. 2022, 12, 508-517. https://doi.org/10.3390/audiolres12050051
Pastucha M, Jedrzejczak WW. Fluctuations of Otoacoustic Emissions and Medial Olivocochlear Reflexes: Tracking One Subject over a Year. Audiology Research. 2022; 12(5):508-517. https://doi.org/10.3390/audiolres12050051
Chicago/Turabian StylePastucha, Malgorzata, and W. Wiktor Jedrzejczak. 2022. "Fluctuations of Otoacoustic Emissions and Medial Olivocochlear Reflexes: Tracking One Subject over a Year" Audiology Research 12, no. 5: 508-517. https://doi.org/10.3390/audiolres12050051
APA StylePastucha, M., & Jedrzejczak, W. W. (2022). Fluctuations of Otoacoustic Emissions and Medial Olivocochlear Reflexes: Tracking One Subject over a Year. Audiology Research, 12(5), 508-517. https://doi.org/10.3390/audiolres12050051








