Otoacoustic Emissions in Non-Mammals
Abstract
1. Introduction
2. The Auditory Epithelia of Non-Mammals
3. Otoacoustic Emissions in Non-Mammalian Land Vertebrate Groups
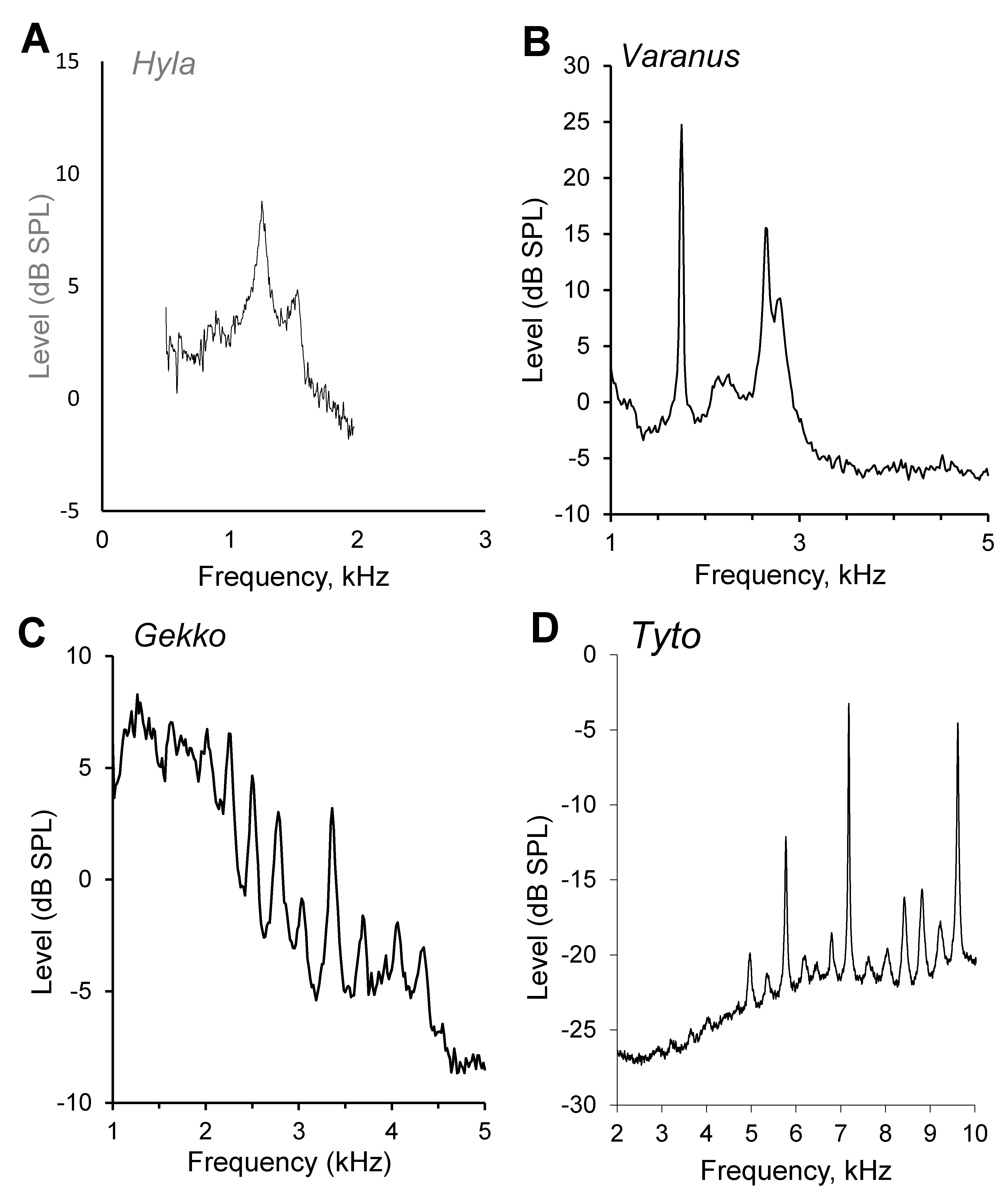
3.1. OAE of Amphibians
3.2. OAE of Turtles and Their Relatives
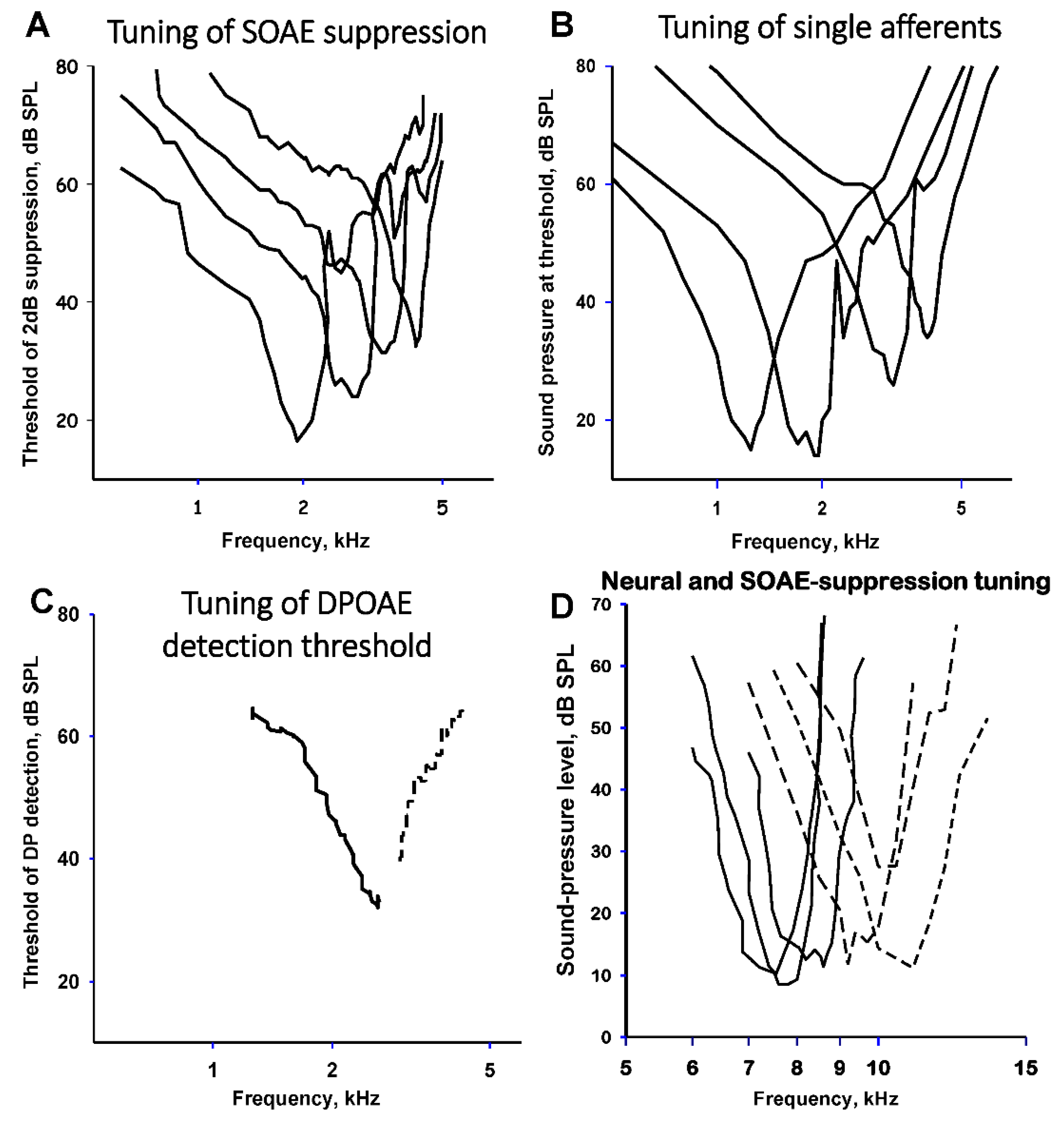
3.3. OAE in Birds and Crocodilians
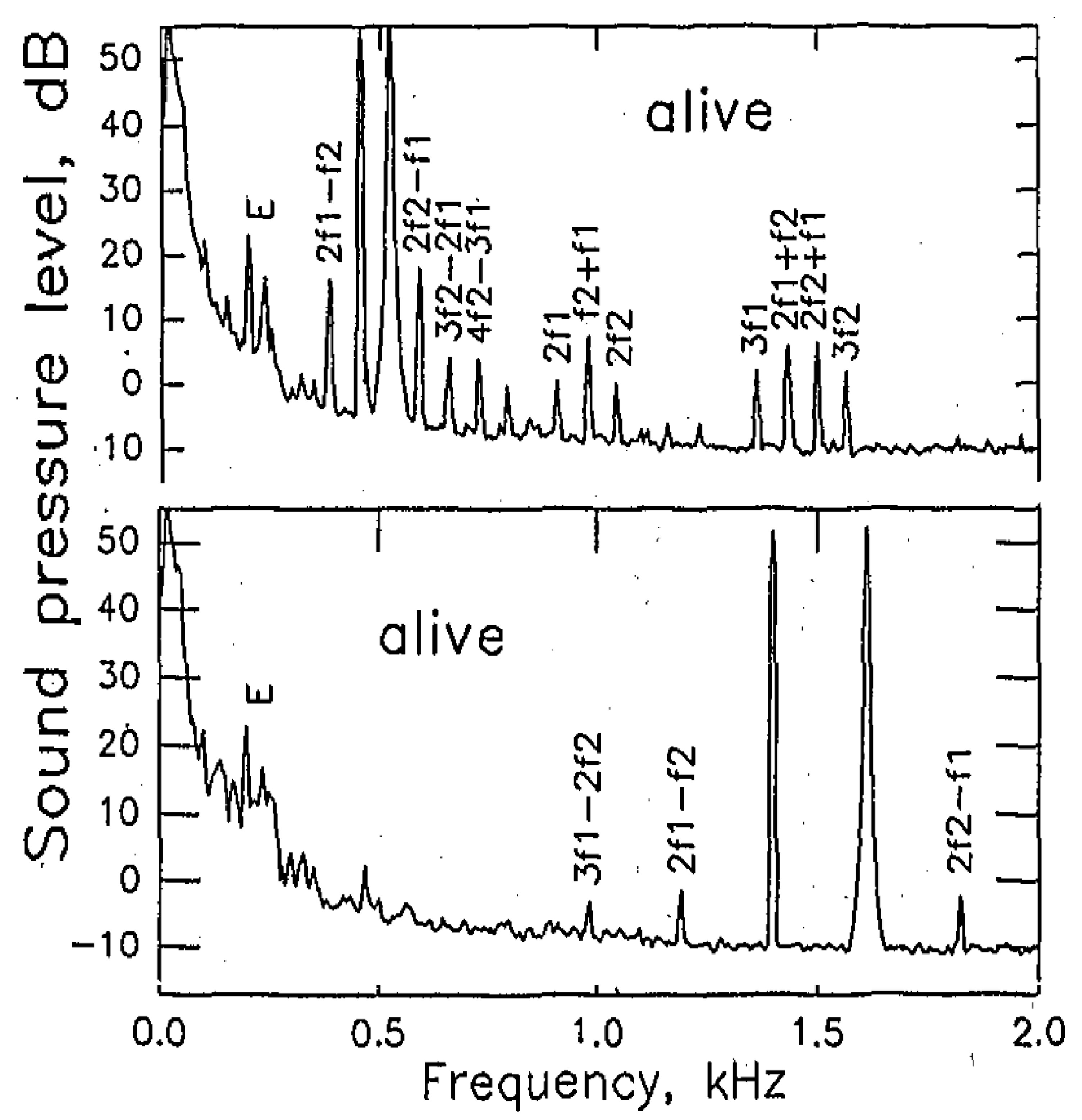
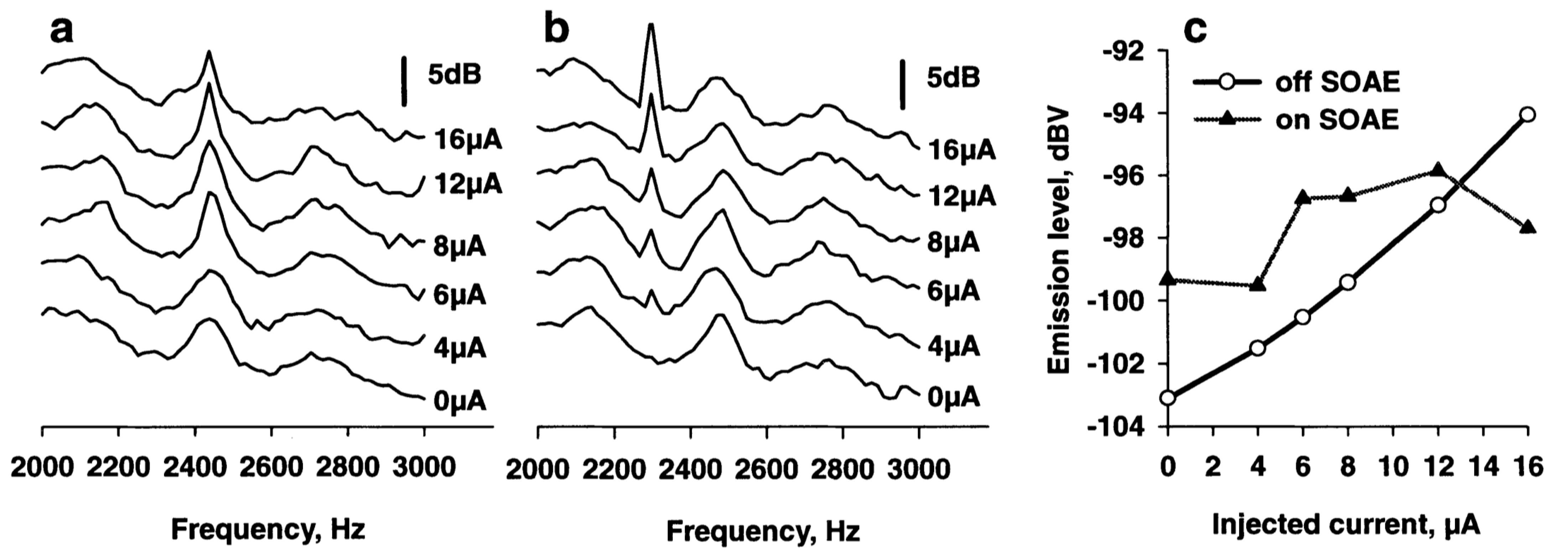
3.4. OAE in Lizards
4. Common Factors Underlying OAE
- The main location of tonal suppressive effects (by frequencies near f1 in non-mammals, but near f2 in mammals);
- Data on the underlying statistics are inconclusive with regard to whether they indicate that the generators are oscillators (mammals, some non-mammalian data) or not (some other non-mammalian data, including some from barn owls). This is complicated by the influence of widely varying peak amplitudes on the analysis;
- Interactions between peaks are much faster in lizards than in mammals. This is responsible for at least some of the spectral broadness of SOAE peaks in lizards, their temperature sensitivity, and the changes of frequency due to suppression/entrainment by added tones.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manley, G.A. Comparative Auditory Neuroscience: Understanding the Evolution and Function of Ears. J. Assoc. Res. Otolaryngol. 2016, 18, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.A.; Gummer, A.W.; Popper, A.N.; Fay, R.R. Understanding the Cochlea; Springer: New York, NY, USA, 2017. [Google Scholar]
- Manley, G.A.; Sienknecht, U.J. The evolution and development of middle ears in land vertebrates. In The Middle Ear: Science, Otosurgery, and Technology; Puria, S., Popper, A.N., Fay, R.R., Eds.; Springer: New York, NY, USA, 2013; pp. 7–30. [Google Scholar] [CrossRef]
- van Dijk, P.; Manley, G.A. The effect of ear canal pressure on spontaneous otoacoustic emissions: Comparison between human and lizard ears. In Concepts and Challenges in the Biophysics of Hearing; Cooper, N.P., Kemp, D.T., Eds.; World Scientific Publishing Company: Singapore, 2009; pp. 196–202. [Google Scholar]
- Van Dijk, P.; Manley, G.A. The Effects of Air Pressure on Spontaneous Otoacoustic Emissions of Lizards. J. Assoc. Res. Otolaryngol. 2013, 14, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Hudspeth, A. Making an Effort to Listen: Mechanical Amplification in the Ear. Neuron 2008, 59, 530–545. [Google Scholar] [CrossRef]
- Manley, G.A. The hearing organs of lizards. In Comparative Hearing: Birds and Reptiles; Springer Handbook of Auditory Research; Dooling, R., Popper, A.N., Fay, R.R., Eds.; Springer: New York, MY, USA, 2000; pp. 139–196. [Google Scholar]
- Fox, J.H. Morphological Correlates of Auditory Sensitivity in Anuran Amphibians. Brain Behav. Evol. 1995, 45, 327–338. [Google Scholar] [CrossRef]
- Manley, G.A.; Van Dijk, P. Otoacoustic emissions in amphibians, lepidosaurs and archosaurs. In Active Processes and Otoacoustic Emissions in Hearing; Springer Handbook of Auditory Research; Manley, G.A., Fay, R.R., Popper, A., Eds.; Springer: New York, NY, USA, 2008; pp. 211–260. [Google Scholar]
- Manley, G.A. Peripheral Hearing Mechanisms in Reptiles and Birds; Springer: Heidelberg, Germany, 1990. [Google Scholar]
- Gleich, O.; Fischer, F.P.; Köppl, C.; Manley, G.A. Hearing organ evolution and specialization: Archosaurs. In Evolution of the Vertebrate Auditory System; Springer Handbook of Auditory Research; Manley, G.A., Popper, A., Fay, R.R., Eds.; Springer: New York, NY, USA, 2004; pp. 224–255. [Google Scholar]
- Fischer, F.P. Quantitative TEM analysis of the barn owl basilar papilla. Heart Res. 1994, 73, 1–15. [Google Scholar] [CrossRef]
- Köppl, C.; Manley, G.A. Unique contributions from comparative auditory research. In Insights from Comparative Hearing Research; Springer Handbook of Auditory Research; Köppl, C., Manley, G.A., Popper, A.N., Fay, R.R., Eds.; Springer: New York, NY, USA, 2014; pp. 1–12. [Google Scholar] [CrossRef]
- Manley, G.A. Evolution of structure and function of the hearing organ of lizards. J. Neurobiol. 2002, 53, 202–211. [Google Scholar] [CrossRef]
- Fettiplace, R.; Fuchs, P.A. Mechanisms of hair cell tuning. Annu. Rev. Physiol. 1999, 61, 809–834. [Google Scholar] [CrossRef]
- Ramanathan, K.; Michael, T.H.; Jiang, G.-J.; Hiel, H.; Fuchs, P.A. A Molecular Mechanism for Electrical Tuning of Cochlear Hair Cells. Science 1999, 283, 215–217. [Google Scholar] [CrossRef]
- Beurg, M.; Gamble, T.; Griffing, A.H.; Fettiplace, R. Atypical tuning and amplification mechanisms in gecko auditory hair cells. Proc. Natl. Acad. Sci. USA 2022, 119, e2122501119. [Google Scholar] [CrossRef]
- Manley, G.A. Diversity in hearing-organ structure and the characteristics of spontaneous otoacoustic emissions in lizards. In Diversity in Auditory Mechanics; Lewis, E.R., Long, G.R., Lyon, R.F., Narins, P.M., Steele, C.R., Eds.; World Scientific Publishing Co.: Singapore, 1997; pp. 32–38. [Google Scholar]
- van Dijk, P.; Manley, G.A. Distortion product otoacoustic emissions in the tree frog Hyla cinerea. Heart Res. 2001, 153, 14–22. [Google Scholar] [CrossRef]
- Van Dijk, P.; Narins, P.M.; Mason, M.J. Physiological vulnerability of distortion product otoacoustic emissions from the amphibian ear. J. Acoust. Soc. Am. 2003, 114, 2044–2048. [Google Scholar] [CrossRef]
- Vassilakis, P.N.; Meenderink, S.W.F.; Narins, P.M. Distortion product otoacoustic emissions provide clues to hearing mechanisms in the frog ear. J. Acoust. Soc. Am. 2004, 116, 3713–3726. [Google Scholar] [CrossRef]
- Gallo, L. Otoakustische Emissionen bei den Reptilien. Ph.D. Thesis, Technical University, Munich, Germany, 1996. [Google Scholar]
- Manley, G.A. Spontaneous otoacoustic emissions in monitor lizards. Heart Res. 2004, 189, 41–57. [Google Scholar] [CrossRef]
- Manley, G.A.; Gallo, L.; Köppl, C. Spontaneous otoacoustic emissions in two gecko species, Gekko gecko and Eublepharis macularius. J. Acoust. Soc. Am. 1996, 99, 1588–1603. [Google Scholar] [CrossRef]
- Taschenberger, G.; A Manley, G. Spontaneous otoacoustic emissions in the barn owl. Heart Res. 1997, 110, 61–76. [Google Scholar] [CrossRef]
- Bergevin, C.; Manley, G.A.; Köppl, C. Otoacoustic Interrelationships of the Barn Owl. Mechanics of Hearing: Protein to Perception. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2015; Volume 1703, p. 090011. [Google Scholar] [CrossRef]
- Manley, G.A.; Gleich, O. Avian primary auditory neurons: The relationship between characteristic frequency and preferred intervals. Die Nat. 1984, 71, 592–594. [Google Scholar] [CrossRef]
- Manley, G.; Schulze, M.; Oeckinghaus, H. Otoacoustic emissions in a song bird. Heart Res. 1987, 26, 257–266. [Google Scholar] [CrossRef]
- Klinke, R.; Smolders, J. Hearing mechanisms in caiman and pigeon. In Comparative Physiology of Sensory Systems; Bolis, L., Keynes, R., Maddrell, S., Eds.; Cambridge University Press: Cambridge, UK, 1984; pp. 195–211. [Google Scholar]
- Manley, G.A.; Gleich, O.; Leppelsack, H.J.; Oeckinghaus, H. Activity patterns of cochlear ganglion neurones in the starling. J. Comp. Physiol. A 1985, 157, 161–181. [Google Scholar] [CrossRef]
- Kettembeil, S.; Manley, G.A.; Siegl, E. Distortion-product otoacoustic emissions and their anaesthesia sensitivity in the European Starling and the chicken. Heart Res. 1995, 86, 47–62. [Google Scholar] [CrossRef]
- Whitehead, M.L.; Lonsbury-Martin, B.L.; Martin, G.K. Evidence for two discrete sources of 2f1-f2 distortion-product otoacoustic emissions in rabbit. I. Differential dependence on stimulus parameters. J. Acoust. Soc. Am. 1992, 91, 1587–1607. [Google Scholar] [CrossRef]
- Taschenberger, G.; Manley, G.A. General characteristics and suppression tuning properties of the distortion-product otoacoustic emission 2f1−f2 in the barn owl. Heart Res. 1998, 123, 183–200. [Google Scholar] [CrossRef]
- Köppl, C. Frequency Tuning and Spontaneous Activity in the Auditory Nerve and Cochlear Nucleus Magnocellularis of the Barn Owl Tyto alba. J. Neurophysiol. 1997, 77, 364–377. [Google Scholar] [CrossRef]
- Engler, S.; Köppl, C.; Manley, G.A.; de Kleine, E.; van Dijk, P. Suppression Tuning of Spontaneous Otoacoustic Emissions in the Barn Owl (Tyto alba). Heart Res. 2020, 385, 107835. [Google Scholar] [CrossRef]
- Köppl, C.; Gleich, O.; Manley, G.A. An auditory fovea in the barn owl cochlea. J. Comp. Physiol. 1993, 171, 695–704. [Google Scholar] [CrossRef]
- Manley, G.A.; Taschenberger, G.; Oeckinghaus, H. Influence of contralateral acoustic stimulation on distortion-product and spontaneous otoacoustic emissions in the barn owl. Heart Res. 1999, 138, 1–12. [Google Scholar] [CrossRef]
- Kaiser, A.; Manley, G.A. Physiology of putative single cochlear efferents in the chicken. J. Neurophysiol. 1994, 72, 2966–2979. [Google Scholar] [CrossRef]
- Bergevin, C.; Fulcher, A.; Richmond, S.; Velenovsky, D.; Lee, J. Interrelationships between spontaneous and low-level stimulus-frequency otoacoustic emissions in humans. Heart Res. 2012, 285, 20–28. [Google Scholar] [CrossRef]
- Rosowski, J.J.; Peake, W.T.; White, J.R. Cochlear non-linearities inferred from two-tone distortion products in the ear canal of the alligator lizard. Heart Res. 1984, 13, 141–158. [Google Scholar] [CrossRef]
- Manley, G.A.; Köppl, C.; Johnstone, B.M. Acoustic distortion products in the ear canal of the bobtail lizard. I: General characteristics. J. Acoust. Soc. Am. 1993, 93, 2820–2933. [Google Scholar] [CrossRef]
- Köppl, C.; Manley, G.A. Acoustic distortion products in the ear canal of the bobtail lizard. II: Suppression tuning characteristics. J. Acoust. Soc. Am. 1993, 93, 2834–2844. [Google Scholar] [CrossRef]
- Manley, G.A.; Köppl, C.; Johnstone, B.M. Components of the 2f1-f2 distortion product in the ear canal of the bobtail lizard. In Mechanics and Biophysics of Hearing; Dallos, P., Geisler, C.D., Matthews, J.W., Ruggero, M., Steele, C.R., Eds.; Springer: Heidelberg, Germany, 1990; pp. 210–217. [Google Scholar]
- Köppl, C.; Manley, G.A. Spontaneous otoacoustic emissions in the bobtail lizard. I: General characteristics. Heart Res. 1993, 71, 157–169. [Google Scholar] [CrossRef]
- Köppl, C.; Manley, G.A. Spontaneous otoacoustic emissions in the bobtail lizard. II: Interactions with external tones. Heart Res. 1994, 72, 159–170. [Google Scholar] [CrossRef]
- Manley, G.A.; Köppl, C. Spontaneous otoacoustic emissions in the bobtail lizard. III: Temperature effects. Heart Res. 1994, 72, 171–180. [Google Scholar] [CrossRef]
- Manley, G.A.; Kirk, D.L.; Köppl, C.; Yates, G.K. In vivo evidence for a cochlear amplifier in the hair-cell bundle of lizards. Proc. Natl. Acad. Sci. USA 2001, 98, 2826–2831. [Google Scholar] [CrossRef]
- Manley, G.A.; Sienknecht, U.; Köppl, C. Calcium Modulates the Frequency and Amplitude of Spontaneous Otoacoustic Emissions in the Bobtail Skink. J. Neurophysiol. 2004, 92, 2685–2693. [Google Scholar] [CrossRef]
- Manley, G.A.; Wartini, A.; Schwabedissen, G.; Siegl, E. Spontaneous otoacoustic emissions in teiid lizards. Heart Res. 2018, 363, 98–108. [Google Scholar] [CrossRef]
- Van Dijk, P.; Manley, G.A.; Gallo, L.; Pavusa, A.; Taschenberger, G. Statistical properties of spontaneous otoacoustic emissions in one bird and three lizard species. J. Acoust. Soc. Am. 1996, 100, 2220–2227. [Google Scholar] [CrossRef]
- van Dijk, P.; Manley, G.A.; Gallo, L. Correlated amplitude fluctuations of spontaneous otoacoustic emissions in five lizard species. J. Acoust. Soc. Amer. 1998, 104, 1559–1564. [Google Scholar] [CrossRef]
- Manley, G.A.; Gallo, L. Otoacoustic emissions, hair cells and myosin motors. J. Acoust. Soc. Am. 1997, 102, 1049–1055. [Google Scholar] [CrossRef]
- Manley, G.A. Evidence for an active process and a cochlear amplifier in non-mammals. J. Neurophysiol. 2001, 86, 541–549. [Google Scholar] [CrossRef][Green Version]
- Manley, G.A.; Kirk, D.L. The Influence of Injected AC and DC Currents on Spontaneous Otoacoustic Emissions in the Bobtail Lizard. J. Assoc. Res. Otolaryngol. 2001, 3, 200–208. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manley, G.A.; Kirk, D.L. BAPTA Induces Frequency Shifts in vivo of Spontaneous Otoacoustic Emissions of the Bobtail Lizard. Audiol. Neurotol. 2005, 10, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.A. The tectorial membrane stabilizes spontaneous otoacoustic emissions. In Biophysics of the Cochlea: From Molecules to Models; Gummer, A.W., Dalhoff, E., Nowotny, M., Scherer, M.P., Eds.; World Scientific Press: Singapore, 2003; pp. 480–487. [Google Scholar]
- Manley, G.A. Spontaneous otoacoustic emissions from free-standing stereovillar bundles of ten species of lizard with small papillae. Heart Res. 2006, 212, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.A. Spontaneous otoacoustic emissions in lizards: A comparison of the skink-like lizard families Cordylidae and Gerrhosauridae. Heart Res. 2009, 255, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.A. Lizard auditory papillae: An evolutionary kaleidoscope. Heart Res. 2011, 273, 59–64. [Google Scholar] [CrossRef]
- Köppl, C.; Forge, A.; Manley, G.A. Low density of membrane particles in auditory hair cells of lizards and birds suggests an absence of somatic motility. J. Comp. Neurol. 2004, 479, 149–155. [Google Scholar] [CrossRef]
- Wit, H.P.; Van Dijk, P.; Manley, G.A. A model for the relation between stimulus frequency and spontaneous otoacoustic emissions in lizard papillae. J. Acoust. Soc. Am. 2012, 132, 3273–3279. [Google Scholar] [CrossRef]
- Wit, H.P.; Manley, G.A.; Van Dijk, P. Modeling the characteristics of spontaneous otoacoustic emissions in lizards. Heart Res. 2019, 385, 107840. [Google Scholar] [CrossRef]
- Manley, G.A.; Köppl, C.; Yates, G.K. Micromechanical basis of high-frequency tuning in the bobtail lizard. In Mechanics of Hearing; Wilson, J.P., Kemp, D., Eds.; Plenum Press: New York, NY, USA, 1989; pp. 143–150. [Google Scholar]
- Holton, T.; Hudspeth, A.J. A Micromechanical Contribution to Cochlear Tuning and Tonotopic Organization. Science 1983, 222, 508–510. [Google Scholar] [CrossRef]
- Frishkopf, L.S.; DeRosier, D.J. Mechanical tuning of free-standing stereociliary bundles and frequency analysis in the alligator lizard cochlea. Heart Res. 1983, 12, 393–404. [Google Scholar] [CrossRef]
- Gelfand, M.; Piro, O.; Magnasco, M.O.; Hudspeth, A.J. Interactions between Hair Cells Shape Spontaneous Otoacoustic Emissions in a Model of the Tokay Gecko’s Cochlea. PLoS ONE 2010, 5, e11116. [Google Scholar] [CrossRef] [PubMed]
- Vilfan, A.; Duke, T. Frequency Clustering in Spontaneous Otoacoustic Emissions from a Lizard’s Ear. Biophys. J. 2008, 95, 4622–4630. [Google Scholar] [CrossRef] [PubMed]
- Bergevin, C.; Salerno, A. Dynamics of Spontaneous Otoacoustic Emissions. Mechanics of Hearing: Protein to Perception. In Aip Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2015; Volume 1703, p. 090024. [Google Scholar] [CrossRef]
- Cheatham, M.A.; Ahmad, A.; Zhou, Y.; Goodyear, R.J.; Dallos, P.; Richardson, G.P. Increased Spontaneous Otoacoustic Emissions in Mice with a Detached Tectorial Membrane. J. Assoc. Res. Otolaryngol. 2015, 17, 81–88. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manley, G.A. Otoacoustic Emissions in Non-Mammals. Audiol. Res. 2022, 12, 260-272. https://doi.org/10.3390/audiolres12030027
Manley GA. Otoacoustic Emissions in Non-Mammals. Audiology Research. 2022; 12(3):260-272. https://doi.org/10.3390/audiolres12030027
Chicago/Turabian StyleManley, Geoffrey A. 2022. "Otoacoustic Emissions in Non-Mammals" Audiology Research 12, no. 3: 260-272. https://doi.org/10.3390/audiolres12030027
APA StyleManley, G. A. (2022). Otoacoustic Emissions in Non-Mammals. Audiology Research, 12(3), 260-272. https://doi.org/10.3390/audiolres12030027




