1. Introduction
Hemifacial microsomia, first comprehensively described by von Arlt in 1881, represents a congenital disorder characterized by unilateral facial underdevelopment. This condition, recognized as the second most common craniofacial birth defect after cleft lip and palate, occurs in approximately 1:3500 to 1:5600 live births [
1]. The spectrum of severity and phenotypic heterogeneity has been further emphasized in subsequent reports, highlighting the complexity of both diagnosis and treatment planning [
2]. The most widely accepted classification system is the OMENS criteria [
3], encompassing Orbital distortion, Mandibular hypoplasia, Ear anomalies, Nerve involvement, and Soft tissue deficiency. For mandibular deformities specifically, the Pruzansky–Kaban classification categorizes severity into three distinct types: Type I (mild hypoplasia), Type II (moderate hypoplasia with morphological abnormalities), and Type III (severe hypoplasia or absence of ramus and temporomandibular joint) [
4,
5]. This system has proven particularly useful in guiding surgical decision-making and staging [
6].
The condition encompasses the oculo–auriculo–vertebral spectrum (OAVS), including Goldenhar syndrome, characterized by additional features such as epibulbar dermoids and vertebral anomalies [
1]. Alternative terminology includes craniofacial microsomia and first–second branchial arch syndrome, reflecting the embryological origin of affected structures. Distinguishing true hemifacial microsomia from hemimandibular hypoplasia with condylar-coronoid collapse has been shown to have important diagnostic and prognostic implications [
7].
While the etiology remains incompletely understood, current evidence suggests multifactorial causation involving genetic mutations and environmental factors, including vascular disruptions during early fetal development [
8]. Other studies have emphasized the potential role of functional impairment and aberrant growth patterns in influencing mandibular asymmetry and progression over time [
9]. Contemporary management requires multidisciplinary collaboration, incorporating advanced surgical techniques, orthodontic intervention, and comprehensive supportive therapy to address both functional and aesthetic concerns [
10,
11,
12].
Several treatment protocols for mandibular hypoplasia in hemifacial microsomia have been proposed, ranging from early functional orthopedic approaches to surgical interventions during growth or adulthood [
13,
14]. Osteogenic distraction has historically represented the cornerstone of vertical ramus augmentation, with significant experience accumulated in pediatric and adolescent populations [
15]. However, despite its widespread application, vector control, device bulkiness, and soft tissue adaptation remain critical challenges [
13,
14].
This technical note illustrates the evolution of our institutional experience in orthodontic-surgical treatment of adult patients with grade I–IIb hemifacial hypoplasia according to the Pruzansky–Kaban classification. Our protocol typically involves two surgical phases: initial vertical augmentation of the affected mandibular ramus with concurrent orthodontic leveling of the maxillary arch to address occlusal compensation, followed by bimaxillary surgery for asymmetry correction and midline centering. While mandibular ramus osteodistraction remains the standard procedure for vertical augmentation in hypoplastic cases, with osteotomy traditionally performed superior to the lingula for inferior alveolar neurovascular bundle preservation, we present an alternative approach utilizing classic unidirectional distraction applied to an Obwegeser-type osteotomy design [
16]. Given the potential for 15–20 mm vertical augmentation while maintaining osseous segment contact, we propose direct ramus vertical augmentation with subsequent stabilization using sagittal osteotomy according to Obwegeser’s original technique. This modification offers reduced surgical complexity and cost compared to distraction protocols, while the maintained bony contact and rigid fixation ensure optimal osteotomy healing.
Our institutional series comprises eighteen cases, with the three presented cases serving as illustrative examples of different techniques for mandibular ramus height augmentation, representing a progressive treatment algorithm adaptable to individual patient requirements. The aim of this technical note is to describe and compare three surgical techniques for mandibular ramus augmentation in hemifacial microsomia, evaluating their effectiveness in relation to anatomical characteristics and clinical complexity, to develop criteria for appropriate surgical technique selection and propose a treatment algorithm based on individual patient features.
2. Materials and Methods
This retrospective study was conducted including patients with hemifacial microsomia who underwent mandibular ramus augmentation between January 2010 and September 2022, with a minimum follow-up period of three years, at IRCCS Ospedale Policlinico San Martino, Genoa, Italy, and Salus Hospital of Tirana, Albania. The study protocol received approval from the institutional review board, and all procedures were performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients or their legal guardians prior to surgical intervention. Patients aged between 13 and 19 years with Pruzansky–Kaban grade I to IIb hemifacial microsomia requiring vertical ramus augmentation were included in the study. Exclusion criteria comprised patients with Pruzansky–Kaban grade III deformity, as these cases necessitate total temporomandibular joint reconstruction and represent a distinct therapeutic challenge beyond the scope of the present investigation. Patients with insufficient follow-up data or those who declined participation were also excluded.
We report our clinical experience in the surgical treatment of patients affected by hemifacial microsomia, classified according to the Pruzansky–Kaban system and distributed based on anatomical severity, age at intervention, and gender. Surgical technique selection was determined through multidisciplinary evaluation considering the degree of mandibular hypoplasia, soft tissue deficiency, and technical feasibility of each approach. The patients included in this retrospective report were equally divided into three groups of six patients each, based on the surgical technique employed. All patients underwent vertical augmentation of the affected mandibular ramus using different surgical strategies derived from, and progressively refining, the original osteotomy of the mandibular ramus described by Obwegeser in 1956. Virtual surgical planning (VSP) was employed in the cases treated with direct vertical augmentation and rigid fixation following sagittal osteotomy. Patient-specific computed tomography (CT) data were segmented to create a three-dimensional model of the mandible. This allowed a comparative evaluation of the height of the two mandibular rami, enabling accurate assessment of the discrepancy and calculation of the vertical augmentation required to achieve mandibular symmetry. Among this cohort of eighteen patients, three representative cases are presented in detail with iconographic documentation, as they illustrate a logical progression toward surgical simplification while preserving functional and aesthetic outcomes.
3. Results
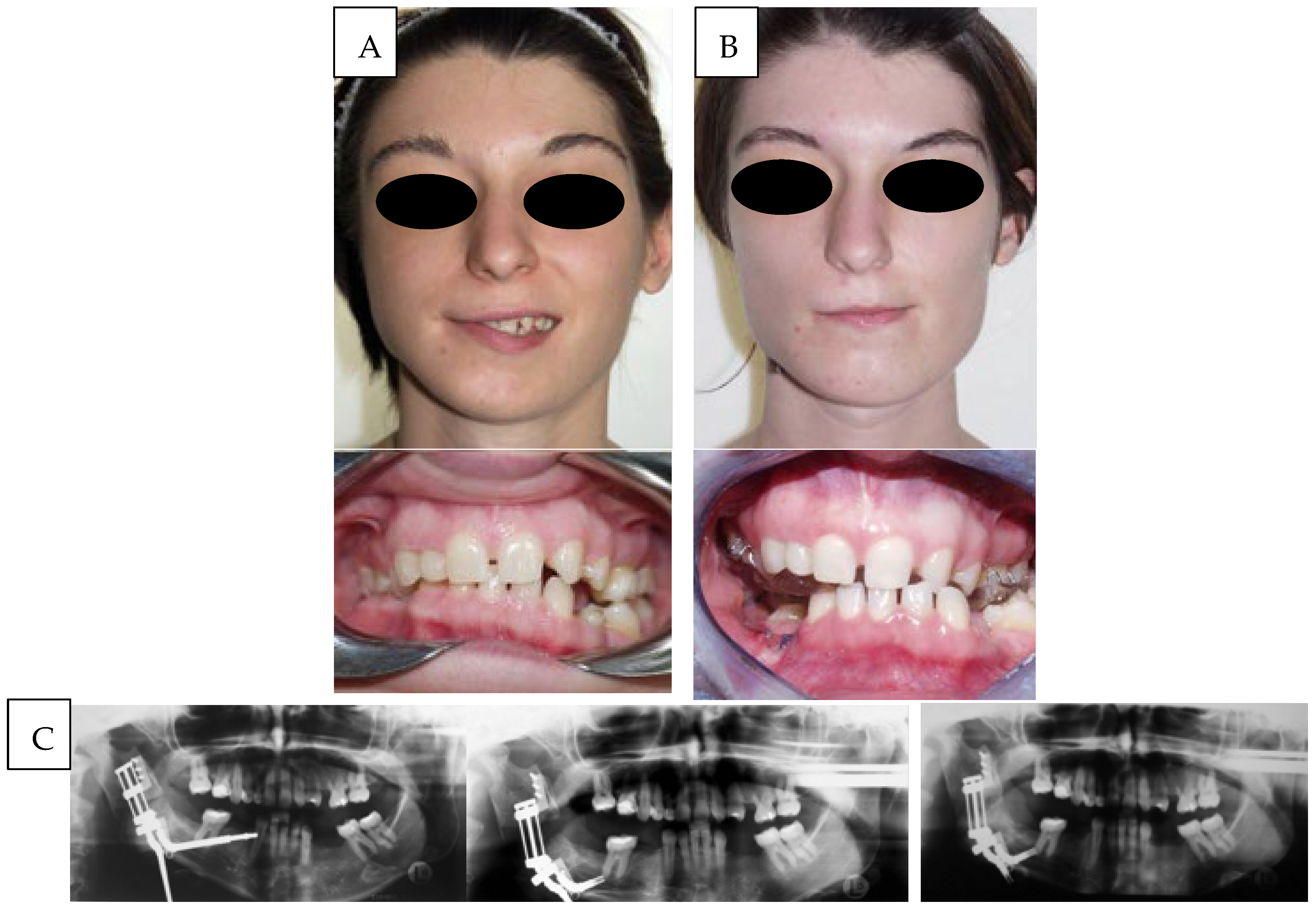
The first case (
Figure 1) involved a patient classified as type IIa according to the Pruzansky–Kaban system, who underwent distraction osteogenesis of the mandibular ramus through an osteotomy performed just above the lingula, thereby preserving the inferior alveolar neurovascular bundle. A bidirectional distractor was applied with the dual purpose of increasing ramus height and correcting mandibular angle inclination. No second-stage maxillomandibular osteotomy was planned. Although the clinical outcome was satisfactory, technical challenges arose due to the placement of the distractor in a reduced anatomical space, further compromised by associated soft tissue hypoplasia. The main difficulties were related to the positioning of the activation arms and to vector control, which depended exclusively on the mechanical structure of the device. Despite the complex three-dimensional movements of the segments, the distraction gap was successfully consolidated with high-quality new bone.
Considering the presence of multiple edentulous spaces, the young age of the patient, and the specific request for a straightforward and time-efficient intervention, a two-vector distraction protocol was selected as previously described. The treatment plan included subsequent orthodontic management and prosthetic rehabilitation to complete the case. The overall treatment duration was approximately one year. The distraction phase lasted 15 days for the vertical component and 6 days for the angular component, with an average daily activation of 1 mm. The consolidation phase of the bone callus required about three months, after which the distractor was removed. During the first postoperative month, an occlusal splint was maintained, followed by progressive orthodontic decompensation and alignment, culminating in the implant-prosthetic phase beginning around the fourth month and continuing until one year after distractor placement.
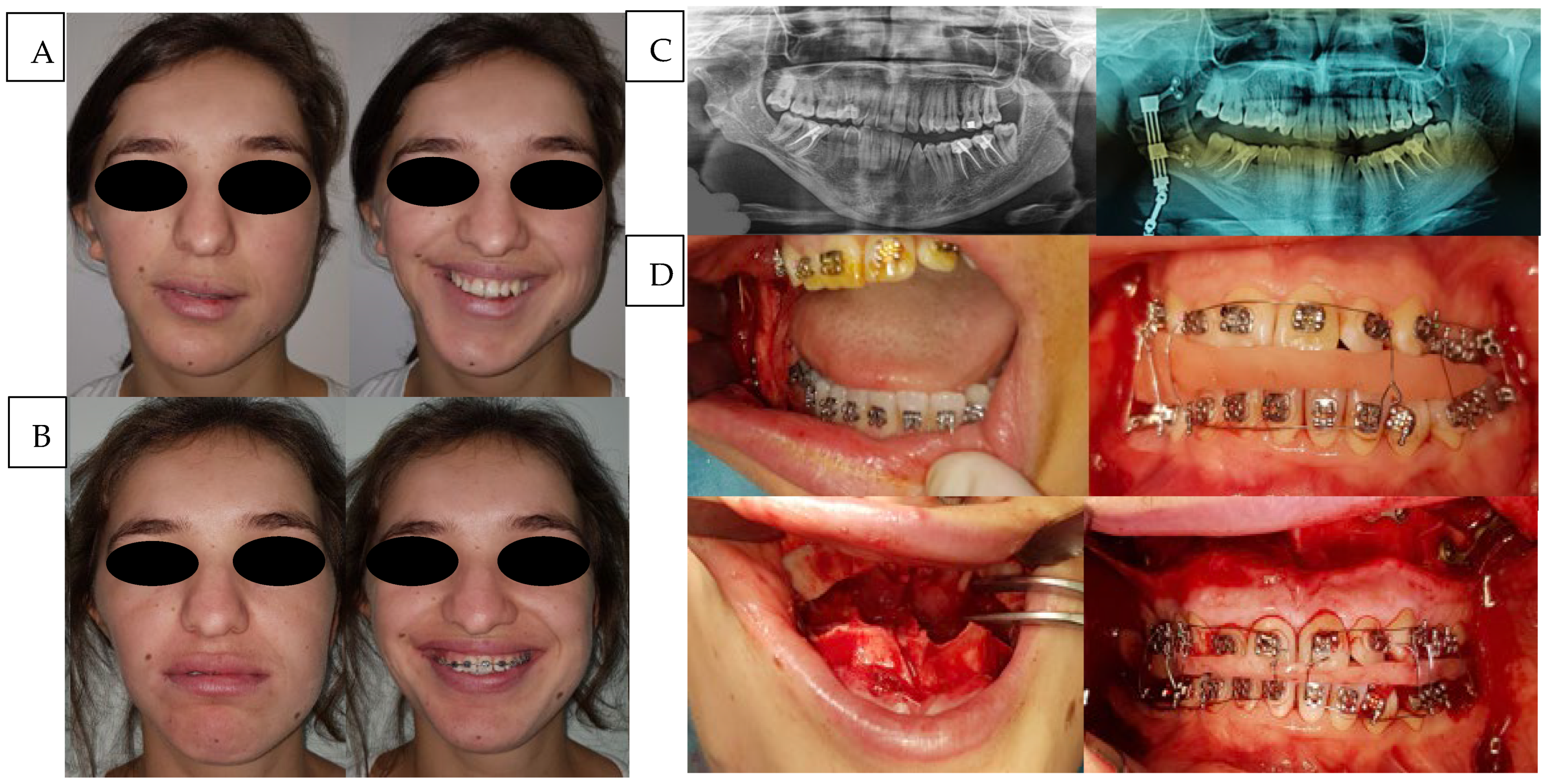
The second case (
Figure 2), classified as type IIb, required a two-stage surgical protocol consisting of initial mandibular ramus distraction followed by maxillomandibular osteotomy. To achieve this, a sagittal ramus osteotomy was performed according to Obwegeser’s 1956 technique, followed by application of a unidirectional distractor. The use of this classical osteotomy for distraction provided two key advantages: enhanced control of the vertical augmentation vector due to continuous cortical contact between the osteotomized segments and facilitated device positioning owing to the more caudal vestibular osteotomy, which simplified fixation.
Ramus vertical augmentation was performed progressively, with an increase ranging between 15 and 25 mm while preserving minimal cortical continuity. The distraction device was of reduced volume compared to that used in prior cases, reflecting the simplified vector control. Vertical augmentation was continued until an aesthetically satisfactory result in terms of vertical symmetry was achieved. Following a stabilization phase like that previously described, the distractor was removed, and orthodontic decompensation and alignment were completed.
Approximately six months after the initial procedure, a maxillomandibular osteotomy was performed to achieve centering and facilitate the post-surgical orthodontic phase. The total treatment duration, from distractor placement to final completion, was approximately one year.
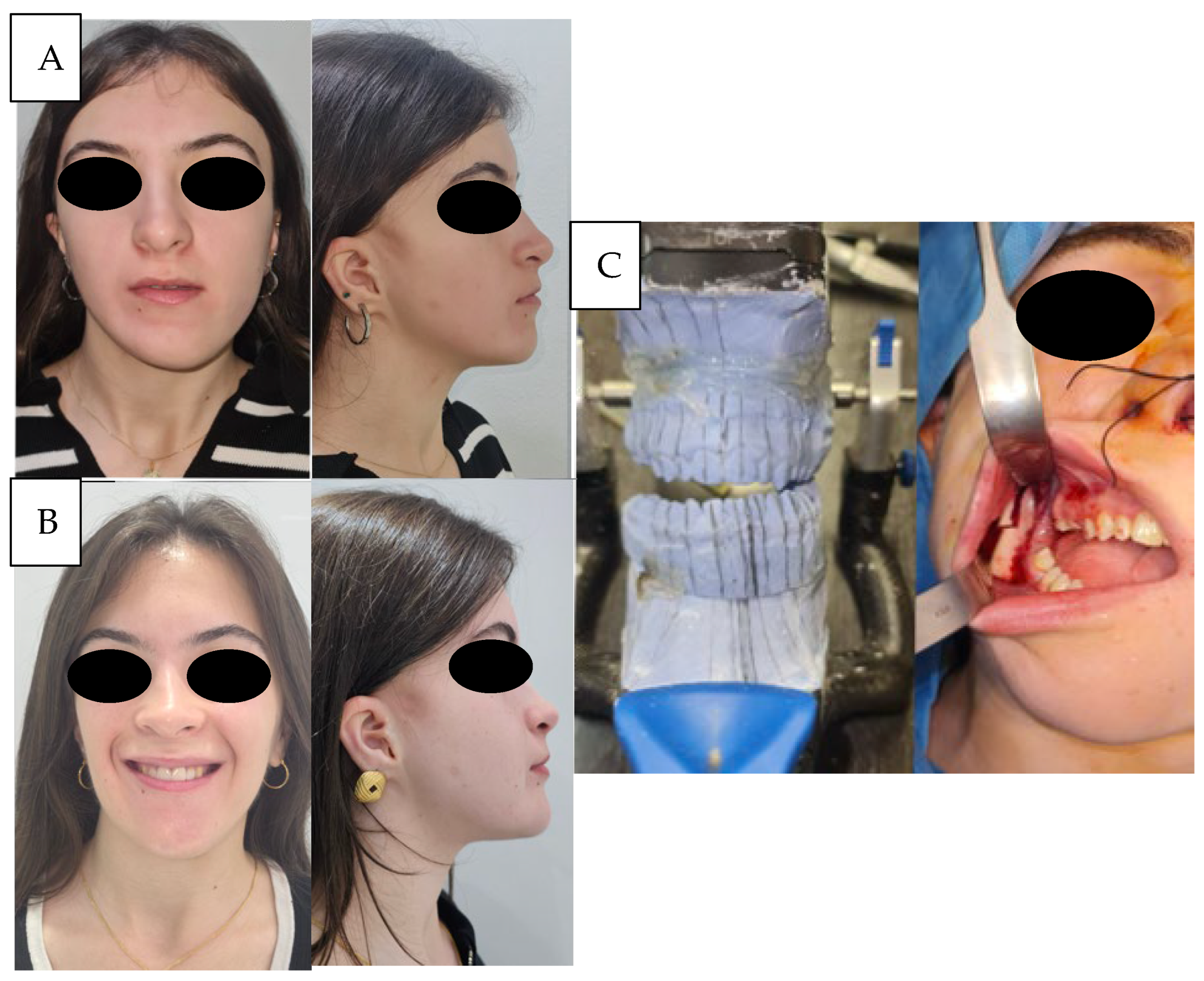
The third case (
Figure 3 and
Figure 4) concerned a patient with type I hemifacial microsomia, characterized by preservation of the temporomandibular joint, auricular malformation, and involvement of the marginal mandibular branch of the facial nerve. A two-stage treatment was planned. In the first stage, sagittal ramus osteotomy, as described by Obwegeser, was performed, and stabilized with titanium osteosynthesis. Fixation was obtained with a L-plate (2.0 system) fixed with its short arm to the distal segment, allowing for easier removal after consolidation. During this period, orthodontic alignment and leveling of the maxillary arch were carried out. In the second stage, the osteosynthesis material was removed, and definitive orthognathic correction was performed with a bilateral sagittal split osteotomy (Obwegeser–Dal Pont) combined with a Le Fort I osteotomy.
All patients included in this series successfully underwent vertical augmentation of the hypoplastic mandibular ramus, achieving augmentations ranging from 15 to 25 mm (mean: 19.93 mm) on one-year post-operative CT scans, depending on the applied technique. The first approach, consisting of a bidirectional distraction after a full-thickness osteotomy above the lingula, provided satisfactory bone regeneration and correction of mandibular angle inclination, though difficulties in device placement within a limited anatomical space and challenges in vector control were consistently observed. The second technique, employing a unidirectional distractor applied to a sagittal osteotomy of the ramus according to Obwegeser, improved control of the vertical augmentation vector due to the preserved cortical contact and allowed easier fixation and removal of the device. The third approach, based on direct vertical augmentation and rigid fixation of the sagittal osteotomy, eliminated the need for distractors, simplified intraoperative management, and reduced overall treatment costs, while still ensuring reliable consolidation with stable bony contact.
Postoperative management was homogeneous across all groups. Patients remained hospitalized for an average of 4.5 days, during which broad-spectrum antibiotic therapy and analgesic support were routinely administered. No postoperative complications were recorded. Wound healing was uneventful in all cases, with no infections, device exposures, or neurovascular injuries observed. For patients treated with distraction, activation protocols were initiated after a latency period of 3 days, with a daily increase of 1 mm, divided into 0.5 mm every 12 h. Distractor activation and consolidation were closely monitored through scheduled clinical and radiographic evaluations at 10 days post-surgery, and subsequently at 1 to 2 months, before device removal. Radiographs confirmed progressive callus formation and consolidation in all distracted segments. Device removal was performed under general anesthesia after consolidation without adverse events. In patients managed with direct fixation, postoperative radiographs confirmed stable osteotomy healing without loss of correction. Overall, the staged treatment protocol, combining initial ramus vertical augmentation with subsequent orthodontic therapy and bimaxillary osteotomy, provided stable correction of facial asymmetry and satisfactory functional and aesthetic outcomes in all patients.
4. Discussion
This study illustrates the progressive refinement of surgical strategies for vertical augmentation of the hypoplastic mandibular ramus in patients with hemifacial microsomia. Our experience reflects an evolution from the use of distraction osteogenesis [
17] toward the reconsideration of classical osteotomy techniques, aided by modern digital planning and computer-assisted surgery.
Distraction osteogenesis has long been considered the cornerstone of mandibular reconstruction in hemifacial microsomia, especially in growing patients, due to its ability to achieve significant skeletal vertical augmentation while simultaneously stretching the surrounding soft tissues [
14,
15]. In our early cases, we adopted a full-thickness osteotomy above the lingula to preserve the inferior alveolar neurovascular bundle, applying bidirectional distractors. However, the use of such devices presented several technical limitations. The upper part of the distractor often had to be placed just below the sigmoid notch, making stabilization with fixation screws technically demanding. Furthermore, the bulk of bidirectional distractors rendered subperiosteal placement particularly challenging in patients with associated soft tissue deficiency.
Another critical limitation of DO is the difficulty in maintaining precise vector control during distraction. Muscle forces acting on the mandibular ramus may lead to medialization of the proximal fragment, a tendency that cannot always be adequately compensated for by the distractor’s mechanical design [
13]. These technical drawbacks highlight the dependency of outcomes on meticulous intraoperative positioning of the device, and partly explain the variability reported in the literature regarding long-term stability of DO in hemifacial microsomia [
7,
9,
17,
18,
19,
20,
21,
22,
23]. To overcome these challenges, we progressively shifted to applying the sagittal ramus osteotomy described by Obwegeser in 1956 as the basis for distraction. This osteotomic scheme allowed distractor placement in a more caudal position along the external cortex of the ramus, thereby facilitating both intraoperative fixation and later removal. Additionally, the technique ensured dual cortical guidance of the proximal segment—vestibular and lingual—for at least 15–20 mm, resulting in superior vector control and more predictable bone formation, as exemplified in our second case.
With the increasing availability of virtual surgical planning and CAD/CAM technologies, we further explored the possibility of predefining the desired vertical gain of the ramus. This enabled us to employ the sagittal osteotomy not only as a means for distraction but also as a stand-alone reconstructive option. In selected patients, predetermined increments could be achieved and stabilized directly with titanium plates and screws, thus eliminating the need for a distractor. Such an approach significantly simplifies the surgical procedure, reduces costs, and decreases patient morbidity, while maintaining satisfactory outcomes. Digital planning enhances accuracy in osteotomy design and allows for highly individualized treatment [
2,
10]. The contemporary management of hemifacial microsomia has further evolved through the integration of advanced technological platforms beyond traditional VSP. The incorporation of robotic-assisted surgical systems and comprehensive CAD/CAM workflows has expanded the therapeutic armamentarium available to address the multifaceted surgical challenges inherent to this condition [
24,
25,
26,
27]. These emerging technologies facilitate enhanced precision in osteotomy execution, improved three-dimensional spatial control, and the fabrication of patient-specific instrumentation and fixation devices. Such innovations represent a natural extension of the computer-assisted surgical paradigm, potentially offering solutions to technical limitations previously encountered with conventional approaches, particularly in anatomically challenging cases where traditional instrumentation proves suboptimal [
18,
24,
28,
29]. In this study, the VSP-assisted design of the sagittal osteotomy of the hypoplastic mandibular ramus allowed a precise evaluation of the maximum achievable vertical augmentation while maintaining bone contact between the medial cortex of the proximal fragment and the vestibular cortex of the distal segment. This assessment made it possible to confirm the feasibility of achieving stable fixation with a single L-shaped plate, avoiding the need for osteodistraction techniques. The superiority of the proposed technique was therefore intended in terms of procedural simplification rather than clinical outcome, which remained comparable among the three approaches analyzed.
From a clinical perspective, the intraoral positioning of a mandibular ramus distractor, particularly in cases requiring bidirectional devices, may present significant technical challenges. The placement of fixation screws in a deep and narrow surgical field can complicate both the initial positioning and, even more, the subsequent removal of the distractor after callus consolidation. In contrast, when applicable, the simplified technique described in this study allows for stable fixation using a single L-shaped plate and facilitates its removal through a less invasive approach. This contributes to reduced operative complexity and improved postoperative management without compromising clinical outcomes.
Patient selection is paramount, as this simplified technique is not universally applicable. More severe forms of hemifacial microsomia (Pruzansky–Kaban type IIb or III) still often require staged approaches with distraction osteogenesis to achieve sufficient skeletal vertical augmentation and soft tissue accommodation [
6,
30,
31]. Moreover, small residual asymmetries are frequently present after ramus vertical augmentation, and in our experience, these can be corrected during subsequent orthognathic procedures, such as maxillomandibular osteotomies, which remain an integral component of the comprehensive treatment pathway. Orthodontic therapy is also essential to optimize occlusion and facial symmetry in the long term [
9,
14]. Our study provides a comparison of different surgical strategies applied within the same institution, illustrating the conceptual progression from device-dependent distraction osteogenesis to simplified osteotomy-based techniques augmented by digital planning. Furthermore, it highlights the practical implications of combining traditional surgical principles with contemporary computer-assisted surgery.
However, some limitations should be acknowledged. The number of detailed cases presented is small; therefore, future prospective studies with larger cohorts and standardized outcome measures are needed to validate the reproducibility of this simplified approach and to compare it directly with conventional distraction osteogenesis, as previously reported in larger retrospective cohorts [
15].
5. Conclusions
In conclusion, this study demonstrates that mandibular ramus vertical augmentation can be effectively achieved through three distinct surgical techniques, each tailored to specific anatomical characteristics and clinical complexity. Our comparative analysis reveals that distraction osteogenesis remains the preferred option for younger patients or more severe deformities requiring gradual soft tissue accommodation, while sagittal ramus osteotomy with virtual surgical planning and rigid fixation offers a simplified, predictable alternative in less severe cases with adequate bone stock. Based on our experience, we propose a treatment algorithm that individualizes technique selection according to patient age, deformity severity (Pruzansky–Kaban classification), and anticipated soft tissue adaptation needs. This patient-centered approach optimizes outcomes while minimizing surgical morbidity and costs, achieving predictable and reproducible results across the spectrum of hemifacial microsomia presentations.
Author Contributions
Conceptualization, F.L. and B.C.B.; methodology, F.L. and B.C.B.; software, A.S.; validation, F.L., B.B. and L.A.V.; formal analysis, A.M.M. and F.A.; investigation, F.L., M.S. and E.B.P.; resources, F.L., B.C.B. and F.A.; data curation, A.S. and E.A.; writing—original draft preparation, F.L. and A.M.M.; writing—review and editing, A.M.M.; visualization, L.A.V. and E.A.; supervision, B.C.B. and B.B.; project administration, F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee CET-Liguria on 11 November 2024, n. 307/2024-DB id 13948.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study and Informed consent for publication was obtained from all identifiable human participants.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gorlin, R.J.; Jue, K.L.; Jacobsen, U.; Goldschmidt, E. Oculoauriculovertebral dysplasia. J. Pediatr. 1963, 63, 991–999. [Google Scholar] [CrossRef]
- Luo, S.; Sun, H.; Bian, Q.; Liu, Z.; Wang, X. The etiology, clinical features, and treatment options of hemifacial microsomia. Oral Dis. 2023, 29, 2449–2462. [Google Scholar] [CrossRef]
- Vento, A.R.; LaBrie, R.A.; Mulliken, J.B. The O.M.E.N.S. classification of hemifacial microsomia. Cleft Palate Craniofac J. 1991, 28, 68–76; discussion 77. [Google Scholar] [CrossRef]
- Pruzansky, S. Not all dwarfed mandibles are alike. Birth Defects 1969, 5, 120–129. [Google Scholar]
- Kaban, L.B.; Mulliken, J.B.; E Murray, J. Three-dimensional approach to analysis and treatment of hemifacial microsomia. Cleft Palate J. 1981, 18, 90–99. [Google Scholar] [PubMed]
- Huh, J.; Park, J.-S.; Sodnom-Ish, B.; Yang, H.J. Growth characteristics and classification systems of hemifacial microsomia: A literature review. Maxillofac. Plast. Reconstr. Surg. 2024, 46, 18. [Google Scholar] [CrossRef]
- Meazzini, M.C.; Mazzoleni, F.; Canzi, G.; Bozzetti, A. Mandibular distraction osteogenesis in hemifacial microsomia: Long-term follow-up. J. Cranio-Maxillofacial Surg. 2005, 33, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.M. Perspectives on craniofacial asymmetry. IV. Hemi-asymmetries. Int. J. Oral Maxillofac. Surg. 1995, 24, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Meazzini, M.C.; Brusati, R.; Caprioglio, A.; Diner, P.; Garattini, G.; Giannì, E.; Lalatta, F.; Poggio, C.; Sesenna, E.; Silvestri, A.; et al. True hemifacial microsomia and hemimandibular hypoplasia with condylar-coronoid collapse: Diagnostic and prognostic differences. Am. J. Orthod. Dentofac. Orthop. 2011, 139, e435–e447. [Google Scholar] [CrossRef]
- Cano-Rosás, M.; Benito-Cano, J.; Benito-Cano, J.; Diosdado-Cano, J.M.; Benito-Duque, P.; Curto, A. Multidisciplinary Treatment of Hemifacial Microsomia: Several Clinical Cases. Clin. Pract. 2024, 14, 2410–2418. [Google Scholar] [CrossRef]
- Laganà, F.; Arcuri, F.; Spinzia, A.; Bianchi, B. Maxillomandibular Advancement for Obstructive Sleep Apnea Syndrome: Long-Term Results of Respiratory Function and Reverse Face-Lift. J. Craniofacial Surg. 2023, 34, 1760–1765. [Google Scholar] [CrossRef]
- Supplement, D.; Beltramini, G.A.; Rossi, D.; Bolzoni, A.; Piva, A.; Laganà, F. Implants outcome inserted in different sites. J. Biol. Regul. Homeost Agents 2020, 34, 13–17. [Google Scholar]
- Posnick, J.C. Surgical correction of mandibular hypoplasia in hemifacial microsomia: A personal perspective. J. Oral Maxillofac. Surg. 1998, 56, 639–650. [Google Scholar] [CrossRef]
- Kaban, L.B.; Padwa, B.L.; Mulliken, J.B. Surgical correction of mandibular hypoplasia in hemifacial microsomia: The case for treatment in early childhood. J. Oral Maxillofac. Surg. 1998, 56, 628–638. [Google Scholar] [CrossRef]
- Bertin, H.; Mercier, J.; Cohen, A.; Giordanetto, J.; Cohen, N.; Lee, S.; Perrin, J.; Corre, P. Surgical correction of mandibular hypoplasia in hemifacial microsomia: A retrospective study in 39 patients. J. Cranio-Maxillofac. Surg. 2017, 45, 1031–1038. [Google Scholar] [CrossRef]
- Trauner, R.; OBWEGESER, H. The surgical correction of mandibular prognathism and retrognathia with consideration of genioplasty. I. Surgical procedures to correct mandibular prognathism and reshaping of the chin. Oral Surg. Oral Med. Oral Pathol. 1957, 10, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Ascenço, A.S.K.; Balbinot, P.; Junior, I.M.; D’Oro, U.; Busato, L.; da Silva Freitas, R. Mandibular distraction in hemifacial microsomia is not a permanent treatment: A long-term evaluation. J. Craniofac. Surg. 2014, 25, 352–354. [Google Scholar] [CrossRef]
- Shakir, S.; Bartlett, S.P. Modern Mandibular Distraction Applications in Hemifacial Microsomia. Clin. Plast. Surg. 2021, 48, 375–389. [Google Scholar] [CrossRef]
- López, D.F.; Acosta, D.M.; A Rivera, D.; Mejía, C.M. Hemifacial microsomia: Treatment alternatives—A systematic review of literature. J. Clin. Pediatr. Dent. 2022, 46, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Suppapinyaroj, C.; Lin, C.-H.; Lo, L.-J.; Ko, E.-C. Outcome of surgical-orthodontic treatment in hemifacial microsomia with and without early mandibular distraction osteogenesis. Int. J. Oral Maxillofac. Surg. 2021, 50, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Polley, J.W.; Figueroa, A.A. Distraction osteogenesis: Its application in severe mandibular deformities in hemifacial microsomia. J. Craniofacial Surg. 1997, 8, 422–430. [Google Scholar] [CrossRef]
- Prior, A.; Allegretti, L.; Melloni, I.; Bovio, M.; Laganà, F.; Ceraudo, M.; Zona, G. Traumatic subarachnoid hemorrhage related to ophthalmic artery avulsion: A case report. Acta Neurochir. 2018, 160, 913–917. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Hwang, K.-G.; Baek, S.-H.; Lee, J.-H.; Kim, T.-W.; Kim, M.-J.; Chang, Y.-I. Original sagittal split osteotomy revisited for mandibular distraction. J. Cranio-Maxillofac. Surg. 2001, 29, 165–173. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Z.; Wang, Y.; Li, X.; Ye, B.; Li, J. The accuracy of virtual-surgical-planning-assisted treatment of hemifacial microsomia in adult patients: Distraction osteogenesis vs. orthognathic surgery. Int. J. Oral Maxillofac. Surg. 2019, 48, 341–346. [Google Scholar] [CrossRef]
- Shi, L.; Liu, W.; Yin, L.; Feng, S.; Xu, S.; Zhang, Z.-Y. Surgical guide assistant mandibular distraction osteogenesis and sagittal split osteotomy in the treatment of hemifacial microsomia. J. Craniofacial Surg. 2015, 26, 498–500. [Google Scholar] [CrossRef]
- Renkema, R.W.; Caron, C.J.; Heike, C.L.; Koudstaal, M.J. A decade of clinical research on clinical characteristics, medical treatments, and surgical treatments for individuals with craniofacial microsomia: What have we learned? J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 1781–1792. [Google Scholar] [CrossRef]
- Kim, B.S.; Zhang, Z.; Sun, M.; Han, W.; Chen, X.; Yan, Y.; Shi, Y.; Xu, H.; Lin, L.; Chai, G. Feasibility of a Robot-Assisted Surgical Navigation System for Mandibular Distraction Osteogenesis in Hemifacial Microsomia: A Model Experiment. J. Craniofacial Surg. 2023, 34, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, Y.; Zhang, Z.; Li, X.; Ye, B.; Li, J. Comprehensive consideration and design with the virtual surgical planning-assisted treatment for hemifacial microsomia in adult patients. J. Craniomaxillofac. Surg. 2018, 46, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Sugar, A.; Evans, P.; Bartlett, S.; Key, S. Virtual planning for corrections of hemifacial microsomia. Innov. Surg. Sci. 2023, 8, 159–183. [Google Scholar] [CrossRef] [PubMed]
- Sesenna, E.; Magri, A.S.; Magnani, C.; Brevi, B.C.; Anghinoni, M.L. Mandibular distraction in neonates: Indications, technique, results. Ital. J. Pediatr. 2012, 38, 7. [Google Scholar] [CrossRef]
- Brevi, B.; Bergonzani, M.; Zito, F.; Varazzani, A.; Sesenna, E. Infant mandibular distraction in absence of ascending ramus: Case series. Oral Maxillofac. Surg. 2021, 25, 401–410. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).