Abstract
Background and objective: Heterotopic ossification (HO) of the plantar fascia is an exceptionally rare condition, with only a few cases mentioned in the literature. In comparison, calcification of the fascia occurs more frequently, especially in cases of chronic plantar fasciitis. Tenex™, a percutaneous ultrasonic tenotomy system initially designed for tendinopathy treatment, may offer a minimally invasive alternative to conventional surgery in selected cases of HO. So, the aim of this case report was to assess the improvement in the pain and in the foot function after a percutaneous ultrasonic debridement. Case presentation: We present the case of an 82-year-old male with a history of hypertension and hyperuricemia, who reported a two-year history of mechanical-type plantar pain described as “walking on a stone.” Radiographs and MRI confirmed heterotopic ossification at the central component of the plantar fascia. Pain and function were assessed with the Foot Function Index (FFI). Under ultrasound and fluoroscopic guidance, percutaneous ultrasonic debridement with Tenex™ was performed following tibial and sural nerve block and conscious sedation. The procedure was completed in 6 min and 29 s of cutting time. After surgery, the patient wore a protective shoe for 3 weeks, followed a relative rest protocol, and received NSAIDs for 5 days. At 48–72 h, the patient reported noticeable pain relief, with significant functional improvement after 1 month. Conclusions: This case shows how Tenex™ effectively treats plantar fascia HO. It led to quick symptom relief and functional recovery. The ultrasonic percutaneous debridement with Tenex™ was a safe and effective option compared to open surgery for this patient. However, more research is needed to set standardized treatment protocols and assess long-term results.
Keywords:
heterotopic ossification; plantar fasciitis; fasciopathy; heel; Tenex; Foot Function Index 1. Introduction
Heterotopic ossification (HO) is defined as the formation of bone within soft tissues where bone usually does not exist. This aberrant tissue repair is most commonly a complication of trauma or surgery, although genetic forms of HO also exist, such as fibrodysplasia ossificans progressiva and progressive osseous heteroplasia [1,2]. It is important to distinguish between the acellular deposition of calcium phosphate, known as calcification, and the formation of mature trabecular bone in ectopic locations, characteristic of heterotopic ossification. The separation from the insertion site further supports its differentiation from a calcaneal spur or enthesophyte [3]. In its acquired form, this condition is more frequently observed in young men in their second and third decades of life, with a male-to-female ratio of approximately 3:2. Classically, HO is associated with a history of trauma or surgery in about 75% of cases. In contrast, the remaining cases are attributed to repetitive microtrauma or chronic mechanical stress [2].
Prophylactic strategies for heterotopic ossification include nonsteroidal anti-inflammatory drugs (NSAIDs), bisphosphonates, and, in selected cases, radiotherapy. HO is often asymptomatic; however, when patients develop pain, functional impairment, or other symptoms, surgical excision may be required to completely remove the ossified tissue [1,2,4,5]. According to the literature, the most commonly affected sites are the elbow, thigh, pelvis, and shoulder [2]. In contrast, reports of heterotopic ossification involving the plantar fascia are exceedingly rare, with calcification being a more frequent finding in this region.
Tenex™ is a minimally invasive system inspired by the phacoemulsification technology traditionally used in cataract surgery. It employs a low-amplitude, high-frequency (25–28 kHz) ultrasonic needle that induces tissue fragmentation within approximately 1 mm of the tip through a localized “jackhammer” effect [6]. Other types of surgery that could be performed include open surgery or minimally invasive surgery; however, these approaches would not allow us to minimize damage to the surrounding healthy connective tissue. In patients with heterotopic ossifications involving soft tissues such as tendons or fascia, Tenex™ may play a novel and clinically valuable role. This technique enables targeted removal of devitalized bone and diseased connective tissue while preserving the integrity of surrounding healthy structures. So, the aim of this case report was to assess the improvement in the pain and in the foot function after a percutaneous ultrasonic debridement.
2. Case Report
We report the case of an 82-year-old male patient. He has a medical history of high blood pressure and high uric acid levels, and he previously underwent a prostatectomy. The patient experienced plantar heel pain for two years, which became worse with weight-bearing activities. The pain was not linked with post-static dyskinesia and intensified when walking barefoot or on hard surfaces. He described the sensation as feeling like “walking on a stone.” For diagnostic purposes, plain radiographs and magnetic resonance imaging (MRI) were performed in order to establish a differential diagnosis and to confirm the presence of heterotopic ossification at the plantar fascia (Figure 1). The diagnosis of heterotopic ossification was established upon identifying a bone formation with visible trabeculae within the substance of the plantar fascia and a clear separation from the bony insertion, allowing differentiation from a calcaneal spur or enthesophyte.
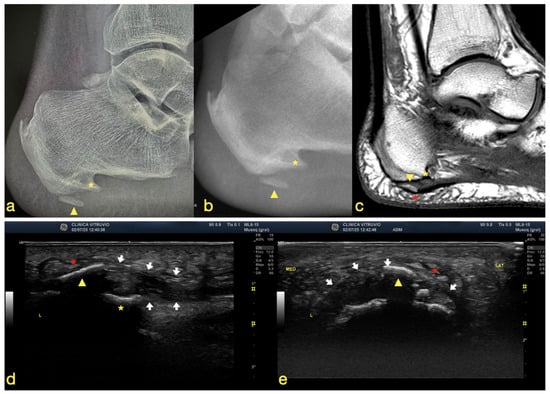
Figure 1.
(a) Plain radiograph, (b) fluoroscopic image, (c) MRI scan, (d) longitudinal (long-axis) ultrasound of the plantar fascia, and (e) transverse (short-axis) ultrasound of the plantar fascia. The yellow asterisk indicates the plantar enthesophyte (heel spur), the yellow arrowhead denotes the intrafascial heterotopic ossification, the white arrows outline the plantar fascia, and the red asterisk highlights the lesion involving the macrocameras of the plantar fat pad.
In addition, functional status and pain intensity were assessed using the Foot Function Index (FFI). Written informed consent was obtained from the patient for both the procedure and publication of this case report.
For the intervention, the patient was placed in the prone position, and anesthesia was performed using the ‘two-for-one’ block technique. This technique involves blocking both the tibial and sural nerves with a single injection, providing effective anesthesia to the foot [7]. Subsequently, with the patient in the supine position, the lower limb was externally rotated, and under conscious sedation, a percutaneous debridement using Tenex™ (Tenex Health Inc., Lake Forest, CA, USA) was carried out (Figure 2).
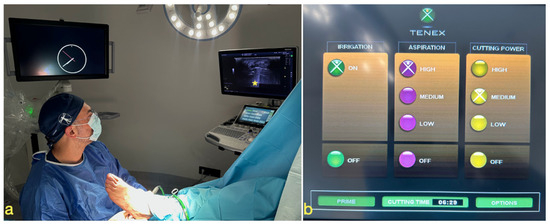
Figure 2.
(a) The patient is positioned supine with the lower limb in slight external rotation to allow for a medial approach. We introduced the Tenex device through a medial approach, with the probe aligned in the long axis, visualizing the plantar fascia in the short axis. The ultrasound machine is placed so that the operator can easily visualize both the puncture site and the monitor. On the ultrasound screen, a yellow asterisk marks the ultrasound artifact, confirming correct device functionality. (b) Display of the Tenex™ system, showing irrigation activated, aspiration set at high power to remove debris, and cutting power set at medium to prevent overheating. The total cutting time is also displayed on the screen and automatically recorded by the device.
The first step consisted of an ultrasound examination of the plantar fascia using a General Electric S7 XDclear system with a 15 MHz linear array transducer in the short axis in order to locate the HO within the central component of the plantar fascia at its insertional zone. Once identified, a medial calcaneal approach was established using a 27-gauge, 40 mm needle to determine the optimal trajectory. An MIS scalpel with a no. 64 blade was then used to perform a small incision guided by the needle, thereby minimizing trauma and potential errors in access.
Next, the Tenex™ system was introduced, connected to a 1000-cc saline irrigation bag and fitted with the TXB handpiece for bone removal. The microtip was positioned over the ossified area, and oscillatory movements were used to fragment and remove the lesion. In addition to ultrasound guidance, fluoroscopic control was employed to verify the correct removal of the ossified tissue. In accordance with the manufacturer’s recommendations, a “duty cycle”, which refers to the proportion of time the device is actively removing tissue, of up to 15 s ON and 45 s OFF (1:3 ratio) was employed, not exceeding a total working time of 10 min as registered by the device (Figure 2). The entire surgical technique can be viewed in the Supplementary Materials (Video S1).
Postoperatively, the patient was instructed to wear a postoperative shoe for the first 3 weeks and to follow a protocol of relative rest, progressively increasing weight-bearing and activity according to pain tolerance. Oral NSAIDs were prescribed for 5 days after the intervention.
3. Results
The Foot Function Index (FFI) showed a marked improvement following the procedure. Preoperatively, the patient scored 37 for pain, 35 for disability, and 4 for activity limitation, with a total score of 76 (33%). At one month postoperatively, the scores decreased to 10, 21, and 0, respectively, yielding a total of 31 (13%). This represented an overall relative improvement of approximately 59% in foot pain and function.
The total cutting time was 6 min and 29 s, during which complete removal of the ossified tissue was achieved (Figure 3). Within 48–72 h after the procedure, the patient reported marked relief of the characteristic sensation of “walking on a stone.” At the 1-month follow-up, he demonstrated a significant improvement in pain symptoms, with progressive recovery of function.
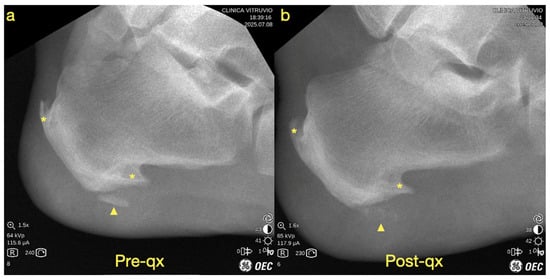
Figure 3.
Fluoroscopic images showing (a) the preoperative view and (b) the postoperative view. The yellow asterisk indicates the calcaneal spurs (enthesophytes), and the yellow arrowhead denotes the heterotopic ossification.
4. Discussion
Heterotopic ossification (HO) of the plantar fascia is a very rare occurrence. Only a few cases have been reported in the literature, and these are usually linked to diffuse idiopathic skeletal hyperostosis (DISH). On the other hand, calcification of the plantar fascia is fairly common. It has been well documented, especially in the context of chronic plantar fasciitis, and can be treated with ultrasound-guided percutaneous lavage [8]. To our knowledge, this is the first reported case in which Tenex™, a percutaneous ultrasonic tenotomy system, has been successfully used to remove HO within the plantar fascia.
Experimental data have provided important insights into the biological effects of ultrasonic debridement. In a collagenase-induced tendinopathy model of the murine Achilles tendon, this technique has demonstrated the ability to selectively remove pathological tendon tissue and normalize collagen profile ex vivo [9]. Similar findings have been confirmed in cadaveric studies of the patellar tendon, where ultrasonic debridement successfully removed diseased tissue while preserving adjacent healthy fibers [10]. Nevertheless, further research is needed to clarify the optimal treatment times and their structural effects. In bovine soleus tendon models, Palee and colleagues reported that longer treatment durations were associated with increased structural disruption, including paratenon separation and fibrous disorganization [11].
Most authors recommend that when using the TX1 microtip for tendinopathy, activation cycles of approximately 5 s are adequate, with a total treatment time between 30 s and 2 min [6,12,13], as seen in Table 1. In contrast, when employing the TXBone microtip, treatment duration is determined by the complete elimination of the ossified tissue. This introduces certain limitations, as the need to entirely remove heterotopic bone may risk collateral damage to adjacent connective tissue. The manufacturer advises not exceeding 10 min of total treatment time. Based on our experience, the lesion size is therefore a critical factor, and lesions larger than approximately 2 × 1 cm may represent a relative contraindication for this approach.

Table 1.
Summary of Tenex™ microtips, their main clinical indications, and recommended activation parameters. The table details the suggested ON/OFF duty cycles and the total treatment dose according to current literature and manufacturer guidelines. TX1 is primarily used for soft tissue tendinopathies, while TXBone (TXB) is designed for the removal of calcifications and heterotopic ossifications.
Despite these limitations, in selected cases such as ours, Tenex™ may represent an attractive alternative to conventional surgery. Standard surgical excision of HO in weight-bearing areas is typically performed through open approaches, which involve removal of both non-viable and viable tissue and are associated with higher complication rates, including painful and functionally limiting scars. By contrast, ultrasonic percutaneous debridement offers the potential for targeted removal of ossified tissue with minimal collateral injury.
An additional proposed mechanism of action of ultrasonic debridement is the ablation of nociceptive nerve endings, which are increased in patients with chronic tendinopathy [14]. This may contribute to symptomatic relief beyond simple removal of pathological tissue. In a clinical series of plantar fasciitis, Encinas and colleagues reported significant improvements in pain and function in 90% of patients treated with Tenex™, with follow-up exceeding two years [15]. Similar outcomes were observed by Rahul Razdan and colleagues, who demonstrated high patient satisfaction at 6 months in a cohort of 100 patients [16]. Although these results are promising, current evidence remains limited. Case series by Patrick J. Sanchez and colleagues have suggested that ultrasonic debridement may occasionally produce transverse lesions in addition to longitudinal ones [17]. Furthermore, there is insufficient data regarding the optimal treatment time for different pathologies and disease stages, the effect of excessive manipulation of the handpiece, and whether the approach should be longitudinal or transverse depending on the structure treated. It is therefore crucial that future research aim to better classify the types of lesions and define treatment durations in order to approach a more optimal therapeutic “dose”.
It is important to note that the follow-up period is short, and future studies should aim to report medium- and long-term outcomes.
5. Conclusions
This case highlights the successful use of Tenex™, a percutaneous ultrasonic tenotomy system, for the removal of heterotopic ossification of the plantar fascia, a condition rarely described in the literature. The procedure resulted in rapid pain relief and functional improvement while avoiding the morbidity associated with open surgical excision. Although this report suggests that ultrasonic percutaneous debridement may represent a safe and effective minimally invasive alternative for selected patients, further studies are needed to validate its long-term outcomes, establish standardized treatment protocols, and define lesion characteristics most suitable for this technique.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/std14040038/s1, Video S1: Surgical technique of ultrasound-guided percutaneous ultrasonic debridement for heterotopic ossification in a plantar fasciopathy.
Author Contributions
Conceptualization, A.F.-G., G.C.-N. and R.M.-S., methodology, A.F.-G., G.C.-N. and R.M.-S.; resources, F.G.; writing—original draft preparation, A.F.-G.; writing—review and editing, A.M.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author. The data supporting reported results can be found at: https://biomecanicavitruvio.com/.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shehab, D.; Elgazzar, A.H.; Collier, B.D. Heterotopic Ossification. J. Nucl. Med. 2002, 43, 346–353. [Google Scholar] [PubMed]
- Meyers, C.; Lisiecki, J.; Miller, S.; Levin, A.; Fayad, L.; Ding, C.; Sono, T.; McCarthy, E.; Levi, B.; James, A.W. Heterotopic Ossification: A Comprehensive Review. JBMR Plus 2019, 3, e10172. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, R.; Sawyer, M.A.M.; Rosier, R.N.; Puzas, J.E. Heterotopic Ossification: Clinical and Cellular Aspects. Calcif. Tissue Int. 1991, 49, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Vanden Bossche, L.; Vanderstraeten, G. Heterotopic ossification: A review. J. Rehabil. Med. 2005, 37, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Legosz, P.; Drela, K.; Pulik, L.; Sarzynska, S.; Maldyk, P. Challenges of heterotopic ossification-Molecular background and current treatment strategies. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.E. Ultrasonic Energy in Tendon Treatment. Oper. Tech. Orthop. 2013, 23, 78–83. [Google Scholar] [CrossRef]
- Fernandez-Gibello, A.; Camunas, G.; Yamak Altinpulluk, E.; Oliver-Fornies, P.; Fajardo Perez, M. A novel ankle compartment block: A “two-for-one” block. J. Clin. Anesth. 2022, 80, 110799. [Google Scholar] [CrossRef] [PubMed]
- Felice Galluccio, G.C.; Gibello, A.F.; Perez, M.F. Ultrasound-Guided Percutaneous Lavage in Calcific Plantar Fasciitis. Asia Pac. J. Pain 2023, 33, 39–40. [Google Scholar] [CrossRef]
- Kamineni, S.; Butterfield, T.; Sinai, A. Percutaneous ultrasonic debridement of tendinopathy—A pilot Achilles rabbit model. J. Orthop. Surg. Res. 2015, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Baria, M.R.; Vasileff, W.K.; Miller, M.; Borchers, J.; Miller, T.L.; Magnussen, R.A.; Durgam, S.S. Percutaneous ultrasonic tenotomy effectively debrides tendons of the extensor mechanism of the knee: A technical note. Knee 2020, 27, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Palee, S.; Lu, C.; Betcher, A.; Yener, U.; Alerte, J.; Howarth, D.; Cooper, L.; Hasanoglu, A.N.; Abd-Elsayed, A.; Wahez, S.E. Tendon modification with percutaneous Ultrasound-Guided Tenotomy using TENEX(R): A histological and macroscopic analysis of a bovine cadaveric model. Interv. Pain Med. 2025, 4, 100590. [Google Scholar] [CrossRef] [PubMed]
- Elattrache, N.S.; Morrey, B.F. Percutaneous Ultrasonic Tenotomy as a Treatment for Chronic Patellar Tendinopathy—Jumper’s Knee. Oper. Tech. Orthop. 2013, 23, 98–103. [Google Scholar] [CrossRef]
- Langer, P.R. Two emerging technologies for Achilles tendinopathy and plantar fasciopathy. Clin. Podiatr. Med. Surg. 2015, 32, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Palee, S.; Yener, U.; Abd-Elsayed, A.; Wahezi, S.E. Is Chronic Tendon Pain Caused by Neuropathy? Exciting Breakthroughs may Direct Potential Treatment. Curr. Pain Headache Rep. 2024, 28, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Encinas, R.; Gauthier, C.; Cantrell, A.C.; Zhang, Y.; Jackson, J.B.; Gross, C.E.; Gonzalez, T.; Scott, D.J. Minimally Invasive Ultrasound Guided Percutaneous Fasciotomy for the Treatment of Chronic Plantar Fasciitis. Foot Ankle Surg. 2024, 9, 2473011424S00366. [Google Scholar] [CrossRef]
- Razdan, E.; Vanderwoude, E. Percutaneous Ultrasonic Fasciotomy: A Novel Approach to Treat Chronic Plantar Fasciitis. J. Vasc. Interv. Radiol. 2015, 26, S40. [Google Scholar] [CrossRef]
- Sanchez, P.J.; Grady, J.F.; Saxena, A. Percutaneous Ultrasonic Tenotomy for Achilles Tendinopathy Is a Surgical Procedure with Similar Complications. J. Foot Ankle Surg. 2017, 56, 982–984. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).