“Pantaloon” Ureteroneocystostomy for Double Ureter Kidney Grafts: A Matched Single-Center Study of Perioperative and Long-Term Outcomes over 14 Years
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patient Selection
2.2. Data Collection
- Recipient variables: Age, sex, BMI, primary kidney disease (diabetic nephropathy, polycystic kidney disease, glomerulonephritis, IgA nephropathy, focal segmental glomerulosclerosis, congenital anomalies, unknown), dialysis vintage, previous transplants;
- Donor variables: Age, sex, donor type (living/deceased), cause of death (for deceased), serum creatinine, kidney anatomy (single/double ureter).
- Transplant variables: Transplant date, HLA mismatches, cold ischemia time (CIT), warm ischemia time, number of arteries/veins, operative time.
- Outcome variables: Delayed graft function (DGF); discharge creatinine; creatinine at 1, 3, 6, 12 months, and annually; ultrasound findings; last creatinine; date of last follow-up; graft loss; patient death.
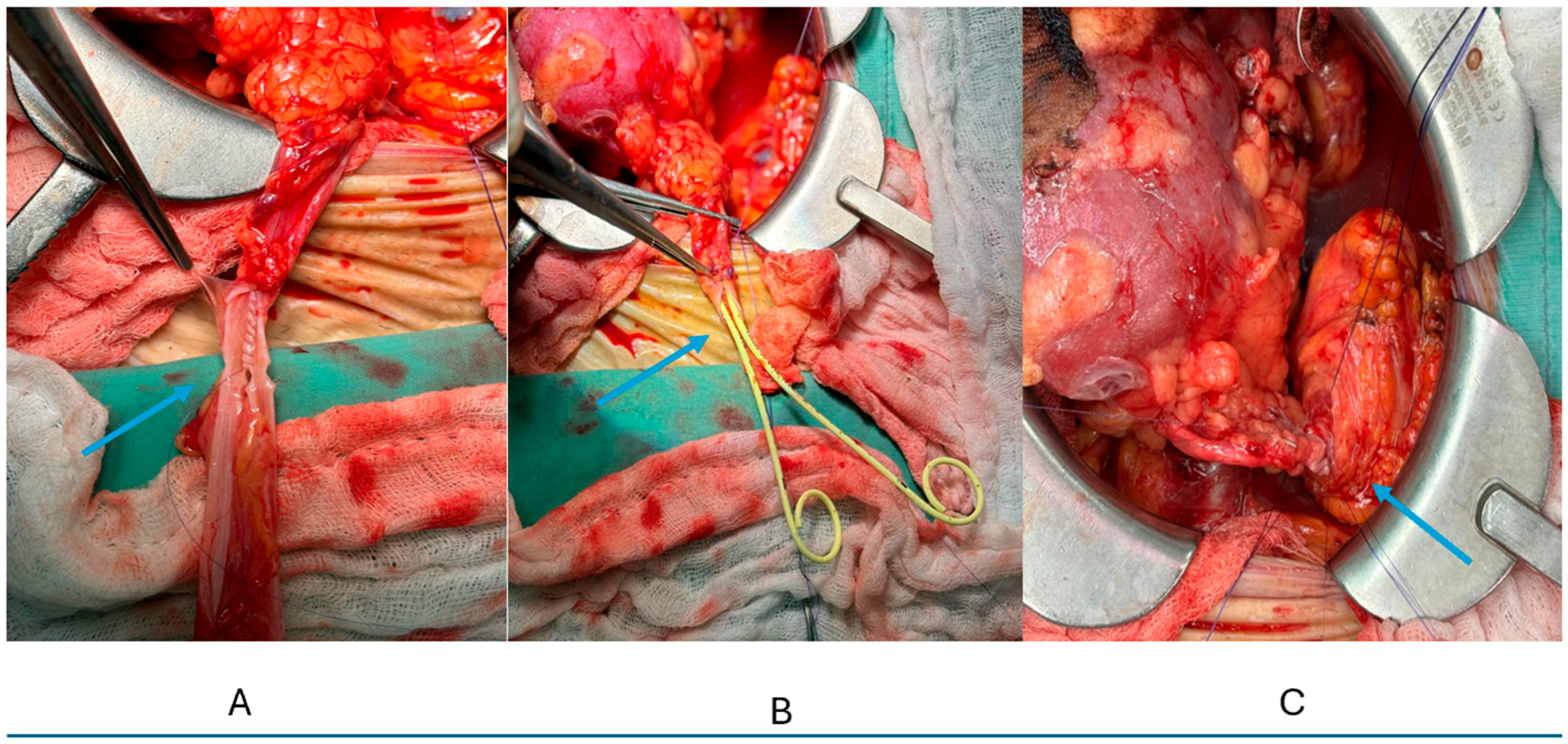
2.3. Surgical Technique (Figure 1)
- Vascular anastomosis: Gibson incision, retroperitoneal approach. Venous anastomosis: end-to-side to external iliac vein using continuous 5-0 Prolene. Arterial anastomosis: end-to-side to external iliac artery using continuous 6-0 Prolene. Multiple arteries were managed by ex–vivo reconstruction when feasible or separate anastomoses.
- Surgical technique:
- 2.1.
- Single ureter technique: Lich–Gregoir ureteroneocystostomy using running 6-0 PDS (PDS II® monofilament polydioxanone suture (Ethicon)), 2–3 cm submucosal tunnel, routine 6Fr double-J stent placement [12].
- 2.2.
- “Pantaloon” technique for double ureters:
- Ureters trimmed to equal length maintaining vascularity;
- Medial walls spatulated at 15 mm;
- Posterior walls approximated with running 6-0 PDS creating a common channel;
- Two 6Fr stents inserted separately;
- Single ureteroneocystostomy as above;
- Bladder closed in two layers (mucosa: 4-0 Vicryl continuous, detrusor: 3-0 Vicryl interrupted);
- Jackson–Pratt drain placed routinely, removed when output < 50 mL/day.
- 2.2.1
- Surgical technique—special consideration: The length of the ureter-to-ureter anastomosis was standardized at a minimum of 1.5 cm to ensure adequate luminal diameter. This length was adjusted based on patient body habitus and ureteral caliber, with larger anastomoses (up to 2.0 cm) created in patients with dilated ureters or larger body habitus to ensure optimal drainage.
- 2.3
- Postoperative stent management: Ureteral stents were routinely removed at 4–6 weeks postoperatively via cystoscopy under local anesthesia. No patients required early removal due to intractable symptoms, and all stents were successfully removed without complications.
- 2.4
- Postoperative stent management: For the management of stent-related symptoms, patients received (1) tamsulosin 0.4 mg daily for alpha blockade starting on postoperative day 1, (2) oxybutynin 5 mg twice daily as needed for bladder irritative symptoms, and (3) prophylactic trimethoprim–sulfamethoxazole 80/400 mg daily while stents remained in place. These medications were discontinued following stent removal unless clinically indicated.
2.4. Definitions and Outcome Assessment
- Urologic complications:
- Urinary leak: Drain creatinine/serum creatinine ratio > 2, confirmed by nuclear renography or CT urography;
- Ureteral stricture: Hydronephrosis with >50% ureteral narrowing requiring intervention;
- Complications graded using Clavien–Dindo classification: Grades I–II (conservative management), Grade III (procedural intervention), Grade IV (organ dysfunction), Grade V (death).
Imaging protocol: Ultrasound with Doppler on postoperative days 1 and 7, then at 1, 3, 6, 12 months, and annually. Additional imaging for rising creatinine (>20% from baseline) or hydronephrosis.
2.5. Immunosuppression
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Transplant Characteristics
3.3. Early Outcomes
3.4. Long-Term Outcomes
3.5. Urologic Complications
4. Discussion
Technical Considerations and Learning Curve
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CAKUT | congenital anomalies of kidney and urinary tract |
| CIT | cold ischemia time |
| CT | computed tomography |
| DGF | delayed graft function |
| eGFR | estimated glomerular filtration rate |
| HLA | human leukocyte antigen |
| IQR | interquartile range |
| IRB | institutional review board |
| PCKD | polycystic kidney disease |
| PDS | polydioxanone suture |
| PRA | panel reactive antibody |
| SD | standard deviation |
References
- Pisani, F.; Iaria, G.; D’Angelo, M.; Rascente, M.; Barletta, A.; Rizza, V.; Laurenzi, C.; Famulari, A. Urologic complications in kidney transplantation. Transpl. Proc. 2005, 37, 2521–2522. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Denis, T.; Gaba, F.; Inouye, B.; White, M.; Yamamoto, T.; Shahbazov, R. Risk Factors and Rates of Occurrence of Ureteral Stricture Formation Following Renal Transplantation: A Literature Review. Exp. Clin. Transplant. 2024, 22, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Neri, F.; Tsivian, M.; Coccolini, F.; Bertelli, R.; Cavallari, G.; Nardo, B.; Fuga, G.; Faenza, A. Urological complications after kidney transplantation: Experience of more than 1,000 transplantations. Transplant. Proc. 2009, 41, 1224–1226. [Google Scholar] [CrossRef] [PubMed]
- Choate, H.R.; Mihalko, L.A.; Choate, B.T. Urologic complications in renal transplants. Transl. Androl. Urol. 2019, 8, 141–147. [Google Scholar] [CrossRef]
- Di Carlo, H.N.; Darras, F.S. Urologic considerations and complications in kidney transplant recipients. Adv. Chronic Kidney Dis. 2015, 22, 306–311. [Google Scholar] [CrossRef]
- Minkovich, M.; Famure, O.; Li, Y.; Ghanekar, A.; Selzner, M.; Kim, S.J.; Lee, J.Y. Ureteral strictures post-kidney transplantation: Trends, impact on patient outcomes, and clinical management. Can. Urol. Assoc. J. 2021, 15, E524–E530. [Google Scholar] [CrossRef]
- Privett, J.T.; Jeans, W.D.; Roylance, J. The incidence and importance of renal duplication. Clin. Radiol. 1976, 27, 521–530. [Google Scholar] [CrossRef]
- Hopkins, L.M. Duplicated collecting system. Am. J. Obstet. Gynecol. 2021, 225, B12–B13. [Google Scholar] [CrossRef]
- Didier, R.A.; Chow, J.S.; Kwatra, N.S.; Retik, A.B.; Lebowitz, R.L. The duplicated collecting system of the urinary tract: Embryology, imaging appearances and clinical considerations. Pediatr Radiol. 2017, 47, 1526–1538. [Google Scholar] [CrossRef]
- Arumugam, S.; Subbiah, N.K.; Mariappan Senthiappan, A. Double Ureter: Incidence, Types, and Its Applied Significance—A Cadaveric Study. Cureus 2020, 12, e7760. [Google Scholar] [CrossRef]
- Cylke, R.; Karpeta, E.; Bieniasz, M.; Kosieradzki, M. Urologic Complications After Transplantation of Kidneys with Duplicated Ureter: A Retrospective Study. Transpl. Proc. 2019, 51, 779–782. [Google Scholar] [CrossRef]
- Barry, J.M. Surgical Techniques of Renal Transplantation. In Kidney Transplantation: Principles and Practice, 6th ed.; Morris, P.J., Knechtle, S.J., Eds.; Elsevier: Amsterdam, The Netherland, 2008; pp. 158–171. [Google Scholar]
- Thrasher, J.B.; Temple, D.R.; Spees, E.K. Extravesical versus Leadbetter-Politano ureteroneocystostomy: A comparison of urological complications in 320 renal transplants. J. Urol. 1990, 144, 1105–1109. [Google Scholar] [CrossRef]
- Apel, H.; Rother, U.; Wach, S.; Schiffer, M.; Kunath, F.; Wullich, B.; Heller, K. Transplant Ureteral Stenosis after Renal Transplantation: Risk Factor Analysis. Urol. Int. 2022, 106, 518–526. [Google Scholar] [CrossRef]
- Lescay, H.A.; Jiang, J.; Leslie, S.W.; Tuma, F. Anatomy, Abdomen and Pelvis Ureter. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Chait, A.; Matasar, K.W.; Fabian, C.E.; Mellins, H.Z. Vascular impressions on the ureters. Am. J. Roentgenol. 1971, 111, 729–749. [Google Scholar] [CrossRef] [PubMed]
- Fernbach, S.K.; Feinstein, K.A.; Spencer, K.; Lindstrom, C.A. Ureteral duplication and its complications. Radiographics 1997, 17, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Haferkamp, A.; Dörsam, J.; Möhring, K.; Wiesel, M.; Staehler, G. Ureteral complications in renal transplantation with more than one donor ureter. Nephrol. Dial. Transplant. 1999, 14, 1521–1524. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alberts, V.P.; Minnee, R.C.; van Donselaar-van der Pant, K.A.; Bemelman, F.J.; Zondervan, P.J.; Laguna Pes, M.P.; Idu, M.M. Duplicated ureters and renal transplantation: A case-control study and review of the literature. Transpl. Proc. 2013, 45, 3239–3244. [Google Scholar] [CrossRef]
- Sulikowski, T.; Zietek, Z.; Ostrowski, M.; Kamiński, M.; Sieńko, J.; Romanowski, M.; Majewski, W.; Ostrowska-Clarck, K.; Domański, L.; Róźański, J.; et al. Experiences in kidney transplantation with duplicated ureters. Transpl. Proc. 2005, 37, 2096–2099. [Google Scholar] [CrossRef]
- Heidari, M.; Gharaati, M.R.; Iran-Pour, E.; Simforoosh, N.; Zare, S.; Basiri, A. Transplantation of kidneys with duplicated ureters. Scand. J. Urol. Nephrol. 2010, 44, 337–340. [Google Scholar] [CrossRef]
- Uchida, J.; Naganuma, T.; Machida, Y.; Kitamoto, K.; Yamazaki, T.; Iwai, T.; Nakatani, T. Modified extravesical ureteroneocystostomy for completely duplicated ureters in renal transplantation. Urol. Int. 2006, 77, 104–106. [Google Scholar] [CrossRef]
- Perico, N.; Cattaneo, D.; Sayegh, M.H.; Remuzzi, G. Delayed graft function in kidney transplantation. Lancet 2004, 364, 1814–1827. [Google Scholar] [CrossRef]
- ANZDATA Registry. 46th Report, Chapter 7: Kidney Transplantation; Australia and New Zealand Dialysis and Transplant Registry: Adelaide, Australia, 2023; Available online: https://www.anzdata.org.au (accessed on 30 January 2023).
- Loupy, A.; Haas, M.; Roufosse, C.; Naesens, M.; Adam, B.; Afrouzian, M.; Akalin, E.; Alachkar, N.; Bagnasco, S.; Becker, J.U.; et al. The Banff 2019 Kidney Meeting Report (I): Updates on and clarification of criteria for T cell-and antibody-mediated rejection. Am. J. Transpl. 2020, 20, 2318–2331. [Google Scholar] [CrossRef]
- Tennak, V.; Nesher, E.; Eizner, S.; Gurevich, M.; Mehjibovsky, V.; Mor, E. Single Anastomosis of Double Ureter Kidneys—Experience in 12 Kidney Transplant Recipients. Transplantation 2018, 102, S542. [Google Scholar] [CrossRef]
- Riedmiller, H.; Gerharz, E.W.; Köhl, U.; Weingärtner, K. Continent urinary diversion in preparation for renal transplantation. Transplantation 2000, 70, 1713–1717. [Google Scholar] [CrossRef]
- Ribeiro, L.; Olsburgh, J.; Sabharwal, T. Long Transplant Ureteric Stents: Better in IR! Cardiovasc. Interv. Radiol. 2025, 48, 1218–1219. [Google Scholar] [CrossRef] [PubMed]
- Memarsadeghi, M.; Heinz-Peer, G.; Helbich, T.H.; Schaefer-Prokop, C.; Kramer, G.; Scharitzer, M.; Prokop, M. Unenhanced multi-detector row CT in patients suspected of having urinary stone disease: Effect of section width on diagnosis. Radiology 2005, 235, 530–536. [Google Scholar] [CrossRef] [PubMed]
- El-Mekresh, M.; Osman, Y.; Ali-El-Dein, B.; El-Diasty, T.; Ghoneim, M.A. Urological complications after living-donor renal transplantation. BJU Int. 2001, 87, 295–306. [Google Scholar] [CrossRef]
- Patel, P.; Rebollo-Mesa, I.; Ryan, E.; Sinha, M.D.; Marks, S.D.; Banga, N.; Macdougall, I.C.; Webb, M.C.; Koffman, G.; Olsburgh, J. Prophylactic ureteric stents in renal transplant recipients: A multicenter randomized controlled trial of early versus late removal. Am. J. Transpl. 2017, 17, 2129–2138. [Google Scholar] [CrossRef]
- Tavakoli, A.; Surange, R.S.; Pearson, R.C.; Parrott, N.R.; Augustine, T.; Riad, H.N. Impact of stents on urological complications and health care expenditure in renal transplant recipients: Results of a prospective, randomized clinical trial. J. Urol. 2007, 177, 2260–2264. [Google Scholar] [CrossRef]
- Visser, I.J.; van der Staaij, J.P.T.; Muthusamy, A.; Willicombe, M.; Lafranca, J.A.; Dor, F.J.M.F. Timing of ureteric stent removal and occurrence of urological complications after kidney transplantation: A systematic review and meta-analysis. J. Clin. Med. 2019, 8, 689. [Google Scholar] [CrossRef]
- Thompson, E.R.; Hosgood, S.A.; Nicholson, M.L.; Wilson, C.H. Early versus late ureteric stent removal after kidney transplantation. Cochrane Database Syst. Rev. 2023, 6, CD011455. [Google Scholar] [CrossRef]
- Vogel, T.; Utech, M.; Schmidt, F.; Keplin, W.H.; Diller, R.; Brockmann, J.; Wolters, H. Double-J versus external ureteral stents in kidney transplantation: A retrospective analysis. Nephro. Urol. Mon. 2015, 7, e27820. [Google Scholar] [CrossRef]
- Oudmaijer, C.A.; Muller, K.; van Straalen, E.; Minnee, R.C.; Kimenai, D.J.; Reinders, M.E.; van de Wetering, J.; IJzermans, J.N.; Terkivatan, T. Long-term Double-J stenting is superior to short-term Single-J stenting in renal transplantation: A randomized controlled trial. PLoS ONE 2025, 20, e0317991. [Google Scholar] [CrossRef]
| Characteristic | Double Ureter (n = 26) | Single Ureter (n = 26) | p-Value |
|---|---|---|---|
| Age, years, median (IQR) | 51 (38–60) | 52 (40–61) | 0.824 |
| Male sex, n (%) | 18 (69.2) | 17 (65.4) | 0.768 |
| BMI, kg/m, median (IQR) | 25.5 (23.8–28.3) | 26.1 (24.2–28.8) | 0.642 |
| Dialysis time, years, median (IQR) | 3.5 (2.0–5.0) | 3.2 (1.8–4.8) | 0.713 |
| Preemptive transplant, n (%) | 2 (7.7) | 3 (11.5) | 0.640 |
| Primary kidney disease, n (%) | 0.892 | ||
| - Diabetes mellitus | 9 (34.6) | 8 (30.8) | |
| - PCKD | 3 (11.5) | 4 (15.4) | |
| - Unknown | 6 (23.1) | 7 (26.9) | |
| - Glomerular disease | 3 (11.5) | 4 (15.4) | |
| - CAKUT | 3 (11.5) | 2 (7.7) | |
| - Other | 2 (7.7) | 1 (3.8) |
| Characteristic | Double Ureter (n = 26) | Single Ureter (n = 26) | p-Value |
|---|---|---|---|
| Donor type, n (%) | 1.000 | ||
| - Living donor | 13 (50.0) | 13 (50.0) | |
| - Deceased donor | 13 (50.0) | 13 (50.0) | |
| Donor age, years, median (IQR) | |||
| - Living donors | 42 (35–50) | 43 (36–51) | 0.812 |
| - Deceased donors | 54 (45–62) | 55 (46–63) | 0.756 |
| HLA mismatches, median (IQR) | |||
| - Living donors | 3 (2–4) | 3 (2–4) | 1.000 |
| - Deceased donors | 5 (4–6) | 5 (4–6) | 1.000 |
| Cold ischemia time, hours | |||
| - Living donors (by definition) | 1 (1–1) | 1 (1–1) | 1.000 |
| - Deceased donors | 10 (8–12) | 11 (9–13) | 0.521 |
| Multiple arteries, n (%) | 8 (30.8) | 7 (26.9) | 0.761 |
| Induction therapy, n (%) | |||
| - Basiliximab (Simulect®) | 20 (76.9) | 19 (73.1) | 0.751 |
| - Thymoglobulin® (Sanofi) | 6 (23.1) | 7 (26.9) | 0.751 |
| Outcome | Double Ureter (n = 26) | Single Ureter (n = 26) | p-Value |
|---|---|---|---|
| Delayed graft function, n (%) | 3 (11.5) | 4 (15.4) | 1.000 |
| - Living donor recipients | 0/13 (0) | 0/13 (0) | 1.000 |
| - Deceased donor recipients | 3/13 (23.1) | 4/13 (30.8) | 1.000 |
| Acute rejection < 90 days, n (%) | 2 (7.7) | 3 (11.5) | 1.000 |
| Discharge creatinine, mg/dL | |||
| - All patients, median (IQR) | 1.26 (0.91–1.82) | 1.31 (0.95–1.89) | 0.724 |
| - Living donors | 1.12 (0.85–1.45) | 1.15 (0.88–1.48) | 0.812 |
| - Deceased donors | 1.68 (1.17–2.80) | 1.72 (1.20–2.85) | 0.867 |
| Urologic complications, n (%) | 1 (3.8) | 2 (7.7) | 1.000 |
| - Urinary leak | 1 (3.8) | 1 (3.8) | |
| - Ureteral stricture < 90 days | 0 (0) | 1 (3.8) | |
| - Vesicoureteral reflux | 0 (0) | 0 (0) | |
| Clavien–Dindo Grade ≥ III, n (%) | 1 (3.8) | 2 (7.7) | 1.000 |
| Outcome | Double Ureter (n = 26) | Single Ureter (n = 26) | p-Value |
|---|---|---|---|
| Follow-up time, months | |||
| - Median (IQR) | 63 (36–96) | 60 (36–92) | 0.812 |
| - Range | 3–168 | 6–164 | |
| Graft function at last F/U | |||
| Last creatinine, mg/dL6 | |||
| - All patients, median (IQR) | 1.25 (1.02–1.72) | 1.28 (1.05–1.68) | 0.891 |
| - Living donor recipients | 1.10 (0.94–1.34) | 1.12 (0.96–1.36) | 0.834 |
| - Deceased donor recipients | 1.45 (1.15–1.94) | 1.48 (1.18–1.92) | 0.912 |
| eGFR, mL/min/1.73 m | |||
| - All patients, median (IQR) | 58 (42–72) | 56 (44–70) | 0.765 |
| Late ureteral stricture, n (%) | 0 (0) | 1 (3.8) | 1.000 |
| Survival outcomes | |||
| 1-year graft survival, % | 100 | 96.2 | 1.000 |
| 5-year graft survival, % | 96.0 | 92.3 | 1.000 |
| - Living donors | 100 | 100 | 1.000 |
| - Deceased donors | 92.3 | 84.6 | 1.000 |
| 1-year patient survival, % | 96.2 | 96.2 | 1.000 |
| 5-year patient survival, % | 80.8 | 84.6 | 0.723 |
| Graft loss, n (%) | 1 (3.8) | 2 (7.7) | 1.000 |
| - Living donor recipients | 0/13 (0) | 0/13 (0) | 1.000 |
| - Deceased donor recipients | 1/13 (7.7) | 2/13 (15.4) | 1.000 |
| Patient death, n (%) | 5 (19.2) | 4 (15.4) | 1.000 |
| - With functioning graft | 5 (19.2) | 3 (11.5) | 0.704 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gravetz, A.; Tennak, V.; Mezhybovsky, V.; Gurevich, M.; Eisner, S.; Bielopolski, D.; Kanani, F.; Nesher, E. “Pantaloon” Ureteroneocystostomy for Double Ureter Kidney Grafts: A Matched Single-Center Study of Perioperative and Long-Term Outcomes over 14 Years. Surg. Tech. Dev. 2025, 14, 31. https://doi.org/10.3390/std14030031
Gravetz A, Tennak V, Mezhybovsky V, Gurevich M, Eisner S, Bielopolski D, Kanani F, Nesher E. “Pantaloon” Ureteroneocystostomy for Double Ureter Kidney Grafts: A Matched Single-Center Study of Perioperative and Long-Term Outcomes over 14 Years. Surgical Techniques Development. 2025; 14(3):31. https://doi.org/10.3390/std14030031
Chicago/Turabian StyleGravetz, Aviad, Vladimir Tennak, Vadym Mezhybovsky, Michael Gurevich, Sigal Eisner, Dana Bielopolski, Fahim Kanani, and Eviatar Nesher. 2025. "“Pantaloon” Ureteroneocystostomy for Double Ureter Kidney Grafts: A Matched Single-Center Study of Perioperative and Long-Term Outcomes over 14 Years" Surgical Techniques Development 14, no. 3: 31. https://doi.org/10.3390/std14030031
APA StyleGravetz, A., Tennak, V., Mezhybovsky, V., Gurevich, M., Eisner, S., Bielopolski, D., Kanani, F., & Nesher, E. (2025). “Pantaloon” Ureteroneocystostomy for Double Ureter Kidney Grafts: A Matched Single-Center Study of Perioperative and Long-Term Outcomes over 14 Years. Surgical Techniques Development, 14(3), 31. https://doi.org/10.3390/std14030031







