Retrospective Validation Study of a Treatment Strategy for Benign Bone Lesions in the Proximal Femur
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Patients
2.3. Surgical Strategy and Fixation Device Selection
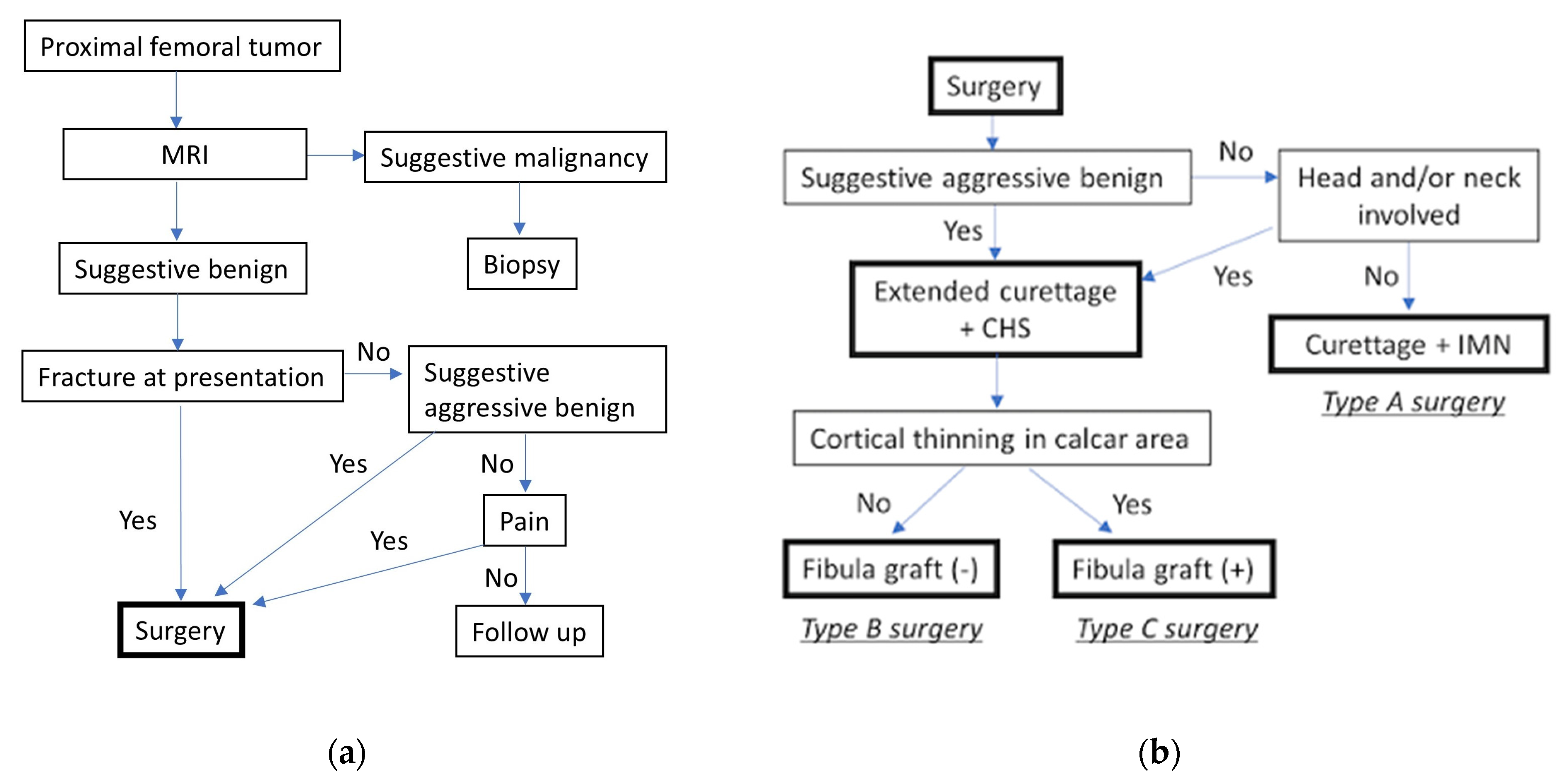

2.4. Surgical Procedure
2.5. Postoperative Rehabilitation and Weight-Bearing Protocol
2.6. Monitoring for Tumor Recurrence
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics and Treatment Distribution
3.2. Operative Outcomes
3.3. Impact of Fibula Grafting on Surgical Outcomes
3.4. Functional Recovery and Complications
3.5. Summary of Key Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CHS | Compression hip screw |
| IMN | Intramedullary nail |
| GCT | Giant cell tumor |
| ABC | Aneurysmal bone cyst |
| FD | Fibrous dysplasia |
| SBC | Simple bone cyst |
| PWB | Partial weight bearing |
| FWB | Full weight bearing |
References
- Shi, J.; Zhao, Z.; Yan, T.; Guo, W.; Yang, R.; Tang, X.; Qu, H.; Dong, S. Surgical treatment of benign osteolytic lesions in the femoral head and neck: A systematic review. BMC Musculoskelet. Disord. 2021, 22, 549. [Google Scholar] [CrossRef]
- George, B.; Abudu, A.; Grimer, R.J.; Carter, S.R.; Tillman, R.M. The treatment of benign lesions of the proximal femur with non-vascularised autologous fibular strut grafts. J. Bone Jt. Surg. Br. 2008, 90, 648–651. [Google Scholar] [CrossRef]
- Maes, D.J.A.; Kaneuchi, Y.; Abudu, A.; Stevenson, J.D. Biological reconstruction of the proximal femur after treatment of benign lesions: Comparison of functional and oncological outcomes for children and adults. Eur. J. Orthop. Surg. Traumatol. 2022, 32, 559–566. [Google Scholar] [CrossRef]
- Jaffe, K.A.; Launer, E.P.; Scholl, B.M. Use of a fibular allograft strut in the treatment of benign lesions of the proximal femur. Am. J. Orthop. 2002, 31, 575–578. [Google Scholar]
- Nakamura, T.; Matsumine, A.; Asanuma, K.; Matsubara, T.; Sudo, A. Treatment of the benign bone tumors including femoral neck lesion using compression hip screw and synthetic bone graft. SICOT J. 2015, 1, 15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.; Li, J.Z.; Lu, X.C.; Zhang, Y.; Zhang, H.S.; Shi, H.L.; Lei, Z.; Feng, G.; Fu, W.P. Intramedullary Nailing Combined with Bone Grafting for Benign Lesions of the Proximal Femur. Orthop. Surg. 2017, 9, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Kushare, I.V.; Colo, D.; Bakhshi, H.; Dormans, J.P. Fibrous dysplasia of the proximal femur: Surgical management options and outcomes. J. Child. Orthop. 2014, 8, 505–511. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Soft Tissue and Bone Tumours; World Health Organization: Geneva, Switzerland, 2020; Volume 3.
- Yenigul, A.E.; Sofulu, O.; Erol, B. Treatment of locally aggressive benign bone tumors by means of extended intralesional curettage without chemical adjuvants. SAGE Open Med. 2022, 10, 20503121221094199. [Google Scholar] [CrossRef]
- Rahman, M.A.; El Masry, A.M.; Azmy, S.I. Review of 16 cases of aneurysmal bone cyst in the proximal femur treated by extended curettage and cryosurgery with reconstruction using autogenous nonvascularized fibula graft. J. Orthop. Surg. 2018, 26, 2309499018783905. [Google Scholar] [CrossRef]
- Errani, C.; Ruggieri, P.; Asenzio, M.A.; Toscano, A.; Colangeli, S.; Rimondi, E.; Rossi, G.; Longhi, A.; Mercuri, M. Giant cell tumor of the extremity: A review of 349 cases from a single institution. Cancer Treat. Rev. 2010, 36, 1–7. [Google Scholar] [CrossRef]
- Wijsbek, A.E.; Vazquez-Garcia, B.L.; Grimer, R.J.; Carter, S.R.; Abudu, A.A.; Tillman, R.M.; Jeys, L. Giant cell tumour of the proximal femur: Is joint-sparing management ever successful? Bone Jt. J. 2014, 96, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Stanton, R.P.; Ippolito, E.; Springfield, D.; Lindaman, L.; Wientroub, S.; Leet, A. The surgical management of fibrous dysplasia of bone. Orphanet J. Rare Dis. 2012, 7 (Suppl. S1), S1. [Google Scholar] [CrossRef] [PubMed]
- Roposch, A.; Saraph, V.; Linhart, W.E. Treatment of femoral neck and trochanteric simple bone cysts. Arch. Orthop. Trauma Surg. 2004, 124, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Erol, B.; Topkar, M.O.; Aydemir, A.N.; Okay, E.; Caliskan, E.; Sofulu, O. A treatment strategy for proximal femoral benign bone lesions in children and recommended surgical procedures: Retrospective analysis of 62 patients. Arch. Orthop. Trauma Surg. 2016, 136, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Panchwagh, Y.; Joshi, S.K.; Sancheti, P.K. Benign Aggressive Lesions of Femoral Head and Neck: Is Salvage Possible? Indian J. Orthop. 2018, 52, 51–57. [Google Scholar] [CrossRef]
- Günther, K.P.; Hartmann, A.; Aikele, P.; Aust, D.; Ziegler, J. Large femoral-neck cysts in association with femoroacetabular impingement: A report of three cases. J. Bone Jt. Surg. 2007, 89, 863–870. [Google Scholar] [CrossRef]
- Friedl, W.; Clausen, J. Experimental examination for optimized stabilisation of trochanteric femur fractures, intra- or extramedullary implant localisation and influence of femur neck component profile on cut-out risk. Chirurg 2001, 72, 1344–1352. [Google Scholar] [CrossRef]
- Grønhaug, K.M.L.; Dybvik, E.; Matre, K.; Östman, B.; Gjertsen, J.E. Intramedullary nail versus sliding hip screw for stable and unstable trochanteric and subtrochanteric fractures: 17,341 patients from the Norwegian Hip Fracture Register. Bone Jt. J. 2022, 104, 274–282. [Google Scholar] [CrossRef]
- Zang, W.; Liu, P.F.; Han, X.F. A comparative study of proximal femoral locking compress plate, proximal femoral nail antirotation and dynamic hip screw in intertrochanteric fractures. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 119–123. [Google Scholar] [CrossRef]
- Abram, S.G.; Pollard, T.C.; Andrade, A.J. Inadequate ‘three-point’ proximal fixation predicts failure of the Gamma nail. Bone Jt. J. 2013, 95, 825–830. [Google Scholar] [CrossRef]
- Hu, Y.C.; Lun, D.X.; Zhao, S.K. Combined anterior and lateral approaches for bone tumors of the femoral neck and head. Orthopedics 2012, 35, e628–e634. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Bai, X.; Chen, W.; Li, Y.; Zhao, L.; Liu, C.; Liu, Z.; Wang, B. Clinical and functional comparison of dynamic hip screws and intramedullary nails for treating proximal femur metastases in older individuals. Chin. J. Cancer Res. 2020, 32, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Zekry, K.M.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Alkhooly, A.; Abd-Elfattah, A.S.; Fouly, E.H.; Ahmed, A.R.; Tsuchiya, H. Treatment of the benign lytic lesions of the proximal femur with synthetic bone graft. J. Orthop. Surg. Res. 2018, 13, 270. [Google Scholar] [CrossRef] [PubMed]
- Matsumine, A.; Myoui, A.; Kusuzaki, K.; Araki, N.; Seto, M.; Yoshikawa, H.; Uchida, A. Calcium hydroxyapatite ceramic implants in bone tumour surgery: A long-term follow-up study. J. Bone Jt. Surg. Br. 2004, 86, 719–725. [Google Scholar] [CrossRef]
| Case | Age | Gender | Diagnosis | Location | Surgery Type | |||
|---|---|---|---|---|---|---|---|---|
| Head | Neck | Trochanter | Sub- Trochanter | |||||
| 1 | 19 | M | FD | + | + | A | ||
| 2 | 24 | F | FD | + | A | |||
| 3 | 56 | F | SBC | + | A | |||
| 4 | 31 | F | SBC | + | A | |||
| 5 | 20 | F | SBC | + | + | A | ||
| 6 | 16 | M | Chondroblastoma | + | B | |||
| 7 | 36 | M | Chondroblastoma | + | + | B | ||
| 8 | 62 | F | GCT | + | + | B | ||
| 9 | 57 | F | SBC | + | + | B | ||
| 10 | 64 | M | SBC | + | + | B | ||
| 11 | 37 | F | SBC | + | + | B | ||
| 12 | 44 | M | GCT | + | + | C | ||
| 13 | 22 | M | GCT | + | + | + | C | |
| 14 | 23 | F | FD | + | + | + | + | C |
| 15 | 42 | F | FD | + | C | |||
| 16 | 31 | F | FD | + | + | + | C | |
| Internal Fixation Device | p-Value | ||
|---|---|---|---|
| IMN (N = 5) | CHS (N = 11) | ||
| Time to PWB (day) | 31 (2–42.5) | 14 (7–30) | 0.78 |
| Time to FWB (day) | 37 (16–68.5) | 30 (28–56) | 1.00 |
| Bleeding (g) | 168 (90–327.5) | 300 (200–1205) | 0.11 |
| Operative time (min) | 110 (95–140.5) | 224 (174–302) | <0.01 |
| Internal Fixation Method | p-Value | ||
|---|---|---|---|
| IMN (Type A, N = 5) | CHS Without Fibula Graft (Type B, N = 6) | ||
| Bleeding (g) | 168 (90–327.5) | 230 (146.75–620) | 0.30 |
| Operative time (min) | 110 (95–140.5) | 178.5 (155.5–299) | <0.05 |
| Authors (Year) | N | Internal Fixation | Type of Graft | Time to Full Wight Bearing (Weeks) | Postoperative Fracture | Tumor Recurrence | Other Complications |
|---|---|---|---|---|---|---|---|
| George et al. 2008 [2] | 17 | No | Autologous fibula | 13.5 | None | 2 (11.7%) | |
| Maes et al. 2021 [3] | 54 | No | Autologous fibula | 12 | 5 (10%) | 5 (9%) | Peroneal nerve palsy (15%) |
| Jaffe et al. 2002 [4] | 15 | CHS | Autologous fibula | 6 | None | None | |
| Nakamura et al. 2015 [5] | 13 | CHS | Synthetic bone | 3 | None | 1 (7.7%) | |
| Zhang et al. 2017 [6] | 32 | No | Allogeneic bone | N. S. | 1 (3.1%) | 2 (6.3%) | |
| 36 | IMN | Allogeneic bone | N. S. | None | 1 (2.8%) | ||
| Erol et al. 2016 [15] | 8 | No | Allogeneic or autologous bone | 6 (hip spica cast for 6 weeks) | None | None | Superficial wound infection (n = 1), skin necrosis (n = 1) |
| 54 | CHS or elastic nail | Allogeneic or autologous bone | |||||
| Our study | 16 | CHS or IMN | Synthetic bone with or without autologous fibula | 5 | None | 2 (12.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinohara, N.; Nagano, S.; Sasaki, H.; Taniguchi, N. Retrospective Validation Study of a Treatment Strategy for Benign Bone Lesions in the Proximal Femur. Surg. Tech. Dev. 2025, 14, 29. https://doi.org/10.3390/std14030029
Shinohara N, Nagano S, Sasaki H, Taniguchi N. Retrospective Validation Study of a Treatment Strategy for Benign Bone Lesions in the Proximal Femur. Surgical Techniques Development. 2025; 14(3):29. https://doi.org/10.3390/std14030029
Chicago/Turabian StyleShinohara, Naohiro, Satoshi Nagano, Hiromi Sasaki, and Noboru Taniguchi. 2025. "Retrospective Validation Study of a Treatment Strategy for Benign Bone Lesions in the Proximal Femur" Surgical Techniques Development 14, no. 3: 29. https://doi.org/10.3390/std14030029
APA StyleShinohara, N., Nagano, S., Sasaki, H., & Taniguchi, N. (2025). Retrospective Validation Study of a Treatment Strategy for Benign Bone Lesions in the Proximal Femur. Surgical Techniques Development, 14(3), 29. https://doi.org/10.3390/std14030029







