The Orthopedic Strategy for Patients with Larsen Syndrome
Abstract
1. Introduction
2. Materials and Methods
3. Results
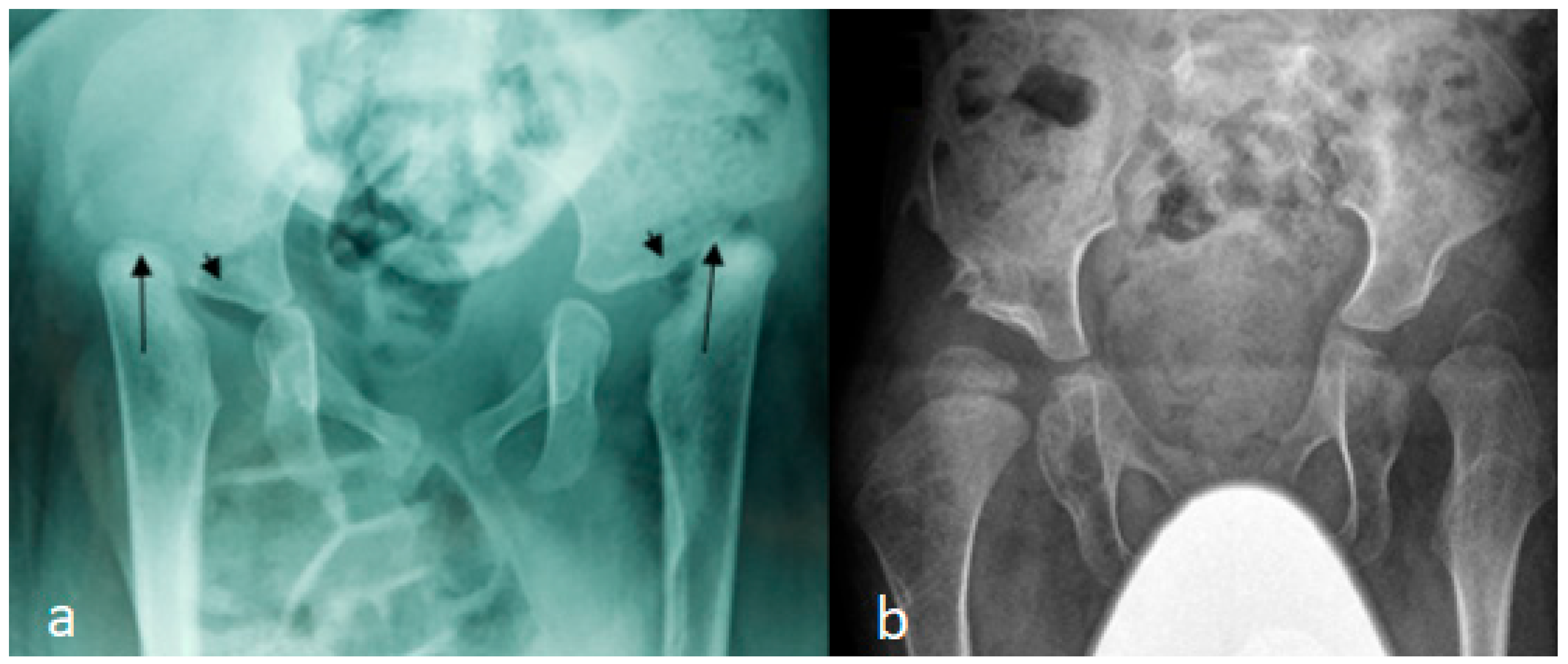
3.1. The Classical Clinical and Radiological Phenotype in Children with Larsen Syndrome
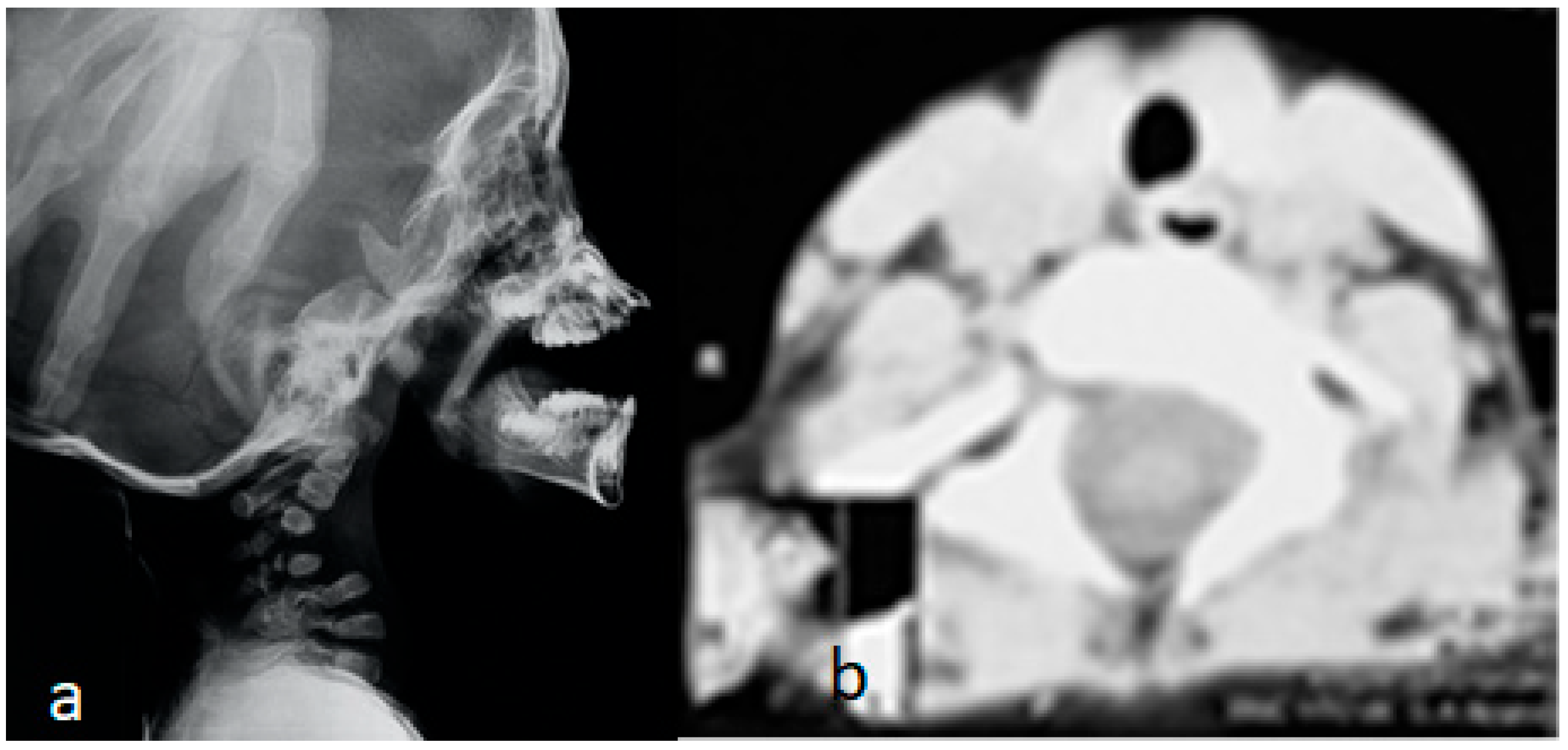
3.2. Congenital Tetraplegia in a Child with Larsen Syndrome
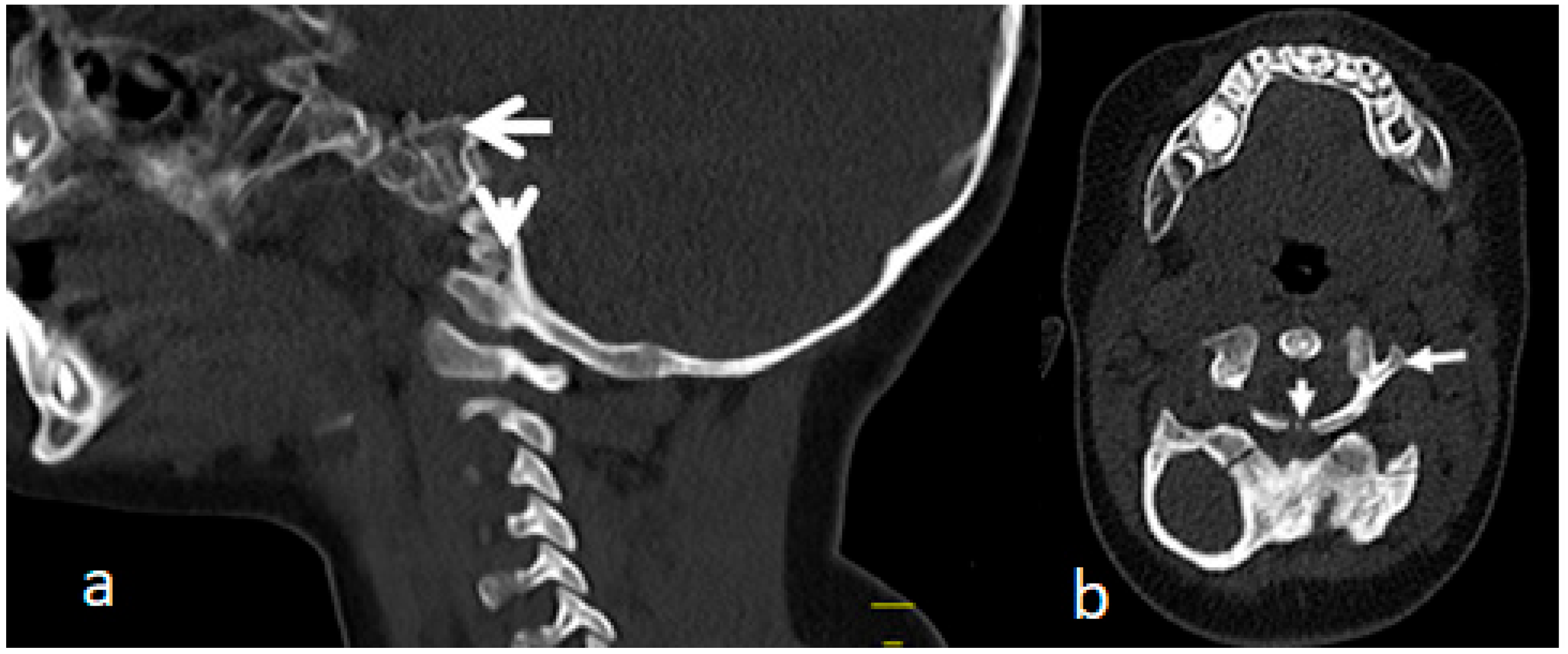
3.3. Acute-Angle Cervico-Thoracic Kyphosis in a Child with Larsen Syndrome
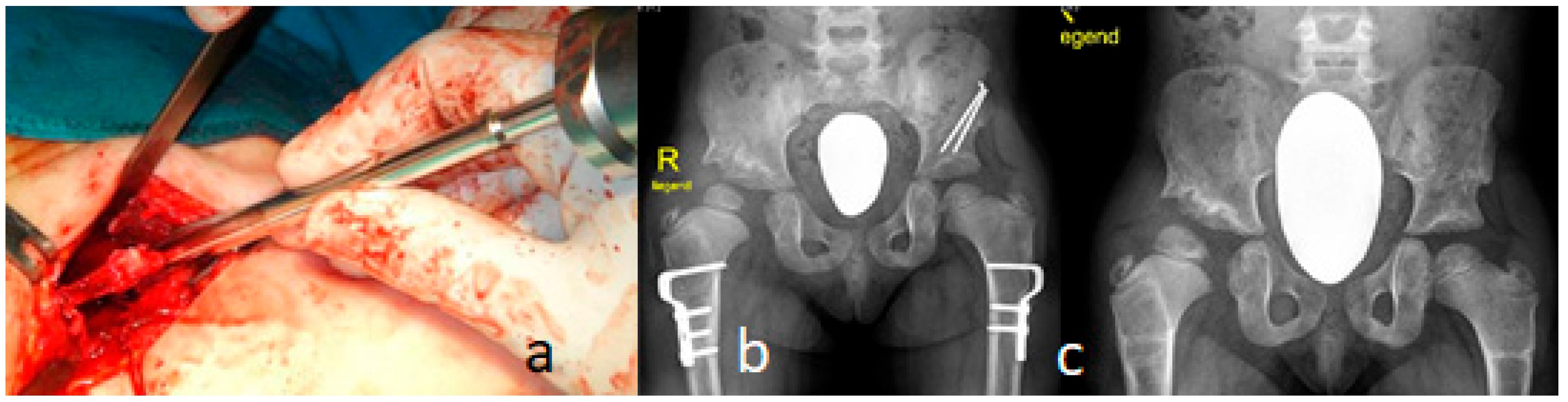
3.4. Surgical Interventions to Correct Hip/Knee Dislocations and Talipes Equinovarus
3.5. Surgical Intervention to Correct Traumatic Tertaplagia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larsen, L.J.; Schottstaedt, E.R.; Bost, F.C. Multiple congenital dislocations associated with characteristic facial abnormality. J. Pediatr. 1950, 37, 574–581. [Google Scholar] [PubMed]
- Klenn, P.J.; Iozzo, R.V. Larsen’s syndrome with novel congenital anomalies. Hum. Pathol. 1991, 22, 1055–1057. [Google Scholar] [PubMed]
- Latta, R.J.; Graham, C.B.; Aase, J.; Scham, S.M.; Smith, D.W. Larsen’s syndrome: A skeletal dysplasia with multiple joint dislocations and unusual facies. J. Pediatr. 1971, 78, 291–298. [Google Scholar] [PubMed]
- Kozlowski, K.; Robertson, F.; Middleton, R. Radiographic findings in Larsen’s syndrome. Australas. Radiol. 1974, 18, 336–344. [Google Scholar]
- De Smet, L.; Legius, E.; Fabry, G.; Fryns, J.P. The Larsen syndrome. The diagnostic contribution of the analysis of the metacarpophalangeal pattern profile. Genet. Couns. 1993, 4, 157–164. [Google Scholar]
- Maack, R.W.; Muntz, H.R. Ossicular abnormality in Larsen’s syndrome: A case report. Am. J. Otolaryngol. 1991, 12, 51–53. [Google Scholar]
- Kaga, K.; Suzuki, J.I.; Kimizuka, M. Temporal bone pathology of two infants with Larsen’s syndrome. Int. J. Pediatr. Otorhinolaryngol. 1991, 22, 257. [Google Scholar]
- Henriksson, P.; Ivarsson, S.; Theander, G. Larsen syndrome and glial profileration in the brain. Acta Paediatr. Scand. 1977, 66, 653. [Google Scholar]
- Goldberg, M.J. The Dysmorphic Child: An Orthopedic Perspective; Raven Press: New York, NY, USA, 1986. [Google Scholar]
- Steel, H.H.; Kohl, E.J. Multiple congenital dislocations associated with other skeletal anomalies (Larsen syndrome) in three siblings. J. Bone Jt. Surg. 1972, 54A, 75–82. [Google Scholar]
- Silverman, F.N. Larsen’s syndrome: Congenital dislocation of the knees and other joints, distinctive facies, and frequently, cleft palate. Ann. Radiol. 1972, 15, 297–328. [Google Scholar]
- Strisciuglio, P.; Sebastio, G.; Andria, G. Severe cardiac anomalies in sibs with Larsen syndrome. J. Med. Genet. 1983, 20, 422–424. [Google Scholar] [PubMed]
- Eletta, R.; Pandey, A.; Dharmasaputra, T.; Moreno, M.; Oparaugo, Y.; Kumar, T.S.; Beker, S. Severe Aortic Root Dilatation in a Patient With Larsen Syndrome. World J. Pediatr. Congenit. Heart Surg. 2023, 14, 532–535. [Google Scholar] [PubMed]
- Micheli, L.J.; Hall, J.E.; Watts, H.G. Spinal instability in Larsen’s syndrome: Report of three cases. J. Bone Jt. Surg. 1976, 58, 562–565. [Google Scholar]
- Weisenbach, J.; Melegh, B. Vertebral anomalies in Larsen‘s syndrome. Pediatr. Radiol. 1996, 26, 682–683. [Google Scholar] [PubMed]
- Lutter, L.D. Larsen syndrome: Clinical features and treatment—A report of two cases. J. Pediatr. Orthop. 1990, 10, 270–274. [Google Scholar]
- Johnston, C.E.; Birch, J.G.; Daniels, J.L. Cervical kyphosis in patients who have Larsen syndrome. J. Bone Jt. Surg. 1996, 78, 538–545. [Google Scholar]
- Zhang, D.; Herring, J.A.; Swaney, S.S.; McClendon, T.B.; Gao, X.; Browne, R.H.; Rathjen, K.E.; Johnston, C.E.; Harris, S.; Cain, N.M.; et al. Mutations responsible for Larsen syndrome cluster in the FLNB protein. J. Med. Genet. 2006, 43, e24. [Google Scholar]
- Hermanns, P.; Unger, S.; Rossi, A.; Perez-Aytes, A.; Cortina, H.; Bonafé, L.; Boccone, L.; Setzu, V.; Dutoit, M.; Sangiorgi, L.; et al. Congenital joint dislocations caused by carbohydrate sulfotransferase 3 deficiency in recessive Larsen syndrome and humero-spinal dysostosis. Am. J. Hum. Genet. 2008, 82, 1368–1374. [Google Scholar]
- Krakow, D.; Robertson, S.P.; King, L.M.; Morgan, T.; Sebald, E.T.; Bertolotto, C.; Wachsmann-Hogiu, S.; Acuna, D.; Shapiro, S.S.; Takafuta, T.; et al. Mutations in the gene encoding filamin B disrupt vertebral segmentation, joint formation and skeletogenesis. Nat. Genet. 2004, 36, 405–410. [Google Scholar]
- De La Rocha, A.; Birch, J.G.; Schiller, J.R. Precocious appearance of the capital femoral nucleus in Larsen Syndrome. J. Bone Jt. Surg. 2012, 94, pe55. [Google Scholar]
- Curtis, B.H.; Fisher, R.L. Congenital hyperextension with anterior subluxation of the knee. Surgical treatment and long-term observations. J. Bone Jt. Surg. 1969, 51, 255–1969. [Google Scholar] [PubMed]
- Rodríguez, A.; Asenjo, B.; Dominguez, R.; Lemaire, R. Larsen syndrome: Multicenter study of 12 new cases. Diagnosis, planning and results of treatment. Acta Orthop. Belg. 1994, 60, 259–273. [Google Scholar] [PubMed]
- Morcuende, J.A.; Dolan, L.A.; Dietz, F.R.; Ponseti, I.V. Radical reduction in the rate of extensive corrective surgery for clubfoot using the Ponseti method. Pediatrics 2004, 113, 376–380. [Google Scholar] [PubMed]
- Gerlach, D.J.; Gurnett, C.A.; Limpaphayom, N.; Alaee, F.; Zhang, Z.; Porter, K.; Kirchhofer, M.; Smyth, M.D.; Dobbs, M.B. Early results of the Ponseti method for the treatment of clubfoot associated with myelomeningocele. J. Bone Jt. Surg. 2009, 91, 1350–1359. [Google Scholar]
- Merrill, R.K.; Ishmael, T.G.; Samdani, A.F.; Hwang, S.W.; Pahys, J.M. Severe Cervical Kyphosis and Spondyloptosis with Myelopathy in Larsen Syndrome: A Report of 2 Cases. JBJS Case Connect. 2021, 11, e21. [Google Scholar]
- McKay, S.D.; Al-Omari, A.; Tomlinson, L.A.; Dormans, J.P. Review of cervical spine anomalies in genetic syndromes. Spine 2012, 37, E269–E277. [Google Scholar]
- Armouti, M.; Hirbawi, H.; Jadaan, M.; Hashem, H.; Muhsen, B.A. Surgical management of cervical kyphosis in larsen syndrome. Case report and review of literature. Ann. Med. Surg. 2022, 75, 103372. [Google Scholar]
- Unger, S.; Lausch, E.; Rossi, A.; Mégarbané, A.; Sillence, D.; Alcausin, M.; Aytes, A.; Mendoza-Londono, R.; Nampoothiri, S.; Afroze, B.; et al. Phenotypic features of carbohydrate sulfotransferase 3 (CHST3) deficiency in 24 patients: Congenital dislocations and vertebral changes as principal diagnostic features. Am. J. Med. Genet. A 2010, 152A, 2543–2549. [Google Scholar]
- Chen, H.; Chang, C.H.; Perrin, E.; Perrin, J.; Opitz, J.M. A lethal, Larsen-like multiple joint dislocation syndrome. Am. J. Med. Genet. 1982, 13, 149–161. [Google Scholar]
- Clayton-Smith, J.; Donnai, D. A further patient with the lethal type of Larsen syndrome. J. Med. Genet. 1988, 25, 499–500. [Google Scholar]
- Kulkarni, M.L.; Mohammed, Z.; Kulkarni, P.M. Larsen syndrome—Lethal variety. Indian J. Pediatr. 2005, 72, 1053–1054. [Google Scholar] [CrossRef] [PubMed]
- Caksen, H.; Kurtoglu, S. Larsen syndrome associated with severe congenital hydrocephalus. Genet. Couns. 2001, 12, 369–372. [Google Scholar] [PubMed]
- Siafaka, A.; Angelis, S.; Piagkou, M.; Apostolopoulos, A.; Troupis, T.; Filippou, D. Larsen Syndrome and Associated Spinal Deformities. Cureus 2023, 15, e41655. [Google Scholar] [CrossRef]
- Furuya, M.; Takeoka, Y.; Yurube, T.; Ito, M.; Suzuki, T.; Kakutani, K.; Uno, K. Two-Staged Surgery for Kyphoscoliosis in Larsen Syndrome with A 30-Year Follow-Up: A Case Report. Spine Surg. Relat. Res. 2023, 8, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sardhara, J.; Raiyani, V.; Saxena, D.; Kumar, A.; Bhaisora, K.S.; Das, K.K.; Mehrotra, A.; Srivastava, A.K.; Behari, S. Craniovertebral junction instability in Larsen syndrome: An institutional series and review of literature. J. Craniovertebr Junction Spine 2020, 11, 276–286. [Google Scholar]
- Deora, H.; Singh, S.; Sardhara, J.; Behari, S. A 360-Degree Surgical Approach for Correction of Cervical Kyphosis and Atlantoaxial Dislocation in the Case of Larsen Syndrome. J. Neurosci. Rural. Pract. 2020, 11, 196–201. [Google Scholar] [CrossRef]
- Hall, C.M.; Elcioglu, N.H.; MacDermot, K.D.; Offiah, A.C.; Winter, R.M. Spondyloepimetaphyseal dysplasia with multiple dislocations (Hall type): Three further cases and evidence of autosomal dominant inheritance. J. Med. Genet. 2002, 39, 666–670. [Google Scholar] [CrossRef][Green Version]
- Escobar, L.F.; Bixler, D.; Weaver, D.D.; Bull, M.J.; Bader, P. Larsen syndrome and craniosynostosis: Idaho syndrome? Dysmorph. Clin. Genet. 1989, 3, 24–27. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaissi, A.A.; Gubin, A.; Ryabykh, S.; Dougales, V.; Al Kaissi, H.; Kircher, S.G.; Grill, F. The Orthopedic Strategy for Patients with Larsen Syndrome. Surg. Tech. Dev. 2025, 14, 10. https://doi.org/10.3390/std14020010
Kaissi AA, Gubin A, Ryabykh S, Dougales V, Al Kaissi H, Kircher SG, Grill F. The Orthopedic Strategy for Patients with Larsen Syndrome. Surgical Techniques Development. 2025; 14(2):10. https://doi.org/10.3390/std14020010
Chicago/Turabian StyleKaissi, Ali Al, Alexander Gubin, Sergey Ryabykh, Vasileios Dougales, Hamza Al Kaissi, Susanne Gerit Kircher, and Franz Grill. 2025. "The Orthopedic Strategy for Patients with Larsen Syndrome" Surgical Techniques Development 14, no. 2: 10. https://doi.org/10.3390/std14020010
APA StyleKaissi, A. A., Gubin, A., Ryabykh, S., Dougales, V., Al Kaissi, H., Kircher, S. G., & Grill, F. (2025). The Orthopedic Strategy for Patients with Larsen Syndrome. Surgical Techniques Development, 14(2), 10. https://doi.org/10.3390/std14020010







