Objective Gait Analysis Using a Single-Point Wearable Sensor to Assess Lumbar Spine Patients Pre- and Postoperatively
Abstract
1. Introduction
1.1. Low Back Pain Is Commonly Caused by Lumbar Spine Pathologies
1.2. Patient-Reported Outcome Measures Have Drawbacks
1.3. Gait Analysis Can Objectively Assess Lumbar Spine Patients
1.4. Single-Point Wearable Sensors Are the Most Clinically Viable Form of Objective Gait Analysis
1.5. Research Problem
2. Methodology
2.1. Study Population
2.2. Wearable Device
2.3. Procedure
2.4. Statistical Analysis
3. Results
3.1. Comparison of Outcome Measures between Groups
3.1.1. Lumbar Spine Patients Had Altered Gait Metrics Preoperatively
3.1.2. Lumbar Spine Patients Demonstrated Reduced Gait Asymmetry and Variability after Surgery
3.2. Changes in Spatiotemporal and Asymmetry Metrics Correlate Well with Changes in the ODI after Surgery
4. Discussion
4.1. Preoperative Assessment of Lumbar Spine Patients Compared with Healthy Controls
4.1.1. Spatiotemporal Gait Metrics
4.1.2. Asymmetry and Variability Gait Metrics
4.2. Changes in Outcome Measures after Surgery and Comparisons with Healthy Controls
4.2.1. Spatiotemporal Gait Metrics
4.2.2. Asymmetry and Variability Gait Metrics
4.2.3. Correlation between Changes in Gait Metrics and Changes in the Oswestry Disability Index
4.3. Justification of Study Techniques
4.4. Strengths and Limitations
4.5. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CoV | Coefficient of variation |
| COVID-19 | Novel coronavirus disease |
| ICC | Intraclass correlation coefficient |
| IMU | Inertial measurement unit |
| LBP | Low back pain |
| LDH | Lumbar disc herniation |
| LSS | Lumbar spinal stenosis |
| MMC | MetaMotionC |
| MLBP | Mechanical low back pain |
| ODI | Oswestry Disability Index |
| p | Probability value |
| PROM | Patient-reported outcome measure |
| r | Pearson’s correlation coefficient |
| SD | Standard deviation |
| VAS | Visual Analogue Scale |
Appendix A. Data Processing Workflow
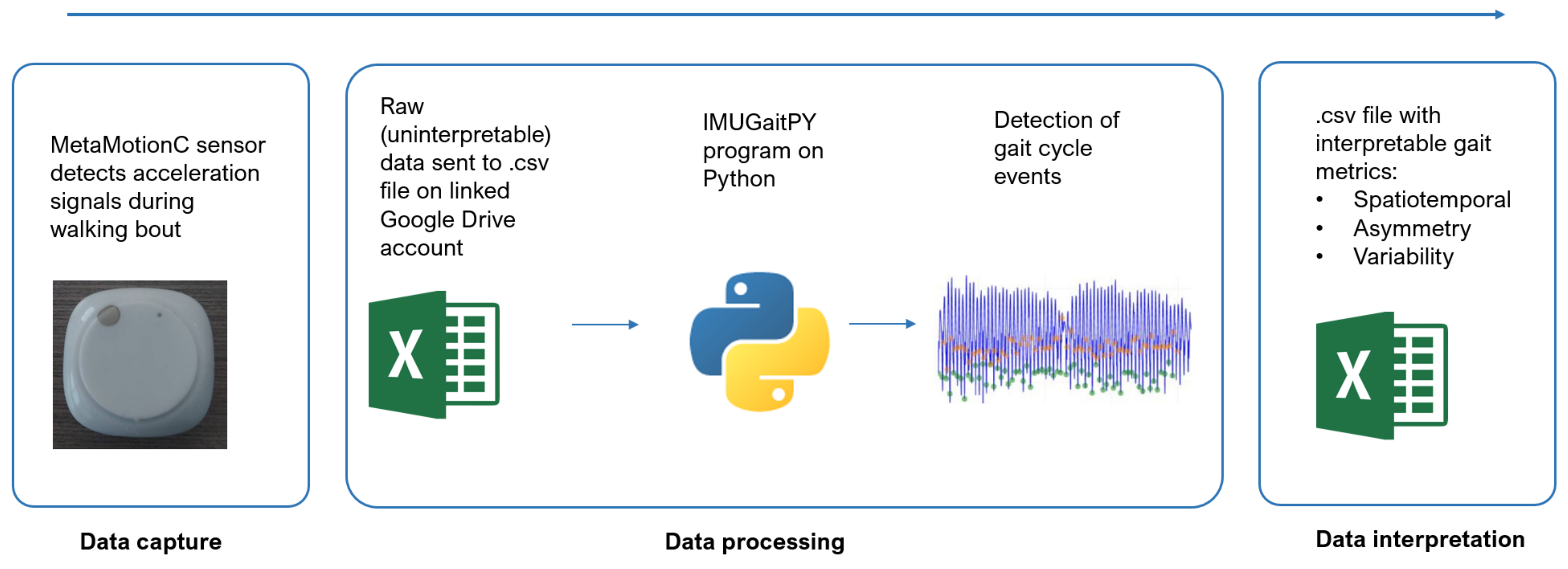
Appendix B. Additional Information Regarding the IMUGaitPY Program
References
- Vos, T.; Allen, C.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef]
- Fairbank, J.C.; Pynsent, P.B. The Oswestry Disability Index. Spine 2000, 25, 2940–2952. [Google Scholar] [CrossRef]
- Mannion, A.F.; Balagué, F.; Pellisé, F.; Cedraschi, C. Pain measurement in patients with low back pain. Nat. Clin. Pract. Rheumatol. 2007, 3, 610–618. [Google Scholar] [CrossRef]
- Stienen, M.N.; Smoll, N.R.; Joswig, H.; Snagowski, J.; Corniola, M.V.; Schaller, K.; Hildebrandt, G.; Gautschi, O.P. Influence of the mental health status on a new measure of objective functional impairment in lumbar degenerative disc disease. Spine J. 2017, 17, 807–813. [Google Scholar] [CrossRef]
- Merrill, R.K.; Zebala, L.P.; Peters, C.; Qureshi, S.A.; McAnany, S.J. Impact of depression on patient-reported outcome measures after lumbar spine decompression. Spine 2018, 43, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Haddas, R.; Ju, K.L.; Belanger, T.; Lieberman, I.H. The use of gait analysis in the assessment of patients afflicted with spinal disorders. Eur. Spine J. 2018, 27, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Betteridge, C.; Mobbs, R.J.; Fonseka, R.D.; Natarajan, P.; Ho, D.; Choy, W.J.; Sy, L.W.; Pell, N. Objectifying clinical gait assessment: Using a single-point wearable sensor to quantify the spatiotemporal gait metrics of people with lumbar spinal stenosis. J. Spine Surg. 2021, 7, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Muro-de-la-Herran, A.; Garcia-Zapirain, B.; Mendez-Zorrilla, A. Gait analysis methods: An overview of wearable and non-wearable systems, highlighting clinical applications. Sensors 2014, 14, 3362–3394. [Google Scholar] [CrossRef] [PubMed]
- Siebert, E.; Prüss, H.; Klingebiel, R.; Failli, V.; Einhäupl, K.M.; Schwab, J.M. Lumbar spinal stenosis: Syndrome, diagnostics and treatment. Nat. Rev. Neurol. 2009, 5, 392–403. [Google Scholar] [CrossRef]
- Raj, P.P. Intervertebral disc: Anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008, 8, 18–44. [Google Scholar] [CrossRef] [PubMed]
- van Kleef, M.; Vanelderen, P.; Cohen, S.P.; Lataster, A.; Van Zundert, J.; Mekhail, N. Pain originating from the lumbar facet joints. Pain Pract. 2010, 10, 459–469. [Google Scholar] [CrossRef]
- Borenstein, D. Mechanical low back pain—A rheumatologist’s view. Nat. Rev. Rheumatol. 2013, 9, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Moon, S.-H.; Suk, K.-S.; Kim, H.-S.; Yang, J.-H.; Lee, H.-M. Lumbar spinal stenosis: Pathophysiology and treatment principle: A narrative review. Asian Spine J. 2020, 14, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Vlaeyen, J.W.S.; Maher, C.G.; Wiech, K.; Van Zundert, J.; Meloto, C.B.; Diatchenko, L.; Battié, M.C.; Goossens, M.; Koes, B.; Linton, S.J. Low back pain. Nat. Rev. Dis. Primers 2018, 4, 52. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.I.; Mirza, S.K.; Spina, N.; Spiker, W.R.; Lawrence, B.; Brodke, D.S. Trends in lumbar fusion procedure rates and associated hospital costs for degenerative spinal diseases in the United States, 2004 to 2015. Spine 2019, 44, 369–376. [Google Scholar] [CrossRef] [PubMed]
- McCormick, J.D.; Werner, B.C.; Shimer, A.L. Patient-reported outcome measures in spine surgery. J. Am. Acad. Orthop. Surg. 2013, 21, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.E.; Bode, R.; Repucci, N.; Becker, J.; Sprangers, M.A.G.; Fayers, P.M. The clinical significance of adaptation to changing health: A meta-analysis of response shift. Qual. Life Res. 2006, 15, 1533–1550. [Google Scholar] [CrossRef] [PubMed]
- Oort, F.J.; Visser, M.R.; Sprangers, M.A. An application of structural equation modeling to detect response shifts and true change in quality of life data from cancer patients undergoing invasive surgery. Qual. Life Res. 2005, 14, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lach, J.; Lo, B.; Yang, G.Z. Toward pervasive gait analysis with wearable sensors: A systematic review. IEEE J. Biomed. Health Inform. 2016, 20, 1521–1537. [Google Scholar] [CrossRef]
- DeFroda, S.F.; Daniels, A.H.; Deren, M.E. Differentiating radiculopathy from lower extremity arthropathy. Am. J. Med. 2016, 129, 1124.e1–1124.e7. [Google Scholar] [CrossRef]
- Stienen, M.N.; Maldaner, N.; Joswig, H.; Corniola, M.V.; Bellut, D.; Prömmel, P.; Regli, L.; Weyerbrock, A.; Schaller, K.; Gautschi, O.P. Objective functional assessment using the “Timed Up and Go” test in patients with lumbar spinal stenosis. Neurosurg. Focus 2019, 46, e4. [Google Scholar] [CrossRef]
- Saleh, M.; Murdoch, G. In defence of gait analysis. Observation and measurement in gait assessment. J. Bone Jt. Surg. 1985, 67, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Cappozzo, A.; Della Croce, U.; Leardini, A.; Chiari, L. Human movement analysis using stereophotogrammetry. Part 1: Theoretical background. Gait Posture 2005, 21, 186–196. [Google Scholar] [PubMed]
- Lamoth, C.J.C.; Stins, J.F.; Pont, M.; Kerckhoff, F.; Beek, P.J. Effects of attention on the control of locomotion in individuals with chronic low back pain. J. Neuroeng. Rehabil. 2008, 5, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Gaßner, H.; Jensen, D.; Marxreiter, F.; Kletsch, A.; Bohlen, S.; Schubert, R.; Muratori, L.M.; Eskofier, B.; Klucken, J.; Winkler, J.; et al. Gait variability as digital biomarker of disease severity in Huntington’s disease. J. Neurol. 2020, 267, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Shahar, R.T.; Agmon, M. Gait analysis using accelerometry data from a single smartphone: Agreement and consistency between a smartphone application and gold-standard gait analysis system. Sensors 2021, 21, 7497. [Google Scholar] [CrossRef]
- Washabaugh, E.P.; Kalyanaraman, T.; Adamczyk, P.G.; Claflin, E.S.; Krishnan, C. Validity and repeatability of inertial measurement units for measuring gait parameters. Gait Posture 2017, 55, 87–93. [Google Scholar] [CrossRef]
- Kluge, F.; Gaßner, H.; Hannink, J.; Pasluosta, C.; Klucken, J.; Eskofier, B.M. Towards mobile gait analysis: Concurrent validity and test-retest reliability of an inertial measurement system for the assessment of spatio-temporal gait parameters. Sensors 2017, 17, 1522. [Google Scholar] [CrossRef] [PubMed]
- Rantalainen, T.; Pirkola, H.; Karavirta, L.; Rantanen, T.; Linnamo, V. Reliability and concurrent validity of spatiotemporal stride characteristics measured with an ankle-worn sensor among older individuals. Gait Posture 2019, 74, 33–39. [Google Scholar] [CrossRef]
- Phan, K.; Mobbs, R.J. Long-term objective physical activity measurements using a wireless accelerometer following minimally invasive transforaminal interbody fusion surgery. Asian Spine J. 2016, 10, 366–369. [Google Scholar] [CrossRef]
- Loske, S.; Nüesch, C.; Byrnes, K.S.; Fiebig, O.; Schären, S.; Mündermann, A.; Netzer, C. Decompression surgery improves gait quality in patients with symptomatic lumbar spinal stenosis. Spine J. 2018, 18, 2195–2204. [Google Scholar] [CrossRef]
- Ghent, F.; Mobbs, R.J.; Mobbs, R.R.; Sy, L.; Betteridge, C.; Choy, W.J. Assessment and post-intervention recovery after surgery for lumbar disk herniation based on objective gait metrics from wearable devices using the Gait Posture Index. World Neurosurg. 2020, 142, e111–e116. [Google Scholar] [CrossRef]
- Mobbs, R.J.; Mobbs, R.R.; Choy, W.J. Proposed objective scoring algorithm for assessment and intervention recovery following surgery for lumbar spinal stenosis based on relevant gait metrics from wearable devices: The Gait Posture index (GPi). J. Spine Surg. 2019, 5, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Suda, Y.; Saitou, M.; Shibasaki, K.; Yamazaki, N.; Chiba, K.; Toyama, Y. Gait analysis of patients with neurogenic intermittent claudication. Spine 2002, 27, 2509–2513. [Google Scholar] [CrossRef] [PubMed]
- Perring, J.; Mobbs, R.; Betteridge, C. Analysis of patterns of gait deterioration in patients with lumbar spinal stenosis. World Neurosurg. 2020, 141, e55–e59. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.E.; Simmonds, M.J.; Etnyre, B.R.; Morris, G.S. Influence of pain distribution on gait characteristics in patients with low back pain: Part 1: Vertical ground reaction force. Spine 2007, 32, 1329–1336. [Google Scholar] [CrossRef]
- Kang, X.; Li, K.; Li, J.; Wei, N.; Yue, S. Abnormal gait and neuromuscular dysfunction analysis in patients with lumbar disc herniation. IFAC-PapersOnLine 2020, 53, 244–249. [Google Scholar] [CrossRef]
- Papadakis, N.C.; Christakis, D.G.; Tzagarakis, G.N.; Chlouverakis, G.I.; Kampanis, N.A.; Stergiopoulos, K.N.; Katonis, P.G. Gait variability measurements in lumbar spinal stenosis patients: Part B. Preoperative versus postoperative gait variability. Physiol. Meas. 2009, 30, 1187–1195. [Google Scholar] [CrossRef]
- Papadakis, N.C.; Christakis, D.G.; Tzagarakis, G.N.; Chlouverakis, G.I.; Kampanis, N.A.; Stergiopoulos, K.N.; Katonis, P.G. Gait variability measurements in lumbar spinal stenosis patients: Part A. Comparison with healthy subjects. Physiol. Meas. 2009, 30, 1171–1186. [Google Scholar] [CrossRef]
- Czech, M.; Patel, S. GaitPy: An open-source python package for gait analysis using an accelerometer on the lower back. J. Open Source Softw. 2019, 4, 1778. [Google Scholar] [CrossRef]
- Nazarahari, M.; Rouhani, H. Detection of daily postures and walking modalities using a single chest-mounted tri-axial accelerometer. Med. Eng. Phys. 2018, 57, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Di Tocco, J.; Raiano, L.; Sabbadini, R.; Massaroni, C.; Formica, D.; Schena, E. A wearable system with embedded conductive textiles and an IMU for unobtrusive cardio-respiratory monitoring. Sensors 2021, 21, 3018. [Google Scholar] [CrossRef] [PubMed]
- Betteridge, C.; Mobbs, R.J.; Ho, D. Proposed objective scoring algorithm for walking performance, based on relevant gait metrics: The Simplified Mobility Score (SMoS™)-observational study. J. Orthop. Surg. Res. 2021, 16, 419. [Google Scholar] [CrossRef]
- Romijnders, R.; Warmerdam, E.; Hansen, C.; Welzel, J.; Schmidt, G.; Maetzler, W. Validation of IMU-based gait event detection during curved walking and turning in older adults and Parkinson’s Disease patients. J. Neuroeng. Rehabil. 2021, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Chiari, L.; Holmstrom, L.; Salarian, A.; Horak, F.B. Validity and reliability of an IMU-based method to detect APAs prior to gait initiation. Gait Posture 2016, 43, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.; Beckbauer, M.; Romijnders, R.; Warmerdam, E.; Welzel, J.; Geritz, J.; Emmert, K.; Maetzler, W. Reliability of IMU-derived static balance parameters in neurological diseases. Int. J. Environ. Res. Public Health 2021, 18, 3644. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-C.; Sugiarto, T.; Lin, Y.-J.; Yang, F.-C.; Lin, Z.-Y.; Sun, C.-T.; Hsu, C.-L.; Chou, K.-N. Multiple-wearable-sensor-based gait classification and analysis in patients with neurological disorders. Sensors 2018, 18, 3397. [Google Scholar] [CrossRef]
- Baghdadi, A.; Cavuoto, L.A.; Crassidis, J.L. Hip and trunk kinematics estimation in gait through kalman filter using IMU data at the ankle. IEEE Sens. J. 2018, 18, 4253–4260. [Google Scholar] [CrossRef]
- Tripuraneni, K.R.; Foran, J.R.H.; Munson, N.R.; Racca, N.E.; Carothers, J.T. A smartwatch paired with a mobile application provides postoperative self-directed rehabilitation without compromising total knee arthroplasty outcomes: A randomized controlled trial. J. Arthroplast. 2021, 36, 3888–3893. [Google Scholar] [CrossRef]
- Heydari, F.; Ebrahim, M.P.; Redoute, J.-M.; Joe, K.; Walker, K.; Yuce, M.R. A chest-based continuous cuffless blood pressure method: Estimation and evaluation using multiple body sensors. Inf. Fusion 2020, 54, 119–127. [Google Scholar] [CrossRef]
- Galna, B.; Lord, S.; Rochester, L. Is gait variability reliable in older adults and Parkinson’s disease? Towards an optimal testing protocol. Gait Posture 2013, 37, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Williams Andrews, A. Normal walking speed: A descriptive meta-analysis. Physiotherapy 2011, 97, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Ardestani, M.M.; Hornby, T.G. Effect of investigator observation on gait parameters in individuals with stroke. J. Biomech. 2020, 100, 109602. [Google Scholar] [CrossRef] [PubMed]
- Robles-García, V.; Corral-Bergantiños, Y.; Espinosa, N.; Jácome, M.A.; García-Sancho, C.; Cudeiro, J.; Arias, P. Spatiotemporal gait patterns during overt and covert evaluation in patients with Parkinson’s disease and healthy subjects: Is there a Hawthorne effect? J. Appl. Biomech. 2015, 31, 189–194. [Google Scholar] [CrossRef]
- Mbientlab. MetaMotionS. 2021. Available online: https://mbientlab.com/metamotions/?gclid=Cj0KCQiA47GNBhDrARIsAKfZ2rCXRMpZxFutmRVfwEbY0yH4sVZiro9DIW0J0Gn3i63A7uqQtxVnj_8aAuljEALw_wcB (accessed on 9 December 2021).




| Inclusion Criteria |
|---|
| Clinical diagnosis of either lumbar spinal stenosis, lumbar disc herniation, or mechanical low back pain |
| Be medically suitable for lumbar spine surgery |
| Have not improved with non-surgical treatment |
| Age greater than 18 years |
| Exclusion criteria |
| Inability to walk independently |
| Women who are pregnant |
| Concurrent serious spinal pathology such as cancer, cauda equina syndrome, spinal fracture, and inflammatory arthritis |
| Present with active Paget’s disease of the spine |
| Presence of significant lumbar scoliosis (Cobb angle ≥ 25°) or other spinal deformities |
| Meyerding classification grade 2 or greater spondylolisthesis |
| Symptomatic hip disease with symptoms reproduced with external or internal rotation of the hip joint |
| Cognitive impairment of inadequate English language skills that interfere with the patient’s ability to give fully informed consent or complete baseline or follow-up assessments |
| Base a Metric | Definition (Units) | Type b | Derivative Metrics c | Definition (Units) | Type b |
|---|---|---|---|---|---|
| Gait velocity | Distance travelled per second (m/s) | Combined spatiotemporal | Gait velocity variability d | Step-to-step variability in gait velocity (sm−1) | Variability |
| Step time | Time between two consecutive contacts of any foot with the ground (s) | Temporal | Step time asymmetry | Average of difference in time between successive steps on the left and right foot (s) | Asymmetry |
| Step time variability d | Step-to-step variability in step time (s−1) | Variability | |||
| Step length | Distance between two consecutive contacts of any foot with the ground (m) | Spatial | Step length asymmetry | Average difference in length between successive steps on the left and right foot (m) | Asymmetry |
| Step length variability d | Step-to-step variability in step length (m−1) | Variability | |||
| Stance time | For each foot the time between the first point of contact with the ground to the last point of contact (s) | Temporal | Stance time asymmetry | Average difference in stance time between successive steps on the left and right foot (s) | Asymmetry |
| Stance time variability d | Step-to-step variability in stance time (s−1) | Variability | |||
| Swing time | For each foot the time between the last point of contact with the ground to the first point of contact (s) | Temporal | Swing time asymmetry | Average difference in swing time between successive steps on the left and right foot (s) | Asymmetry |
| Swing time variability d | Step-to-step variability in swing time (s−1) | Variability | |||
| Single support time | Time where only one foot is in contact with the ground (s) | Temporal | Single support time asymmetry | Average difference in single support time between successive steps on the left and right foot (s) | Asymmetry |
| Single support time variability d | Step-to-step variability in single support time (s−1) | Variability | |||
| Double support time | Time where both feet are in contact with the ground (s) | Temporal | Double support time asymmetry | Average difference in double support time between successive steps (s) | Asymmetry |
| Double support time variability d | Step-to-step variability in double support time (s−1) | Variability |
| Demographic Variables | Lumbar Spine Patients (n = 12) | Healthy Controls (n = 24) |
|---|---|---|
| Continuous mean {[range, (SD)]} | ||
| Age (years) | 61.0 [41–86 (16.8)] | 60.7 [42–91 (13.6)] |
| BMI (kg/m2) | 27.9 [19.5–36.4 (5.45)] | 26.7 [21.1–39.9 (4.59)] |
| Height (m) | 1.70 [1.50–1.88 (0.115)] | 1.65 [1.50–1.79 (0.0892)] |
| Categorical [n, (percentage of total)] | ||
| Gender | ||
| Male | 5 (41.7) | 10 (41.7) |
| Female | 7 (58.3) | 14 (58.3) |
| Daily smoker | 0 (0) | 1 (4.17) |
| Diabetic | 0 (0) | 1 (4.17) |
| Fall in previous year | 2 (16.7) | 2 (8.33) |
| Pathology [n, (percentage of total)] | ||
| Lumbar spinal stenosis | 6 (50.0) | - |
| Lumbar disc herniation | 4 (33.3) | - |
| Discogenic and/or facetogenic mechanical Low back pain | 2 (16.7) | - |
| Metric | Preoperative | Postoperative | Healthy Controls |
|---|---|---|---|
| ODI | 42.4 (19.0) | 22.8 (18.3) | - |
| VAS | 7.00 (5.50–8.00) | 1.50 (0–4.50) | - |
| Spatiotemporal | |||
| Gait velocity (ms−1) | 1.03 (0.308) | 1.13 (0.358) | 1.29 (0.197) |
| Step time (ms) | 573 (537–616) | 573 (556–673) | 514 (38.9) |
| Step length (mm) | 591 (120) | 637 (153) | 656 (97.6) |
| Stance (ms) | 741 (124) | 736 (130) | 642 (49.8) |
| Swing (ms) | 464 (112) | 440 (78.7) | 389 (27.9) |
| Double support time (ms) | 284 (31.2) | 296 (52.8) | 257 (21.4) |
| Single support time (ms) | 446 (413–545) | 448 (82.6) | 390 (28.8) |
| Asymmetry | |||
| Step time (ms) | 43.2 (9.08–77.4) | 27.1 (19.8–73.5) | 37.1 (26.6–60.8) |
| Step length (mm) | 59.6 (43.9–114) | 49.2 (41.2–69.5) | 57.0 (44.6–75.2) |
| Stance time (ms) | 63.1 (22.7–89.5) | 25.0 (20.7–90.6) | 32.4 (25.3–49.5) |
| Swing (ms) | 61.7 (23.8–95.3) | 24.5 (19.1–90.4) | 29.6 (25.7–57.8) |
| Single support time (ms) | 65.6 (35.5–144) | 32.8 (22.4–90.2) | 34.9 (28.1–63.7) |
| Double support time (ms) | 15.0 (11.6–28.4) | 10.6 (7.26–22.7) | 12.9 (8.18–16.8) |
| Variability | |||
| Gait velocity (sm−1) | 10.8 (2.38) | 8.53 (1.97) | 10.2 (3.49) |
| Step time (s−1) | 13.2 (9.69–17.5) | 6.03 (3.73–8.88) | 11.8 (6.00) |
| Step length (m−1) | 12.5 (8.13–20.5) | 8.31 (7.92–13. 9) | 9.40 (7.61–11.2) |
| Stance time (s−1) | 9.49 (6.30–12.1) | 6.72 (5.30–9.31) | 8.67 (4.17) |
| Swing time (s−1) | 20.0 (11.6) | 8.26 (6.14–14.7) | 13.4 (7.05–21.4) |
| Single support time (s−1) | 44.2 (24.1) | 16.8 (12.1–36.0) | 22.6 (10.4–35.1) |
| Double support time (s−1) | 17.9 (7.67–28.1) | 6.12 (4.63–19.0) | 10.2 (5.71–16.6) |
| Within Patients | Patients–Controls | ||
|---|---|---|---|
| Metric | Postoperative–Preoperative | Preoperative | Postoperative |
| ODI | −46.2 (0.01) | - | - |
| VAS | −78.6 (0.001) | - | - |
| Spatiotemporal | |||
| Gait velocity | 9.71 (0.195) | −20.2 (0.008) | −12.4 (0.095) |
| Step time | −0.000103 (0.468) | 10.3 (0.006) | 11.5 (0.001) |
| Step length | 7.78 (0.123) | −9.91 (0.121) | −2.90 (0.643) |
| Stance time | −0.67 (0.828) | 15.4 (0.003) | 14.6 (0.005) |
| Swing time | 5.17 (0.193) | 19.3 (0.002) | 13.1 (0.026) |
| Single support time | 0.448 (0.065) | 14.4 (0.001) | 14.9 (0.044) |
| Double support time | 4.23 (0.255) | 10.5 (0.027) | 15.2 (0.002) |
| Asymmetry | |||
| Step time | −37.3 (0.066) | 16.4 (0.063) | −27.0 (0.983) |
| Step length | −17.4 (0.016) | 4.56 (0.097) | −13.7 (0.904) |
| Stance time | −60.4 (0.053) | 94.8 (0.037) | −22.8 (0.594) |
| Swing time | −60.3 (0.039) | 108 (0.036) | −17.2 (0.699) |
| Single limb support | −50.0 (0.012) | 88.0 (0.009) | −6.02 (0.650) |
| Double limb support | −29.3 (0.027) | 16.3 (0.017) | −17.8 (0.845) |
| Variability | |||
| Gait velocity | −21.0 (0.011) | 5.88 (0.564) | −16.4 (0.134) |
| Step time | −54.3 (0.001) | 11.9 (0.110) | −48.9 (0.094) |
| Step length | −33.5 (0.011) | 33.0 (0.019) | −11.6 (0.929) |
| Stance | −29.2 (0.023) | 9.46 (0.171) | −22.5 (0.550) |
| Swing | −58.7 (0.004) | 49.3 (0.182) | −38.4 (0.265) |
| Single limb support | −62.0 (0.001) | 95.6 (0.009) | −25.7 (0.751) |
| Double limb support | −65.8 (0.014) | 75.5 (0.048) | −40.0 (0.675) |
| Gait Metric | Correlation Coefficient | p-Value |
|---|---|---|
| Spatiotemporal | ||
| Gait velocity | −0.914 | <0.001 |
| Step time | 0.557 | 0.060 |
| Step length | −0.862 | <0.001 |
| Stance | 0.902 | <0.001 |
| Swing | 0.835 | 0.001 |
| Single limb support | 0.869 | <0.001 |
| Double support * | 0.445 | 0.147 |
| Asymmetry | ||
| Step time * | 0.581 | 0.047 |
| Step length * | 0.434 | 0.158 |
| Stance * | 0.666 | 0.018 |
| Swing * | 0.623 | 0.030 |
| Single limb support * | 0.606 | 0.037 |
| Double limb support * | 0.438 | 0.155 |
| Variability | ||
| Gait velocity * | 0.459 | 0.134 |
| Step time * | 0.452 | 0.140 |
| Step length variability | 0.596 | 0.041 |
| Stance * | 0.434 | 0.158 |
| Swing * | 0.434 | 0.158 |
| Single limb support * | 0.175 | 0.586 |
| Double limb support * | 0.231 | 0.470 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseka, R.D.; Natarajan, P.; Maharaj, M.M.; Koinis, L.; Sy, L.; Mobbs, R.J. Objective Gait Analysis Using a Single-Point Wearable Sensor to Assess Lumbar Spine Patients Pre- and Postoperatively. Surg. Tech. Dev. 2024, 13, 58-75. https://doi.org/10.3390/std13010004
Fonseka RD, Natarajan P, Maharaj MM, Koinis L, Sy L, Mobbs RJ. Objective Gait Analysis Using a Single-Point Wearable Sensor to Assess Lumbar Spine Patients Pre- and Postoperatively. Surgical Techniques Development. 2024; 13(1):58-75. https://doi.org/10.3390/std13010004
Chicago/Turabian StyleFonseka, R Dineth, Pragadesh Natarajan, Monish Movin Maharaj, Lianne Koinis, Luke Sy, and Ralph Jasper Mobbs. 2024. "Objective Gait Analysis Using a Single-Point Wearable Sensor to Assess Lumbar Spine Patients Pre- and Postoperatively" Surgical Techniques Development 13, no. 1: 58-75. https://doi.org/10.3390/std13010004
APA StyleFonseka, R. D., Natarajan, P., Maharaj, M. M., Koinis, L., Sy, L., & Mobbs, R. J. (2024). Objective Gait Analysis Using a Single-Point Wearable Sensor to Assess Lumbar Spine Patients Pre- and Postoperatively. Surgical Techniques Development, 13(1), 58-75. https://doi.org/10.3390/std13010004






