Abstract
Background: Although robot-assisted laparoscopic surgery has become more in popular, it remains unclear what clinical advantages it offers over conventional laparoscopic surgery. Objective: This (systematic) umbrella review aims to synthesize and compare the clinical outcomes of robot-assisted laparoscopic surgery versus conventional laparoscopic surgery. Methods: A systematic literature search was conducted in PubMed and Scopus. All systematic reviews and meta-analyses published in the past five years that compared the clinical outcomes for cholecystectomy, colectomy, hysterectomy, nephrectomy, and/or prostatectomy were included. The quality of all included reviews was assessed with the AMSTAR 2 quality assessment tool. Each review’s study characteristics and primary sources were extracted, along with the quantitative and qualitative data for blood loss, rate of conversion to open surgery, hospitalization costs, incisional hernia rate, intraoperative complication rate, postoperative complication rate, length of hospital stay, operative time, readmission rate, and wound infection. Results: Fifty-two systematic reviews and (network) meta-analyses were included in this umbrella review, covering more than 1,288,425 patients from 1046 primary sources published between 1996 and 2022. The overall quality of the included reviews was assessed to be low or critically low. Robot-assisted laparoscopic surgery yielded comparable results to conventional laparoscopic surgery in terms of blood loss, conversion to open surgery rate, intraoperative complication rate, postoperative complication rate, readmission rate, and wound infection rate for most surgical procedures. While the hospitalization costs of robot-assisted laparoscopic surgery were higher and the operative times of robot-assisted laparoscopic surgery were longer than conventional laparoscopic surgery, robot-assisted laparoscopic surgery reduced the length of hospital stay of patients in nearly all cases. Conclusion: Robot-assisted laparoscopic surgery achieved comparable results with conventional laparoscopic surgery for cholecystectomy, colectomy, hysterectomy, nephrectomy, and prostatectomy based on ten clinical outcomes.
Keywords:
robotic surgery; laparoscopy; cholecystectomy; colectomy; hysterectomy; nephrectomy; prostatectomy 1. Introduction
The first demonstration of a laparoscopic instrument dates back to 1901 by surgeon Georg Kelling, but it took several decades before the laparoscopic approach was introduced in the operating theatre [1,2]. From 1960 onwards, laparoscopic surgery advanced quickly and, despite some resistance at first [3], developed into an independent surgical approach. Since the twenty-first century, laparoscopic surgery has become a preferred surgical procedure and the scope of its applicability continues to expand. New technologies enabled even more advances, such as robot-assisted laparoscopic surgery, novel instrument designs, and enhanced imaging capabilities [1].
Even though laparoscopic surgery has proven to be beneficial for patients compared to open surgery regarding the reduction in length of hospital stay and infection rates in procedures such as cholecystectomy [4] and colorectal surgery [5,6], it remains unclear what clinical advantages robot-assisted laparoscopic surgery (RALS) has over conventional laparoscopic surgery (CLS) for the patient. RALS is associated with high acquisition, training, instrumentation, and maintenance costs [7]. RALS systems are therefore affordable only for wealthy surgical centres with a large volume of patients [8]. New developments within laparoscopic instrumentation, such as modular, cleanable, and, therefore, reusable components, may pave the way for more affordable RALS systems in the future [9]. Technical advantages of RALS when compared to CLS include immersive 3D viewings, improved ergonomics, and enhanced dexterity due to features such as tremor filtration, motion scaling, and instrument articulation [10,11]. The question remains whether these technical advantages have also resulted into improved clinical outcomes for patients.
As the field of RALS develops rapidly, this (systematic) umbrella review provides an updated, comprehensive analysis of clinical outcomes for five surgical procedures (colectomy, cholecystectomy, hysterectomy, nephrectomy, and prostatectomy) and synthesizes current evidence on the use of RALS and CLS.
2. Methods
The extensive research and the ongoing debate about whether RALS or CLS yields better clinical outcomes justifies the conduction of an umbrella review [12,13]. An umbrella review systematically identifies and collects data from multiple systematic reviews and meta-analyses on a given subject [14,15,16]. This umbrella review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17]. This section outlines the methodology used, including the databases utilized, the search queries established, the eligibility criteria formulated for the inclusion and exclusion of identified studies, the PRISMA evaluation process, the quality assessment with AMSTAR 2, the data extraction method, and the structuring of the extracted data. All systematic reviews and meta-analyses published in the past five years that compared the clinical outcomes of CLS and RALS for cholecystectomy, colectomy, hysterectomy, nephrectomy, and/or prostatectomy were included.
2.1. Search Strategy
The MEDLINE and EMBASE databases were searched with PubMed and Scopus on the 11th of February 2023 (by S.C.). A search strategy was developed to identify systematic reviews and (network) meta-analyses published in the past five years (1 January 2018 up to 1 January 2023) and written in the English language that compared RALS versus CLS for five specific surgical procedures related to abdominal-pelvic organ removal. The following surgical procedures, which are commonly executed within minimally invasive surgery (RALS and CLS) [4,18,19,20,21], were selected: colectomy (partial or complete), cholecystectomy, hysterectomy, nephrectomy, and prostatectomy. A separate search query was formulated for each surgical procedure (Table 1, Table 2, and Table A7). Search results were filtered on study type, systematic reviews and (network) meta-analyses, and year of publication. It was decided to include reviews published within the past five years only to consolidate the latest research and data, particularly given the rapid advancements in RALS.

Table 1.
PubMed search strategy for colectomy.

Table 2.
Scopus search strategy for colectomy.
2.2. Eligibility Criteria
The established eligibility criteria were based on the Population, Intervention, Comparison, Outcomes, and Study design (PICOS) principle [22]. Articles that included human adults (P), compared CLS with RALS for colectomy and/or cholecystectomy and/or hysterectomy and/or nephrectomy and/or prostatectomy (I, C), and reported at least one of the clinical outcomes of interest (O) with the following outcomes were considered.
Primary outcomes: Conversion to open surgery rate, hospitalization costs, intraoperative complication rate, postoperative complication rate, length of hospital stay, operative time.
Secondary outcomes: Intraoperative blood loss, incisional hernia rate, readmission rate and/or wound infection rate, in a systematic review or (network) meta-analysis (S).
In case a systematic review or meta-analysis covered multiple surgical procedures of which one (or more) was of interest, the review was included and only the relevant data were extracted.
Additionally, the following exclusion criteria were established:
- Studies that focused on certain comorbidities (e.g., obesity);
- Studies that reported none of the clinical outcomes of interest;
- Studies that did not compare the outcomes of CLS and RALS separately, but combined RALS and CLS into one minimally invasive surgery group instead;
- Descriptive studies that defined protocols or methods;
- Studies that researched the effects of intervention timing;
- Studies that focused on recovery programs (after RALS or CLS);
- Studies that focused on pre-operative difficulty prediction scores; and
- Studies of which full text was unavailable.
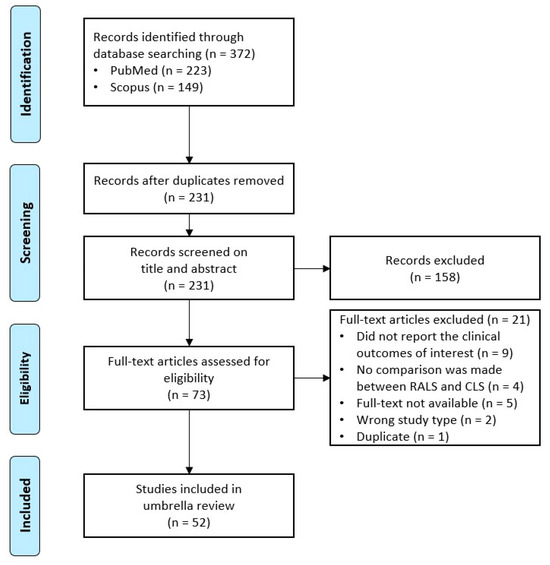
Figure 1.
PRISMA flow diagram depicting the number of papers identified, included, and excluded [17].
The quality of all included systematic reviews and meta-analyses was assessed by means of the AMSTAR 2 quality assessment tool [23,24,25]. Instead of generating an overall score, AMSTAR 2 utilizes a quality rating system [23]. This rating system expresses the level of confidence in the findings of a systematic review. The 16 items of AMSTAR 2 are split into critical (7) and non-critical (9) flaws, which are listed in Table 3 and File S2.

Table 3.
The AMSTAR 2 quality assessment grouped in critical and non-critical flaws [23].
2.3. Data Extraction
Four types of tables were constructed for data extraction: (1) A table with the study characteristics of included reviews, (2) tables with the quantitative findings for the included outcomes, (3) tables with qualitative data provided in studies, and (4) a table (along with graphs) with the final or overall synthesized findings from the reviews. A data extraction Excel sheet was created based on the standardized data extraction tool [16], ensuring similar data extraction across all included studies.
Qualitative data were extracted and processed into tables (cholecystectomy, Table A2; colectomy, Table A3; hysterectomy, Table A4; nephrectomy, Table A5; prostatectomy, Table A6). The quantitative data that were extracted from the included reviews are listed in separate tables (cholecystectomy, Table 6; colectomy, Tables 7–9; hysterectomy, Table 10; nephrectomy, Table 11; prostatectomy, Table 12). Some meta-analyses performed a general analysis to compare RALS and CLS for a given surgical category (e.g., colectomy), while others focused their analyses on specific subgroups (such as single- or multiple-incision laparoscopy or left hemicolectomy). In meta-analyses that conducted general analyses, only the pooled results were extracted (regardless of any subgroup analyses). In cases where meta-analytic studies only performed subgroup analyses, data were extracted and included in the quantitative table along with additional information specifying the scope of these data. This is because pooled results are preferred as they include larger sample sizes [14].
Summary of Evidence
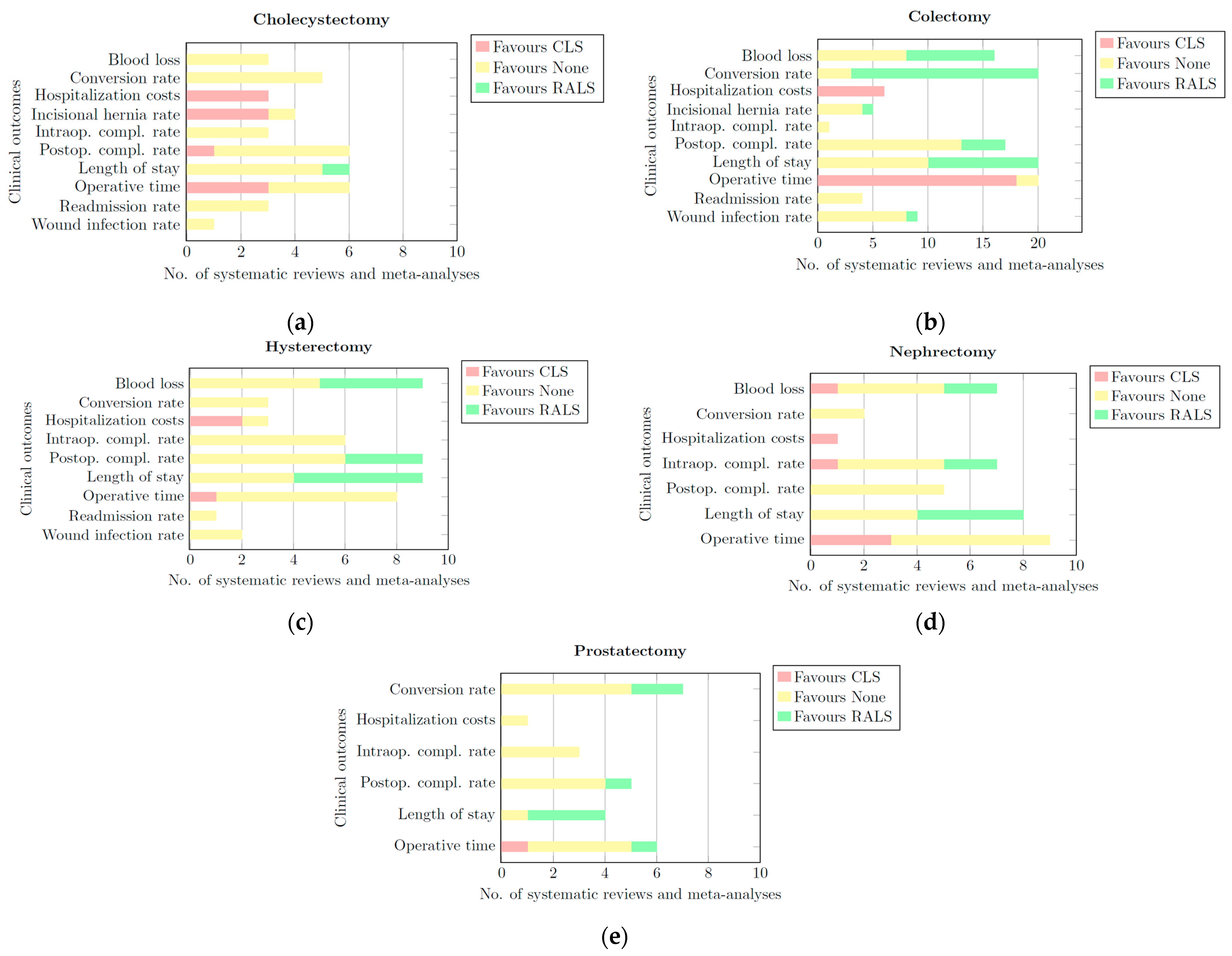
Per clinical outcome, for each surgical category, horizontally stacked bar charts were constructed as shown in Figure 2. Each bar chart was split into three categories: CLS, RALS, and None.
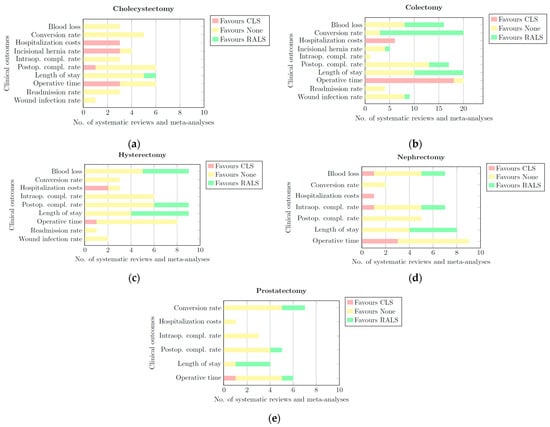
Figure 2.
Bar charts of all quantitative and qualitative data per clinical outcome for: (a) cholecystectomy; (b) colectomy; (c) hysterectomy; (d) nephrectomy; (e) prostatectomy. Each bar chart has three categorieS. CLS: This portion of the bar is coloured red. The length of this part represents the number of systematic reviews and meta-analyses that provided quantitative or qualitative data showing a significant difference in favour of CLS for a given clinical outcome.; None: This portion of the bar is coloured yellow. The length of this part of the bar represents the number of systematic reviews and meta-analyses that provided quantitative or qualitative data showing that RALS and CLS derived comparable results for a given clinical outcome. RALS: This portion of the bar is coloured green. The length of this part of the bar represents the number of systematic reviews and meta-analyses that provided quantitative or qualitative data showing a significant difference in favour of RALS for a given clinical outcome.
- CLS: This portion of the bar is coloured red. The length of this part of the bar represents the number of systematic reviews and meta-analyses that provided quantitative or qualitative data showing a significant difference in favour of CLS for a given clinical outcome.
- None: This portion of the bar is coloured yellow. The length of this part of the bar represents the number of systematic reviews and meta-analyses that provided quantitative or qualitative data showing that RALS and CLS had comparable results for a given clinical outcome.
- RALS: This portion of the bar is coloured green. The length of this part of the bar represents the number of systematic reviews and meta-analyses that provided quantitative or qualitative data showing a significant difference in favour of RALS for a given clinical outcome.
These bar charts synthesize the findings of all reviews listed in the quantitative and qualitative tables. Based on these bar charts, conclusions were drawn in a final table (Table 13). Per surgical category and in general, it was indicated whether the data of all reviews showed comparable results (i.e., ‘None’) or significant benefits in favour of RALS or CLS for each clinical outcome. In cases with insufficient evidence to favour one method over the other, it was indicated in the table as ‘RALS/None’ or ‘CLS/None’. The results were presented in a stoplight format: red denotes a preference for CLS, yellow indicates comparable outcomes between CLS and RALS, and green indicates that RALS is the superior option for that particular outcome. A grey background was used in RALS/None and CLS/None cases.
2.4. Corrected Covered Area
The corrected covered area (CCA) indicates how much overlap exists between the data of the included systematic reviews and meta-analysis [26]. High levels of overlap should generate more consistent conclusions [25]. An example is given in Table 4, where primary source 3 is included in three different systematic reviews, while primary source 1 is included in two reviews and primary source 2 is included only once. Calculating the percentage of overlap (which would be 66%) does not take into account multiple inclusions of a single source, but CCA does. The CCA is calculated with Equation (1).
where N is the total amount of included articles (the ticked boxes in Table 4), r is the number of primary sources (the number of rows in Table 4), and c is the number of systematic reviews (the number of columns in Table 4). The CCA for the example given in Table 4 is:

Table 4.
A citation matrix. Primary source 1 is included in systematic reviews 1 and 3 and primary source 3 is included in all three systematic reviews. CCA accounts for higher degrees of overlap.
The CCA score ranges between 0–100% and the overlap interpretation is given in Table 5 [26]. CCA scores were calculated for each surgical category using a citation matrix.

Table 5.
CCA scores and the associated overlap interpretation [26].
3. Results
3.1. PRISMA Flow Diagram
A final search was conducted on PubMed and Scopus on 11 February 2023. In total, 372 records were initially identified and exported to the EndNote X9 citation manager. During the screening phase, 141 duplicates were removed via the in-built Find Duplicate feature of EndNote X9 and additional manual searching for duplicates was conducted. During the title and abstract screening, 158 records were excluded according to the inclusion and exclusion criteria. During the eligibility phase, the full text of 73 reviews were reviewed and 21 of these reviews were excluded: Nine records did not report data on the outcomes of interest, four records did not compare RALS with CLS, the full text was not available for five records, two records were excluded because of their study type, and one record was excluded as it was a duplicate. A full list of the records that were excluded during the full-text review, with the reason(s) for exclusion, is provided in the Appendix (Table A8).
The remaining 52 articles are included in this umbrella review. The inclusion and exclusion process of all articles is schematically represented in the PRISMA flow diagram in Figure 1.
3.2. Study Characteristics
The characteristics of all included reviews are listed in Table S1 (available in the list of Supplementary Materials). In total, 38 out of the 52 records are meta-analyses, 7 records are systematic reviews and 7 are network meta-analyses. Colectomy was the most researched procedure (22/52 records) and prostatectomy was the least researched (8/52) among the included reviews. Out of the 52 records, a total of 1,288,425 patients were included from 1046 primary sources. In total, 151,599 patients were treated with RALS, and 970,563 patients were treated with CLS. Some reviews included patients treated with open surgery as well, and five reviews did not provide complete data regarding the number of RALS, CLS, and/or the total amount of patients [27,28,29,30,31]. As a result, the total number of patients is slightly higher than the combined number of patients in the RALS and CLS groups. The year of publication of the primary sources ranged between 1996 and 2022. The citation matrices, listing all the primary sources of the included reviews, are included in the list of Supplementary Materials (Table S2).
3.3. Clinical Outcomes
The results of all five surgical categories are addressed below.
3.3.1. Cholecystectomy
Seven studies on cholecystectomy were included, of which one was a systematic review [32], five were meta-analyses [33,34,35,36,37], and one was a network meta-analysis [38]. The corrected covered area (overlap) of these six studies was 3.7% and this is considered to be slight (Table A1). Specific subgroup analyses were conducted for the number of ports or incisions in RALS and CLS in [32,34,35,36,38]. One study [33] conducted both general and subgroup analyses. The quality of all seven studies, based on the AMSTAR 2 quality assessment, was considered to be low [32,37] or critically low [33,34,35,36,38]. AMSTAR 2 scores are listed in Table S3 (available in the list of Supplementary Materials).
In Table 6, quantitative data retrieved from the seven papers are synthesized. It is important to note that the meta-analytic data on blood loss and postoperative complications presented by [37] (indicated with an a in Table 6) was based on a single study only, which precludes the assessment of heterogeneity. Therefore, the (non)-significance of these findings should be interpreted with caution and be considered of limited value.
No significant differences were observed between RALS and CLS cholecystectomy for almost all clinical outcomes. The only significant differences measured were in favour of CLS. The hospitalization costs were measured in three publications, which all concluded that RALS hospitalization costs are significantly higher compared to CLS hospitalization costs [33,34,35]. The incisional hernia rate was significantly lower in patients treated by CLS compared to RALS [33,34,36]. In one review, the operative time was found to be significantly longer in RALS procedures compared to CLS [33], but these results were not obtained in two other systematic reviews.
Qualitative data, listed in Table A2, were retrieved from two studies [32,38]. Similar to the quantitative studies, the results did not show any differences between RALS and CLS for most of the clinical outcomes. In a network meta-analysis, a ranking of five competing interventions for cholecystectomy (RALS, single-incision CLS, three-port CLS, four-port CLS, and mini laparoscopy) was formulated (quantitative data from this network meta-analysis were not included, which is further elaborated in the dPliscussion. The ranking of the surgical interventions indicated which approach scored best per clinical outcome. RALS was ranked the highest regarding postoperative pain and length of hospital stay [38]. For postoperative complications and operative time, CLS (three-port and four-port) ranked highest [38]. In one study [32], there were longer operative times when patients were treated with RALS compared to CLS.
3.3.2. Colectomy
Twenty-three reviews on colectomy were included [30,31,37,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58]. Of the twenty-three reviews, two were systematic reviews [30,57], eighteen were meta-analyses [31,37,39,40,42,43,44,45,46,47,49,50,51,52,53,55,56,58], and three were network meta-analyses [41,48,54]. The corrected covered area of the 23 reviews was 2.6% (slight overlap). Furthermore, all studies scored either low or critically low on the AMSTAR 2 quality rating (all AMSTAR 2 Quality Assessment results are available for download in the list of Supplementary Materials). Within colectomy, multiple indications for surgery and surgical procedures exist. Regarding the indications for surgery, thirteen reviews included colorectal cancer surgery studies only [30,31,41,43,44,45,46,47,48,54,55,57,58], two reviews focused on resections indicated by diverticular diseases [39,42], and the remaining seven reviews included studies related to colectomy for any or multiple indications [37,40,49,50,51,52,53]. As for the surgical procedures themselves, nine studies focused on left or right hemicolectomy [50,51,52,53,54,55,56,57,58], four on total mesorectal excisions [31,46,47,48], two on complete mesocolic excisions [30,45], three on colorectal resections [41,43,44], two on diverticular resections [39,42], and three on multiple surgical procedures related to the colon [37,40,49].
The quantitative data that were extracted from all (network) meta-analyses can be found in Table 7, Table 8 and Table 9. In Table 7 the clinical outcomes of blood loss, conversion to open surgery rate, hospitalization costs, incisional hernia rate, and intraoperative complication rate are listed. Fifteen studies reported data on blood loss, of which eight studies observed no significant differences between RALS and CLS, but seven studies found significantly less blood loss in colectomies performed with RALS compared to CLS.
Regarding the conversion to open surgery rate, 15 out of 17 meta-analyses observed a significantly lower conversion to open surgery rate when patients were treated for colectomy with RALS compared to CLS. The other two meta-analyses observed no significant differences. One meta-analysis included only one primary source for its analysis of this outcome and should therefore be regarded as limited in evidential value (indicated in Table 7 with a c).
All six reviews that reported on hospitalization costs noted that the costs of RALS were significantly higher in comparison to CLS. Similar rates for incisional hernia were observed between RALS and CLS in four meta-analyses.
Only one meta-analysis considered the rate of intraoperative complications between RALS and CLS and found no significant differences. However, this finding was based on one primary source only, and therefore has limited evidential value (indicated in Table 7 with a c). The quantitative data collected about the postoperative (or overall) complication rate, the length of hospital stay, and the operative time are reported in Table 8.
Sixteen meta-analyses reported the postoperative (or overall) complication rate. In twelve out of sixteen reviews, no significant differences in complication rates were observed after colectomy performed by either RALS or CLS. These twelve studies included data from 26,029 patients. However, four studies, including 76,341 patients, did show a significantly lower complication rate in favour of RALS.
Ten out of eighteen studies that compared and analysed the length of hospital stay after colectomy by RALS or CLS reported similar outcomes. The other eight meta-analyses found that colectomy performed by RALS resulted in significantly shorter hospital stays.
The operative time of RALS was found to be significantly longer compared to CLS in sixteen meta-analyses. Only two studies observed no significant differences, but both lacked data: one study failed to report how many primary sources and patients were included in their analysis [54] and the other one considered a rather small group of patients [37].
The quantitative data collected on 30-day readmission rate and rate of wound infection can be found in Table 9. None of the four reviews that reported on the 30-day readmission rate found any significant differences between RALS and CLS.
Lastly, eight meta-analyses compared the rates of wound infection after CLS and RALS colectomy. All but one analysis found no significant differences between RALS and CLS. The one study that did find a significantly lower infection rate in favour of RALS comprised eight primary sources that together included 51,445 patients [50]. These results were obtained in patients undergoing a left hemicolectomy. The same authors conducted a similar analysis with patients undergoing a right hemicolectomy. In this study, covering 7698 patients, comparable results were obtained in wound infection rates between RALS and CLS colectomy [55].
Qualitative data were retrieved from two systematic reviews [30,57] and one meta-analysis [45]. Although this meta-analysis analysed clinical outcomes that were not included in this umbrella review, it did systematically review some clinical outcomes that were of interest [45]. These and the findings from [30,57] are summarized in Table A3. Blood loss, incisional hernia rates, and length of hospital stay were observed to be less or shorter for colectomies executed with RALS. The operative time was observed to be longer in the case of RALS in two studies. As for the postoperative complication and wound infection rates, comparable results were obtained.
3.3.3. Hysterectomy
Ten reviews reported outcomes related to hysterectomy [29,37,59,60,61,62,63,64,65,66]. Seven reviews were meta-analyses [29,37,60,61,62,63,66], two were network meta-analyses [64,65], and one was a systematic review [59]. All reviews scored either critically low [59,60,61,62,63,64,65,66] or low [37,64,66] on the AMSTAR 2 quality assessment, except for one meta-analysis [29]. This is the only study in which the quality was assessed to be high, having one non-critical and no critical flaws. With the use of a citation matrix, the corrected covered area was calculated to be 2.9% (Table A1), which indicates the existence of only a slight overlap between the primary sources of the included reviews.
Five studies specifically focused on radical hysterectomy procedures [61,63,64,65,66], three studies reviewed hysterectomy in general [29,59,62], and one study focused on single-site hysterectomy [60].
All quantitative data extracted from the included reviews are outlined in Table 10. In general, most studies found no significant differences in any of the outcomes. Seven studies analysed and compared the blood loss during a hysterectomy performed by either RALS or CLS, four of which reported no significant difference (although some pointed out a statistically non-significant favour for RALS). Three studies did observe a significantly lower blood loss when hysterectomies were performed by RALS.
Three studies reported the conversion to open surgery rate and none of these reviews found any significant differences. There were little data available regarding hospitalization costs. The only two meta-analyses reporting on hospitalization costs of RALS and CLS hysterectomy based their analysis on only one primary source [29,37]. Hence, these outcomes should be interpreted with caution (indicated in Table 10 with a d). In one study [29], it was shown that the costs of CLS hysterectomy were significantly lower compared to RALS, in contrast to another study [37] where no significant differences between RALS and CLS hysterectomy were found.
The intraoperative complication rate was analysed in six reviews and none measured any significant differences between RALS and CLS hysterectomy. The results of the postoperative complication rate, which was also defined in six reviews, were mixed. Two studies reported a significant difference in favour of RALS; the other four did not observe any significant difference.
Seven studies documented the length of hospital stay after RALS or CLS hysterectomy. Four of these reviews noted a significantly shorter hospital stay when patients underwent RALS compared to CLS. The other three studies did not observe any significant differences between the length of hospital stay after RALS or CLS hysterectomy but the results were in slight favour of RALS.
All data available on the operation time, 30-day readmission rate, and the rate of wound infections were non-significant. Hence, the results between RALS and CLS on these clinical outcomes were comparable.
Qualitative data were extracted from three reviews [59,60,64]. Ref. [59] is a systematic review. Ref. [60] is a meta-analysis, but it did not analyse the clinical outcomes of interest. However, this study was still included as it systematically reviewed and compared the postoperative complication rates and operative time of CLS and RALS hysterectomy. These qualitative data are included in Table A4. Finally, Ref. [64] is a network meta-analysis, which reported data that could not be included in the quantitative table (see also Discussion Section 4.2.10). Therefore, its outcomes are included in Table A4 as well.
In one review, blood loss was reported to be less in RALS hysterectomy compared to CLS. RALS was associated with higher hospitalization costs in one review. Two out of three reviews described comparable results in postoperative complication rates between RALS and CLS. However, a third review observed lower postoperative complication rates with RALS.
Regarding the operative time, two out of three reviews found comparable results between RALS and CLS hysterectomy. A third review did find a significantly shorter operative time when patients were treated with CLS hysterectomy. Lastly, one study reported similar lengths of hospital stays between RALS and CLS hysterectomy, and one study reported a shorter length of hospital stay after RALS.
3.3.4. Nephrectomy
Nine systematic reviews and (network) meta-analyses about nephrectomy were included [28,37,67,68,69,70,71,72,73]. Six papers were meta-analyses [37,68,69,70,71,72], two were systematic reviews [28,67], and one was a network meta-analysis [73]. The corrected covered area (an indication of the overlap of primary sources) was 1.4%, which is considered as a slight overlap (Table A1). The quality assessment scores of the nine reviews based on AMSTAR 2 were low for two reviews [37,67] and critically low for seven reviews [28,68,69,70,71,72,73].
Within nephrectomy, multiple surgical procedures and various indications exist. The surgical procedures discussed in the nine included reviews are radical nephrectomy [67,70], nephroureterectomy [67,68], (living) donor nephrectomy [71,73], partial nephrectomy [67], and nephrectomy in general [28,37,69,72]. Four reviews specifically included primary sources with renal cell carcinoma patients [28,69,70,72]; all other publications did not specify the indication for nephrectomy.
The quantitative data of all nine reviews are summarized in Table 11. Most reviews did not observe a significant difference between RALS nephrectomy and CLS nephrectomy. For blood loss specifically, one study reported significantly less blood loss when nephrectomies were performed by CLS [71]. All other reviews observed no significant differences in blood loss between the two surgical modalities. No significant differences were reported regarding the conversion to open surgery rate and postoperative complication rate either. One study observed significantly higher costs associated with RALS nephrectomy compared to CLS nephrectomy [70]. Regarding the intraoperative complication rate, four out of five reviews did not observe any significant differences between RALS and CLS, but one study [73] did: a significantly lower intraoperative complication rate was observed in CLS nephrectomy compared to RALS nephrectomy.
The length of hospital stay was noted to be comparable between RALS and CLS nephrectomy in four out of six studies. The other two studies reported a significantly shorter hospital stay after RALS nephrectomy [37,70]. It should be noted that the findings of [37] were based on one primary source only; hence, these outcomes are of limited value (indicated with an e in Table 10).
Lastly, six reviews collected and analysed data on operative time. Four out of these six reviews found no significant differences, but two reviews observed a significantly longer operative time when nephrectomy was conducted with the use of RALS [70,71].
As for the qualitative data, the synthesized findings are summarized in Table A5. In terms of blood loss, two reviews indicated that RALS has been found to be a more favourable option [28,68]. Regarding intraoperative complications, two out of three reviews noted fewer intraoperative complications during RALS [67,68]. Operative time was found to be longer in RALS procedures in one review, but comparable in two others. Two reviews claimed that the length of hospital stay was shorter after RALS nephrectomy [28,68].
3.3.5. Prostatectomy
Eight reviews were included that researched RALS and CLS prostatectomy. This included six meta-analyses [37,59,74,75,76,77] and two systematic reviews [27,67]. The corrected covered area was slight (3.1%). All reviews scored critically low on the AMSTAR quality assessment, except for two reviews that scored low [37,67]. Three meta-analyses specifically examined radical prostatectomy as a treatment for prostate cancer [59,74,75]. Three other meta-analyses focused on simple prostatectomy for the treatment of large benign prostatic hyperplasia [27,76,77]. In two studies, no additional information was provided on the indication of surgery and the specific surgical procedures executed [37,67].
Table 12 shows the quantitative data that were extracted from all the meta-analyses. Among the five studies reporting on blood loss after RALS or CLS prostatectomy, four found no significant difference between the two procedures. However, one study reported that significantly less blood was lost during RALS procedures [59].
Data on the conversion to open surgery rate was analysed in one meta-analysis [77]. Comparable results were obtained between RALS and CLS conversion rates during prostatectomy. Of the two studies reporting on the intraoperative complication rate, no significant differences were observed.
Regarding the postoperative complication rates, three out of four studies found no significant differences between RALS and CLS. One study investigated minor and major complications separately and found no significant differences between the two procedures in either category [77]. Only one study [75] reported a significantly lower postoperative complication rate in favour of RALS.
The length of hospital stay was assessed in two studies. In both studies, the length of hospital stay was significantly shorter after RALS prostatectomy in comparison with CLS prostatectomy. Lastly, the operative time was reported to be comparable between RALS and CLS in four out of five reviews. One study found a significantly shorter operative time during RALS prostatectomies [59]. It should be noted that one study [37] included only one primary source for its analyses (indicated with an f in Table 12); these outcomes are therefore of limited value.
Table A5 contains the qualitative data extracted from two systematic reviews [27,67]. In these two reviews, one reported no significant differences between RALS and CLS prostatectomy, while the other observed less blood loss during RALS prostatectomy. The same applies to the length of hospital stay; one reported no significant differences and the other observed a shorter stay after RALS prostatectomy. Comparable results were obtained regarding the intraoperative complications. Lastly, the operative time was shorter for CLS prostatectomies.
3.4. Summary of Data
All the data that were collected are synthesized in bar charts (Figure 2) and in Table 13. On most of the clinical outcomes of interest, RALS obtained comparable results to CLS. The hospitalization costs of RALS were higher compared to CLS across all but one surgical category, and the length of hospital stay was shorter or tended to be shorter when patients were treated by RALS in all but one surgical category. Furthermore, the operative time was in general longer in RALS cases when compared to CLS.

Table 6.
All quantitative data extracted from the included studies regarding cholecystectomy. A primary or secondary outcome is indicated with (I) or (II) respectively. The last column denotes whether the synthesized outcome favours CLS, RALS, or shows no significant differences. This distinction is highlighted using different cell colours: red represents a significant difference in favour of CLS, and yellow indicates no significant difference. a The result of the corresponding meta-analysis is based on one primary source only. Abbreviations: RALS = robot-assisted laparoscopic surgery, CLS = conventional laparoscopic surgery, RE = random effect model, FE = fixed effect model, CI = confidence interval, SR = single-incision robot surgery, MR = multiple-incision robot surgery, SL = single-incision laparoscopic surgery, ML = multiple-incision laparoscopic surgery, all = both single- and multiple-incision laparoscopic and robot surgery, OR = Odds Ratio, RR = Risk Ratio, RD = Risk Difference, MD = Mean Difference, N/A = not applicable or available.
Table 6.
All quantitative data extracted from the included studies regarding cholecystectomy. A primary or secondary outcome is indicated with (I) or (II) respectively. The last column denotes whether the synthesized outcome favours CLS, RALS, or shows no significant differences. This distinction is highlighted using different cell colours: red represents a significant difference in favour of CLS, and yellow indicates no significant difference. a The result of the corresponding meta-analysis is based on one primary source only. Abbreviations: RALS = robot-assisted laparoscopic surgery, CLS = conventional laparoscopic surgery, RE = random effect model, FE = fixed effect model, CI = confidence interval, SR = single-incision robot surgery, MR = multiple-incision robot surgery, SL = single-incision laparoscopic surgery, ML = multiple-incision laparoscopic surgery, all = both single- and multiple-incision laparoscopic and robot surgery, OR = Odds Ratio, RR = Risk Ratio, RD = Risk Difference, MD = Mean Difference, N/A = not applicable or available.
| Blood loss (II) | |||||||||||
| Author (year) | Ref. | Subgroup analysis | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Mean Difference/Odds Ratio [95%-CI] | Heterogeneity (I2) | Favours | ||
| Han et al., (2018) | [33] | all | 5/769 | 442 | 327 | RE | MD | −0.95 | [−3.69; 1.79] | 0% | None |
| Roh et al., (2018) | [37] | all | 1/136 | 83 | 53 | FE | MD | −2.23 | [−49.84; 45.38] | N/A | None a |
| Sun et al., (2018a) | [34] | SR vs. ML | 2/258 | 129 | 129 | FE | OR | 1.63 | [0.40; 6.56] | 0% | None |
| Conversion to open surgery rate (I) | |||||||||||
| Author (year) | Ref. | Subgroup analysis | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Risk Ratio/Odds Ratio [95%-CI] | Heterogeneity (I2) | Favours | ||
| Han et al., (2018) | [33] | all | 22/2771 | 1214 | 1557 | RE | RR | 0.53 | [0.26; 1.07] | 36% | None |
| Roh et al., (2018) | [37] | all | 2/146 | 70 | 76 | FE | OR | 0.85 | [0.18; 4.05] | N/A | None |
| Sun et al., (2018a) | [34] | SR vs. ML | 6/1537 | 715 | 822 | FE | OR | 1.30 | [0.71; 2.37] | 0% | None |
| Sun et al., (2018b) | [35] | SR vs. SL | 5/301 | 139 | 162 | FE | OR | 0.52 | [0.14; 1.96] | 0% | None |
| Hospitalization costs (I) | |||||||||||
| Author (year) | Ref. | Subgroup analysis | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Mean Difference [95%-CI] | Heterogeneity (I2) | Favours | ||
| Han et al., (2018) | [33] | all | 6/1176 | 456 | 720 | RE | MD | 3246 | [2416; 4075] | 96% | CLS |
| Sun et al., (2018a) | [34] | SR vs. ML | 2/643 | 177 | 466 | RE | MD | 3510 | [310; 6710] | 99% | CLS |
| Sun et al., (2018b) | [35] | SR vs. SL | 2/196 | 89 | 107 | FE | MD | 3700 | [3610; 3790] | 0% | CLS |
| Incisional hernia rate (II) | |||||||||||
| Author (year) | Ref. | Subgroup analysis | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Risk Ratio/Odds Ratio/Risk Difference [95%-CI] | Heterogeneity (I2) | Favours | ||
| Han et al., (2018) | [33] | all | 7/1499 | 676 | 823 | RE | RR | 3.22 | [1.54; 6.76] | 0% | CLS |
| Sun et al., (2018a) | [34] | SR vs. ML | 4/1381 | 622 | 759 | FE | OR | 4.23 | [1.87; 9.58] | 0% | CLS |
| Wang et al., (2021) | [36] | SR vs. SL | 15/916 | 534 | 382 | FE | RD | 0.05 | [0.02; 0.07] | 0% | CLS |
| Intraoperative complication rate (I) | |||||||||||
| Author (year) | Ref. | Subgroup analysis | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Risk Ratio/Odds Ratio [95%-CI] | Heterogeneity (I2) | Favours | ||
| Han et al., (2018) | [33] | All | 13/422 | 211 | 211 | RE | RR | 0.95 | [0.60; 1.50] | 2% | None |
| Sun et al., (2018b) | [35] | SR vs. SL | 4/219 | 101 | 118 | FE | OR | 0.48 | [0.17; 1.39] | 0% | None |
| Postoperative complication rate (I) | |||||||||||
| Author (year) | Ref. | Subgroup analysis | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Risk Ratio/Odds Ratio/Risk Difference [95%-CI] | Heterogeneity (I2) | Favours | ||
| Han et al., (2018) | [33] | all | 16/1859 | 817 | 1042 | RE | RR | 0.78 | [0.40; 1.52] | 28% | None |
| Roh et al., (2018) | [37] | all | 1/136 | 83 | 53 | RE | OR | 1.29 | [0.23; 7.31] | N/A | None a |
| Sun et al., (2018a) | [34] | SR vs. ML | 6/1536 | 714 | 822 | RE | OR | 1.11 | [0.35; 3.51] | 76% | None |
| Sun et al., (2018b) | [35] | SR vs. SL | 6/633 | 305 | 328 | FE | OR | 0.62 | [0.21; 1.86] | 0% | None |
| Wang et al., (2021) | [36] | SR vs. SL | 16/3161 | 1509 | 1652 | FE | RD | 0.01 | [−0.00; 0.03] | 44% | None |
| Length of hospital stay (days) (I) | |||||||||||
| Author (year) | Ref. | Subgroup analysis | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Mean Difference [95%-CI] | Heterogeneity (I2) | Favours | ||
| Han et al., (2018) | [33] | all | 17/3514 | 1602 | 1912 | RE | MD | −0.20 | [−0.49; 0.08] | 92% | None |
| Roh et al., (2018) | [37] | all | 3/216 | 123 | 93 | RE | MD | 0.07 | [−0.28; 0.42] | 0% | None |
| Sun et al., (2018a) | [34] | SR vs. ML | 4/1441 | 652 | 789 | RE | MD | −0.02 | [−0.60; 0.57] | 93% | None |
| Sun et al., (2018b) | [35] | SR vs. SL | 4/521 | 247 | 274 | FE | MD | −0.01 | [−0.21; 0.19] | 0% | None |
| Operative time (min) (I) | |||||||||||
| Author (year) | Ref. | Subgroup analysis | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Mean Difference [95%-CI] | Heterogeneity (I2) | Favours | ||
| Han et al., (2018) | [33] | all | 21/3640 | 1653 | 1987 | RE | MD | 13.14 | [4.97; 21.50] | 94% | CLS |
| Roh et al., (2018) | [37] | all | 4/302 | 163 | 139 | RE | MD | 10.09 | [−6.04; 26.21] | 85% | None |
| Sun et al., (2018a) | [34] | SR vs. ML | 2/697 | 424 | 273 | FE | MD | −3.06 | [−7.61; 1.49] | 0% | None |
| Sun et al., (2018b) | [35] | SR vs. SL | 5/551 | 267 | 284 | RE | MD | 17.32 | [−8.93; 43.57] | 97% | None |
| 30-day readmission rate (II) | |||||||||||
| Author (year) | Ref. | Subgroup analysis | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Risk Ratio/Odds Ratio [95%-CI] | Heterogeneity (I2) | Favours | ||
| Han et al., (2018) | [33] | all | 6/1420 | 811 | 609 | RE | RR | 1.21 | [0.62; 2.35] | 0% | None |
| Sun et al., (2018b) | [35] | SR vs. SL | 3/412 | 211 | 201 | FE | OR | 0.70 | [0.09; 5.63] | 0% | None |
| Wound infection rate (II) | |||||||||||
| Author (year) | Ref. | Subgroup analysis | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Odds Ratio [95%-CI] | Heterogeneity (I2) | Favours | ||
| Sun et al., (2018a) | [34] | SR vs. ML | 4/1319 | 606 | 713 | FE | OR | 1.92 | [0.86; 4.32] | 18% | None |

Table 7.
All quantitative data extracted from the included meta-analyses regarding colectomy (1/3). A primary or secondary outcome is indicated with (I) or (II) respectively. The last column denotes whether the synthesized outcome favours CLS, RALS, or shows no significant differences. This distinction is highlighted using different cell colours: red represents a significant difference in favour of CLS, yellow indicates no significant difference, and green indicates a significant difference in favour of RALS. c The result of the corresponding meta-analysis is based on one primary source only. Abbreviations: RALS = robot-assisted laparoscopic surgery, CLS = conventional laparoscopic surgery, RE = random effect model, FE = fixed effect model, CMH = Cochran–Mantel–Haenszel method, CI = confidence interval, OR = Odds Ratio, RR = Risk Ratio, MD = Mean Difference, SMD = Standardized Mean Difference, N/A = not applicable or available.
Table 7.
All quantitative data extracted from the included meta-analyses regarding colectomy (1/3). A primary or secondary outcome is indicated with (I) or (II) respectively. The last column denotes whether the synthesized outcome favours CLS, RALS, or shows no significant differences. This distinction is highlighted using different cell colours: red represents a significant difference in favour of CLS, yellow indicates no significant difference, and green indicates a significant difference in favour of RALS. c The result of the corresponding meta-analysis is based on one primary source only. Abbreviations: RALS = robot-assisted laparoscopic surgery, CLS = conventional laparoscopic surgery, RE = random effect model, FE = fixed effect model, CMH = Cochran–Mantel–Haenszel method, CI = confidence interval, OR = Odds Ratio, RR = Risk Ratio, MD = Mean Difference, SMD = Standardized Mean Difference, N/A = not applicable or available.
| Blood loss (II) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | (Standardized) Mean Difference [95%-CI] | Heterogeneity (I2) | Favours | ||
| Bianchi et al., (2022) | [40] | proctocolectomy, proctectomy | 3/194 | 105 | 89 | RE | MD | 57.99 | [−65.20; 181.17] | 81% | None |
| Sheng et al., (2018) | [41] | - | 40/12,825 | 129 | 6749 | RE | MD | −21.12 | [−175.07; 33.17] | N/A | None |
| Cuk et al., (2022) | [43] | - | 7/635 | 218 | 417 | RE | MD | −0.33 | [−16.54; 15.88] | 75% | None |
| Flynn et al., (2022) | [46] | total mesorectal excision | 30/- | N/A | N/A | RE | SMD | −0.12 | [−0.32; 0.08] | 93% | None |
| Gavriilidis et al., (2020) | [47] | total mesorectal excision | 16/3210 | N/A | N/A | RE | MD | 10.48 | [−15.50; 36.46] | 84% | None |
| Jones et al., (2018) | [31] | total mesorectal excision | 18/3002 | 1393 | 1609 | RE | SMD | −0.10 | [−0.26; 0.05] | 74% | None |
| Roh et al., (2018) | [37] | - | 2/136 | 64 | 72 | FE | MD | −20.10 | [−33.44; −6.75] | 0% | RALS |
| Solaini et al., (2022) | [50] | left hemicolectomy | 3/411 | 118 | 293 | RE | MD | −19.77 | [−39.10; −0.43] | 79% | RALS |
| Genova et al., (2021) | [51] | right hemicolectomy | 15/1413 | 536 | 877 | RE | MD | −12.14 | [−19.08; −5.20] | 18% | RALS |
| Lauka et al., (2020) | [52] | right hemicolectomy | 13/1379 | 523 | 856 | RE | MD | −8.68 | [−17.27; −0.08] | 46% | RALS |
| Ma et al., (2019) | [53] | right hemicolectomy | 8/694 | 234 | 460 | FE | MD | −16.89 | [−24.80; −8.98] | 35% | RALS |
| Rausa et al., (2019) | [54] | right hemicolectomy | -/- | N/A | N/A | RE | MD | 0.40 | [−28.00; 28.00] | 89% | None |
| Solaini et al., (2018) | [55] | right hemicolectomy | 8/888 | N/A | N/A | N/A | SMD | −0.19 | [−0.51; 0.12] | 77% | None |
| Tschann et al., (2022) | [56] | right hemicolectomy | 12/- | N/A | N/A | RE | MD | −10.03 | [−18.45; −1.61] | 65% | RALS |
| Zhu et al., (2021) | [58] | right hemicolectomy | 5/454 | 194 | 260 | FE | MD | −13.43 | [−20.65; −6.21] | 33% | RALS |
| Conversion to open surgery rate (I) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE/CMH | Risk Ratio/Odds Ratio [95%-CI] | Heterogeneity (I2) | Favours | ||
| Larkins et al., (2022) | [39] | diverticular resection | 8/13,190 | 3182 | 10,008 | RE | OR | 0.57 | [0.49; 0.66] | 0% | RALS |
| Bianchi et al., (2022) | [40] | sub(total) colectomy | 3/10,042 | 364 | 9678 | RE | OR | 0.17 | [0.04; 0.82] | 38% | RALS |
| Bianchi et al., (2022) | [40] | proctocolectomy, proctectomy | 4/240 | 128 | 112 | RE | OR | 0.45 | [0.09; 2.26] | 0% | None |
| Giuliani et al., (2022) | [42] | - | 9/3927 | 1922 | 2005 | FE | OR | 0.56 | [0.45; 0.70] | 31% | RALS |
| Cuk et al., (2022) | [43] | - | 17/10,906 | 1554 | 9352 | FE | OR | 0.31 | [0.23; 0.41] | 41% | RALS |
| Flynn et al., (2022) | [46] | total mesorectal excision | 44/9799 | 4476 | 5323 | CMH | OR | 0.34 | [0.27; 0.43] | 0% | RALS |
| Gavriilidis et al., (2020) | [47] | total mesorectal excision | 17/3381 | N/A | N/A | FE | OR | 0.26 | [0.17; 0.38] | 0% | RALS |
| Jones et al., (2018) | [31] | total mesorectal excision | 24/4961 | 2379 | 2582 | RE | OR | 0.40 | [0.29; 0.55] | 0% | RALS |
| Roh et al., (2018) | [37] | - | 4/226 | 110 | 116 | FE | OR | 0.25 | [0.07; 0.91] | 24% | RALS |
| Solaini et al., (2022) | [50] | left hemicolectomy | 9/52,058 | 13,281 | 38,777 | RE | RR | 0.53 | [0.50; 0.57] | 0% | RALS |
| Genova et al., (2021) | [51] | right hemicolectomy | 28/13,057 | 1777 | 11,280 | RE | OR | 0.46 | [0.34; −0.63] | 0% | RALS |
| Lauka et al., (2020) | [52] | right hemicolectomy | 21/9324 | 1519 | 7805 | RE | RR | 0.47 | [0.27; 0.81] | 33% | RALS |
| Ma et al., (2019) | [53] | right hemicolectomy | 9/800 | 336 | 464 | FE | OR | 0.34 | [0.15; 0.75] | 0% | RALS |
| Rausa et al., (2019) | [54] | right hemicolectomy | -/- | N/A | N/A | RE | RR | 1.70 | [0.53; 5.90] | 23% | None |
| Solaini et al., (2018) | [55] | right hemicolectomy | 10/7843 | N/A | N/A | N/A | RR | 0.59 | [0.38; 0.91] | 5% | RALS |
| Tschann et al., (2022) | [56] | right hemicolectomy | 19/- | N/A | N/A | RE | OR | 0.65 | [0.46; 0.93] | 14% | RALS |
| Zhu et al., (2021) | [58] | right hemicolectomy | 9/1084 | 488 | 596 | FE | OR | 0.30 | [0.17; 0.54] | 43% | RALS |
| Hospitalization costs (I) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | (Standardized) Mean Difference [95%-CI] | Heterogeneity (I2) | Favours | ||
| Roh et al., (2018) | [37] | - | 1/70 | 35 | 35 | RE | MD | 1.92 | [1.09; 2.74] | N/A | CLS c |
| Genova et al., (2021) | [51] | right hemicolectomy | 9/8660 | 875 | 7785 | RE | MD | 2589.46 | [972.72; 4206.21] | 94% | CLS |
| Lauka et al., (2020) | [52] | right hemicolectomy | 6/528 | 206 | 322 | RE | MD | 3185.50 | [720.98; 5650.02] | 95% | CLS |
| Rausa et al., (2019) | [54] | right hemicolectomy | 4/- | N/A | N/A | RE | SMD | 0.60 | [0.33; 0.86] | 66% | CLS |
| Solaini et al., (2018) | [55] | right hemicolectomy | 5/659 | N/A | N/A | N/A | SMD | 0.52 | [0.04; 1.00] | 84% | CLS |
| Tschann et al., (2022) | [56] | right hemicolectomy | 5/- | N/A | N/A | RE | MD | 2660 | [150; 5170] | 96% | CLS |
| Incisional hernia rate (II) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Risk Ratio/Odds Ratio [95%-CI] | Heterogeneity (I2) | Favours | ||
| Ravindra et al., (2022) | [44] | - | 2/684 | 342 | 342 | RE | RR | 0.93 | [0.05; 17.20] | 60% | None |
| Genova et al., (2021) | [51] | right hemicolectomy | 6/985 | 346 | 639 | RE | OR | 0.63 | [0.33; 1.19] | 0% | None |
| Solaini et al., (2018) | [55] | right hemicolectomy | 5/708 | N/A | N/A | N/A | RR | 0.38 | [0.07; 2.50] | 0% | None |
| Tschann et al., (2022) | [56] | right hemicolectomy | 3/- | N/A | N/A | RE | OR | 0.66 | [0.35; 1.28] | 0% | None |
| Intraoperative complication rate (I) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Odds Ratio [95%-CI] | Heterogeneity (I2) | Favours | ||
| Roh et al., (2018) | [37] | - | 1/34 | 18 | 16 | FE | OR | 4.29 | [0.43; 43.14] | N/A | None c |

Table 8.
All quantitative data extracted from the included meta-analyses regarding colectomy (2/3). A primary or secondary outcome is indicated with (I) or (II) respectively. The last column denotes whether the synthesized outcome favours CLS, RALS, or shows no significant differences. This distinction is highlighted using different cell colours: red represents a significant difference in favour of CLS, yellow indicates no significant difference, and green indicates a significant difference in favour of RALS. Abbreviations: RALS = robot-assisted laparoscopic surgery, CLS = conventional laparoscopic surgery, RE = random effect model, FE = fixed effect model, CMH = Cochran–Mantel–Haenszel method, CI = confidence interval, OR = Odds Ratio, RR = Risk Ratio, HG = Hedge’s G, MD = Mean Difference, SMD = Standardized Mean Difference, N/A = not applicable or available.
Table 8.
All quantitative data extracted from the included meta-analyses regarding colectomy (2/3). A primary or secondary outcome is indicated with (I) or (II) respectively. The last column denotes whether the synthesized outcome favours CLS, RALS, or shows no significant differences. This distinction is highlighted using different cell colours: red represents a significant difference in favour of CLS, yellow indicates no significant difference, and green indicates a significant difference in favour of RALS. Abbreviations: RALS = robot-assisted laparoscopic surgery, CLS = conventional laparoscopic surgery, RE = random effect model, FE = fixed effect model, CMH = Cochran–Mantel–Haenszel method, CI = confidence interval, OR = Odds Ratio, RR = Risk Ratio, HG = Hedge’s G, MD = Mean Difference, SMD = Standardized Mean Difference, N/A = not applicable or available.
| Postoperative complication rate (I) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE/CMH | Risk Ratio/Odds Ratio [95%-CI] | Heterogeneity (I2) | Favours | ||
| Larkins et al., (2022) | [39] | diverticular resection | 6/1384 | 663 | 721 | RE | OR | 0.74 | [0.49; 1.13] | 0% | None |
| Bianchi et al., (2022) | [40] | (sub)total colectomy | 3/10,042 | 364 | 9678 | RE | OR | 0.86 | [0.54; 1.38] | 19% | None |
| Bianchi et al., (2022) | [40] | proctocolectomy, proctectomy | 5/345 | 161 | 184 | RE | OR | 0.66 | [0.22; 1.73] | 0% | None |
| Sheng et al., (2018) | [41] | - | 40/12,825 | 129 | 6749 | RE | OR | 0.79 | [0.28; 2.13] | N/A | None |
| Giuliani et al., (2022) | [42] | - | 8/1453 | 686 | 767 | FE | OR | 0.76 | [0.58; 1.01] | 0% | None |
| Cuk et al., (2022) | [43] | - | 20/13,799 | 1740 | 12,059 | FE | OR | 0.85 | [0.73; 1.00] | 10% | RALS |
| Flynn et al., (2022) | [46] | total mesorectal excision | 43/9520 | 4317 | 5203 | CMH | OR | 0.84 | [0.76; 0.92] | 47% | RALS |
| Jones et al., (2018) | [31] | total mesorectal excision | 21/4833 | 2315 | 2518 | RE | OR | 0.92 | [0.75; 1.12] | 39% | None |
| Rausa et al., (2019) | [48] | total mesorectal excision | 22/- | N/A | N/A | RE | RR | 1.10 | [0.91; 1.30] | 0% | None |
| Flynn et al., (2021) | [49] | proctocolectomy with IPAA | 4/240 | 128 | 112 | CMH | OR | 0.65 | [0.38; 1.12] | 0% | None |
| Solaini et al., (2022) | [50] | left hemicolectomy | 10/52,061 | 13,330 | 38,731 | RE | RR | 0.86 | [0.83; 0.90] | 0% | RALS |
| Lauka et al., (2020) | [52] | right hemicolectomy | 16/- | N/A | N/A | RE | RR | 0.91 | [0.80; 1.04] | 0% | None |
| Ma et al., (2019) | [53] | right hemicolectomy | 11/961 | 402 | 559 | FE | OR | 0.73 | [0.52; 1.01] | 1% | RALS |
| Rausa et al., (2019) | [54] | right hemicolectomy | -/- | N/A | N/A | RE | RR | 1.00 | [0.66; 1.50] | 20% | None |
| Solaini et al., (2018) | [55] | right hemicolectomy | 10/7843 | N/A | N/A | N/A | RR | 0.95 | [0.50; 1.11] | 0% | None |
| Zhu et al., (2021) | [58] | right hemicolectomy | 5/854 | 383 | 471 | FE | OR | 0.83 | [0.60; 1.14] | 0% | None |
| Length of hospital stay (days) (I) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | (Standardized) Mean Difference [95%-CI] | Heterogeneity (I2) | Favours | ||
| Bianchi et al., (2022) | [40] | (sub)total colectomy | 2/102 | 38 | 64 | RE | MD | −1.86 | [−3.99; 0.26] | 0% | None |
| Bianchi et al., (2022) | [40] | proctocolectomy, proctectomy | 4/299 | 138 | 161 | RE | MD | −0.13 | [−1.80; 2.06] | 70% | None |
| Sheng et al., (2018) | [41] | - | 40/12,825 | 129 | 6749 | RE | MD | −0.34 | [−2.93; 2.21] | N/A | None |
| Giuliani et al., (2022) | [42] | - | 7/1426 | 683 | 743 | FE | SMD | −0.21 | [−0.32; −0.11] | 45% | RALS |
| Cuk et al., (2022) | [43] | - | 17/4626 | 981 | 3645 | RE | MD | −0.58 | [−1.37; 0.21] | 91% | None |
| Ravindra et al., (2022) | [44] | - | 12/1973 | 872 | 1101 | FE | SMD | −0.10 | [−0.19; −0.01] | 0% | RALS |
| Flynn et al., (2022) | [46] | total mesorectal excision | 39/- | N/A | N/A | RE | SMD | −0.22 | [−0.33; −0.11] | 83% | RALS |
| Gavriilidis et al., (2020) | [47] | total mesorectal excision | 23/4509 | N/A | N/A | RE | MD | −0.58 | [−1.24; 0.09] | 68% | None |
| Jones et al., (2018) | [31] | total mesorectal excision | 24/5010 | 2409 | 2601 | RE | SMD | −0.15 | [−0.27; −0.03] | 74% | RALS |
| Roh et al., (2018) | [37] | - | 4/226 | 110 | 116 | RE | MD | −0.54 | [−2.16; 1.08] | 54% | None |
| Solaini et al., (2022) | [50] | left hemicolectomy | 9/52,333 | 13,378 | 38,955 | RE | MD | −0.28 | [−0.63; 0.06] | 89% | None |
| Genova et al., (2021) | [51] | right hemicolectomy | 34/16,010 | 2059 | 13,951 | RE | MD | −0.50 | [−0.85; −0.15] | 58% | RALS |
| Lauka et al., (2020) | [52] | right hemicolectomy | 22/4945 | 1218 | 3727 | RE | MD | −0.60 | [−1.01; −0.19] | 64% | RALS |
| Ma et al., (2019) | [53] | right hemicolectomy | 10/7535 | 534 | 7001 | RE | MD | −0.61 | [−1.15; −0.06] | 52% | RALS |
| Rausa et al., (2019) | [54] | right hemicolectomy | -/- | N/A | N/A | RE | MD | 2.90 | [−0.70; 6.50] | 80% | None |
| Solaini et al., (2018) | [55] | right hemicolectomy | 10/7968 | N/A | N/A | N/A | SMD | −0.09 | [−0.30; 0.06] | 67% | None |
| Tschann et al., (2022) | [56] | right hemicolectomy | 20/- | N/A | N/A | RE | MD | −0.84 | [−1.38; −0.29] | 87% | RALS |
| Zhu et al., (2021) | [58] | right hemicolectomy | 4/442 | 188 | 254 | FE | MD | −0.23 | [−0.73; 0.28] | 0% | None |
| Operative time (min) (I) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | (Standardized) Mean Difference/Hedge’s G [95%-CI] | Heterogeneity (I2) | Favours | ||
| Larkins et al., (2022) | [39] | diverticular resection | 6/3675 | 1812 | 1863 | RE | HG | 0.43 | [0.04; 0.81] | 95% | CLS |
| Bianchi et al., (2022) | [40] | (sub)total colectomy | 2/102 | 38 | 64 | RE | MD | 104.64 | [18.42; 190.87] | 58% | CLS |
| Bianchi et al., (2022) | [40] | proctocolectomy, proctectomy | 4/299 | 138 | 161 | RE | MD | 38.88 | [18.70; 59.06] | 36% | CLS |
| Sheng et al., (2018) | [41] | - | 40/12,825 | 129 | 6749 | RE | MD | 65.69 | [38.01; 94.10] | N/A | CLS |
| Giuliani et al., (2022) | [42] | - | 8/1453 | 686 | 767 | FE | SMD | 0.49 | [0.38; 0.60] | 94% | CLS |
| Cuk et al., (2022) | [43] | - | 19/5184 | 1229 | 3955 | RE | MD | 42.99 | [28.37; 57.60] | 97% | CLS |
| Flynn et al., (2022) | [46] | total mesorectal excision | 41/- | N/A | N/A | RE | SMD | 0.82 | [0.60; 1.04] | 96% | CLS |
| Gavriilidis et al., (2020) | [47] | total mesorectal excision | 26/4734 | N/A | N/A | RE | MD | 50.35 | [31.70; 70.69] | 97% | CLS |
| Jones et al., (2018) | [31] | total mesorectal excision | 27/5449 | 2601 | 2848 | RE | SMD | 0.65 | [0.43; 0.87] | 93% | CLS |
| Roh et al., (2018) | [37] | - | 4/226 | 110 | 116 | RE | MD | 23.83 | [−11.87; 59.53] | 94% | None |
| Solaini et al., (2022) | [50] | left hemicolectomy | 10/52,439 | 13,438 | 39,001 | RE | MD | 39.08 | [17.26; 60.91] | 97% | CLS |
| Genova et al., (2021) | [51] | right hemicolectomy | 35/16,292 | 2178 | 14,114 | RE | MD | 56.43 | [45.43; 67.43] | 91% | CLS |
| Lauka et al., (2020) | [52] | right hemicolectomy | 22/11,664 | 1523 | 10,141 | RE | MD | 45.36 | [31.75; 58.97] | 95% | CLS |
| Ma et al., (2019) | [53] | right hemicolectomy | 12/7740 | 656 | 7084 | RE | MD | 43.60 | [26.71; 60.48] | 92% | CLS |
| Rausa et al., (2019) | [54] | right hemicolectomy | -/- | N/A | N/A | RE | MD | −24.00 | [−70.00; 21.00] | 90% | None |
| Solaini et al., (2018) | [55] | right hemicolectomy | 11/8257 | 869 | 7388 | N/A | SMD | 0.99 | [0.60; 1.40] | 95% | CLS |
| Tschann et al., (2022) | [56] | right hemicolectomy | 22/- | N/A | N/A | RE | MD | 42.01 | [32.96; 51.06] | 89% | CLS |
| Zhu et al., (2021) | [58] | right hemicolectomy | 6/522 | 255 | 267 | RE | MD | 65.20 | [53.40; 77.01] | 55% | CLS |

Table 9.
All quantitative data extracted from the included meta-analyses regarding colectomy (3/3). A primary or secondary outcome is indicated with (I) or (II) respectively. The last column denotes whether the synthesized outcome favours CLS, RALS, or shows no significant differences. This distinction is highlighted using different cell colours: pink represents a significant difference in favour of CLS, yellow indicates no significant difference, and green indicates a significant difference in favour of RALS. Abbreviations: RALS = robot-assisted laparoscopic surgery, CLS = conventional laparoscopic surgery, RE = random effect model, FE = fixed effect model, CMH = Cochran–Mantel–Haenszel method, CI = confidence interval, OR = Odds Ratio, RR = Risk Ratio, HG = Hedge’s G, MD = Mean Difference, SMD = Standardized Mean Difference, N/A = not applicable or available.
Table 9.
All quantitative data extracted from the included meta-analyses regarding colectomy (3/3). A primary or secondary outcome is indicated with (I) or (II) respectively. The last column denotes whether the synthesized outcome favours CLS, RALS, or shows no significant differences. This distinction is highlighted using different cell colours: pink represents a significant difference in favour of CLS, yellow indicates no significant difference, and green indicates a significant difference in favour of RALS. Abbreviations: RALS = robot-assisted laparoscopic surgery, CLS = conventional laparoscopic surgery, RE = random effect model, FE = fixed effect model, CMH = Cochran–Mantel–Haenszel method, CI = confidence interval, OR = Odds Ratio, RR = Risk Ratio, HG = Hedge’s G, MD = Mean Difference, SMD = Standardized Mean Difference, N/A = not applicable or available.
| 30-day readmission rate (II) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE/CMH | Risk Ratio/Odds Ratio [95%-CI] | Heterogeneity (I2) | Favours | ||
| Ravindra et al., (2022) | [44] | - | 7/797 | 327 | 470 | FE | RR | 0.89 | [0.50; 1.60] | 6% | None |
| Gavriilidis et al., (2020) | [47] | total mesorectal excision | 4/508 | N/A | N/A | FE | OR | 1.17 | [0.54; 2.56] | 68% | None |
| Flynn et al., (2021) | [49] | proctocolectomy with IPAA | 3/207 | 112 | 95 | CMH | OR | 0.73 | [0.35; 1.55] | 0% | None |
| Genova et al., (2021) | [51] | right hemicolectomy | 12/8691 | 1072 | 7619 | RE | OR | 0.98 | [0.53; 1.82] | 38% | None |
| Wound infection rate (II) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Risk Ratio/Odds Ratio [95%-CI] | Heterogeneity (I2) | Favours | ||
| Sheng et al., (2018) | [41] | - | 40/12,825 | 129 | 6749 | RE | OR | 1.09 | [0.11; 8.45] | N/A | None |
| Cuk et al., (2022) | [43] | - | 15/4598 | 940 | 3658 | FE | OR | 0.81 | [0.55; 1.20] | 0% | None |
| Ravindra et al., (2022) | [44] | - | 11/1796 | 822 | 974 | FE | RR | 1.00 | [0.65; 1.53] | 0% | None |
| Rausa et al., (2019) | [48] | total mesorectal excision | 17/- | N/A | N/A | RE | RR | 1.50 | [0.86; 2.60] | 0% | None |
| Solaini et al., (2022) | [50] | left hemicolectomy | 8/51,445 | 13,061 | 38,384 | RE | RR | 0.78 | [0.70; 0.87] | 0% | RALS |
| Solaini et al., (2018) | [55] | right hemicolectomy | 8/7698 | N/A | N/A | N/A | RR | 0.67 | [0.42; 1.11] | 0% | None |
| Tschann et al., (2022) | [56] | right hemicolectomy | 16/- | N/A | N/A | RE | OR | 0.87 | [0.64; 1.19] | 0% | None |
| Zhu et al., (2021) | [58] | right hemicolectomy | 5/709 | 329 | 380 | FE | OR | 0.65 | [0.34; 1.25] | 0% | None |

Table 10.
All quantitative data extracted from the included meta-analyses regarding hysterectomy. A primary or secondary outcome is indicated with (I) or (II) respectively. The last column denotes whether the synthesized outcome favours CLS, RALS, or shows no significant differences. This distinction is highlighted using different cell colours: red represents a significant difference in favour of CLS, yellow indicates no significant difference, and green indicates a significant difference in favour of RALS. d The result of the corresponding meta-analysis is based on one primary source only. Abbreviations: RALS = robot-assisted laparoscopic surgery, CLS = conventional laparoscopic surgery, RE = random effect model, FE = fixed effect model, OR = Odds Ratio, RR = Risk Ratio, MD = Mean Difference, N/A = not applicable or available.
Table 10.
All quantitative data extracted from the included meta-analyses regarding hysterectomy. A primary or secondary outcome is indicated with (I) or (II) respectively. The last column denotes whether the synthesized outcome favours CLS, RALS, or shows no significant differences. This distinction is highlighted using different cell colours: red represents a significant difference in favour of CLS, yellow indicates no significant difference, and green indicates a significant difference in favour of RALS. d The result of the corresponding meta-analysis is based on one primary source only. Abbreviations: RALS = robot-assisted laparoscopic surgery, CLS = conventional laparoscopic surgery, RE = random effect model, FE = fixed effect model, OR = Odds Ratio, RR = Risk Ratio, MD = Mean Difference, N/A = not applicable or available.
| Blood loss (II) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Mean Difference [95%-CI] | Heterogeneity (I2) | Favours | ||
| Prodromidou et al., (2020) | [60] | single-site hysterectomy | 5/287 | 125 | 162 | RE | MD | −10.84 | [−20.35; −1.32] | 55% | RALS |
| Kampers et al., (2022) | [61] | radical hysterectomy | 5/343 | 139 | 204 | RE | MD | −30.89 | [−114.46; 52.69] | - | None |
| Marchand et al., (2021) | [62] | - | 2/196 | 111 | 85 | FE | MD | −85.27 | [−124.09; −46.45] | 0% | RALS |
| Zhang et al., (2019) | [63] | radical hysterectomy | 8/640 | 283 | 357 | RE | MD | −22.25 | [−81.38; 36.87] | 89% | None |
| Jin et al., (2018) | [65] | radical hysterectomy | 5/- | N/A | N/A | RE | MD | −40.39 | [−117.75; 35.97] | 96% | None |
| Lawrie et al., (2019) | [29] | - | 1/95 | 47 | 48 | RE | MD | 7.00 | [−18.26; 32.26] | N/A | None d |
| Roh et al., (2018) | [37] | - | 5/478 | 235 | 243 | FE | MD | −5.57 | [−8.81; −2.32] | 14% | RALS |
| Conversion to open surgery rate (I) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Risk Ratio/Odds Ratio [95%-CI] | Heterogeneity (I2) | Favours | ||
| Zhang et al., (2019) | [63] | radical hysterectomy | 3/176 | 98 | 78 | RE | OR | 0.66 | [0.09; 4.67] | 30% | None |
| Lawrie et al., (2019) | [29] | - | 3/269 | 134 | 135 | RE | RR | 1.17 | [0.24; 5.77] | 0% | None |
| Roh et al., (2018) | [37] | - | 4/368 | 184 | 184 | FE | OR | 0.46 | [0.15; 1.44] | 33% | None |
| Hospitalization costs (I) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Mean Difference [95%-CI] | Heterogeneity (I2) | Favours | ||
| Lawrie et al., (2019) | [29] | - | 1/97 | 61 | 36 | RE | MD | 1564.00 | [1079.57; 2048.43] | N/A | CLS d |
| Roh et al., (2018) | [37] | - | 1/74 | 38 | 36 | RE | MD | 0.09 | [−0.43; 0.61] | N/A | None d |
| Intraoperative complication rate (I) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Risk Ratio/Odds Ratio [95%-CI] | Heterogeneity (I2) | Favours | ||
| Marchand et al., (2021) | [62] | - | 4/708 | 359 | 349 | RE | RR | 1.15 | [0.30; 4.35] | 36% | None |
| Zhang et al., (2019) | [63] | radical hysterectomy | 7/588 | 249 | 339 | RE | OR | 1.17 | [0.44; 3.10] | 0% | None |
| Jin et al., (2018) | [65] | radical hysterectomy | 3/- | N/A | N/A | FE | OR | 0.83 | [0.16; 4.34] | 63% | None |
| Hwang et al., (2020) | [66] | radical hysterectomy | 23/2855 | 986 | 1869 | FE | OR | 0.86 | [0.48; 1.55] | 0% | None |
| Lawrie et al., (2019) | [29] | - | 5/487 | 256 | 231 | RE | RR | 1.05 | [0.31; 3.56] | 28% | None |
| Roh et al., (2018) | [37] | - | 3/316 | 158 | 158 | FE | OR | 1.11 | [0.48; 2.53] | 48% | None |
| Postoperative complication rate (I) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Risk Ratio/Odds Ratio [95%-CI] | Heterogeneity (I2) | Favours | ||
| Marchand et al., (2021) | [62] | - | 4/708 | 359 | 349 | RE | RR | 0.93 | [0.50; 1.75] | 59% | None |
| Zhang et al., (2019) | [63] | radical hysterectomy | 9/678 | 305 | 373 | RE | OR | 0.66 | [0.39; 1.12] | 31% | None |
| Jin et al., (2018) | [65] | radical hysterectomy | 2/- | N/A | N/A | FE | OR | 0.42 | [0.20; 0.87] | 0% | RALS |
| Hwang et al., (2020) | [66] | radical hysterectomy | 23/2855 | 986 | 1869 | FE | OR | 0.94 | [0.64; 1.38] | 0% | None |
| Lawrie et al., (2019) | [29] | - | 5/533 | 291 | 242 | RE | RR | 0.82 | [0.42; 1.59] | 51% | None |
| Roh et al., (2018) | [37] | - | 3/316 | 158 | 158 | RE | OR | 0.96 | [0.28; 3.25] | 72% | None |
| Length of hospital stay (days) (I) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Mean Difference [95%-CI] | Heterogeneity (I2) | Favours | ||
| Prodromidou et al., (2020) | [60] | single-site hysterectomy | 4/328 | 119 | 209 | RE | MD | −0.32 | [−0.44; −0.19] | 0% | RALS |
| Kampers et al., (2022) | [61] | radical hysterectomy | 5/343 | 139 | 204 | RE | MD | −0.96 | [−2.33; 0.41] | - | None |
| Marchand et al., (2021) | [62] | - | 3/246 | 136 | 110 | RE | MD | −1.20 | [−2.01; −0.38] | 91% | RALS |
| Zhang et al., (2019) | [63] | radical hysterectomy | 9/678 | 305 | 373 | RE | MD | −0.24 | [−1.33; 0.85] | 87% | None |
| Jin et al., (2018) | [65] | radical hysterectomy | 4/- | N/A | N/A | RE | MD | −1.01 | [−2.82; 0.80] | 92% | None |
| Lawrie et al., (2019) | [29] | - | 2/192 | 108 | 84 | RE | MD | −0.30 | [−0.53; −0.07] | 0% | RALS |
| Roh et al., (2018) | [37] | - | 5/425 | 212 | 213 | RE | MD | −0.56 | [−1.04; −0.09] | 73% | RALS |
| Operative time (min) (I) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Mean Difference [95%-CI] | Heterogeneity (I2) | Favours | ||
| Kampers et al., (2022) | [61] | radical hysterectomy | 5/343 | 139 | 204 | RE | MD | 30.84 | [−0.72; 62.40] | - | None |
| Zhang et al., (2019) | [63] | radical hysterectomy | 9/678 | 305 | 373 | RE | MD | 18.10 | [−14.94; 51.13] | 93% | None |
| Jin et al., (2018) | [65] | radical hysterectomy | 5/- | N/A | N/A | RE | MD | −8.24 | [−61.56; 45.07] | 97% | None |
| Lawrie et al., (2019) | [29] | - | 2/148 | 73 | 75 | RE | MD | 41.18 | [−6.17; 88.53] | 80% | None |
| Roh et al., (2018) | [37] | - | 5/410 | 205 | 205 | RE | MD | −1.24 | [−32.57; 30.09] | 95% | None |
| 30-day readmission rate (II) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Risk Ratio [95%-CI] | Heterogeneity (I2) | Favours | ||
| Lawrie et al., (2019) | [29] | - | 2/220 | 122 | 98 | RE | RR | 0.46 | [0.14; 1.48] | 0% | None |
| Wound infection rate (II) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Risk Ratio [95%-CI] | Heterogeneity (I2) | Favours | ||
| Marchand et al., (2021) | [62] | - | 3/340 | 183 | 157 | FE | RR | 1.43 | [0.50; 4.00] | 0% | None |
| Lawrie et al., (2019) | [29] | - | 4/367 | 195 | 172 | RE | RR | 0.62 | [0.13; 2.88] | 2% | None |

Table 11.
All quantitative data extracted from the included meta-analyses regarding nephrectomy. A primary or secondary outcome is indicated with (I) or (II) respectively. The last column denotes whether the synthesized outcome favours CLS, RALS, or shows no significant differences. This distinction is highlighted using different cell colours: red represents a significant difference in favour of CLS, yellow indicates no significant difference, and green indicates a significant difference in favour of RALS. e The result of the corresponding meta-analysis is based on one primary source only. Abbreviations: RALS = robot-assisted laparoscopic surgery, CLS = conventional laparoscopic surgery, RE = random effect model, FE = fixed effect model, CI = confidence interval, OR = Odds Ratio, MD = Mean Difference, SMD = Standardized Mean Difference, N/A = not applicable or available.
Table 11.
All quantitative data extracted from the included meta-analyses regarding nephrectomy. A primary or secondary outcome is indicated with (I) or (II) respectively. The last column denotes whether the synthesized outcome favours CLS, RALS, or shows no significant differences. This distinction is highlighted using different cell colours: red represents a significant difference in favour of CLS, yellow indicates no significant difference, and green indicates a significant difference in favour of RALS. e The result of the corresponding meta-analysis is based on one primary source only. Abbreviations: RALS = robot-assisted laparoscopic surgery, CLS = conventional laparoscopic surgery, RE = random effect model, FE = fixed effect model, CI = confidence interval, OR = Odds Ratio, MD = Mean Difference, SMD = Standardized Mean Difference, N/A = not applicable or available.
| Blood loss (II) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Mean Difference [95%-CI] | Heterogeneity (I2) | Favours | ||
| Li et al., (2020) | [69] | - | 6/1372 | 532 | 840 | RE | MD | 1.83 | [−18.61; 22.27] | 74% | None |
| Crocerossa et al., (2021) | [70] | radical nephrectomy | 5/1135 | 511 | 624 | RE | MD | 2.18 | [−26.69; 31.04] | 84% | None |
| Wang et al., (2019) | [71] | donor nephrectomy | 4/324 | 130 | 194 | FE | MD | 28.30 | [10.24; 46.37] | 0% | CLS |
| Sharma et al., (2022) | [72] | partial nephrectomy | 5/969 | N/A | N/A | RE | MD | −16.98 | [−52.03; 18.08] | 80% | None |
| Xiao et al., (2020) | [73] | donor nephrectomy | -/- | N/A | N/A | N/A | MD | 2.60 | [−52.57; 55.09] | N/A | None |
| Conversion to open surgery rate (I) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Odds Ratio [95%-CI] | Heterogeneity (I2) | Favours | ||
| Li et al., (2020) | [69] | - | 4/1334 | 516 | 813 | RE | OR | 2.67 | [0.69; 10.33] | 51% | None |
| Wang et al., (2019) | [71] | donor nephrectomy | 2/190 | 96 | 94 | RE | OR | 0.57 | [0.11; 2.93] | 0% | None |
| Hospitalization costs (I) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Mean Difference [95%-CI] | Heterogeneity (I2) | Favours | ||
| Crocerossa et al., (2021) | [70] | radical nephrectomy | 4/50,990 | 13,296 | 37,694 | RE | MD | 4.70 | [3.58; 5.82] | 67% | CLS |
| Intraoperative complication rate (I) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Odds Ratio [95%-CI] | Heterogeneity (I2) | Favours | ||
| Li et al., (2020) | [69] | - | 4/- | N/A | N/A | RE | OR | 1.13 | [0.61; 2.12] | 51% | None |
| Crocerossa et al., (2021) | [70] | radical nephrectomy | 4/7138 | 5421 | 1717 | RE | OR | 1.01 | [0.17; 6.03] | 95% | None |
| Sharma et al., (2022) | [72] | partial nephrectomy | 3/- | N/A | N/A | FE | OR | 0.57 | [0.27; 1.22] | 0% | None |
| Xiao et al., (2020) | [73] | donor nephrectomy | -/- | N/A | N/A | N/A | OR | 22.5 | [1.59; 630.10] | N/A | CLS |
| Postoperative complication rate (I) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Odds Ratio [95%-CI] | Heterogeneity (I2) | Favours | ||
| Li et al., (2020) | [69] | - | 6/- | N/A | N/A | FE | OR | 1.07 | [0.68; 1.67] | 0% | None |
| Crocerossa et al., (2021) | [70] | radical nephrectomy | 7/33,397 | 10,617 | 22,780 | RE | OR | 0.93 | [0.70; 1.23] | 83% | None |
| Wang et al., (2019) | [71] | donor nephrectomy | 5/369 | 145 | 224 | FE | OR | 1.12 | [0.52; 2.44] | 0% | None |
| Xiao et al., (2020) | [73] | donor nephrectomy | -/- | N/A | N/A | N/A | OR | 1.15 | [0.44; 3.07] | N/A | None |
| Length of hospital stay (days) (I) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Mean Difference [95%-CI] | Heterogeneity (I2) | Favours | ||
| Li et al., (2020) | [69] | - | 7/1832 | 762 | 1070 | RE | MD | −0.34 | [−0.68; −0.00] | 85% | None |
| Crocerossa et al., (2021) | [70] | radical nephrectomy | 7/26,100 | 8528 | 17,572 | RE | MD | −0.84 | [−1.52; −0.16] | 99% | RALS |
| Wang et al., (2019) | [71] | donor nephrectomy | 7/514 | 250 | 264 | RE | MD | −6.79 | [−17.25; 3.66] | 81% | None |
| Sharma et al., (2022) | [72] | partial nephrectomy | 5/969 | N/A | N/A | RE | MD | −0.36 | [−1.04; 0.32] | 93% | None |
| Xiao et al., (2020) | [73] | donor nephrectomy | -/- | N/A | N/A | N/A | MD | −0.01 | [−0.66; 0.69] | N/A | None |
| Roh et al., (2018) | [37] | - | 1/45 | 15 | 30 | RE | MD | −1.00 | [−1.38; −0.62] | N/A | RALS e |
| Operative time (min) (I) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | (Standardized) Mean Difference [95%-CI] | Heterogeneity (I2) | Favours | ||
| Li et al., (2020) | [69] | - | 6/1372 | 532 | 840 | RE | MD | 29.05 | [−0.31; 58.41] | 93% | None |
| Crocerossa et al., (2021) | [70] | radical nephrectomy | 5/1328 | 511 | 817 | RE | MD | 37.44 | [3.94; 70.94] | 94% | CLS |
| Wang et al., (2019) | [71] | donor nephrectomy | 7/510 | 249 | 261 | RE | SMD | 0.53 | [0.20; 0.85] | 59% | CLS |
| Sharma et al., (2022) | [72] | partial nephrectomy | 5/969 | N/A | N/A | RE | MD | −11.74 | [−38.17; 14.69] | 93% | None |
| Xiao et al., (2020) | [73] | donor nephrectomy | -/- | N/A | N/A | N/A | MD | 16.06 | [−13.46; 46.82] | N/A | None |
| Roh et al., (2018) | [37] | - | 1/45 | 15 | 30 | RE | MD | 15.87 | [−4.79; 36.53] | N/A | None e |

Table 12.
All quantitative data extracted from the included meta-analyses regarding prostatectomy. A primary or secondary outcome is indicated with (I) or (II) respectively. The last column denotes whether the synthesized outcome favours CLS, RALS, or shows no significant differences. This distinction is highlighted using different cell colours: red represents a significant difference in favour of CLS, yellow indicates no significant difference, and green indicates a significant difference in favour of RALS. * In [77], minor and major postoperative complications were reported separately, of which both are included. f The result of the corresponding meta-analysis is based on one primary source only. Abbreviations: RALS = robot-assisted laparoscopic surgery, CLS = conventional laparoscopic surgery, RE = random effect model, FE = fixed effect model, CI = confidence interval, OR = Odds Ratio, MD = Mean Difference, SMD = Standardized Mean Difference, N/A = not applicable or available.
Table 12.
All quantitative data extracted from the included meta-analyses regarding prostatectomy. A primary or secondary outcome is indicated with (I) or (II) respectively. The last column denotes whether the synthesized outcome favours CLS, RALS, or shows no significant differences. This distinction is highlighted using different cell colours: red represents a significant difference in favour of CLS, yellow indicates no significant difference, and green indicates a significant difference in favour of RALS. * In [77], minor and major postoperative complications were reported separately, of which both are included. f The result of the corresponding meta-analysis is based on one primary source only. Abbreviations: RALS = robot-assisted laparoscopic surgery, CLS = conventional laparoscopic surgery, RE = random effect model, FE = fixed effect model, CI = confidence interval, OR = Odds Ratio, MD = Mean Difference, SMD = Standardized Mean Difference, N/A = not applicable or available.
| Blood loss (II) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | (Standardized) Mean Difference [95%-CI] | Heterogeneity (I2) | Favours | ||
| Du et al., (2018) | [59] | radical prostatectomy | 5/3185 | 1466 | 1692 | RE | SMD | −0.31 | [−0.61; −0.01] | 87% | RALS |
| Carbonara et al., (2021) | [74] | radical prostatectomy | 10/4722 | 2328 | 2394 | RE | MD | −53.19 | [−116.11; 9.74] | 97% | None |
| Wang et al., (2019) | [75] | radical prostatectomy | 9/1914 | 912 | 1002 | RE | SMD | −0.38 | [−0.84; 0.08] | 95% | None |
| Pandolfo et al., (2022) | [76] | simple prostatectomy | 5/2006 | 828 | 1178 | RE | MD | −23.33 | [−85.93; 39.27] | 89% | None |
| Roh et al., (2018) | [37] | - | 1/120 | 60 | 60 | FE | MD | −32.10 | [−81.36; 17.16] | N/A | None f |
| Conversion to open surgery rate (I) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Odds Ratio [95%-CI] | Heterogeneity (I2) | Favours | ||
| Li et al., (2022) | [77] | simple prostatectomy | 4/1878 | 728 | 1150 | RE | OR | 0.89 | [0.55; 1.45] | 0% | None |
| Roh et al., (2018) | [37] | - | 1/112 | 52 | 60 | FE | OR | 2.00 | [0.61; 6.55] | N/A | None f |
| Intraoperative complication rate (I) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Odds Ratio [95%-CI] | Heterogeneity (I2) | Favours | ||
| Li et al., (2022) | [77] | simple prostatectomy | 5/1928 | 753 | 1175 | RE | OR | 1.16 | [0.70; 1.92] | 0% | None |
| Postoperative complication rate (I) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Risk Ratio/Odds Ratio [95%-CI] | Heterogeneity (I2) | Favours | ||
| Carbonara et al., (2021) | [74] | radical prostatectomy | 9/5585 | 3048 | 2537 | RE | OR | 1.03 | [0.78; 1.34] | 37% | None |
| Wang et al., (2019) | [75] | radical prostatectomy | 8/5155 | 3975 | 1180 | RE | OR | 0.61 | [0.46; 0.81] | 35% | RALS |
| Pandolfo et al., (2022) | [76] | simple prostatectomy | 5/2006 | 828 | 1178 | RE | RR | 1.66 | [0.94; 2.91] | 66% | None |
| Li et al., (2022)—minor compl. | [77] | simple prostatectomy | 3/1810 | 696 | 1114 | RE | OR | 2.22 | [0.96; 5.00] | 72% | None * |
| Li et al., (2022)—major compl. | [77] | simple prostatectomy | 3/1810 | 696 | 1114 | RE | OR | 2.38 | [0.99; 5.56] | 15% | None * |
| Length of hospital stay (days) (I) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | Mean Difference [95%-CI] | Heterogeneity (I2) | Favours | ||
| Pandolfo et al., (2022) | [76] | simple prostatectomy | 4/1767 | 674 | 1093 | RE | MD | −1.44 | [−2.48; −0.40] | 97% | RALS |
| Li et al., (2022) | [77] | simple prostatectomy | 4/1767 | 674 | 1093 | RE | MD | −1.20 | [−2.32; −0.09] | 99% | RALS |
| Operative time (min) (I) | |||||||||||
| Author (year) | Ref. | Surgical specifications | No. studies/participants | Participants RALS | Participants CLS | RE/FE | (Standardized) Mean Difference [95%-CI] | Heterogeneity (I2) | Favours | ||
| Du et al., (2018) | [59] | radical prostatectomy | 7/4604 | 1795 | 2809 | RE | SMD | −0.71 | [−1.25; −0.18] | 97% | RALS |
| Carbonara et al., (2021) | [74] | radical prostatectomy | 9/3541 | 2190 | 1351 | RE | MD | −16.36 | [−46.33; 13.60] | 99% | None |
| Pandolfo et al., (2022) | [76] | simple prostatectomy | 5/2003 | 828 | 1175 | RE | MD | 19.14 | [−4.12; 42.39] | 95% | None |
| Li et al., (2022) | [77] | simple prostatectomy | 5/1928 | 753 | 1175 | RE | MD | 24.34 | [−0.82; 49.50] | 96% | None |
| Roh et al., (2018) | [37] | - | 1/120 | 60 | 60 | RE | MD | 8.90 | [−1.27; 19.07] | N/A | None f |

Table 13.
This overview summarizes all the included data. Per surgical category, the clinical outcomes favour either RALS, CLS, None, or in between (RALS/None and CLS/None). Colours are used in a stoplight format to emphasize these findings: red denotes a preference for CLS, yellow indicates comparable outcomes between CLS and RALS, and green indicates that RALS is the superior option for that particular outcome. A grey background was used for RALS/None and CLS/None cases. The final row indicates whether the clinical outcomes favour RALS, CLS, or None across all surgical procedures. A primary or secondary outcome is indicated with (I) or (II) respectively.
Table 13.
This overview summarizes all the included data. Per surgical category, the clinical outcomes favour either RALS, CLS, None, or in between (RALS/None and CLS/None). Colours are used in a stoplight format to emphasize these findings: red denotes a preference for CLS, yellow indicates comparable outcomes between CLS and RALS, and green indicates that RALS is the superior option for that particular outcome. A grey background was used for RALS/None and CLS/None cases. The final row indicates whether the clinical outcomes favour RALS, CLS, or None across all surgical procedures. A primary or secondary outcome is indicated with (I) or (II) respectively.
| Category | Blood Loss (II) | Conversion Rate (I) | Hospitalization Costs (I) | Incisional Hernia Rate (II) | Intraoperative Complication Rate (I) | Postoperative Complication Rate (I) | Length of Hospital Stay (I) | Operative Time (I) | Readmission Rate (II) | Wound Infection Rate (II) |
|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystectomy | None | None | CLS | CLS | None | None | None | CLS/None | None | None |
| Colectomy | RALS/None | RALS | CLS | None | None | None | RALS/None | CLS | None | None |
| Hysterectomy | RALS/None | None | CLS | - | None | None | RALS/None | None | None | None |
| Nephrectomy | None | None | - | - | None | None | RALS/None | CLS/None | - | - |
| Prostatectomy | None | None | CLS | - | None | None | RALS | None | - | - |
| General | None | None | CLS | - | None | None | RALS | CLS | None | None |
4. Discussion
4.1. Costs and Operative Time
The evidence on the use of RALS and CLS and a comprehensive analysis of ten clinical outcomes for five surgical procedures has been compiled in this umbrella review. In general, it has been demonstrated that RALS yielded comparable results to CLS on blood loss, conversion to open surgery rates, intraoperative complication rates, postoperative complication rates, readmission rates, and wound infection rates for most surgical procedures. While the hospitalization costs associated with RALS were greater than the costs of CLS and the operative times of RALS were longer than CLS, it was demonstrated that RALS shortened hospitalization stays in nearly all cases. The available data on incisional hernia rates were lacking.
RALS has been proven to achieve comparable clinical results to CLS; however, there are two important drawbacks associated with RALS: the increased expenses of RALS and the increased operative time. Many of the included reviews emphasized that RALS is more expensive than CLS [31,33,34,35,36,37,42,44,46,49,51,52,53,54,55,59,69,70,71,74,75,76,77]. The substantially higher costs are hard to justify as the clinical outcomes barely showed any significant advantages compared to CLS. However, the increased set-up and consumable costs are ultimately mitigated due to shorter hospital stays of RALS patients [57]. Furthermore, the costs of RALS systems may decrease in the future due to competition and innovations [10,44].
Secondly, it is frequently stressed that the surgeon’s experience and learning curve is not taken into account in the current literature and these shortcomings can negatively impact the clinical findings of RALS [49,50,52,53,54,55,56,57,58,70,77]. Overcoming these limitations might demonstrate stronger clinical benefits for RALS patients, which would justify the higher costs.
Finally, it should be noted that although the clinical outcomes investigated in this study may not reveal significant advancements (except for a shorter length of hospital stay), physicians can still benefit from utilizing RALS systems. Numerous studies emphasized the superior ergonomics, enhanced dexterity, and stable 3D high-definition visualization that RALS can provide, as well as tremor filtration (by filtering out high-frequency movements), providing seven degrees of freedom and scaling down movements of the surgeon, which allows surgeons to perform exaggerated movements which are translated to microscopic manoeuvres [34,37,45,48,57,58,62,63,74,77]. Even though it was assumed that these advantages would translate into improved clinical outcomes, which could not be confirmed in this umbrella review, they can still be beneficial for physicians. For instance, the posture and muscle strain was analysed in thirteen surgeons during colorectal procedures and was found to be less demanding during RALS [78]. Furthermore, the ergonomics of RALS systems directly impacted efficiency and efficacy by reducing cervical strain [62].
It was even observed that the mean heart rate of surgeons was significantly lower when utilizing RALS systems, as opposed to CLS approaches for performing the same surgical procedures [79]. Therefore, when assessing the feasibility and justifying the costs of RALS in surgical settings, it is important to consider clinical and non-clinical aspects that impact both patients and physicians. In this umbrella review, non-clinical aspects and the role of physicians were not taken into account.
The second major drawback of RALS is the increased operative time. In this study, it was found that the operative time was either comparable between RALS and CLS or longer when patients were treated by RALS. However, the available data on operative time was inconsistent. While some studies defined operative time as the complete duration of a patient’s treatment, including preparation time in the operating room, others only considered the time from skin incision to skin closure [34,47]. Moreover, some studies did not provide any definition of operative time. This lack of consistency creates uncertainty regarding the actual duration of operations and makes it difficult to compare RALS and CLS operative times from different reviews.
Some reviews pointed out that the operative time of RALS procedures was longer, due to docking and set-up of the systems and the learning curve of the surgeon and the rest of the team [53,54,56,57,59,67]. Since RALS is not yet a routine procedure at some surgical sites, these factors can prolong the operative time [80]. Moreover, several studies pointed out that complex patients are more likely to be treated with RALS which induces a selection bias [39,45,49,56].
RALS, with its technical advantages, has the potential to expand the boundaries of minimally invasive surgery, such that even the most complex cases are treated by minimally invasive surgery which would otherwise have required an open surgery approach [39]. These factors do have implications on the operative time.
4.2. Reflection on CCA Scores
The CCA indicates to what extent the primary sources of systematic reviews and meta-analyses overlap. If the CCA score is high, indicating a high overlap, the conclusions drawn in the reviews should be consistent. If the CCA score is low, indicating a small overlap, discrepancies in the conclusions drawn by the individual reviews are explainable [81]. The CCA scores of the reviews for cholecystectomy, colectomy, hysterectomy, nephrectomy, and prostatectomy were all less than 5% (Table A1); hence, there was only a slight overlap of primary sources between the systematic reviews and meta-analyses within the five surgical categories. Although the overlap was slight, the conclusions drawn by the reviews are similar. In this case, the low CCA scores suggest that a wide variety of primary sources have been incorporated, which enhances the generalizability of the findings.
4.3. Limitations
The limitations of this umbrella review are discussed in the following sections.
4.3.1. Summarization Table
Table 13 was constructed to formulate overall conclusions. For instance, the hospitalization costs of cholecystectomy were analysed in three reviews. All three reviews concluded that the costs were significantly higher with RALS (which can be easily observed in the bar charts of Figure 2). Therefore, it was concluded in Table 13 that the hospitalization costs are in favour of CLS. Regarding the length of hospital stay of prostatectomy patients, three reviews concluded that RALS shortened the length of hospitalization and one review did not observe any significant differences (as can be easily observed in Figure 2e). Based on these four reviews, the results were in favour of RALS, which was entered into Table 13. However, this approach imposes strong limitations, as some reviews included many more patients and primary sources than others. These quantitative differences were not taken into account.
One might think that combining all data and conducting statistical analysis of the entire pool of patients would be more evident, but performing statistical analyses based on merged meta-analytic data is not allowed. Umbrella reviews are meant to provide a high level of overview and reach intuitive conclusions [82], instead of performing statistical analysis of the total group. Therefore, this method and the way Table 13 was derived was considered to be appropriate. However, these results should be interpreted with caution and readers should be aware of the limitations.
4.3.2. Previous Work
A similar umbrella review has already been conducted and published in 2021. This umbrella review examined and compared the data of systematic reviews and meta-analyses of common laparoscopic and robot-assisted surgeries as well. The study was conducted in 2021 and reviewed papers published between January 2017 and January 2019 [21]. Despite the similarities between this umbrella review and that of [21], the present review is of added value: the umbrella review from Muaddi et al. lacked a systematic approach and failed to properly synthesize the extracted data. In addition, they did not conduct a quality assessment of the included publications (AMSTAR 2 for example) and included incomplete supplementary documents (comments were not processed). Even more, since its publication, many more systematic reviews and meta-analyses have been conducted. Therefore, the current umbrella review holds more significance due to its higher methodological quality and the inclusion of a wider range of publications. Another study [83] suggests that an optimal search in a systematic review and meta-analysis requires exploring at least four databases instead of the two that we used. Comparing the articles from different combinations of four databases with the current results may be interesting.
4.3.3. Selection of Surgical Procedures
To narrow the scope of this umbrella review, it was decided to include systematic reviews and meta-analyses that reported data on either cholecystectomy, colectomy, hysterectomy, nephrectomy, and/or prostatectomy only. As explained in the method section, reviews that analysed multiple surgical procedures of which one (or more) covered one of these five surgical procedures were still included. Only relevant data were extracted from these reviews.
Based on several sources and data, as elaborated in Section 2.2, it was decided to focus on these five specific surgical procedures. However, there are many more procedures that are frequently executed with robotic systems, such as inguinal hernia repair, Roux-en-Y gastric bypass, and hepatectomy. The decision to focus only on these five surgical procedures limits the value of the analysis. Ideally, all commonly executed robotic procedures would have been included. However, given the time frame of this review, including a broader range of surgical procedures was not possible.
It should be noted that some of the laparoscopic procedures (e.g., cholecystectomy and hysterectomy) are sometimes executed in an outpatient setting. This makes the length of the hospital stay a less relevant metric when comparing to the robotic approach executed within the operating room.
4.3.4. Publication Date of Primary Sources
Since RALS has not been around very long, it is likely that RALS development will progress and the learning curve, experience and applications will advance. By including papers published within the past five years only, it was assumed that this umbrella review could provide an overview of and insights into more recently achieved results and data. However, while the search query filtered out systematic reviews and meta-analyses published before 2018, the publication dates of primary sources were not taken into account. During extraction of the study characteristics (a Table with the Study Characteristics is available for download in the list of Supplementary Materials), it was determined that two reviews included primary sources published back in 1996 and 1998. Therefore, the data in this umbrella review was based on primary sources that are published in a much wider time frame than originally intended. Nonetheless, most of the reviews conducted their analyses on primary sources published between 2010 and 2021 (835 out of 1046). A graph depicting the publication year of all 1046 primary sources was generated to provide detailed awareness of this limitation (Figure A1).
4.3.5. Heterogeneity
Many reviews indicated that there was high variability in the data, indicating heterogeneity. Calculations of heterogeneity (I2) were extracted along with the quantitative data. Based on the data presented in the results section, it is apparent that there was a high level of heterogeneity in most cases. This outcome is not surprising considering the large amounts of data that were aggregated in the reviews, which included significant variations in surgical techniques, procedures and approaches, the experience of surgeons, and patient demographics (such as their condition, stage of disease, and age). High heterogeneity can indicate that the results of the studies being analysed are quite diverse, and it may be challenging to draw definitive conclusions from them. However, most reviews used appropriate statistical methods to account for high heterogeneity (random-effect model). Thus, despite the heterogeneity, the conclusions drawn from the reviews are still valid, although the findings should be interpreted with caution.
4.3.6. AMSTAR 2 Quality Assessment
Remarkably, all included studies, except for [29], were assessed to be of critically low or low quality based on the AMSTAR 2 quality assessment [23]. In Figure 3 an overview was created to reflect on the criteria that most reviews failed to fulfil. The AMSTAR 2 quality assessment was published in 2017. A possible explanation could be that systematic reviews and meta-analyses published shortly after the release of AMSTAR 2 were not aware of certain criteria that were added to AMSTAR 2. For instance, criterion 10 “Did the review authors report on the sources of funding for the studies included in the review?” was added to the original AMSTAR quality assessment list. However, all systematic reviews and meta-analyses (even the reviews published many years later) failed to meet this criterion.
Figure 3.
This bar chart illustrates the fulfilment of each criterion of the AMSTAR 2 quality assessment. Each bar is split into a percentage of the reviews that met the criterion (green) and the percentage that did not (red). The criteria in bold on the x-axis are critical criteria, the others are non-critical criteria. The results of the AMSTAR 2 quality assessment can be downloaded from the list of Supplementary Materials.
Similarly, 94% of all reviews included failed to fulfil criterion 7: “Did the review authors provide a list of excluded studies and justify the exclusions?”. Many reviews reported on the number of records excluded and provided the reason for exclusions during the full-text review phase as well (using PRISMA flow diagrams). But the AMSTAR 2 quality assessment requires a full list of records that were excluded during full-text reviewing, along with the reason for exclusion per record. Many reviews failed to do so.
It should be considered that the quality of 51 out of the 52 systematic reviews and meta-analyses was low or critically low. This impacts the quality of the data extracted and presented within this umbrella review. Therefore, it could be suggested to rate the reviews and the meta-analyses based on the quality of the scientific methods before being included.
4.3.7. Study Type of Primary Sources
Another limitation of this umbrella review was the study type of primary sources and the associated quality of evidence. In general, random controlled trials (RCTs) are considered the gold standard for evaluating the effectiveness of interventions [84]. However, the number of RCTs conducted that compare RALS and CLS is very scarce. Almost all systematic reviews and meta-analyses included in this umbrella review indicated the lack of RCTs among their primary sources and the implications this has for the quality of evidence. Since this umbrella review is based on the data from the primary sources of systematic reviews and meta-analyses, the same implications apply to the results presented in this study. Therefore, the results should be interpreted with caution. To increase the quality of evidence among RALS and CLS research, many more RCTs should be conducted in the near future.
4.3.8. Quantitative/Qualitative Data
In some rare cases, quantitative data provided in (network) meta-analyses were not extracted as such, but translated to qualitative data first (e.g., [38,45,68]). The statistical analyses conducted in these reviews did not match the quantitative data format adhered to in this umbrella review. Including the various statistical analyses would make this umbrella review overly detailed and potentially confusing. Thus, the decision was made to translate the quantitative data into qualitative data (along with qualitative data already formulated in these reviews) and to incorporate these results in the qualitative tables. By these means, important findings of these reviews were included without needlessly complicating the overview of the results.
5. Conclusions
In conclusion, this umbrella review synthesized the data of 52 systematic reviews and meta-analyses, including 1046 primary sources published between 1996 and 2022, that reported data from more than 1,288,425 patients. RALS yielded comparable results to CLS in terms of blood loss, conversion to open surgery rate, intraoperative complication rate, postoperative complication rate, readmission rate, and wound infection rate for cholecystectomy, colectomy, hysterectomy, nephrectomy, and prostatectomy. Additionally, RALS significantly reduced the length of hospital stay compared to patients treated by CLS. However, RALS is also associated with significantly higher costs and longer operative times (although this may be affected by confounding factors such as preparation time, surgeon’s experience, and learning curve). Based on the quantitative and qualitative data collected in this umbrella review, RALS obtained promising and consistent results. Future work should evaluate procedure-specific outcomes as well, in order to provide a complete overview of the advantages and disadvantages of the use of RALS as compared to CLS. This approach will enable a better understanding of the potential benefits of RALS in specific surgical procedures. Finally, it is suggested that more research, and especially RCTs, are required to prove that RALS is as safe and reliable as CLS, and to improve the quality of evidence.
Supplementary Materials
The following supporting information can be downloaded: https://www.mdpi.com/article/10.3390/std13010003/s1, Table S1: Study characteristics; Table S2: Primary sources and citation matrices; Table S3: AMSTAR 2 Quality Assessment results; File S1: PDF of the AMSTAR 2 Quality Assessment item list. File S2: PRISMA-2020 checklist.
Author Contributions
Conceptualization, S.C. and T.H.; methodology, S.C.; software, S.C.; validation, T.H., S.C. and J.C.-A.; formal analysis, S.C.; investigation, S.C.; resources, T.H.; data curation, S.C.; writing—original draft preparation, S.C.; writing—review and editing, T.H. and J.C.-A.; visualization, S.C.; supervision, T.H.; project administration, T.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
This umbrella review retrieved data from papers on public databases only. Therefore, informed consent and ethical approval were not required.
Acknowledgments
We would like to thank Bart van Straten for his support during the data collection and structuring of the work.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Corrected covered area (CCA) of each surgical procedure group. The CCA score indicates the overlap between the included papers. CCA is further elaborated in Section 2.4.
Table A1.
Corrected covered area (CCA) of each surgical procedure group. The CCA score indicates the overlap between the included papers. CCA is further elaborated in Section 2.4.
| Surgical Category | N | r | c | CCA Score |
|---|---|---|---|---|
| Cholecystectomy | 197 | 161 | 7 | 3.7% |
| Colectomy | 556 | 354 | 23 | 2.6% |
| Hysterectomy | 186 | 148 | 10 | 2.9% |
| Nephrectomy | 248 | 223 | 9 | 1.4% |
| Prostatectomy | 195 | 160 | 8 | 3.1% |
Appendix B

Table A2.
All qualitative data extracted from the included studies regarding cholecystectomy. A primary or secondary outcome is indicated with (I) or (II) respectively. The last column denotes whether the synthesized outcome favours CLS, RALS, or shows no significant differences. This distinction is highlighted using different cell colours: red represents a significant favour towards CLS, yellow indicates no significant differences observed, and green indicates a significant difference in favour of RALS. Abbreviations: RALS = robot-assisted laparoscopic surgery, CLS = conventional laparoscopic surgery.
Table A2.
All qualitative data extracted from the included studies regarding cholecystectomy. A primary or secondary outcome is indicated with (I) or (II) respectively. The last column denotes whether the synthesized outcome favours CLS, RALS, or shows no significant differences. This distinction is highlighted using different cell colours: red represents a significant favour towards CLS, yellow indicates no significant differences observed, and green indicates a significant difference in favour of RALS. Abbreviations: RALS = robot-assisted laparoscopic surgery, CLS = conventional laparoscopic surgery.
| Conversion to open surgery rate (I) | |||
|---|---|---|---|
| Author (year) | Ref. | Synthesized finding | Favours |
| Shenoy et al., (2021) | [32] | Comparable results in conversion to open surgery rates were observed between RALS and CLS. | None |
| Incisional hernia rate (II) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Shenoy et al., (2021) | [32] | Incisional hernia rate did not differ significantly between RALS and CLS. | None |
| Intraoperative complication rate (I) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Shenoy et al., (2021) | [32] | No significant differences were observed between RALS and CLS. | None |
| Postoperative complication rate (I) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Lin et al., (2023) | [38] | Based on ranking probabilities, the best surgical options for reducing postoperative complications are: three-port (61.3%) and four-port (21.8%) laparoscopy. | CLS |
| Operative time (I) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Shenoy et al., (2021) | [32] | Operative time was longer in cholecystectomy performed by RALS compared to CLS. | CLS |
| Lin et al., (2023) | [38] | The first ranking probabilities for reducing operation time showed that the three-port laparoscopic technique had the shortest operation time, followed by four-port. | CLS |
| Length of hospital stay (I) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Shenoy et al., (2021) | [32] | The length of hospital stay between RALS and CLS was comparable for cholecystectomy. | None |
| Lin et al., (2023) | [38] | The first ranking probabilities for reducing hospital stay (days) are: robotic (32.3%) followed by three-port (29.0%). | RALS |
| Readmission rate (II) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Shenoy et al., (2021) | [32] | The readmission rate after RALS and CLS cholecystectomy was comparable. | None |

Table A3.
All qualitative data extracted from the included studies regarding colectomy. A primary or secondary outcome is indicated with (I) or (II) respectively. The last column denotes whether the synthesized outcome favours CLS, RALS, or shows no significant differences. This distinction is highlighted using different cell colours: red represents a significant favour towards CLS, yellow indicates no significant differences were observed, and green indicates a significant difference in favour of RALS. Abbreviations: RALS = robot-assisted laparoscopic surgery, CLS = conventional laparoscopic surgery.
Table A3.
All qualitative data extracted from the included studies regarding colectomy. A primary or secondary outcome is indicated with (I) or (II) respectively. The last column denotes whether the synthesized outcome favours CLS, RALS, or shows no significant differences. This distinction is highlighted using different cell colours: red represents a significant favour towards CLS, yellow indicates no significant differences were observed, and green indicates a significant difference in favour of RALS. Abbreviations: RALS = robot-assisted laparoscopic surgery, CLS = conventional laparoscopic surgery.
| Blood loss (II) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Cuk et al., (2023) | [45] | RALS reduced intraoperative blood loss compared to CLS. | RALS |
| Conversion to open surgery rate (I) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Cuk et al., (2023) | [45] | No differences in conversion rates between RALS and CLS were observed. | None |
| Petz et al., (2021) | [30] | RALS showed lower conversion rates compared to CLS. | RALS |
| Waters et al., (2020) | [57] | Patients undergoing RALS have a lower conversion to open surgery rate compared to CLS. | RALS |
| Incisional hernia rate (II) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Waters et al., (2020) | [57] | Patients undergoing RALS colectomy have a significantly lower incisional hernia rate compared to CLS colectomy. | RALS |
| Postoperative complication rate (I) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Petz et al., (2021) | [30] | No differences in postoperative complication rates were found. | None |
| Operative time (I) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Petz et al., (2021) | [30] | In all the comparative studies included, the operative time of RALS was significantly longer than CLS. | CLS |
| Waters et al., (2020) | [57] | RALS operative time was found to be significantly longer compared to LRH in thirteen studies. | CLS |
| Length of hospital stay (I) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Cuk et al., (2023) | [45] | The RALS group had a shorter hospital stay compared to the CLS group. | RALS |
| Waters et al., (2020) | [57] | Patients undergoing RALS experience a significantly shorter hospital stay compared to CLS. | RALS |
| Wound infection rate (II) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Waters et al., (2020) | [57] | No significant differences in wound infection rates were observed between CLS and RALS among ten included studies. | None |

Table A4.
All qualitative data extracted from the studies included regarding hysterectomy. A primary or secondary outcome is indicated with (I) or (II) respectively. The last column denotes whether the synthesized outcome favours CLS, RALS, or shows no significant differences. This distinction is highlighted using different cell colours: red represents a significant favour towards CLS, yellow indicates no significant differences were observed, and green indicates a significant difference in favour of RALS. Abbreviations: RALS = robot-assisted laparoscopic surgery, CLS = conventional laparoscopic surgery.
Table A4.
All qualitative data extracted from the studies included regarding hysterectomy. A primary or secondary outcome is indicated with (I) or (II) respectively. The last column denotes whether the synthesized outcome favours CLS, RALS, or shows no significant differences. This distinction is highlighted using different cell colours: red represents a significant favour towards CLS, yellow indicates no significant differences were observed, and green indicates a significant difference in favour of RALS. Abbreviations: RALS = robot-assisted laparoscopic surgery, CLS = conventional laparoscopic surgery.
| Blood loss (II) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Alshowaikh et al., (2021) | [59] | The blood loss between CLS and RALS hysterectomy was comparable. | None |
| Guo et al., (2023) | [64] | On a SUCRA ranking of five surgical approaches, the RALS approach scored best. The laparoscopic approach was ranked second. | RALS |
| Hospitalization costs (I) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Alshowaikh et al., (2021) | [59] | The cost associated with RALS was higher than the costs of CLS hysterectomy. | CLS |
| Postoperative complication rate (I) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Alshowaikh et al., (2021) | [59] | The overall complication rate was comparable between RALS and CLS hysterectomy. | None |
| Prodromidou et al., (2020) | [60] | No differences in either major or overall postoperative complication rates were observed between RALS and CLS hysterectomy. | None |
| Guo et al., (2023) | [64] | Among a SUCRA ranking of five surgical approaches, RALS was ranked higher than CLS regarding the overall complication rate. | RALS |
| Operative time (I) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Alshowaikh et al., (2021) | [59] | The operative time between CLS and RALS hysterectomy was comparable. | None |
| Prodromidou et al., (2020) | [60] | Neither the total operative time nor the operative time (pre-surgical procedures excluded) showed any differences between RALS and CLS. | None |
| Guo et al., (2023) | [64] | The operative time, compared between five surgical approaches with a SUCRA ranking, is the shortest for open surgery. The second best is laparoscopic surgery. The operative time of RALS is ranked fourth. | CLS |
| Length of hospital stay (I) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Alshowaikh et al., (2021) | [59] | No statistical differences were observed between RALS and CLS hysterectomy for the length of hospital stay. | None |
| Guo et al., (2023) | [64] | Among a SUCRA ranking of five surgical approaches, the RALS proved to be the preferred approach for the shortest hospital stay. The laparoscopic approach was ranked second. | RALS |

Table A5.
All qualitative data extracted from the included studies regarding nephrectomy. A primary or secondary outcome is indicated with (I) or (II) respectively. The last column denotes whether the synthesized outcome favours CLS, RALS, or shows no significant differences. This distinction is highlighted using different cell colours: red represents a significant favour towards CLS, yellow indicates no significant differences were observed, and green indicates a significant difference in favour of RALS. Abbreviations: RALS = robot-assisted laparoscopic surgery, CLS = conventional laparoscopic surgery.
Table A5.
All qualitative data extracted from the included studies regarding nephrectomy. A primary or secondary outcome is indicated with (I) or (II) respectively. The last column denotes whether the synthesized outcome favours CLS, RALS, or shows no significant differences. This distinction is highlighted using different cell colours: red represents a significant favour towards CLS, yellow indicates no significant differences were observed, and green indicates a significant difference in favour of RALS. Abbreviations: RALS = robot-assisted laparoscopic surgery, CLS = conventional laparoscopic surgery.
| Blood loss (II) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Veccia et al., (2020) | [68] | Lower blood losses were observed in patients in the RALS group. | RALS |
| Tang et al., (2020) | [28] | There was less blood loss in RALS partial nephrectomy compared to CLS. | RALS |
| Intraoperative complication rate (I) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Zahid et al., (2022) | [67] | Radical nephrectomy with RALS was associated with fewer perioperative complications. | RALS |
| Veccia et al., (2020) | [68] | RALS had the lowest rate of intraoperative complications. | RALS |
| Tang et al., (2020) | [28] | RALS and CLS obtained similar results on the intraoperative complications rate after partial nephrectomy. | None |
| Postoperative complication rate (I) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Tang et al., (2020) | [28] | (Major) postoperative complication rates after CLS or RALS partial nephrectomy were comparable. | None |
| Operative time (I) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Zahid et al., (2022) | [67] | Radical nephrectomy with RALS was associated with longer operative time | CLS |
| Veccia et al., (2020) | [68] | The operative time for RALS and CLS nephroureterectomy was comparable. | None |
| Tang et al., (2020) | [28] | Comparable results in operative time were observed between RALS and CLS. | None |
| Length of hospital stay (I) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Veccia et al., (2020) | [68] | The length of hospital stay was statistically significantly shorter for the RALS group compared to CLS. | RALS |
| Tang et al., (2020) | [28] | The length of hospital stay was shorter after a partial nephrectomy performed with RALS compared to CLS. | RALS |

Table A6.
All qualitative data extracted from the included studies regarding prostatectomy. A primary or secondary outcome is indicated with (I) or (II) respectively. The last column denotes whether the synthesized outcome favours CLS, RALS, or shows no significant differences. This distinction is highlighted using different cell colours: red represents a significant favour towards CLS, yellow indicates no significant differences were observed, and green indicates a significant difference in favour of RALS. Abbreviations: RALS = robot-assisted laparoscopic surgery, CLS = conventional laparoscopic surgery.
Table A6.
All qualitative data extracted from the included studies regarding prostatectomy. A primary or secondary outcome is indicated with (I) or (II) respectively. The last column denotes whether the synthesized outcome favours CLS, RALS, or shows no significant differences. This distinction is highlighted using different cell colours: red represents a significant favour towards CLS, yellow indicates no significant differences were observed, and green indicates a significant difference in favour of RALS. Abbreviations: RALS = robot-assisted laparoscopic surgery, CLS = conventional laparoscopic surgery.
| Blood loss (II) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Zahid et al., (2022) | [67] | Less blood loss was observed during RALS as compared to other approaches. | RALS |
| Kordan et al., (2020) | [27] | Blood loss was comparable between RALS and CLS, with slightly less blood loss in favour of RALS. | None |
| Intraoperative complication rate (I) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Zahid et al., (2022) | [67] | One study reported similar intraoperative complications. | None |
| Operative time (I) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Kordan et al., (2020) | [27] | Operative time was shorter for CLS simple prostatectomy procedures compared to RALS. | CLS |
| Length of hospital stay (I) | |||
| Author (year) | Ref. | Synthesized finding | Favours |
| Zahid et al., (2022) | [67] | RALS showed a shorter length of hospital stay compared to other conventional procedures. | RALS |
| Kordan et al., (2020) | [27] | Length of hospital stay was comparable between RALS and CLS simple prostatectomy. | None |
Appendix C

Table A7.
The search queries established for the other four surgical procedures. The final search queries are composed in a similar manner as demonstrated in Table 1 and Table 2 for colectomy.
| Surgical Procedure | PubMed | Results | Scopus | Results |
|---|---|---|---|---|
| Cholecystectomy | “cholecystectom*”[Title/Abstract] OR “gall bladder resection*”[Title/Abstract] OR “gall bladder surger*”[Title/Abstract] OR “gall bladder remov*”[Title/Abstract] OR cholecystectomy[MeSH Terms] | 16 | TITLE-ABS(“cholecystectom*” OR “gall bladder resection*” OR “gall bladder surger*” OR “gall bladder remov*”) | 11 |
| Hysterectomy | “hysterectomy*”[Title/Abstract] OR “uterus resection*”[Title/Abstract] OR “uterus surger*”[Title/Abstract] OR “uterus remov*”[Title/Abstract] OR hysterectomy[MeSH Terms] | 46 | TITLE-ABS(“hysterectom*” OR “uterus resection *” OR “uterus surger*” OR “uterus remov*”) | 33 |
| Nephrectomy | “nephrectom*”[Title/Abstract] OR “kidney resection*”[Title/Abstract] OR “kidney surger*”[Title/Abstract] OR “kidney remov*”[Title/Abstract] OR nephrectomy[MeSH Terms] | 38 | TITLE-ABS(“nephrectom *” OR “kidney resection *” OR “kidney surger*” OR “kidney remov*”) | 28 |
| Prostatectomy | “prostatectom*”[Title/Abstract] OR “prostate resection*”[Title/Abstract] OR “prostate surger*”[Title/Abstract] OR “prostate remov*”[Title/Abstract] OR prostatectomy[MeSH Terms] | 64 | TITLE-ABS(“prostatectom *” OR “prostate resection *” OR “prostate surger*” OR “prostate remov*”) | 48 |
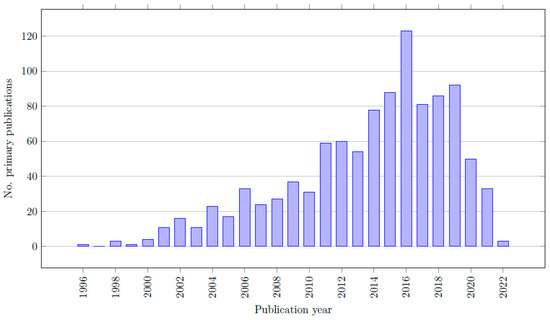
Figure A1.
Bar chart of the number of primary sources published per year.
Figure A1.
Bar chart of the number of primary sources published per year.
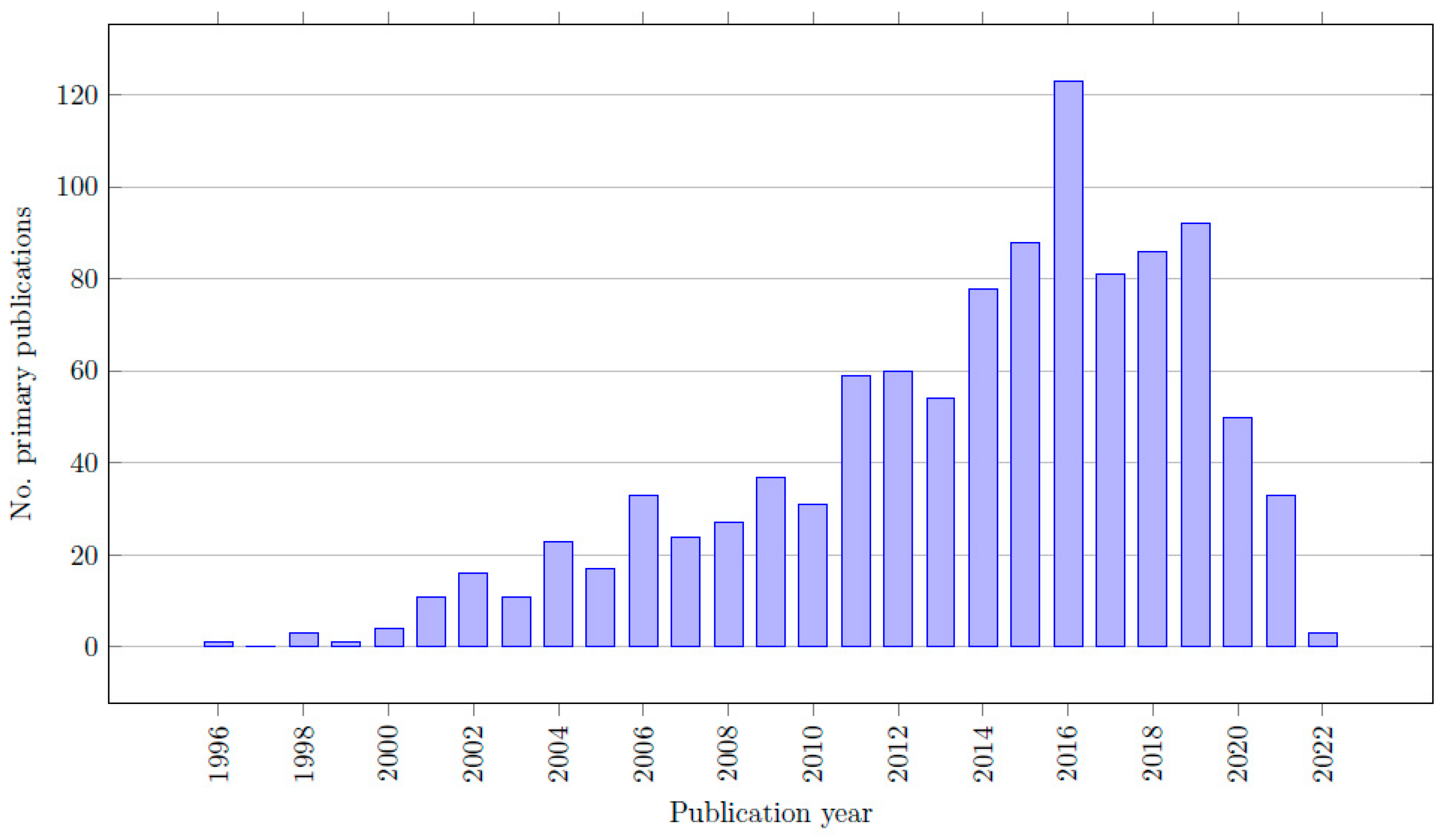

Table A8.
List of all papers that were excluded during the full-text review phase.
Table A8.
List of all papers that were excluded during the full-text review phase.
| No. | Author (Year) | Ref. | Reason for Exclusion |
|---|---|---|---|
| 1 | Alkatout et al., (2022) | [85] | This paper does not compare RALS and CLS. The paper evaluated the outcomes of different Versius systems. |
| 2 | Charalambides et al., (2022) | [86] | This paper does not compare RALS with CLS. |
| 3 | Toh et al., (2020) | [87] | Wrong study type. This review does not have a methodology, is not systematic, and only reviews some outcomes of a few randomly selected papers. |
| 4 | Oweira et al., (2023) | [88] | Full text was not available. |
| 5 | Zhu et al., (2021) | [58] | This paper does not compare RALS with CLS. The paper compared two different Da Vinci systems instead. |
| 6 | Leitoa et al., (2023) | [89] | The clinical outcomes of interest were not reported in this paper. |
| 7 | Kampers et al., (2021) | [90] | The clinical outcomes of interest were not reported in this paper. |
| 8 | Nitecki et al., (2020) | [91] | The clinical outcomes of interest were not reported in this paper. |
| 9 | Marra et al., (2019) | [92] | Full text was not available. |
| 10 | Behbehani et al., (2019) | [93] | The clinical outcomes of interest were not reported in this paper. |
| 11 | Behbehani et al., (2020) | [94] | The clinical outcomes of interest were not reported in this paper. |
| 12 | Kostakis et al., (2019) | [95] | The clinical outcomes of interest were not reported in this paper. |
| 13 | Hinojosa-Gonzalez et al., (2023) | [96] | Full text was not available. |
| 14 | Lin et al., (2021) | [97] | Full text was not available. Publication was removed. |
| 15 | Zahid et al., (2023) | [67] | This review is excluded as it is a duplicate of [67]. Ref. [67] was included. |
| 16 | Cacciamai et al., (2018) | [76] | Full text was not available. |
| 17 | Ficarra et al., (2018) | [98] | The clinical outcomes of interest were not reported in this paper. |
| 18 | Cao et al., (2019) | [85] | This paper does not compare RALS with CLS. Instead, RALS and CLS patients formed one experimental group, which was compared with an open prostatectomy control group. |
| 19 | Sridharan et al., (2018) | [99] | The clinical outcomes of interest were not reported in this paper. |
| 20 | Moretti et al., (2022) | [70] | Wrong study type. This paper is a reverse systematic review that includes all primary sources of identified systematic reviews, which should not be included in an umbrella review. |
| 21 | Marra et al., (2019) | [100] | The clinical outcomes of interest were not reported in this paper. |
References
- Alkatout, I.; Mechler, U.; Mettler, L.; Pape, J.; Maass, N.; Biebl, M.; Gitas, G.; Lagan, A.S.; Freytag, D. The Development of Laparoscopy—A Historical Overview. Front. Surg. 2021, 8, 799442. [Google Scholar] [CrossRef]
- Spaner, S.J.; Warnock, G.L. A brief history of endoscopy, laparoscopy, and laparoscopic surgery. J. Laparoendosc. Adv. Surg. Tech. 1997, 7, 369–373. [Google Scholar] [CrossRef]
- Litynski, G.S. Kurt Semm and the fight against skepticism: Endoscopic hemostasis, laparoscopic appendectomy, and Semm’s impact on the “laparoscopic revolution”. JSLS J. Soc. Laparoendosc. Surg. 1998, 2, 309. [Google Scholar]
- Tiwari, M.M.; Reynoso, J.F.; High, R.; Tsang, A.W.; Oleynikov, D. Safety, efficacy, and cost-effectiveness of common laparoscopic procedures. Surg. Endosc. 2011, 25, 1127–1135. [Google Scholar] [CrossRef]
- Colon Cancer Laparoscopic or Open Resection Study Group. Laparoscopic surgery versus open surgery for colon cancer: Short-term outcomes of a randomised trial. Lancet Oncol. 2005, 6, 477–484. [Google Scholar] [CrossRef]
- Braga, M.; Vignali, A.; Gianotti, L.; Zuliani, W.; Radaelli, G.; Gruarin, P.; Dellabona, P.; Di Carlo, V. Laparoscopic versus open colorectal surgery: A randomized trial on short-term outcome. Ann. Surg. 2002, 236, 759. [Google Scholar] [CrossRef]
- Lotan, Y. Is robotic surgery cost-effective: No. Curr. Opin. Urol. 2012, 22, 66–69. [Google Scholar] [CrossRef]
- Gkegkes, I.D.; Mamais, I.A.; Iavazzo, C. Robotics in general surgery: A systematic cost assessment. J. Minimal Access Surg. 2017, 13, 243. [Google Scholar] [CrossRef]
- Hardon, S.F.; Schilder, F.; Bonjer, J.; Dankelman, J.; Horeman, T. A new modular mechanism that allows full detachability and cleaning of steerable laparoscopic instruments. Surg. Endosc. 2019, 33, 3484–3493. [Google Scholar] [CrossRef]
- Matsuyama, T.; Kinugasa, Y.; Nakajima, Y.; Kojima, K. Robotic-assisted surgery for rectal cancer: Current state and future perspective. Ann. Gastroenterol. Surg. 2018, 2, 406–412. [Google Scholar] [CrossRef]
- Sinno, A.K.; Fader, A.N. Robotic-assisted surgery in gynecologic oncology. Fertil. Steril. 2014, 102, 922–932. [Google Scholar] [CrossRef]
- Becker, L.A.; Oxman, A.D. Chapter 22: Overviews of Reviews, Version 5.1.0 ed.; 2011; Available online: www.handbook.cochrane.org (accessed on 1 January 2023).
- Cant, R.; Ryan, C.; Kelly, M.A. A Nine-Step Pathway to Conduct an Umbrella Review of Literature. J. Contrib. 2022, 32, 31–34. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Radua, J. Ten simple rules for conducting umbrella reviews. Evid. Based Ment. Health 2018, 21, 95–100. [Google Scholar] [CrossRef]
- Aromataris, E.; Fernandez, R.; Godfrey, C.M.; Holly, C.; Khalil, H.; Tungpunkom, P. Summarizing systematic reviews: Methodological development, conduct and reporting of an umbrella review approach. JBI Evid. Implement. 2015, 13, 132–140. [Google Scholar] [CrossRef]
- Aromataris, E.; Munn, Z. JBI Manual for Evidence Synthesis. JBI 2020. Available online: https://synthesismanual.jbi.global (accessed on 1 January 2023).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Sheetz, K.H.; Claflin, J.; Dimick, J.B. Trends in the adoption of robotic surgery for common surgical procedures. JAMA Netw. Open 2020, 3, e1918911. [Google Scholar] [CrossRef]
- Lee, J.R. Anesthetic considerations for robotic surgery. Korean J. Anesthesiol. 2014, 66, 3–11. [Google Scholar] [CrossRef]
- Anderson, J.E.; Chang, D.C.; Parsons, J.K.; Talamini, M.A. The first national examination of outcomes and trends in robotic surgery in the United States. J. Am. Coll. Surg. 2012, 215, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Muaddi, H.; El Hafid, M.; Choi, W.J.; Lillie, E.; de Mestral, C.; Nathens, A.; Stukel, T.A.; Karanicolas, P.J. Clinical outcomes of robotic surgery compared to conventional surgical approaches (laparoscopic or open): A systematic overview of reviews. Ann. Surg. 2021, 273, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg. Med. J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Pieper, D.; Koensgen, N.; Breuing, J.; Ge, L.; Wegewitz, U. How is AMSTAR applied by authors--a call for better reporting. BMC Med. Res. Methodol. 2018, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Zhang, M.; Beal, M.; Ghomrawi, H.M.K. An umbrella review comparing computer-assisted and conventional total joint arthroplasty: Quality assessment and summary of evidence. BMJ Surg. Interv. Health Technol. 2020, 2, e000016. [Google Scholar] [CrossRef] [PubMed]
- Pieper, D.; Antoine, S.-L.; Mathes, T.; Neugebauer, E.A.M.; Eikermann, M. Systematic review finds overlapping reviews were not mentioned in every other overview. J. Clin. Epidemiol. 2014, 67, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Kordan, Y.; Canda, A.E.; Köseoğlu, E.; Balbay, D.; Laguna, M.P.; de la Rosette, J. Robotic-assisted simple prostatectomy: A systematic review. J. Clin. Med. 2020, 9, 1798. [Google Scholar] [CrossRef]
- Tang, A.B.; Lamaina, M.; Childers, C.P.; Mak, S.S.; Ruan, Q.; Begashaw, M.M.; Bergman, J.; Booth, M.S.; Shekelle, P.G.; Wilson, M.; et al. Perioperative and Long-Term Outcomes of Robot-Assisted Partial Nephrectomy: A Systematic Review. Am. Surg. 2020, 87, 21–29. [Google Scholar] [CrossRef]
- Lawrie, T.A.; Liu, H.; Lu, D.; Dowswell, T.; Song, H.; Wang, L.; Shi, G. Robot-assisted surgery in gynaecology. Cochrane Database Syst. Rev. 2019, 4, Cd011422. [Google Scholar] [CrossRef]
- Petz, W.; Borin, S.; Fumagalli Romario, U. Updates on robotic cme for right colon cancer: A qualitative systematic review. J. Pers. Med. 2021, 11, 550. [Google Scholar] [CrossRef]
- Jones, K.; Qassem, M.G.; Sains, P.; Baig, M.K.; Sajid, M.S. Robotic total meso-rectal excision for rectal cancer: A systematic review following the publication of the ROLARR trial. World J. Gastrointest. Oncol. 2018, 10, 449–464. [Google Scholar] [CrossRef]
- Shenoy, R.; Mederos, M.A.; Ye, L.; Mak, S.S.; Begashaw, M.M.; Booth, M.S.; Shekelle, P.G.; Wilson, M.; Gunnar, W.; Maggard-Gibbons, M.; et al. Intraoperative and postoperative outcomes of robot-assisted cholecystectomy: A systematic review. Syst. Rev. 2021, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Shan, X.; Yao, L.; Yan, P.; Li, M.; Hu, L.; Tian, H.; Jing, W.; Du, B.; Wang, L.; et al. Robotic-assisted versus laparoscopic cholecystectomy for benign gallbladder diseases: A systematic review and meta-analysis. Surg. Endosc. 2018, 32, 4377–4392. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Zhang, J.; Zhang, C.; Shi, Y. Single-site robotic cholecystectomy versus multi-port laparoscopic cholecystectomy: A systematic review and meta-analysis. Am. J. Surg. 2018, 216, 1205–1211. [Google Scholar] [CrossRef]
- Sun, N.; Zhang, J.L.; Zhang, C.S.; Li, X.H.; Shi, Y. Single-incision robotic cholecystectomy versus single-incision laparoscopic cholecystectomy: A systematic review and meta-analysis. Medicine 2018, 97, e12103. [Google Scholar] [CrossRef]
- Wang, W.; Sun, X.; Wei, F. Laparoscopic surgery and robotic surgery for single-incision cholecystectomy: An updated systematic review. Updates Surg. 2021, 73, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.F.; Nam, S.H.; Kim, J.M. Robot-assisted laparoscopic surgery versus conventional laparoscopic surgery in randomized controlled trials: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0191628. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, J.; Li, X.; Li, Y.; Su, S. Comparative outcomes of single-incision laparoscopic, mini-laparoscopic, four-port laparoscopic, three-port laparoscopic, and single-incision robotic cholecystectomy: A systematic review and network meta-analysis. Updates Surg. 2023, 75, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Larkins, K.; Mohan, H.; Apte, S.S.; Chen, V.; Rajkomar, A.; Larach, J.T.; Smart, P.; Heriot, A.; Warrier, S. A systematic review and meta-analysis of robotic resections for diverticular disease. Color. Dis 2022, 24, 1105–1116. [Google Scholar] [CrossRef]
- Bianchi, G.; Gavriilidis, P.; Martínez-Pérez, A.; de’Angelis, G.L.; Uzzan, M.; Sobhani, I.; Coccolini, F.; Schena, C.A.; Carra, M.C.; Spinoglio, G.; et al. Robotic multiquadrant colorectal procedures: A single-center experience and a systematic review of the literature. Front. Surg. 2022, 9, 991704. [Google Scholar] [CrossRef]
- Sheng, S.; Zhao, T.; Wang, X. Comparison of robot-assisted surgery, laparoscopic-assisted surgery, and open surgery for the treatment of colorectal cancer A network meta-analysis. Medicine 2018, 97, e11817. [Google Scholar] [CrossRef]
- Giuliani, G.; Guerra, F.; Coletta, D.; Giuliani, A.; Salvischiani, L.; Tribuzi, A.; Caravaglios, G.; Genovese, A.; Coratti, A. Robotic versus conventional laparoscopic technique for the treatment of left-sided colonic diverticular disease: A systematic review with meta-analysis. Int. J. Color. Dis. 2022, 37, 101–109. [Google Scholar] [CrossRef]
- Cuk, P.; Kjær, M.D.; Mogensen, C.B.; Nielsen, M.F.; Pedersen, A.K.; Ellebæk, M.B. Short-term outcomes in robot-assisted compared to laparoscopic colon cancer resections: A systematic review and meta-analysis. Surg. Endosc. 2022, 36, 32–46. [Google Scholar] [CrossRef]
- Ravindra, C.; Igweonu-Nwakile, E.O.; Ali, S.; Paul, S.; Yakkali, S.; Teresa Selvin, S.; Thomas, S.; Bikeyeva, V.; Abdullah, A.; Radivojevic, A.; et al. Comparison of Non-Oncological Postoperative Outcomes Following Robotic and Laparoscopic Colorectal Resection for Colorectal Malignancy: A Systematic Review and Meta-Analysis. Cureus 2022, 14, e27015. [Google Scholar] [CrossRef]
- Cuk, P.; Jawhara, M.; Al-Najami, I.; Helligsø, P.; Pedersen, A.K.; Ellebæk, M.B. Robot-assisted versus laparoscopic short- and long-term outcomes in complete mesocolic excision for right-sided colonic cancer: A systematic review and meta-analysis. Tech. Coloproctol. 2023, 27, 171–181. [Google Scholar] [CrossRef]
- Flynn, J.; Larach, J.T.; Kong, J.C.H.; Rahme, J.; Waters, P.S.; Warrier, S.K.; Heriot, A. Operative and oncological outcomes after robotic rectal resection compared with laparoscopy: A systematic review and meta-analysis. ANZ J. Surg. 2022, 93, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Gavriilidis, P.; Wheeler, J.; Spinelli, A.; de’Angelis, N.; Simopoulos, C.; Di Saverio, S. Robotic vs. laparoscopic total mesorectal excision for rectal cancers: Has a paradigm change occurred? A systematic review by updated meta-analysis. Color. Dis. 2020, 22, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Rausa, E.; Bianco, F.; Kelly, M.E.; Aiolfi, A.; Petrelli, F.; Bonitta, G.; Sgroi, G. Systemic review and network meta-analysis comparing minimal surgical techniques for rectal cancer: Quality of total mesorectum excision, pathological, surgical, and oncological outcomes. J. Surg. Oncol. 2019, 119, 987–998. [Google Scholar] [CrossRef]
- Flynn, J.; Larach, J.T.; Kong, J.C.H.; Warrier, S.K.; Heriot, A. Robotic versus laparoscopic ileal pouch-anal anastomosis (IPAA): A systematic review and meta-analysis. Int. J. Color. Dis. 2021, 36, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Solaini, L.; Bocchino, A.; Avanzolini, A.; Annunziata, D.; Cavaliere, D.; Ercolani, G. Robotic versus laparoscopic left colectomy: A systematic review and meta-analysis. Int. J. Color. Dis. 2022, 37, 1497–1507. [Google Scholar] [CrossRef]
- Genova, P.; Pantuso, G.; Cipolla, C.; Latteri, M.A.; Abdalla, S.; Paquet, J.C.; Brunetti, F.; de’Angelis, N.; Di Saverio, S. Laparoscopic versus robotic right colectomy with extra-corporeal or intra-corporeal anastomosis: A systematic review and meta-analysis. Langenbeck’s Arch. Surg. 2021, 406, 1317–1339. [Google Scholar] [CrossRef]
- Lauka, L.; Brunetti, F.; Beghdadi, N.; Notarnicola, M.; Sommacale, D.; de’Angelis, N. Advantages of robotic right colectomy over laparoscopic right colectomy beyond the learning curve: A systematic review and meta-analysis. Ann. Laparosc. Endosc. Surg. 2020, 5, 20–33. [Google Scholar] [CrossRef]
- Ma, S.; Chen, Y.; Chen, Y.; Guo, T.; Yang, X.; Lu, Y.; Tian, J.; Cai, H. Short-term outcomes of robotic-assisted right colectomy compared with laparoscopic surgery: A systematic review and meta-analysis. Asian J. Surg. 2019, 42, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Rausa, E.; Kelly, M.E.; Asti, E.; Aiolfi, A.; Bonitta, G.; Bonavina, L. Right hemicolectomy: A network meta-analysis comparing open, laparoscopic-assisted, total laparoscopic, and robotic approach. Surg. Endosc. 2019, 33, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Solaini, L.; Bazzocchi, F.; Cavaliere, D.; Avanzolini, A.; Cucchetti, A.; Ercolani, G. Robotic versus laparoscopic right colectomy: An updated systematic review and meta-analysis. Surg. Endosc. 2018, 32, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Tschann, P.; Szeverinski, P.; Weigl, M.P.; Rauch, S.; Lechner, D.; Adler, S.; Girotti, P.N.C.; Clemens, P.; Tschann, V.; Presl, J.; et al. Short-and Long-Term Outcome of Laparoscopic-versus Robotic-Assisted Right Colectomy: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 2387. [Google Scholar] [CrossRef] [PubMed]
- Waters, P.S.; Cheung, F.P.; Peacock, O.; Heriot, A.G.; Warrier, S.K.; O’Riordain, D.S.; Pillinger, S.; Lynch, A.C.; Stevenson, A.R.L. Successful patient-oriented surgical outcomes in robotic vs. laparoscopic right hemicolectomy for cancer—A systematic review. Color. Dis. 2020, 22, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.L.; Xu, X.; Pan, Z.J. Comparison of clinical efficacy of robotic right colectomy and laparoscopic right colectomy for right colon tumor: A systematic review and meta-analysis. Medicine 2021, 100, e27002. [Google Scholar] [CrossRef] [PubMed]
- Alshowaikh, K.; Karpinska-Leydier, K.; Amirthalingam, J.; Paidi, G.; Iroshani Jayarathna, A.I.; Salibindla, D.; Ergin, H.E. Surgical and Patient Outcomes of Robotic Versus Conventional Laparoscopic Hysterectomy: A Systematic Review. Cureus 2021, 13, e16828. [Google Scholar] [CrossRef]
- Prodromidou, A.; Spartalis, E.; Tsourouflis, G.; Dimitroulis, D.; Nikiteas, N. Robotic versus laparoendoscopic single-site hysterectomy: A systematic review and meta-analysis. J. Robot. Surg. 2020, 14, 679–686. [Google Scholar] [CrossRef]
- Kampers, J.; Gerhardt, E.; Sibbertsen, P.; Flock, T.; Hertel, H.; Klapdor, R.; Jentschke, M.; Hillemanns, P. Perioperative morbidity of different operative approaches in early cervical carcinoma: A systematic review and meta-analysis comparing minimally invasive versus open radical hysterectomy. Arch. Gynecol. Obstet. 2022, 306, 295–314. [Google Scholar] [CrossRef]
- Marchand, G.; Taher Masoud, A.; Ware, K.; Govindan, M.; King, A.; Ruther, S.; Brazil, G.; Calteux, N.; Coriell, C.; Ulibarri, H.; et al. Systematic review and meta-analysis of all randomized controlled trials comparing gynecologic laparoscopic procedures with and without robotic assistance. Eur. J. Obs. Gynecol. Reprod. Biol. 2021, 265, 30–38. [Google Scholar] [CrossRef]
- Zhang, S.S.; Ding, T.; Cui, Z.H.; Lv, Y.; Jiang, R.A. Efficacy of robotic radical hysterectomy for cervical cancer compared with that of open and laparoscopic surgery: A separate meta-analysis of high-quality studies. Medicine 2019, 98, e14171. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Tian, S.; Wang, H.; Zhang, J.; Cheng, Y.; Yao, Y. Outcomes associated with different surgical approaches to radical hysterectomy: A systematic review and network meta-analysis. Int. J. Gynaecol. Obs. 2023, 160, 28–37. [Google Scholar] [CrossRef]
- Jin, Y.M.; Liu, S.S.; Chen, J.; Chen, Y.N.; Ren, C.C. Robotic radical hysterectomy is superior to laparoscopic radical hysterectomy and open radical hysterectomy in the treatment of cervical cancer. PLoS ONE 2018, 13, e0193033. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Kim, B.W.; Kim, S.R.; Kim, J.H. Robotic Radical Hysterectomy Is Not Superior to Laparoscopic Radical Hysterectomy in Perioperative Urologic Complications: A Meta-Analysis of 23 Studies. J. Minim. Invasive Gynecol. 2020, 27, 38–47. [Google Scholar] [CrossRef]
- Zahid, A.; Ayyan, M.; Farooq, M.; Cheema, H.A.; Shahid, A.; Naeem, F.; Ilyas, M.A.; Sohail, S. Robotic surgery in comparison to the open and laparoscopic approaches in the field of urology: A systematic review. J. Robot. Surg. 2023, 17, 11–29. [Google Scholar] [CrossRef]
- Veccia, A.; Antonelli, A.; Francavilla, S.; Simeone, C.; Guruli, G.; Zargar, H.; Perdoná, S.; Ferro, M.; Carrieri, G.; Hampton, L.J.; et al. Robotic versus other nephroureterectomy techniques: A systematic review and meta-analysis of over 87,000 cases. World J. Urol. 2020, 38, 845–852. [Google Scholar] [CrossRef]
- Li, J.; Peng, L.; Cao, D.; Cheng, B.; Gou, H.; Li, Y.; Wei, Q. Comparison of Perioperative Outcomes of Robot-Assisted vs. Laparoscopic Radical Nephrectomy: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 551052. [Google Scholar] [CrossRef] [PubMed]
- Crocerossa, F.; Carbonara, U.; Cantiello, F.; Marchioni, M.; Ditonno, P.; Mir, M.C.; Porpiglia, F.; Derweesh, I.; Hampton, L.J.; Damiano, R.; et al. Robot-assisted Radical Nephrectomy: A Systematic Review and Meta-analysis of Comparative Studies. Eur. Urol. 2021, 80, 428–439. [Google Scholar] [CrossRef]
- Wang, H.; Chen, R.; Li, T.; Peng, L. Robot-assisted laparoscopic vs. laparoscopic donor nephrectomy in renal transplantation: A meta-analysis. Clin. Transplant. 2019, 33, e13451. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, A.P.; Tyagi, S.; Bora, G.S.; Mavuduru, R.S.; Devana, S.K.; Singh, S.K. Robot-assisted partial nephrectomy for moderate to highly complex renal masses. A systematic review and meta-analysis. Indian J. Urol. 2022, 38, 174–183. [Google Scholar]
- Xiao, Q.; Fu, B.; Song, K.; Chen, S.; Li, J.; Xiao, J. Comparison of surgical techniques in living donor nephrectomy: A systematic review and Bayesian network meta-analysis. Ann. Transplant. 2020, 25, e926677-1. [Google Scholar] [CrossRef] [PubMed]
- Carbonara, U.; Srinath, M.; Crocerossa, F.; Ferro, M.; Cantiello, F.; Lucarelli, G.; Porpiglia, F.; Battaglia, M.; Ditonno, P.; Autorino, R. Robot-assisted radical prostatectomy versus standard laparoscopic radical prostatectomy: An evidence-based analysis of comparative outcomes. World J. Urol. 2021, 39, 3721–3732. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, Q.; Wang, S. A meta-analysis of robot assisted laparoscopic radical prostatectomy versus laparoscopic radical prostatectomy. Open Med. 2019, 14, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Cacciamani, G.E.; Medina, L.G.; Gill, T.; Abreu, A.; Sotelo, R.; Artibani, W.; Gill, I.S. Impact of Surgical Factors on Robotic Partial Nephrectomy Outcomes: Comprehensive Systematic Review and Meta-Analysis. J. Urol. 2018, 200, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Li, K.P.; Chen, S.Y.; Yang, L. Laparoscopic simple prostatectomy versus robot-assisted simple prostatectomy for large benign prostatic hyperplasia: A systematic review and meta-analysis of comparative trials. J. Robot. Surg. 2022, 17, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Dalager, T.; Jensen, P.T.; Eriksen, J.R.; Jakobsen, H.L.; Mogensen, O.; Sgaard, K. Surgeons’ posture and muscle strain during laparoscopic and robotic surgery. J. Br. Surg. 2020, 107, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk, J.; Zandbergen, H.R.; Keet, S.W.M.; Martijnse, I.; Van Montfort, G.; Peters, R.J.A.; Svircevic, V.; Bouwman, R.A.; Baeten, C.G.M.I.; Bouvy, N.D. Relax, it’s just laparoscopy! A prospective randomized trial on heart rate variability of the surgeon in robot-assisted versus conventional laparoscopic cholecystectomy. Dig. Surg. 2014, 31, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.M.; Dimou, F.; Weber, J.; Almhanna, K.; Hoffe, S.; Shridhar, R.; Karl, R.; Meredith, K. Defining the learning curve for robotic-assisted esophagogastrectomy. J. Gastrointest. Surg. 2013, 17, 1346–1351. [Google Scholar] [CrossRef]
- Hennessy, E.A.; Johnson, B.T. Examining overlap of included studies in meta-reviews: Guidance for using the corrected covered area index. Res. Synth. Methods 2020, 11, 134–145. [Google Scholar] [CrossRef]
- Choi, G.J.; Kang, H. The umbrella review: A useful strategy in the rain of evidence. Korean J. Pain 2022, 35, 127–128. [Google Scholar] [CrossRef]
- Bramer, W.M.; Rethlefsen, M.L.; Kleijnen, J.; Franco, O.H. Optimal database combinations for literature searches in systematic reviews: A prospective exploratory study. Syst. Rev. 2017, 6, 245. [Google Scholar] [CrossRef]
- Akobeng, A.K. Understanding randomised controlled trials. Arch. Dis. Child. 2005, 90, 840–844. [Google Scholar] [CrossRef]
- Alkatout, I.; Salehiniya, H.; Allahqoli, L. Assessment of the Versius Robotic Surgical System in Minimal Access Surgery: A Systematic Review. J. Clin. Med. 2022, 11, 3754. [Google Scholar] [CrossRef] [PubMed]
- Charalambides, M.; Mavrou, A.; Jennings, T.; Powar, M.P.; Wheeler, J.; Davies, R.J.; Fearnhead, N.S.; Simillis, C. A systematic review of the literature assessing operative blood loss and postoperative outcomes after colorectal surgery. Int. J. Color. Dis. 2022, 37, 47–69. [Google Scholar] [CrossRef] [PubMed]
- Toh, J.W.T.; Phan, K.; Kim, S.H. Robotic colorectal surgery: More than a fantastic toy? Innov. Surg. Sci. 2020, 3, 65–68. [Google Scholar] [CrossRef]
- Oweira, H.; Reissfelder, C.; Elhadedy, H.; Rahbari, N.; Mehrabi, A.; Fattal, W.; Khan, J.S.; Chaouch, M.A. Robotic colectomy with CME versus laparoscopic colon resection with or without CME for colon cancer: A systematic review and meta-analysis. Ann. R. Coll. Surg. Engl. 2023, 105, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Leitao, M.M.; Kreaden, U.S.; Laudone, V.; Park, B.J.; Pappou, E.P.; Davis, J.W.; Rice, D.C.; Chang, G.J.; Rossi, E.C.; Hebert, A.E.; et al. The RECOURSE Study: Long-term Oncologic Outcomes Associated With Robotically Assisted Minimally Invasive Procedures for Endometrial, Cervical, Colorectal, Lung, or Prostate Cancer: A Systematic Review and Meta-analysis. Ann. Surg. 2023, 277, 387–396. [Google Scholar] [CrossRef]
- Kampers, J.; Gerhardt, E.; Sibbertsen, P.; Flock, T.; Klapdor, R.; Hertel, H.; Jentschke, M.; Hillemanns, P. Protective operative techniques in radical hysterectomy in early cervical carcinoma and their influence on disease-free and overall survival: A systematic review and meta-analysis of risk groups. Arch. Gynecol. Obstet. 2021, 304, 577–587. [Google Scholar] [CrossRef]
- Nitecki, R.; Ramirez, P.T.; Frumovitz, M.; Krause, K.J.; Tergas, A.I.; Wright, J.D.; Rauh-Hain, J.A.; Melamed, A. Survival after Minimally Invasive vs. Open Radical Hysterectomy for Early-Stage Cervical Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2020, 6, 1019–1027. [Google Scholar] [CrossRef]
- Marra, A.R.; Puig-Asensio, M.; Edmond, M.B.; Schweizer, M.L.; Bender, D. Infectious complications of laparoscopic and robotic hysterectomy: A systematic literature review and meta-analysis. Int. J. Gynecol. Cancer 2019, 29, 518–530. [Google Scholar] [CrossRef]
- Behbehani, S.; Suarez-Salvador, E.; Buras, M.; Magtibay, P.; Magrina, J. Mortality Rates in Laparoscopic and Robotic Gynecologic Oncology Surgery: A Systemic Review and Meta-analysis. J. Minim. Invasive Gynecol. 2019, 26, 1253–1267.e1254. [Google Scholar] [CrossRef]
- Behbehani, S.; Suarez-Salvador, E.; Buras, M.; Magtibay, P.; Magrina, J. Mortality Rates in Benign Laparoscopic and Robotic Gynecologic Surgery: A Systematic Review and Meta-analysis. J. Minim. Invasive Gynecol. 2020, 27, 603–612.e601. [Google Scholar] [CrossRef]
- Kostakis, I.D.; Sran, H.; Uwechue, R.; Chandak, P.; Olsburgh, J.; Mamode, N.; Loukopoulos, I.; Kessaris, N. Comparison Between Robotic and Laparoscopic or Open Anastomoses: A Systematic Review and Meta-Analysis. Robot. Surg. 2019, 6, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa-Gonzalez, D.E.; Roblesgil-Medrano, A.; Tellez-Giron, V.C.; Torres-Martinez, M.; Galindo-Garza, C.A.; Estrada-Mendizabal, R.J.; Alanis-Garza, C.; Gonzalez-Bonilla, E.A.; Flores-Villalba, E. Robotic-assisted versus laparoscopic living donor nephrectomy for renal transplantation: A systematic review and meta-analysis. Ann. R. Coll. Surg. Engl. 2023, 105, 7–13. [Google Scholar] [CrossRef]
- Lin, P.; Wu, M.; Gu, H.; Tu, L.; Liu, S.; Yu, Z.; Chen, Q.; Liu, C. Comparison of outcomes between laparoscopic and robot-assisted partial nephrectomy for complex renal tumors: RENAL score >=7 or maximum tumor size >4 cm. Minerva Urol. Nephrol. 2021, 73, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, V.; Crestani, A.; Inferrera, A.; Novara, G.; Rossanese, M.; Subba, E.; Giannarini, G. Positive surgical margins after partial nephrectomy: A systematic review and meta-analysis of comparative studies. Kidney Cancer 2018, 2, 133–145. [Google Scholar] [CrossRef]
- Sridharan, K.; Sivaramakrishnan, G. Prostatectomies for localized prostate cancer: A mixed comparison network and cumulative meta-analysis. J. Robot. Surg. 2018, 12, 633–639. [Google Scholar] [CrossRef]
- Marra, A.R.; Puig-Asensio, M.; Edmond, M.B.; Schweizer, M.L.; Nepple, K.G. Infectious Complications of Conventional Laparoscopic vs. Robotic Laparoscopic Prostatectomy: A Systematic Literature Review and Meta-Analysis. J. Endourol. 2019, 33, 179–188. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).