Mucosal-Associated Lymphoid Tissue Lymphoma in Southeast Asia: A 15-Year Retrospective Multicenter Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Data Collection and Variables
2.3. Study Endpoints and Follow-Up
2.4. Ethical Considerations
2.5. Statistical Analysis
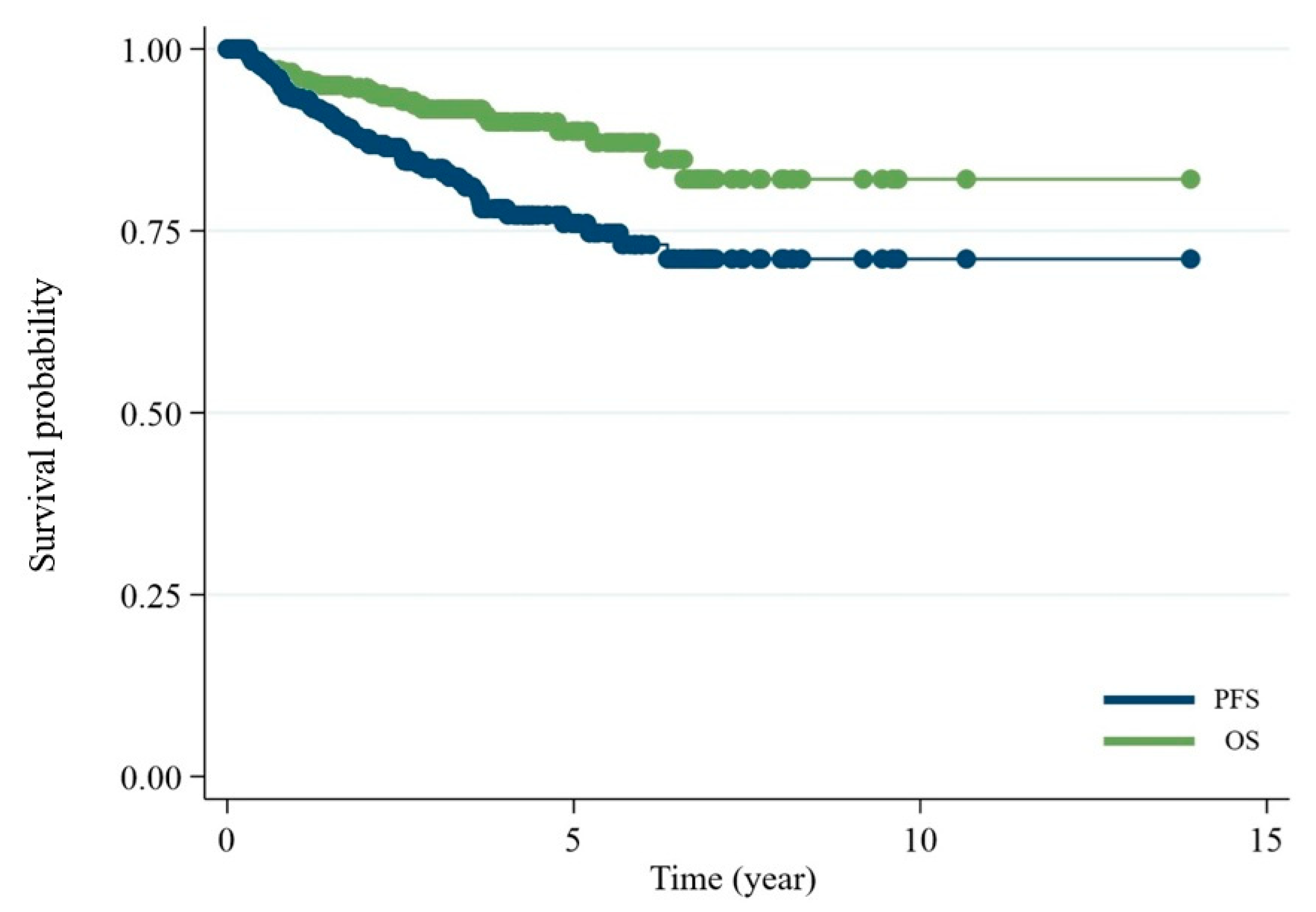
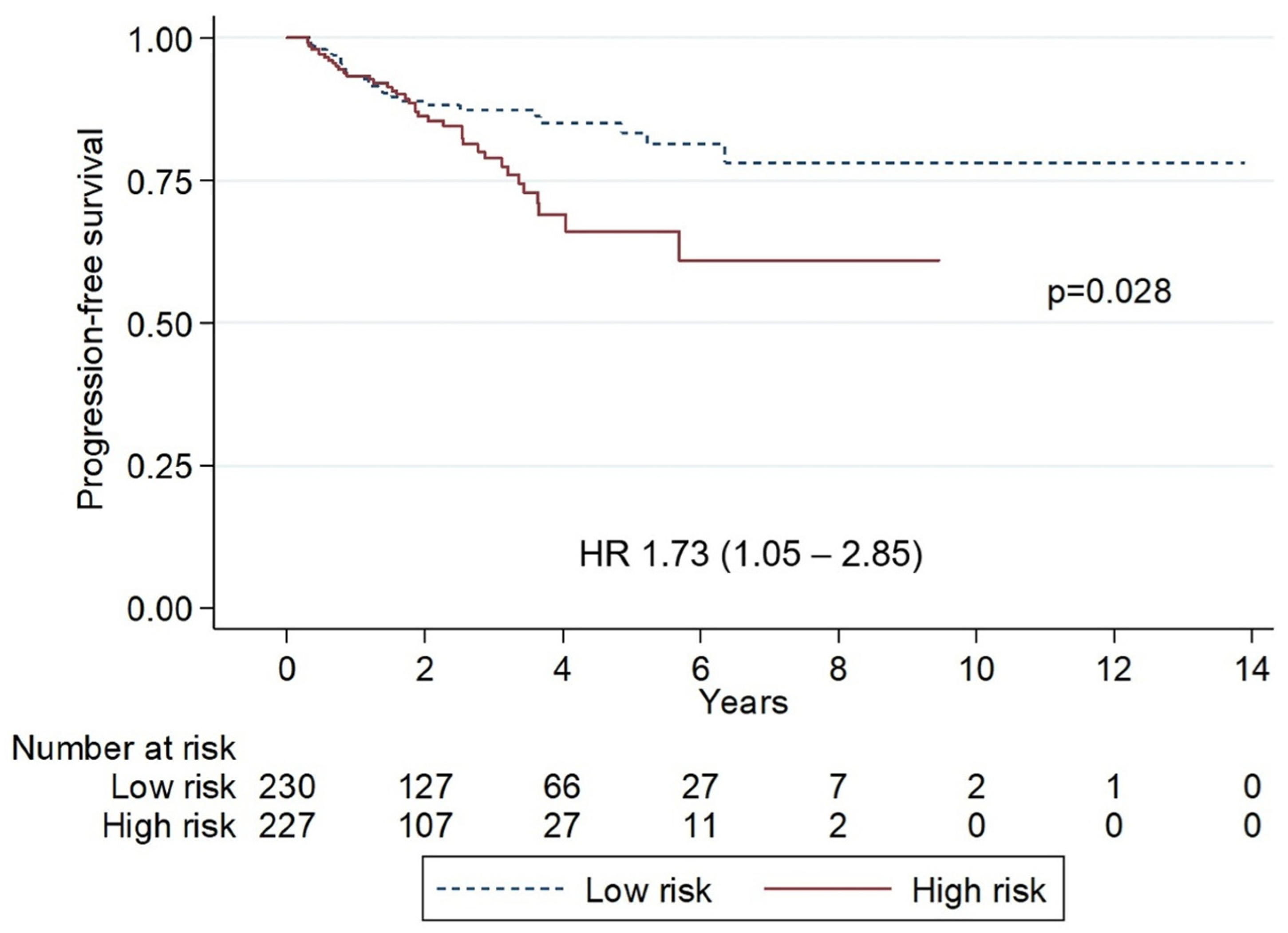
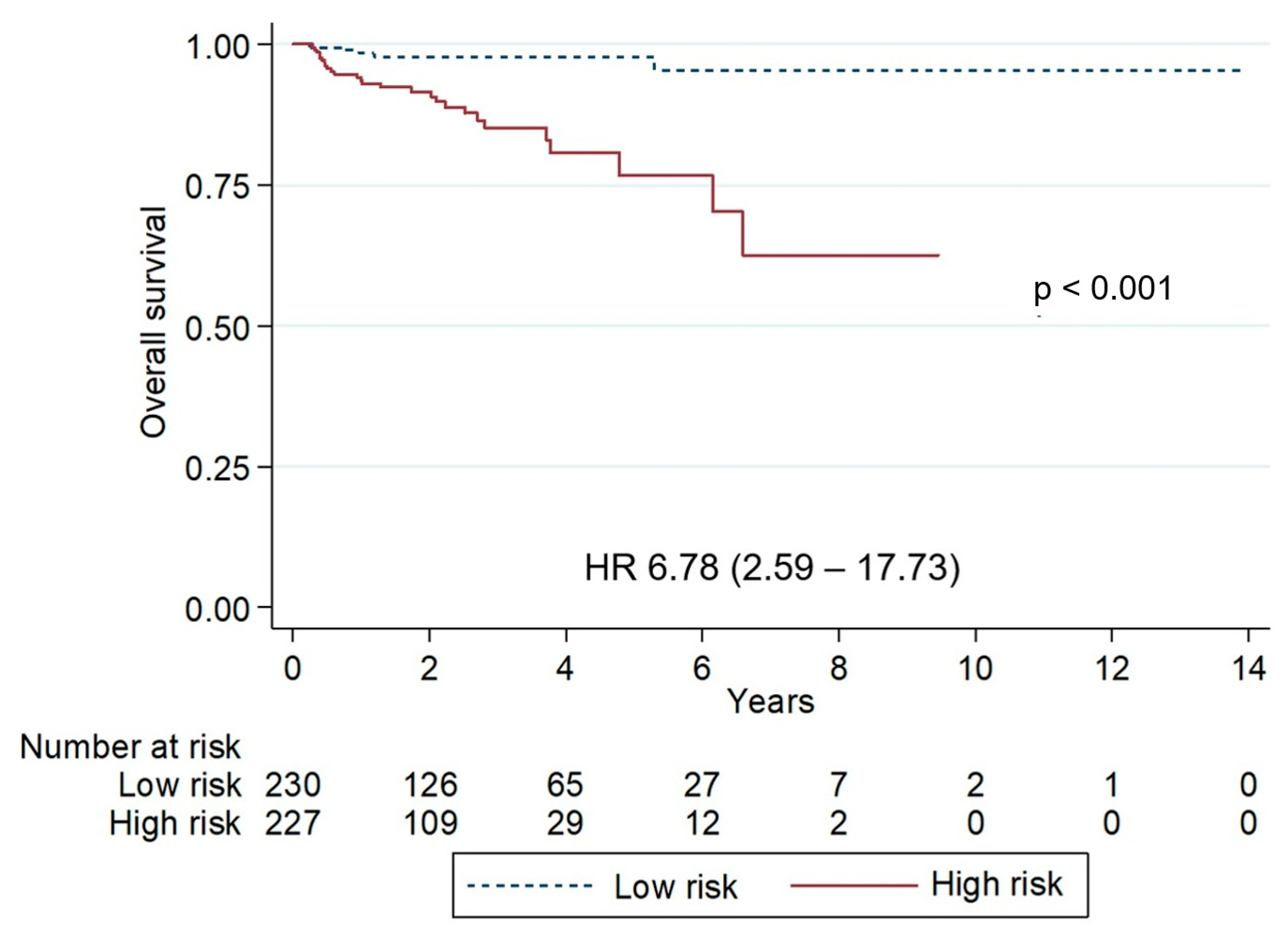
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MALT | mucosal-associated lymphoid tissue |
| TLSG | Thai Lymphoma Study Group |
| MZL | Marginal-zone lymphoma |
| OS | Overall survival |
| PFS | Progression-free survival |
| EMZL | Extranodal marginal-zone lymphoma |
| NMZL | Nodal marginal-zone lymphoma |
| SMZL | Splenic marginal-zone lymphoma |
| PCMZL | Primary cutaneous marginal-zone lymphoma |
| DLBCL | Diffuse large B-cell lymphoma |
| ECOG | Eastern Cooperative Oncology Group |
| LDH | Lactate dehydrogenase |
| CMT | Chemotherapy |
| RT | Radiation |
| R | Rituximab |
| aHR | Adjusted hazard ratio |
| HR | Hazard ratio |
| CI | Confidence interval |
| HBsAg | Hepatitis B surface antigen |
| Anti-HBc Ab | anti-hepatitis B core antibody |
| OAML | Ocular adnexa MALT lymphoma |
| MALT-IPI | MALT Lymphoma International Prognostic Index |
| SEER | Surveillance, Epidemiology, and End Results |
References
- Armitage, J.O.; Weisenburger, D.D. New approach to classifying non-Hodgkin’s lymphomas: Clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J. Clin. Oncol. 1998, 16, 2780–2795. [Google Scholar] [CrossRef]
- Sindel, A.; Al-Juhaishi, T.; Yazbeck, V. Marginal Zone Lymphoma: State-of-the-Art Treatment. Curr. Treat. Options Oncol. 2019, 20, 90. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.; De, O.; Berti, E.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Luminari, S.; Cesaretti, M.; Marcheselli, L.; Rashid, I.; Madrigali, S.; Maiorana, A.; Federico, M. Decreasing incidence of gastric MALT lymphomas in the era of anti-Helicobacter pylori interventions: Results from a population-based study on extranodal marginal zone lymphomas. Ann. Oncol. 2010, 21, 855–859. [Google Scholar] [CrossRef]
- Sriskandarajah, P.; Dearden, C.E. Epidemiology and environmental aspects of marginal zone lymphomas. Best Pract. Res. Clin. Haematol. 2017, 30, 84–91. [Google Scholar] [CrossRef]
- Cerhan, J.R.; Habermann, T.M. Epidemiology of Marginal Zone Lymphoma. Ann. Lymphoma 2021, 5, 1. [Google Scholar] [CrossRef]
- The Non-Hodgkin’s Lymphoma Classification Project. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood 1997, 89, 3909–3918. [Google Scholar] [CrossRef]
- Cheah, C.Y.; Seymour, J.F. Marginal zone lymphoma: 2023 update on diagnosis and management. Am. J. Hematol. 2023, 98, 1645–1657. [Google Scholar] [CrossRef]
- Zucca, E.; Bertoni, F. The spectrum of MALT lymphoma at different sites: Biological and therapeutic relevance. Blood 2016, 127, 2082–2092. [Google Scholar] [CrossRef]
- Du, M.Q. MALT lymphoma: Genetic abnormalities, immunological stimulation and molecular mechanism. Best Pract. Res. Clin. Haematol. 2017, 30, 13–23. [Google Scholar] [CrossRef]
- Inagaki, H. Mucosa-associated lymphoid tissue lymphoma: Molecular pathogenesis and clinicopathological significance. Pathol. Int. 2007, 57, 474–484. [Google Scholar] [CrossRef]
- Intragumtornchai, T.; Bunworasate, U.; Wudhikarn, K.; Lekhakula, A.; Julamanee, J.; Chansung, K.; Sirijerachai, C.; Norasetthada, L.; Nawarawong, W.; Khuhapinant, A.; et al. Non-Hodgkin lymphoma in South East Asia: An analysis of the histopathology, clinical features, and survival from Thailand. Hematol. Oncol. 2018, 36, 28–36. [Google Scholar] [CrossRef]
- Cheson, B.D.; Pfistner, B.; Juweid, M.E.; Gascoyne, R.D.; Specht, L.; Horning, S.J.; Coiffier, B.; Fisher, R.I.; Hagenbeek, A.; Zucca, E.; et al. Revised response criteria for malignant lymphoma. J. Clin. Oncol. 2007, 25, 579–586. [Google Scholar] [CrossRef]
- Riley, R.D.; Snell, K.I.; Ensor, J.; Burke, D.L.; Harrell, F.E., Jr.; Moons, K.G.; Collins, G.S. Minimum sample size for developing a multivariable prediction model: PART II—Binary and time-to-event outcomes. Stat. Med. 2019, 38, 1276–1296. [Google Scholar] [CrossRef]
- Alderuccio, J.P.; Reis, I.M.; Habermann, T.M.; Link, B.K.; Thieblemont, C.; Conconi, A.; Larson, M.C.; Cascione, L.; Zhao, W.; Cerhan, J.R.; et al. Revised MALT-IPI: A new predictive model that identifies high-risk patients with extranodal marginal zone lymphoma. Am. J. Hematol. 2022, 97, 1529–1537. [Google Scholar] [CrossRef]
- Bursac, Z.; Gauss, C.H.; Williams, D.K.; Hosmer, D.W. Purposeful selection of variables in logistic regression. Source Code Biol. Med. 2008, 3, 17. [Google Scholar] [CrossRef]
- Sun, J.; Yang, Q.; Lu, Z.; He, M.; Gao, L.; Zhu, M.; Sun, L.; Wei, L.; Li, M.; Liu, C.; et al. Distribution of lymphoid neoplasms in China: Analysis of 4,638 cases according to the World Health Organization classification. Am. J. Clin. Pathol. 2012, 138, 429–434. [Google Scholar] [CrossRef]
- Oh, S.Y.; Suh, C. Non-gastric marginal zone B-cell lymphoma in Korea: Clinical features, treatment, and prognostic factors. Korean J. Intern. Med. 2010, 25, 227–236. [Google Scholar] [CrossRef]
- Ferreri, A.J.M.; Dolcetti, R.; Du, M.Q.; Doglioni, C.; Resti, A.G.; Politi, L.S.; De Conciliis, C.; Radford, J.; Bertoni, F.; Zucca, E.; et al. Ocular adnexal MALT lymphoma: An intriguing model for antigen-driven lymphomagenesis and microbial-targeted therapy. Ann. Oncol. 2008, 19, 835–846. [Google Scholar] [CrossRef]
- Ferreri, A.J.M.; Guidoboni, M.; Ponzoni, M.; De Conciliis, C.; Dell’Oro, S.; Fleischhauer, K.; Caggiari, L.; Lettini, A.A.; Dal Cin, E.; Ieri, R.; et al. Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. J. Natl. Cancer Inst. 2004, 96, 586–594. [Google Scholar] [CrossRef]
- Seresirikachorn, K.; Norasetthada, L.; Ausayakhun, S.; Apivatthakakul, A.; Tangchittam, S.; Pruksakorn, V.; Wudhikarn, K.; Wiwatwongwana, D. Clinical presentation and treatment outcomes of primary ocular adnexal MALT lymphoma in Thailand. Blood Res. 2018, 53, 307–313. [Google Scholar] [CrossRef]
- Thamgrang, T.; Prayongratana, K.; Norasetthada, L.; Rattanathammethee, T.; Lekhakula, A.; Julamanee, J.; Bunworasate, U.; Wudhikarn, K.; Chuncharunee, S.; Niparuck, P.; et al. Epidemiology and Prognostic Index of Ocular Adnexal Extranodal Marginal Zone Lymphoma of Mucosa-Associated Lymphoid Tissue (MALT) in Thailand: Results from the Thai Lymphoma Study Group (TLSG). Blood 2022, 140 (Suppl. 1), 11960–11961. [Google Scholar] [CrossRef]
- Zucca, E.; Conconi, A.; Pedrinis, E.; Cortelazzo, S.; Motta, T.; Gospodarowicz, M.K.; Patterson, B.J.; Ferreri, A.J.M.; Ponzoni, M.; Devizzi, L.; et al. Nongastric marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Blood 2003, 101, 2489–2495. [Google Scholar] [CrossRef]
- Thieblemont, C.; Berger, F.; Dumontet, C.; Moullet, I.; Bouafia, F.; Felman, P.; Salles, G.; Coiffier, B. Mucosa-associated lymphoid tissue lymphoma is a disseminated disease in one third of 158 patients analyzed. Blood 2000, 95, 802–806. [Google Scholar] [CrossRef]
- de Boer, J.P.; Hiddink, R.F.; Raderer, M.; Antonini, N.; Aleman, B.M.P.; Boot, H.; de Jong, D. Dissemination patterns in non-gastric MALT lymphoma. Haematologica 2008, 93, 201–206. [Google Scholar] [CrossRef]
- Thieblemont, C.; Cascione, L.; Conconi, A.; Kiesewetter, B.; Raderer, M.; Gaidano, G.; Martelli, M.; Laszlo, D.; Coiffier, B.; Guillermo, A.L.; et al. A MALT lymphoma prognostic index. Blood 2017, 130, 1409–1417. [Google Scholar] [CrossRef]
- Zucca, E.; Conconi, A.; Martinelli, G.; Bouabdallah, R.; Tucci, A.; Vitolo, U.; Martelli, M.; Pettengell, R.; Salles, G.; Sebban, C.; et al. Final Results of the IELSG-19 Randomized Trial of Mucosa-Associated Lymphoid Tissue Lymphoma: Improved Event-Free and Progression-Free Survival with Rituximab Plus Chlorambucil Versus Either Chlorambucil or Rituximab Monotherapy. J. Clin. Oncol. 2017, 35, 1905–1912. [Google Scholar] [CrossRef]
- Alderuccio, J.P.; Florindez, J.A.; Reis, I.M.; Zhao, W.; Lossos, I.S. Treatments and Outcomes in Stage I Extranodal Marginal Zone Lymphoma in the United States. Cancers 2021, 13, 1803. [Google Scholar] [CrossRef]
- Endrizzi, L.; Fiorentino, M.V.; Salvagno, L.; Segati, R.; Pappagallo, G.L.; Fosser, V. Serum lactate dehydrogenase (LDH) as a prognostic index for non-Hodgkin’s lymphoma. Eur. J. Cancer Clin. Oncol. 1982, 18, 945–949. [Google Scholar] [CrossRef]
- Epperla, N.; Welkie, R.L.; Torka, P.; Shouse, G.; Karmali, R.; Shea, L.; Anampa-Guzmán, A.; Oh, T.S.; Reaves, H.; Tavakkoli, M.; et al. Impact of early relapse within 24 months after first-line systemic therapy (POD24) on outcomes in patients with marginal zone lymphoma: A US multisite study. J. Hematol. Oncol. 2023, 16, 49. [Google Scholar] [CrossRef]
- Luminari, S.; Merli, M.; Rattotti, S.; Tarantino, V.; Marcheselli, L.; Cavallo, F.; Varettoni, M.; Bianchi, B.; Merli, F.; Tedeschi, A.; et al. Early progression as a predictor of survival in marginal zone lymphomas: An analysis from the FIL-NF10 study. Blood 2019, 134, 798–801. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Number | % | |
|---|---|---|---|
| Age | Median, year (range) | 60 (17–95) | |
| <65 years | 295 | 64.6% | |
| ≥65 years | 162 | 35.4% | |
| Gender | Female | 197 | 43.1% |
| Male | 260 | 56.9% | |
| Type of EMZL | Ocular adnexa | 225 | 49.2% |
| Stomach | 59 | 12.9% | |
| Sinonasal | 57 | 12.5% | |
| Salivary gland | 19 | 4.2% | |
| Thyroid | 16 | 3.5% | |
| Lung/Pleura | 24 | 5.3% | |
| Breast | 8 | 1.8% | |
| Small intestine | 16 | 3.5% | |
| Large intestine | 9 | 2.0% | |
| Skin/Subcutaneous | 4 | 0.9% | |
| Others | 5 | 1.1% | |
| Missing | 15 | 3.3% | |
| Number of extranodal sites | 1 | 379 | 82.9% |
| 2 | 60 | 13.1% | |
| 3 | 17 | 3.7% | |
| 4 | 1 | 0.2% | |
| ECOG | 0 | 237 | 51.9% |
| 1 | 169 | 37.0% | |
| 2 | 36 | 7.9% | |
| 3 | 11 | 2.4% | |
| 4 | 4 | 0.9% | |
| B-symptoms | Presence | 103 | 22.5% |
| Absent | 354 | 77.5% | |
| HBV status (HBsAg or Anti-HBc Ab) | Positive | 104 | 22.8% |
| Negative | 353 | 77.2% | |
| Ann Arbor staging | I–II | 295 | 64.6% |
| III IV | 162 | 35.4% | |
| LDH | Normal | 345 | 75.5% |
| High | 112 | 24.5% | |
| Variables | PFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | |
| Age | ||||||
| ≥65 years | 0.93 | 0.54–1.59 | 0.792 | 3.27 | 1.60–6.68 | 0.001 |
| Gender | ||||||
| Male | 1.02 | 0.63–1.66 | 0.935 | 1.53 | 0.74–3.17 | 0.255 |
| Type of EMZL | ||||||
| Ocular adnexa | 1.04 | 0.64–1.69 | 0.882 | 0.54 | 0.26–1.12 | 0.096 |
| Number of extranodal sites | ||||||
| 2 | 1.84 | 1.00–3.37 | 0.048 | 1.55 | 0.63–3.82 | 0.337 |
| 3–4 | 4.15 | 1.94–8.86 | <0.001 | 3.07 | 0.91–10.30 | 0.070 |
| ECOG | ||||||
| 1 | 1.53 | 0.90–2.60 | 0.117 | 1.45 | 0.62–3.44 | 0.393 |
| 2 | 1.73 | 0.71–4.17 | 0.225 | 2.67 | 0.85–8.40 | 0.093 |
| 3–4 | 5.46 | 1.89–15.75 | 0.002 | 22.08 | 8.32–58.58 | <0.001 |
| HBsAg or Anti-HBc Ab | ||||||
| Positive | 1.32 | 0.75–2.34 | 0.335 | 0.76 | 0.29–1.99 | 0.576 |
| LDH | ||||||
| High | 1.33 | 0.76–2.31 | 0.319 | 1.95 | 0.94–4.07 | 0.074 |
| Advanced stage | ||||||
| Yes | 1.77 | 1.09–2.88 | 0.022 | 1.73 | 0.86–3.48 | 0.121 |
| B-symptoms | ||||||
| Yes | 1.59 | 0.94–2.69 | 0.085 | 2.10 | 1.03–4.30 | 0.043 |
| Variables | PFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | |
| Age ≥ 65 years | 0.86 | 0.5–1.49 | 0.591 | 2.48 | 1.18–5.21 | 0.017 * |
| Number of extranodal sites 2–4 | 2.13 | 1.25–3.62 | 0.005 * | 1.39 | 0.62–3.11 | 0.418 |
| ECOG 2–4 | 1.65 | 0.81–3.38 | 0.169 | 3.5 | 1.57–7.79 | 0.002 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prayongratana, K.; Thamgrang, T.; Laoruangroj, C.; Norasetthada, L.; Rattanathammethee, T.; Bunworasate, U.; Wudhikarn, K.; Julamanee, J.; Noiperm, P.; Chuncharunee, S.; et al. Mucosal-Associated Lymphoid Tissue Lymphoma in Southeast Asia: A 15-Year Retrospective Multicenter Study. Hematol. Rep. 2025, 17, 63. https://doi.org/10.3390/hematolrep17060063
Prayongratana K, Thamgrang T, Laoruangroj C, Norasetthada L, Rattanathammethee T, Bunworasate U, Wudhikarn K, Julamanee J, Noiperm P, Chuncharunee S, et al. Mucosal-Associated Lymphoid Tissue Lymphoma in Southeast Asia: A 15-Year Retrospective Multicenter Study. Hematology Reports. 2025; 17(6):63. https://doi.org/10.3390/hematolrep17060063
Chicago/Turabian StylePrayongratana, Kannadit, Tanapun Thamgrang, Chonlada Laoruangroj, Lalita Norasetthada, Thanawat Rattanathammethee, Udomsak Bunworasate, Kitsada Wudhikarn, Jakrawadee Julamanee, Panarat Noiperm, Suporn Chuncharunee, and et al. 2025. "Mucosal-Associated Lymphoid Tissue Lymphoma in Southeast Asia: A 15-Year Retrospective Multicenter Study" Hematology Reports 17, no. 6: 63. https://doi.org/10.3390/hematolrep17060063
APA StylePrayongratana, K., Thamgrang, T., Laoruangroj, C., Norasetthada, L., Rattanathammethee, T., Bunworasate, U., Wudhikarn, K., Julamanee, J., Noiperm, P., Chuncharunee, S., Niparuck, P., Khuhapinant, A., Siritanaratkul, N., Kanya, P., Chansung, K., Sirijerachai, C., Jit-Uaekul, D., Chaloemwong, J., Kanitsap, N., ... Intragumtornchai, T. (2025). Mucosal-Associated Lymphoid Tissue Lymphoma in Southeast Asia: A 15-Year Retrospective Multicenter Study. Hematology Reports, 17(6), 63. https://doi.org/10.3390/hematolrep17060063








