Abstract
Background and Clinical Significance: Mastocytosis and mast cell activation syndrome (MCAS) include conditions in which patients manifest signs, symptoms, and laboratory findings consistent with mast cell activation and can only be diagnosed in the presence of specific criteria. Mutations of ZRSR2, a gene involved in RNA splicing, are not closely associated with mast cell disorders, but rather with myelodysplastic syndromes development. Case Presentation: We report a case of a 37-year-old man who was referred to our institution for anaphylaxis after a bee sting and elevated serum tryptase levels (17.8 ng/mL in the first sample and 19.2 ng/mL in the second sample). Complete blood count was unremarkable. Bone marrow biopsy showed signs of dysplasia and some CD25+ mast cells. ASO-qPCR and targeted myeloid NGS analysis did not detect the KIT p.D816V mutation, but rather showed the presence of a pathogenetic variant of the ZRSR2 gene (p.S447_R448del) with a variant allele frequency of 7.4%. Mastocytosis could not be diagnosed based on the established diagnostic criteria. The patient’s symptoms were not recurrent and tryptase release was not event-related; therefore, a diagnosis of MCAS could not be made either. Taken together, these findings led to the diagnosis of clonal hematopoiesis of indeterminate potential (CHIP). A watch and wait strategy consisting of clinical evaluations, blood tests, and cardiovascular risk assessment was initiated. Conclusions: This case report highlights the importance of combining clinical and laboratory findings, hematopathology, and molecular analyses to establish the most probable diagnosis in challenging cases. It also underscores the possible relevance of identifying predisposing conditions, such as CHIP, in order to guide counseling and follow-up strategy.
1. Introduction
Mastocytosis is a condition characterized by proliferation and tissue infiltration of clonal mast cells, which accumulate in one or multiple organs. The disease is associated with a huge variety of clinical manifestations [1]. The two main subtypes of mastocytosis, namely cutaneous mastocytosis (typical of children) and systemic mastocytosis (SM), can manifest with or without skin involvement. SM is categorized into five variants: indolent systemic mastocytosis (ISM), smoldering systemic mastocytosis (SSM), systemic mastocytosis with an associated hematologic neoplasm (SM-AHN), aggressive systemic mastocytosis (ASM), and mast cell leukemia (MCL) [2,3]. The diagnosis of mastocytosis is confirmed if patients manifest one major criterion and one minor criterion or meet three minor criteria [2,3,4]. Conversely, mast cell activation syndrome (MCAS) includes a number of conditions in which patients have signs and symptoms consistent with mast cell activation, have evidence of systemic mast cell-mediator release, but do not harbor mastocytosis criteria [1,5]. Symptoms of mast cell activation must be severe, systemic, and recurrent. An event-related mast cell-mediator release and regression of symptoms with anti-mediator therapy are required [6]. The cases of MCAS where an underlying mastocytosis is found are termed clonal MCAS or primary MCAS [5,7].
The oncogenic KIT p.D816V mutation is detectable in >80% of all patients with mastocytosis. Other somatic gene alterations implicated in some cases of advanced systemic mastocytosis include mutations in TET2, SRSF2, ASXL1, RUNX1, CBL, JAK2, NRAS, and KRAS [4]. In contrast, mutations of ZRSR2, a gene involved in RNA splicing, are not closely associated with mast cell disorders, but rather with myelodysplastic syndromes (MDS) development [8,9].
2. Case Report
We report a case of a 37-year-old Caucasian man with an unremarkable clinical history who was referred to our institution for anaphylaxis after a bee sting. During the event, the patient experienced cutaneous erythema, hypotension, and tachycardia. The patient had already been seen by an allergy specialist, who trained the patient in the use of an adrenaline autoinjector and suggested hematological evaluation to exclude mastocytosis. When evaluated at our institution, the patient was asymptomatic and physical examination was negative, in particular for cutaneous lesions, rash, and dermographism. Blood tests were unremarkable except for elevated levels of serum tryptase in two different blood samples: 17.8 ng/mL in the first sample (performed 16 days after anaphylaxis) and 19.2 ng/mL in the second sample, collected one month after the first one (upper limit of normal: 15 ng/mL) (Table 1). Serum tryptase was elevated, including in a third sample collected three months after the second one. Abdominal ultrasound was negative for hepatomegaly and splenomegaly.

Table 1.
Patient’s blood tests.
Considering that serum tryptase elevation and allergic reactions to Hymenoptera stings are a relatively common manifestation in mast cell disorders, a bone marrow aspiration and biopsy were performed. Morphological examination of the bone marrow blood smear revealed the presence of moderate trilinear dysplasia, such as micromegakaryocytes and megakaryocytes with hypolobated nuclei, cytoplasmic hypogranulation in granulocytic lineage, and cytoplasmic and internuclear bridges in the erythroid lineage (Figure 1). The KIT p.D816V mutation assessed by ASO-qPCR was negative and the bone marrow karyotype was normal. PDGFRA, PDGFRB, FGFR1, and PCM1::JAK2 gene fusions were also tested using specific FISH probes and were negative. Targeted myeloid next-generation sequencing (NGS) analysis showed the presence of a pathogenetic variant of the ZRSR2 gene (p.S447_R448del) with a variant allele frequency of 7.4%. Interestingly, NGS analysis did not detect any mutations in all the other KIT exons (2, 8–11, 13, 17, 18) analyzed nor in other genes previously reported in patients with advanced systemic mastocytosis (TET2, SRSF2, ASXL1, RUNX1, CBL, JAK2, NRAS, and KRAS). Bone marrow histology did not show the presence of aggregates of more than 15 mast cells per high-power field, but several spindle-shaped CD117-positive mast cells with partial expression of CD25 were detected (Figure 2A,B). Spindle-shaped mast cells were <25% of the total mast cells. It was not possible to assess CD30 expression by immunohistochemistry nor to perform rare-event flow cytometry to detect aberrant mast cells. Not having performed these tests represents a limitation, as it prevented a more detailed characterization of bone marrow mast cell population. Bone marrow cellularity was within normal limits (30–40%), and signs of dysplasia were confirmed in >10% of megakaryocytic, granulocytic, and erythroid lineages. The percentage of myeloid blasts in the bone marrow was <1%.
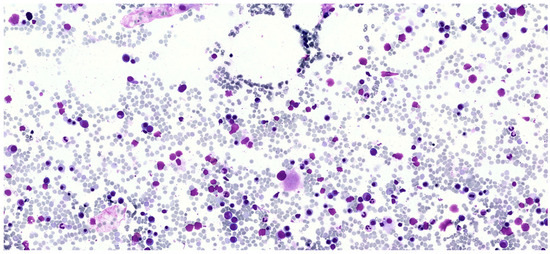
Figure 1.
Bone marrow smear for cytomorphological examination. The image shows dysplastic features observed in the patient’s bone marrow smear, including a dysplastic megakaryocyte and cytoplasmic as well as internuclear bridges in the erythroid lineage.
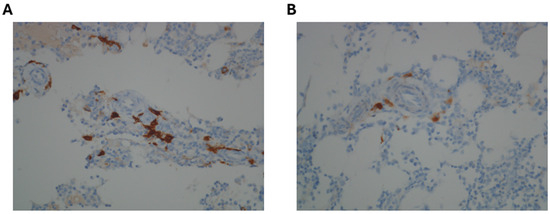
Figure 2.
Immunohistochemical staining performed on bone marrow biopsy. (A) Immunohistochemical staining for CD117: CD117 positivity, combined with morphological evaluation, allows for the effective identification of mast cells. (B) Immunohistochemical staining for CD25: the figure shows the partial positivity for CD25 in some spindle-shaped mast cells, which suggests the presence of a mast cell disorder.
According to mastocytosis diagnostic criteria [1,2,3,4,5] (Table 2), the patient had one/two minor criteria for the disease, but no major criteria. More precisely, spindle-shaped mastocytes were present, but they were <25% of the total mast cells and, therefore, the first minor criterion was not respected. The second minor criterion was not respected either since the KIT p.D816V mutation was negative. The third minor criterion was respected since the patient’s mast cells expressed CD25. The fourth minor criterion was borderline since serum tryptase levels were persistently elevated and very close to 20 ng/mL, though not >20 ng/mL as required by the diagnostic criteria. On these grounds, the diagnosis of clonal mast cell disorder could not be established and ZRSR2 mutation was attributed to clonal hematopoiesis of indeterminate potential (CHIP) [1,2,3,4,5]. Given the detection of CHIP, a watch and wait strategy with a clinical evaluation and blood tests every six months to monitor development of cytopenias was initiated, together with periodic cardiovascular risk assessment. Two years after initial presentation, the complete blood count and differential are unremarkable, and the patient is asymptomatic.

Table 2.
Diagnostic criteria of systemic mastocytosis.
3. Discussion
With mastocytosis excluded, the three main differential diagnoses considered in this patient were MCAS, a myelodysplastic syndrome, or CHIP. Another diagnostic hypothesis could have been hereditary alpha tryptasemia (HαT), which can be associated with elevated serum tryptase levels. However, in the absence of a positive family history and other suggestive symptoms (gastrointestinal, respiratory, musculoskeletal, or neurological), this hypothesis was ruled out and genetic testing of TPSAB1 gene was not performed. The clinical presentation, serum tryptase elevation, and the presence of spindle-shaped CD25+ mast cells suggested the presence of a MCAS, but the patient did not have recurrent symptoms [5,6,7]. Tryptase levels measurement delayed from the anaphylactic reaction, rather than during or immediately after the event, represents a limitation in the diagnostic process, potentially compromising the accuracy of the differential diagnosis. Indeed, confirmation of a diagnosis of MCAS requires evidence of an event-related rise in tryptase levels (Table 3). Therefore, it will be crucial to obtain the patient’s immediate tryptase measurements in case of future anaphylactic episodes. Moreover, although serum tryptase levels were consistently elevated in the patient, they did not reach the threshold for one of the minor criteria of mastocytosis [2,3]. In this respect, it is also important to consider that serum tryptase levels are not specific to mastocytosis but can also be elevated in up to 25% of MDS [10]. Moreover, dysplastic bone marrow features were observed during both the bone marrow smear observation and the histological analysis of the bone marrow biopsy. In addition, mutations of ZRSR2 are not reported to be implicated in mastocytosis, whereas they occur in other myeloid neoplasms, such as MDS [8,9]. Interestingly, ZRSR2 mutations in patients with a diagnosis of MDS are associated with male sex and with an indolent clinical phenotype, and our patient closely reflected this description [11]. However, in the absence of peripheral cytopenias, the diagnosis of MDS could not be made and the ZRSR2 mutation was ascribed to clonal CHIP [12]. Therefore, although the initial clinical presentation of the patient was suggestive of mastocytosis/MCAS, and some bone marrow findings were reminiscent of MDS, these hypotheses were not confirmed by the integration of molecular, morphological, and biochemical findings. Instead, the integration of all findings led to the diagnosis of CHIP, with incidental dysplastic bone marrow features.

Table 3.
Diagnostic criteria of mast cell activation syndrome.
ZRSR2, located on the X chromosome (Xp22.1), encodes a component of the RNA spliceosome complex (Figure 3) [13]. Splice gene mutations, mainly involving SF3B1, SRSF2, ZRSR2, and U2AF35, are among the most common molecular alterations detected in MDS and also in patients harboring CHIP [13,14]. In contrast to mutations in other genes involved in the splicing process, which are typically clustered in a limited number of hotspot regions, mutations in ZRSR2 have been found to be more broadly distributed throughout the gene [15]. Alterations affecting the arginine-serine-rich domain, especially the R446 amino acid residue, have been reported in the context of MDS, suggesting the relevance of this gene region in MDS development [15]. Interestingly, previous studies demonstrated that ZRSR2 loss of function, which is typical of MDS and other myeloid neoplasms, can impair inflammation and immune response pathways (i.e., Toll-like receptors signaling), providing a possible explanation for the dysregulated reaction to a bee sting that was observed in this patient, regardless of the specific hematologic diagnosis [8,16].
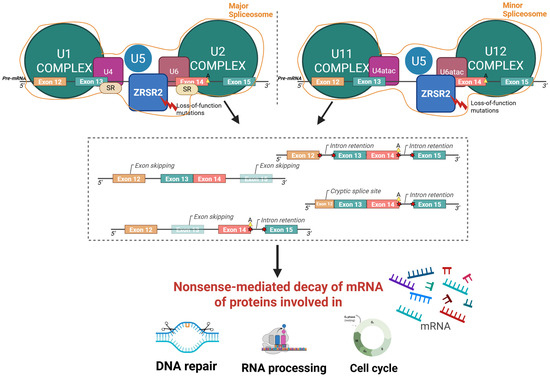
Figure 3.
Graphical representation of the function and biological consequences of the ZRSR2 mutation. Loss-of-function mutations in ZRSR2 gene impair the spliceosome complex, leading to abnormal splicing events such as exon skipping, intron retention, and cryptic splice sites formation. These aberrant splicing patterns trigger nonsense-mediated decay of mRNA, ultimately altering the production of proteins essential for DNA repair, RNA processing, and cell cycle regulation. Major spliceosome complex: assembly of U1/U2/U4/U5/U6 small nuclear ribonucleoproteins (snRNPs); minor spliceosome complex: assembly of U11/U12/U4atac/U6atac snRNPs; SR: serine/arginine-rich proteins; A: branchpoint adenosine; 5′/3′: RNA strand orientation; red bolt: loss-of-function mutations in ZRSR2; red stars: aberrant splicing events. Created using BioRender; Gaidano, G (2025) https://BioRender.com/p5j4m85 (accessed on 3 November 2025).
The rarity and the overlapping definitions of mast cell disorders make this case a diagnostic challenge, with a substantial risk of under- or overdiagnosis [5,7]. This case highlighted that the most common symptoms and laboratory findings of mastocytosis (i.e., anaphylaxis after a bee sting and elevated tryptase levels) do not always lead to mastocytosis/MCAS diagnosis, further underscoring the need for bone marrow evaluation in suspected cases. The current diagnosis of CHIP harboring a ZRSR2 pathogenetic mutation can lead to two different clinical scenarios: (i) the association between CHIP and the occasional finding of dysplastic bone marrow may represent an early detection of a subsequent MDS, or (ii) the typical clinical presentation of mastocytosis/MCAS and the finding of CD25+ spindle-shaped mast cells in the bone marrow may represent an early detection of a subsequent overt mastocytosis/MCAS predisposed by a CHIP ZRSR2-mutated clone.
4. Conclusions
This case report highlights the synergistic role in hematology of combining hematopathology with molecular analyses to establish the most probable diagnosis in challenging cases, especially when the clinical presentation is subtle or ambiguous. It also underscores the possible importance of identifying potential predisposing conditions, such as CHIP, in order to guide follow-up strategies in patients considered at risk of developing overt hematologic diseases.
Clinical monitoring for potential new episodes of anaphylaxis, along with regular assessment of complete blood count and serum tryptase levels, will be continued over time to further clarify this challenging scenario. In the event of an anaphylactic episode, tryptase levels will also be measured during and shortly after the acute phase. If clinically indicated (i.e., presence of suggestive symptoms), genetic testing will be considered to rule out HαT. If new cytopenias emerge, bone marrow re-evaluation will be performed, also including rare-event flow cytometry for aberrant mast cell detection and CD30 immunohistochemical staining. The size of the ZRSR2-mutated myeloid clone will also be closely monitored.
Author Contributions
Conceptualization, R.D., G.G. and R.M.; writing-original draft preparation, R.D., N.M., A.A., G.G. and R.M.; writing-review and editing: R.D., N.M., A.A., S.M.S., M.R.N., A.P., G.G. and R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Molecular bases of disease dissemination in lymphoid malignancies to optimize curative therapeutic strategies (5 × 1000 No. 21198)”.
Institutional Review Board Statement
Ethical review and approval were granted by the Ethical committee (Approval Code: CE 120/19; Approval Date: 27 January 2020).
Informed Consent Statement
Written informed consent was obtained from the patient to publish this report in accordance with the journal’s patient consent policy.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
This work was supported by AIL Novara VCO ODV, Novara, Italy.
Conflicts of Interest
The authors have no conflicts of interests regarding this manuscript.
References
- Leguit, R.J.; Wang, S.A.; George, T.I.; Tzankov, A.; Orazi, A. The international consensus classification of mastocytosis and related entities. Virchows Arch. 2023, 482, 99–112. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.-M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Akin, C.; Metcalfe, D.D. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood 2017, 129, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Hartmann, K.; Bonadonna, P.; Niedoszytko, M.; Triggiani, M.; Arock, M.; Brockow, K. Mast Cell Activation Syndromes: Collegium Internationale Allergologicum Update 2022. Int. Arch. Allergy Immunol. 2022, 183, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Akin, C.; Valent, P.; Metcalfe, D.D. Mast cell activation syndrome: Proposed diagnostic criteria. J. Allergy Clin. Immunol. 2010, 126, 1099–1104.e4. [Google Scholar] [CrossRef] [PubMed]
- Weiler, C.R.; Austen, K.F.; Akin, C.; Barkoff, M.S.; Bernstein, J.A.; Bonadonna, P.; Butterfield, J.H.; Carter, M.; Fox, C.C.; Maitland, A.; et al. AAAAI Mast Cell Disorders Committee Work Group Report: Mast cell activation syndrome (MCAS) diagnosis and management. J. Allergy Clin. Immunol. 2019, 144, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, C.; Martínez-Valiente, C.; Cordón, L.; Liquori, A.; Fernández-González, R.; Pericuesta, E.; Sandoval, J.; Cervera, J.; Gutiérrez-Adán, A.; Sanjuan-Pla, A. Concurrent Zrsr2 mutation and Tet2 loss promote myelodysplastic neoplasm in mice. Leukemia 2022, 36, 2509–2518. [Google Scholar] [CrossRef] [PubMed]
- Chiereghin, C.; Travaglino, E.; Zampini, M.; Saba, E.; Saitta, C.; Riva, E.; Bersanelli, M.; Della Porta, M.G. The Genetics of Myelodysplastic Syndromes: Clinical Relevance. Genes 2021, 12, 1144. [Google Scholar] [CrossRef] [PubMed]
- Sprinzl, B.; Greiner, G.; Uyanik, G.; Arock, M.; Haferlach, T.; Sperr, W.R.; Valent, P.; Hoermann, G. Genetic Regulation of Tryptase Production and Clinical Impact: Hereditary Alpha Tryptasemia, Mastocytosis and Beyond. Int. J. Mol. Sci. 2021, 22, 2458. [Google Scholar] [CrossRef] [PubMed]
- Bernard, E.; Hasserjian, R.P.; Greenberg, P.L.; Ossa, J.E.A.; Creignou, M.; Tuechler, H.; Gutierrez-Abril, J.; Domenico, D.; Medina-Martinez, J.S.; Levine, M.; et al. Molecular taxonomy of myelodysplastic syndromes and its clinical implications. Blood 2024, 144, 1617–1632. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Meggendorfer, M.; Zampini, M.; Tettamanti, M.; Riva, E.; Travaglino, E.; Bersanelli, M.; Mandelli, S.; Galbussera, A.A.; Mosca, E.; et al. Clinical relevance of clonal hematopoiesis in persons aged ≥80 years. Blood 2021, 138, 2093–2105. [Google Scholar] [CrossRef] [PubMed]
- Yacout, M.; Katamesh, B.; Jabban, Y.; He, R.; Viswanatha, D.; Jevremovic, D.; Greipp, P.; Bessonen, K.; Palmer, J.; Foran, J.; et al. Characterisation and prognostic impact Of ZRSR2 mutations in myeloid neoplasms. Leukemia 2024, 38, 2727–2730. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.M.; Saadatagah, S.; Niroula, A.; Yu, B.; Hornsby, W.E.; Ganesh, S.; Lannery, K.; Schuermans, A.; Honigberg, M.C.; Bick, A.G.; et al. Long-term longitudinal analysis of 4,187 participants reveals insights into determinants of clonal hematopoiesis. Nat. Commun. 2024, 15, 7858. [Google Scholar] [CrossRef] [PubMed]
- Damm, F.; Kosmider, O.; Gelsi-Boyer, V.; Renneville, A.; Carbuccia, N.; Hidalgo-Curtis, C.; Della Valle, V.; Couronné, L.; Scourzic, L.; Chesnais, V.; et al. Mutations affecting mRNA splicing define distinct clinical phenotypes and correlate with patient outcome in myelodysplastic syndromes. Blood 2012, 119, 3211–3218. [Google Scholar] [CrossRef] [PubMed]
- Togami, K.; Chung, S.S.; Madan, V.; Booth, C.A.; Kenyon, C.M.; Cabal-Hierro, L.; Taylor, J.; Kim, S.S.; Griffin, G.K.; Ghandi, M.; et al. Sex-Biased ZRSR2 Mutations in Myeloid Malignancies Impair Plasmacytoid Dendritic Cell Activation and Apoptosis. Cancer Discov. 2022, 12, 522–541. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
