PD-1 Expression Promotes Immune Evasion in B-ALL
Abstract
1. Introduction
2. Methods Details
2.1. Mouse Model for Natural Immune Stress-Driven Leukemia
2.2. B-ALL Patient-Derived Xenograft (PDX) Models
2.3. Leukemic Pax5+/− Pro-B-Cell Culture
2.4. Anti-PD1 Inhibitor In Vitro Experiments
2.5. Viability Assays
2.6. BM Transplantation Experiments
2.7. Preclinical Therapeutics
3. Results
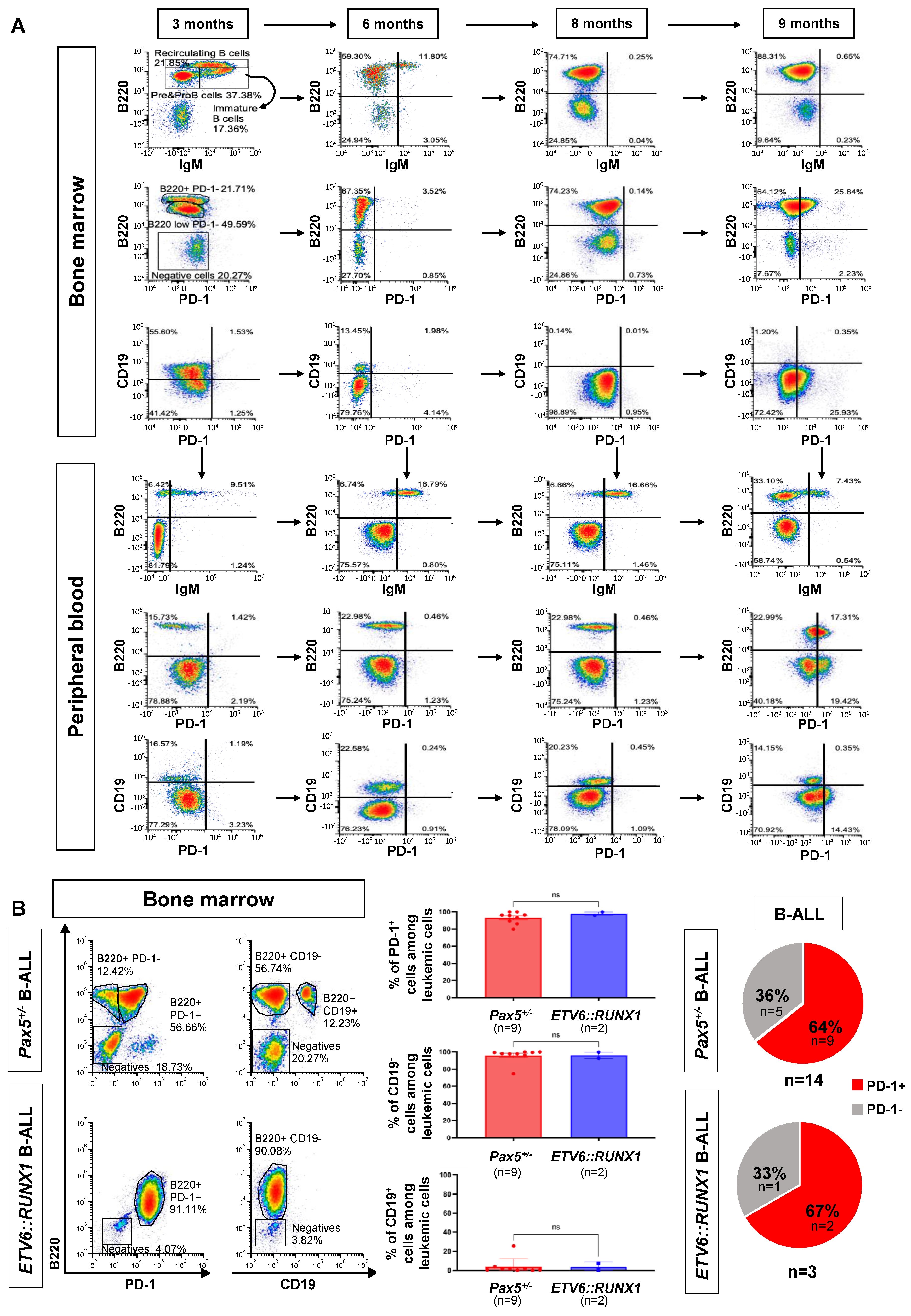
3.1. B-ALL Development Screens in Genetically Predisposed Mice Identify Cancer Cell-Autonomous Upregulation of the Inhibitory Molecule PD-1
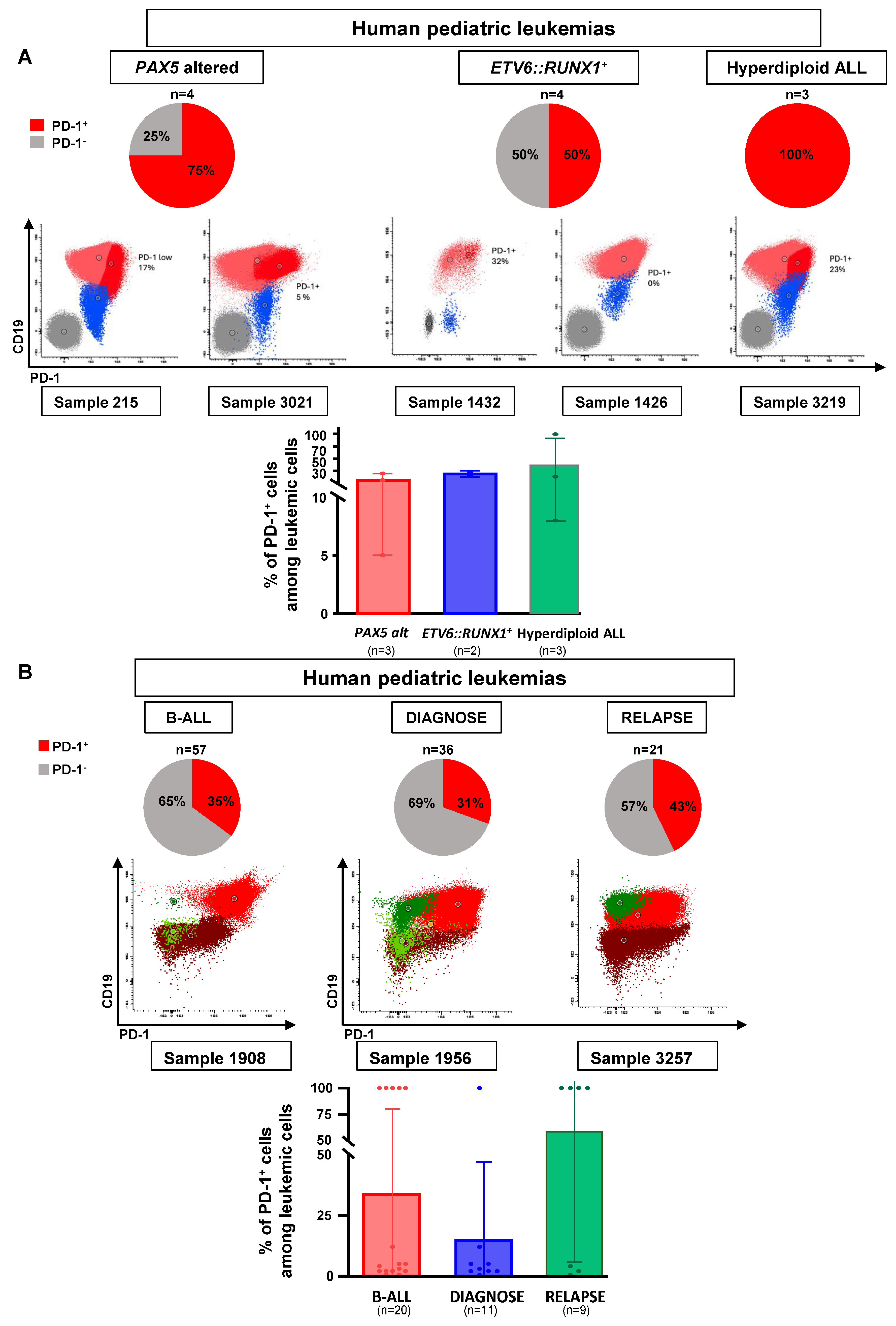
3.2. PD-1 Expression in Human B-ALL
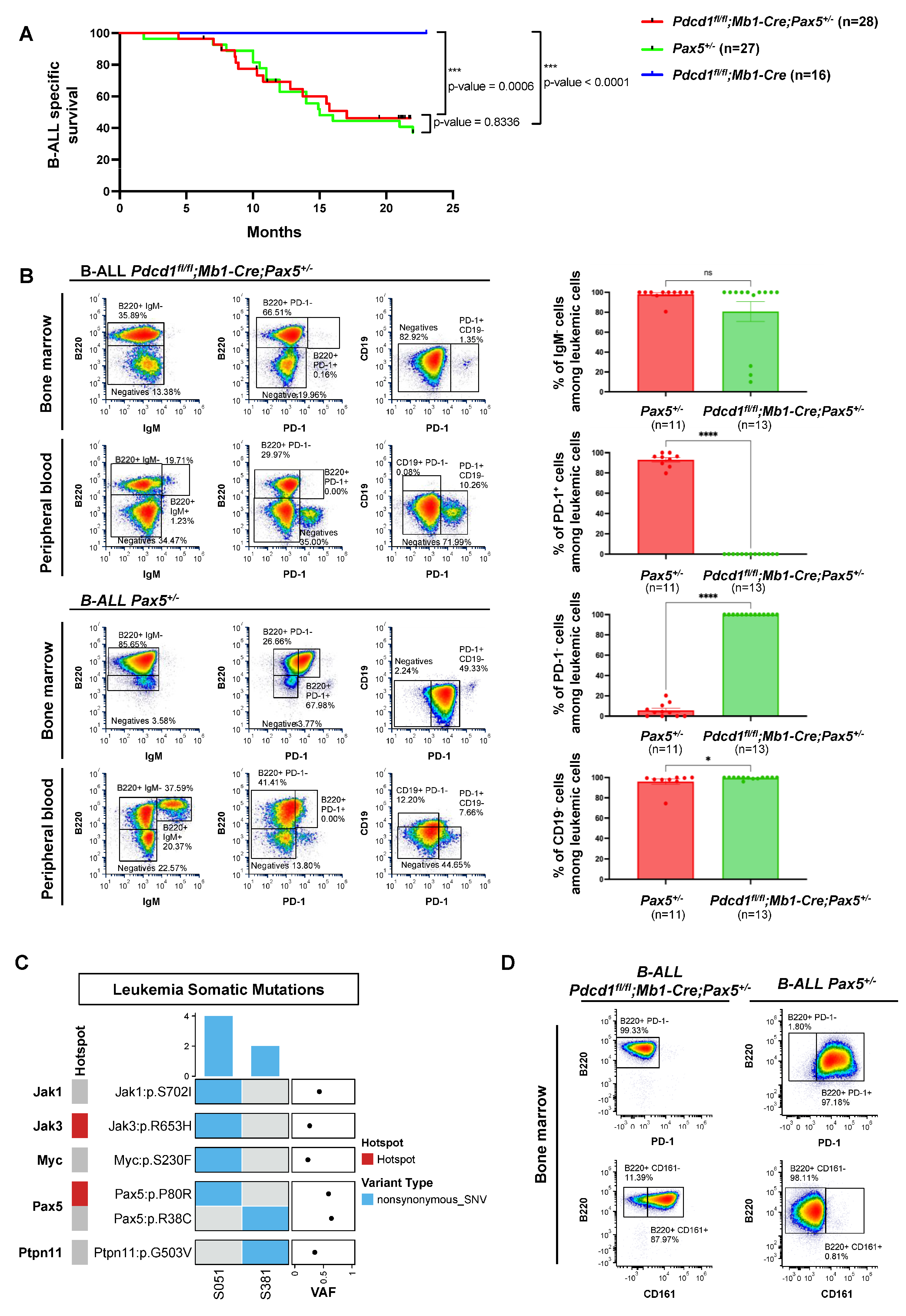
3.3. PD-1 Is Not Required for Pax5-Dependent B-Cell Leukemogenesis
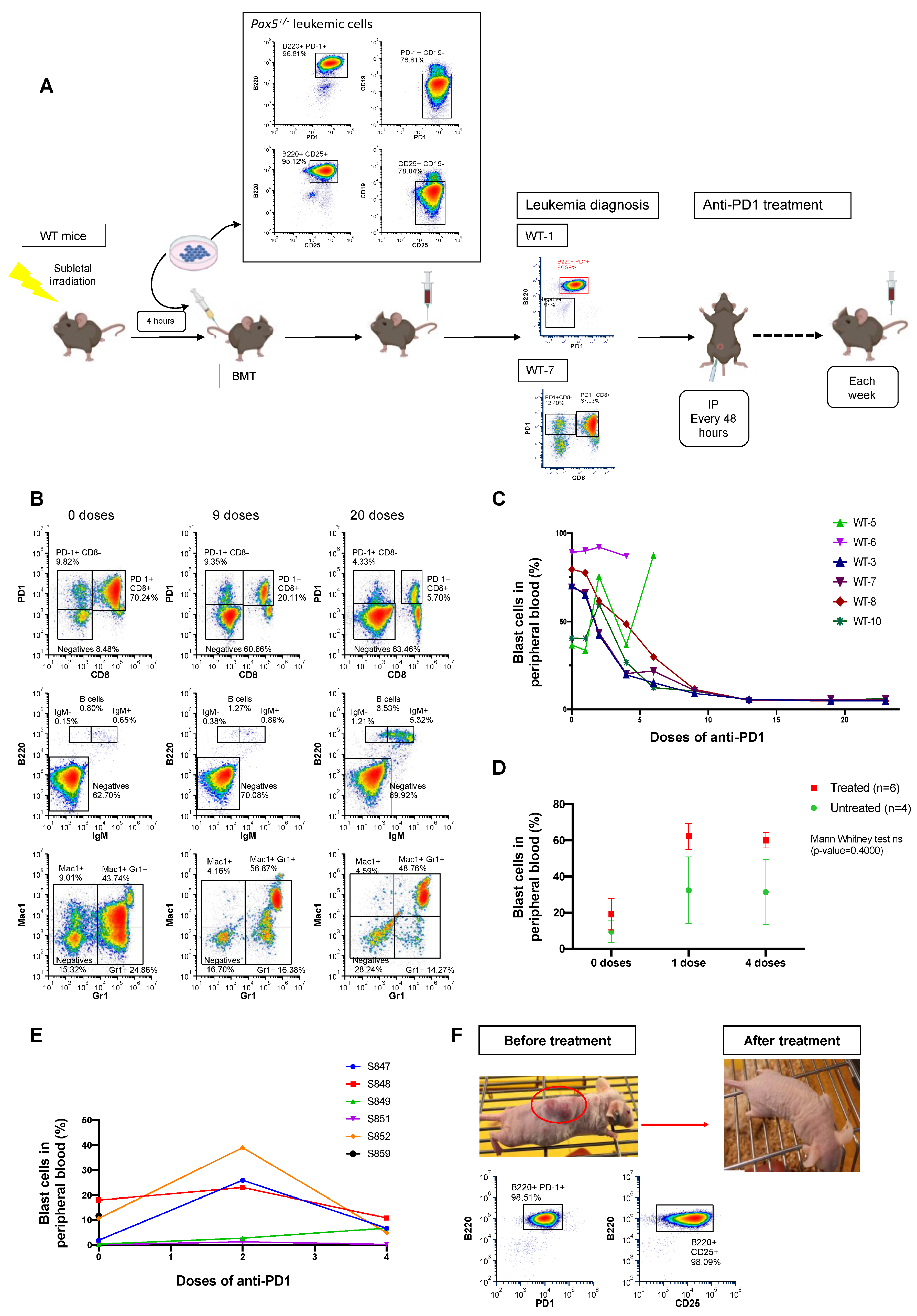
3.4. Anti-PD1 Treatment Restores the Immune Capacity to Eliminate PD-1-Positive Tumor Cells in Mice Engrafted with B-ALL
3.5. PD-1 Targeting Sensitizes PD-1-Positive B-ALLs to NK Cell-Mediated Killing
3.6. PD-1 Directly Inhibits the Antitumor Activity of NK Cells on B-ALL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Data and Code Availability
Resource Availability
Acknowledgments
Conflicts of Interest
References
- Mullighan, C.G.; Goorha, S.; Radtke, I.; Miller, C.B.; Coustan-Smith, E.; Dalton, J.D.; Girtman, K.; Mathew, S.; Ma, J.; Pounds, S.B.; et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature 2007, 446, 758–764. [Google Scholar] [CrossRef]
- Grobner, S.N.; Worst, B.C.; Weischenfeldt, J.; Buchhalter, I.; Kleinheinz, K.; Rudneva, V.A.; Johann, P.D.; Balasubramanian, G.P.; Segura-Wang, M.; Brabetz, S.; et al. The landscape of genomic alterations across childhood cancers. Nature 2018, 555, 321–327. [Google Scholar] [CrossRef]
- Holmfeldt, L.; Wei, L.; Diaz-Flores, E.; Walsh, M.; Zhang, J.; Ding, L.; Payne-Turner, D.; Churchman, M.; Andersson, A.; Chen, S.C.; et al. The genomic landscape of hypodiploid acute lymphoblastic leukemia. Nat. Genet. 2013, 45, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Iacobucci, I.; Mullighan, C.G. Genetic Basis of Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2017, 35, 975–983. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Alexandrov, L.B.; Edmonson, M.N.; Gawad, C.; Zhou, X.; Li, Y.; Rusch, M.C.; Easton, J.; Huether, R.; et al. Pan-cancer genome and transcriptome analyses of 1699 paediatric leukaemias and solid tumours. Nature 2018, 555, 371–376. [Google Scholar] [CrossRef]
- Roberts, K.G.; Morin, R.D.; Zhang, J.; Hirst, M.; Zhao, Y.; Su, X.; Chen, S.C.; Payne-Turner, D.; Churchman, M.L.; Harvey, R.C.; et al. Genetic alterations activating kinase and cytokine receptor signaling in high-risk acute lymphoblastic leukemia. Cancer Cell 2012, 22, 153–166. [Google Scholar] [CrossRef]
- Gu, Z.; Churchman, M.; Roberts, K.; Li, Y.; Liu, Y.; Harvey, R.C.; McCastlain, K.; Reshmi, S.C.; Payne-Turner, D.; Iacobucci, I.; et al. Genomic analyses identify recurrent MEF2D fusions in acute lymphoblastic leukaemia. Nat. Commun. 2016, 7, 13331. [Google Scholar] [CrossRef] [PubMed]
- Iacobucci, I.; Li, Y.; Roberts, K.G.; Dobson, S.M.; Kim, J.C.; Payne-Turner, D.; Harvey, R.C.; Valentine, M.; McCastlain, K.; Easton, J.; et al. Truncating Erythropoietin Receptor Rearrangements in Acute Lymphoblastic Leukemia. Cancer Cell 2016, 29, 186–200. [Google Scholar] [CrossRef]
- Roberts, K.G.; Li, Y.; Payne-Turner, D.; Harvey, R.C.; Yang, Y.L.; Pei, D.; McCastlain, K.; Ding, L.; Lu, C.; Song, G.; et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N. Engl. J. Med. 2014, 371, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Okuno, Y.; Kawashima, N.; Muramatsu, H.; Okuno, T.; Wang, X.; Kataoka, S.; Sekiya, Y.; Hamada, M.; Murakami, N.; et al. MEF2D-BCL9 Fusion Gene Is Associated with High-Risk Acute B-Cell Precursor Lymphoblastic Leukemia in Adolescents. J. Clin. Oncol. 2016, 34, 3451–3459. [Google Scholar] [CrossRef]
- Zhang, J.; McCastlain, K.; Yoshihara, H.; Xu, B.; Chang, Y.; Churchman, M.L.; Wu, G.; Li, Y.; Wei, L.; Iacobucci, I.; et al. Deregulation of DUX4 and ERG in acute lymphoblastic leukemia. Nat. Genet. 2016, 48, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef] [PubMed]
- Bastian, L.; Schroeder, M.P.; Eckert, C.; Schlee, C.; Tanchez, J.O.; Kampf, S.; Wagner, D.L.; Schulze, V.; Isaakidis, K.; Lazaro-Navarro, J.; et al. PAX5 biallelic genomic alterations define a novel subgroup of B-cell precursor acute lymphoblastic leukemia. Leukemia 2019, 33, 1895–1909. [Google Scholar] [CrossRef]
- Cree, I.A. The WHO Classification of Haematolymphoid Tumours. Leukemia 2022, 36, 1701–1702. [Google Scholar] [CrossRef]
- Gocho, Y.; Kiyokawa, N.; Ichikawa, H.; Nakabayashi, K.; Osumi, T.; Ishibashi, T.; Ueno, H.; Terada, K.; Oboki, K.; Sakamoto, H.; et al. A novel recurrent EP300-ZNF384 gene fusion in B-cell precursor acute lymphoblastic leukemia. Leukemia 2015, 29, 2445–2448. [Google Scholar] [CrossRef]
- Gu, Z.; Churchman, M.L.; Roberts, K.G.; Moore, I.; Zhou, X.; Nakitandwe, J.; Hagiwara, K.; Pelletier, S.; Gingras, S.; Berns, H.; et al. PAX5-driven subtypes of B-progenitor acute lymphoblastic leukemia. Nat. Genet. 2019, 51, 296–307. [Google Scholar] [CrossRef]
- Lilljebjorn, H.; Fioretos, T. New oncogenic subtypes in pediatric B-cell precursor acute lymphoblastic leukemia. Blood 2017, 130, 1395–1401. [Google Scholar] [CrossRef]
- Lilljebjorn, H.; Henningsson, R.; Hyrenius-Wittsten, A.; Olsson, L.; Orsmark-Pietras, C.; von Palffy, S.; Askmyr, M.; Rissler, M.; Schrappe, M.; Cario, G.; et al. Identification of ETV6-RUNX1-like and DUX4-rearranged subtypes in paediatric B-cell precursor acute lymphoblastic leukaemia. Nat. Commun. 2016, 7, 11790. [Google Scholar] [CrossRef]
- van Engelen, N.; Roest, M.; van Dijk, F.; Sonneveld, E.; Bladergroen, R.; van Reijmersdal, S.V.; van der Velden, V.H.J.; Hoogeveen, P.G.; Kors, W.A.; Waanders, E.; et al. A novel germline PAX5 single exon deletion in a pediatric patient with precursor B-cell leukemia. Leukemia 2023, 37, 1908–1911. [Google Scholar] [CrossRef]
- Yasuda, T.; Tsuzuki, S.; Kawazu, M.; Hayakawa, F.; Kojima, S.; Ueno, T.; Imoto, N.; Kohsaka, S.; Kunita, A.; Doi, K.; et al. Recurrent DUX4 fusions in B cell acute lymphoblastic leukemia of adolescents and young adults. Nat. Genet. 2016, 48, 569–574. [Google Scholar] [CrossRef]
- Fischer, U.; Yang, J.J.; Ikawa, T.; Hein, D.; Vicente-Duenas, C.; Borkhardt, A.; Sanchez-Garcia, I. Cell Fate Decisions: The Role of Transcription Factors in Early B-cell Development and Leukemia. Blood Cancer Discov. 2020, 1, 224–233. [Google Scholar] [CrossRef]
- Duployez, N.; Jamrog, L.A.; Fregona, V.; Hamelle, C.; Fenwarth, L.; Lejeune, S.; Helevaut, N.; Geffroy, S.; Caillault, A.; Marceau-Renaut, A.; et al. Germline PAX5 mutation predisposes to familial B-cell precursor acute lymphoblastic leukemia. Blood 2021, 137, 1424–1428. [Google Scholar] [CrossRef]
- Shah, S.; Schrader, K.A.; Waanders, E.; Timms, A.E.; Vijai, J.; Miething, C.; Wechsler, J.; Yang, J.; Hayes, J.; Klein, R.J.; et al. A recurrent germline PAX5 mutation confers susceptibility to pre-B cell acute lymphoblastic leukemia. Nat. Genet. 2013, 45, 1226–1231. [Google Scholar] [CrossRef]
- Fregona, V.; Bayet, M.; Bouttier, M.; Largeaud, L.; Hamelle, C.; Jamrog, L.A.; Prade, N.; Lagarde, S.; Hebrard, S.; Luquet, I.; et al. Stem cell-like reprogramming is required for leukemia-initiating activity in B-ALL. J. Exp. Med. 2024, 221, e20230279. [Google Scholar] [CrossRef]
- Zhu, X.; He, F.; Zeng, H.; Ling, S.; Chen, A.; Wang, Y.; Yan, X.; Wei, W.; Pang, Y.; Cheng, H.; et al. Identification of functional cooperative mutations of SETD2 in human acute leukemia. Nat. Genet. 2014, 46, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Mar, B.G.; Bullinger, L.B.; McLean, K.M.; Grauman, P.V.; Harris, M.H.; Stevenson, K.; Neuberg, D.S.; Sinha, A.U.; Sallan, S.E.; Silverman, L.B.; et al. Mutations in epigenetic regulators including SETD2 are gained during relapse in paediatric acute lymphoblastic leukaemia. Nat. Commun. 2014, 5, 3469. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, R.P.; Schoenmakers, E.F.; van Reijmersdal, S.V.; Hehir-Kwa, J.Y.; van Kessel, A.G.; van Leeuwen, F.N.; Hoogerbrugge, P.M. High-resolution genomic profiling of childhood ALL reveals novel recurrent genetic lesions affecting pathways involved in lymphocyte differentiation and cell cycle progression. Leukemia 2007, 21, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Martin-Lorenzo, A.; Auer, F.; Chan, L.N.; Garcia-Ramirez, I.; Gonzalez-Herrero, I.; Rodriguez-Hernandez, G.; Bartenhagen, C.; Dugas, M.; Gombert, M.; Ginzel, S.; et al. Loss of Pax5 Exploits Sca1-BCR-ABL(p190) Susceptibility to Confer the Metabolic Shift Essential for pB-ALL. Cancer Res. 2018, 78, 2669–2679. [Google Scholar] [CrossRef]
- Rodriguez-Hernandez, G.; Casado-Garcia, A.; Isidro-Hernandez, M.; Picard, D.; Raboso-Gallego, J.; Aleman-Arteaga, S.; Orfao, A.; Blanco, O.; Riesco, S.; Prieto-Matos, P.; et al. The Second Oncogenic Hit Determines the Cell Fate of ETV6-RUNX1 Positive Leukemia. Front. Cell Dev. Biol. 2021, 9, 704591. [Google Scholar] [CrossRef]
- Liu, G.J.; Cimmino, L.; Jude, J.G.; Hu, Y.; Witkowski, M.T.; McKenzie, M.D.; Kartal-Kaess, M.; Best, S.A.; Tuohey, L.; Liao, Y.; et al. Pax5 loss imposes a reversible differentiation block in B-progenitor acute lymphoblastic leukemia. Genes. Dev. 2014, 28, 1337–1350. [Google Scholar] [CrossRef]
- Cobaleda, C.; Schebesta, A.; Delogu, A.; Busslinger, M. Pax5: The guardian of B cell identity and function. Nat. Immunol. 2007, 8, 463–470. [Google Scholar] [CrossRef]
- Cobaleda, C.; Jochum, W.; Busslinger, M. Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors. Nature 2007, 449, 473–477. [Google Scholar] [CrossRef]
- Cobaleda, C.; Vicente-Duenas, C.; Sanchez-Garcia, I. Infectious triggers and novel therapeutic opportunities in childhood B cell leukaemia. Nat. Rev. Immunol. 2021, 21, 570–581. [Google Scholar] [CrossRef]
- Cobaleda, C.; Vicente-Duenas, C.; Sanchez-Garcia, I. An immune window of opportunity to prevent childhood B cell leukemia. Trends Immunol. 2021, 42, 371–374. [Google Scholar] [CrossRef]
- Martin-Lorenzo, A.; Hauer, J.; Vicente-Duenas, C.; Auer, F.; Gonzalez-Herrero, I.; Garcia-Ramirez, I.; Ginzel, S.; Thiele, R.; Constantinescu, S.N.; Bartenhagen, C.; et al. Infection Exposure is a Causal Factor in B-cell Precursor Acute Lymphoblastic Leukemia as a Result of Pax5-Inherited Susceptibility. Cancer Discov. 2015, 5, 1328–1343. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Duenas, C.; Janssen, S.; Oldenburg, M.; Auer, F.; Gonzalez-Herrero, I.; Casado-Garcia, A.; Isidro-Hernandez, M.; Raboso-Gallego, J.; Westhoff, P.; Pandyra, A.A.; et al. An intact gut microbiome protects genetically predisposed mice against leukemia. Blood 2020, 136, 2003–2017. [Google Scholar] [CrossRef] [PubMed]
- Casado-Garcia, A.; Isidro-Hernandez, M.; Oak, N.; Mayado, A.; Mann-Ran, C.; Raboso-Gallego, J.; Aleman-Arteaga, S.; Buhles, A.; Sterker, D.; Sanchez, E.G.; et al. Transient Inhibition of the JAK/STAT Pathway Prevents B-ALL Development in Genetically Predisposed Mice. Cancer Res. 2022, 82, 1098–1109. [Google Scholar] [CrossRef] [PubMed]
- Isidro-Hernandez, M.; Casado-Garcia, A.; Oak, N.; Aleman-Arteaga, S.; Ruiz-Corzo, B.; Martinez-Cano, J.; Mayado, A.; Sanchez, E.G.; Blanco, O.; Gaspar, M.L.; et al. Immune stress suppresses innate immune signaling in preleukemic precursor B-cells to provoke leukemia in predisposed mice. Nat. Commun. 2023, 14, 5159. [Google Scholar] [CrossRef]
- Cobaleda, C.; Vicente-Duenas, C.; Ramirez-Orellana, M.; Sanchez-Garcia, I. Revisiting the concept of childhood preleukemia. Trends Cancer 2022, 8, 887–889. [Google Scholar] [CrossRef]
- Iraolagoitia, X.L.; Spallanzani, R.G.; Torres, N.I.; Araya, R.E.; Ziblat, A.; Domaica, C.I.; Sierra, J.M.; Nunez, S.Y.; Secchiari, F.; Gajewski, T.F.; et al. NK Cells Restrain Spontaneous Antitumor CD8+ T Cell Priming through PD-1/PD-L1 Interactions with Dendritic Cells. J. Immunol. 2016, 197, 953–961. [Google Scholar] [CrossRef]
- Quatrini, L.; Mariotti, F.R.; Munari, E.; Tumino, N.; Vacca, P.; Moretta, L. The Immune Checkpoint PD-1 in Natural Killer Cells: Expression, Function and Targeting in Tumour Immunotherapy. Cancers 2020, 12, 3285. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Sierra, J.M.; Secchiari, F.; Nunez, S.Y.; Iraolagoitia, X.L.R.; Ziblat, A.; Friedrich, A.D.; Regge, M.V.; Santilli, M.C.; Torres, N.I.; Gantov, M.; et al. Tumor-Experienced Human NK Cells Express High Levels of PD-L1 and Inhibit CD8(+) T Cell Proliferation. Front. Immunol. 2021, 12, 745939. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, T.J.; Biederstadt, A.; Rezvani, K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat. Rev. Cancer 2022, 22, 557–575. [Google Scholar] [CrossRef]
- Hobeika, E.; Thiemann, S.; Storch, B.; Jumaa, H.; Nielsen, P.J.; Pelanda, R.; Reth, M. Testing gene function early in the B cell lineage in mb1-cre mice. Proc. Natl. Acad. Sci. USA 2006, 103, 13789–13794. [Google Scholar] [CrossRef]
- Rodriguez-Hernandez, G.; Hauer, J.; Martin-Lorenzo, A.; Schafer, D.; Bartenhagen, C.; Garcia-Ramirez, I.; Auer, F.; Gonzalez-Herrero, I.; Ruiz-Roca, L.; Gombert, M.; et al. Infection Exposure Promotes ETV6-RUNX1 Precursor B-cell Leukemia via Impaired H3K4 Demethylases. Cancer Res. 2017, 77, 4365–4377. [Google Scholar] [CrossRef]
- Urbanek, P.; Wang, Z.Q.; Fetka, I.; Wagner, E.F.; Busslinger, M. Complete block of early B cell differentiation and altered patterning of the posterior midbrain in mice lacking Pax5/BSAP. Cell 1994, 79, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Hernandez, G.; Opitz, F.V.; Delgado, P.; Walter, C.; Alvarez-Prado, A.F.; Gonzalez-Herrero, I.; Auer, F.; Fischer, U.; Janssen, S.; Bartenhagen, C.; et al. Infectious stimuli promote malignant B-cell acute lymphoblastic leukemia in the absence of AID. Nat. Commun. 2019, 10, 5563. [Google Scholar] [CrossRef] [PubMed]
- Holmfeldt, L.; Mullighan, C.G. Generation of human acute lymphoblastic leukemia xenografts for use in oncology drug discovery. Curr. Protoc. Pharmacol. 2015, 68, 14–32. [Google Scholar] [CrossRef]
- Nutt, S.L.; Morrison, A.M.; Dorfler, P.; Rolink, A.; Busslinger, M. Identification of BSAP (Pax-5) target genes in early B-cell development by loss- and gain-of-function experiments. EMBO J. 1998, 17, 2319–2333. [Google Scholar] [CrossRef]
- Witkowski, M.T.; Lee, S.; Wang, E.; Lee, A.K.; Talbot, A.; Ma, C.; Tsopoulidis, N.; Brumbaugh, J.; Zhao, Y.; Roberts, K.G.; et al. NUDT21 limits CD19 levels through alternative mRNA polyadenylation in B cell acute lymphoblastic leukemia. Nat. Immunol. 2022, 23, 1424–1432. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, W.; Xuan, L.; Yu, Y.; Zheng, W.; Tao, F.; Nemechek, J.; He, C.; Ma, W.; Han, X.; et al. PD-1 signalling defines and protects leukaemic stem cells from T cell receptor-induced cell death in T cell acute lymphoblastic leukaemia. Nat. Cell Biol. 2023, 25, 170–182. [Google Scholar] [CrossRef]
- Case, M.; Matheson, E.; Minto, L.; Hassan, R.; Harrison, C.J.; Bown, N.; Bailey, S.; Vormoor, J.; Hall, A.G.; Irving, J.A. Mutation of genes affecting the RAS pathway is common in childhood acute lymphoblastic leukemia. Cancer Res. 2008, 68, 6803–6809. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.M., Jr.; Bakan, C.E.; Mishra, A.; Hofmeister, C.C.; Efebera, Y.; Becknell, B.; Baiocchi, R.A.; Zhang, J.; Yu, J.; Smith, M.K.; et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: A therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 2010, 116, 2286–2294. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.; Hodgins, J.J.; Marathe, M.; Nicolai, C.J.; Bourgeois-Daigneault, M.C.; Trevino, T.N.; Azimi, C.S.; Scheer, A.K.; Randolph, H.E.; Thompson, T.W.; et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Investig. 2018, 128, 4654–4668. [Google Scholar] [CrossRef]
- Dang, J.; Wei, L.; de Ridder, J.; Su, X.; Rust, A.G.; Roberts, K.G.; Payne-Turner, D.; Cheng, J.; Ma, J.; Qu, C.; et al. PAX5 is a tumor suppressor in mouse mutagenesis models of acute lymphoblastic leukemia. Blood 2015, 125, 3609–3617. [Google Scholar] [CrossRef]
- Hasim, M.S.; Marotel, M.; Hodgins, J.J.; Vulpis, E.; Makinson, O.J.; Asif, S.; Shih, H.Y.; Scheer, A.K.; MacMillan, O.; Alonso, F.G.; et al. When killers become thieves: Trogocytosed PD-1 inhibits NK cells in cancer. Sci. Adv. 2022, 8, eabj3286. [Google Scholar] [CrossRef]
- Dahan, R.; Sega, E.; Engelhardt, J.; Selby, M.; Korman, A.J.; Ravetch, J.V. FcgammaRs Modulate the Anti-tumor Activity of Antibodies Targeting the PD-1/PD-L1 Axis. Cancer Cell 2015, 28, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Awadasseid, A.; Zhou, Y.; Zhang, K.; Tian, K.; Wu, Y.; Zhang, W. Current studies and future promises of PD-1 signal inhibitors in cervical cancer therapy. Biomed. Pharmacother. 2023, 157, 114057. [Google Scholar] [CrossRef]
- Escudero, A.; Takagi, M.; Auer, F.; Friedrich, U.A.; Miyamoto, S.; Ogawa, A.; Imai, K.; Pascual, B.; Vela, M.; Stepensky, P.; et al. Clinical and immunophenotypic characteristics of familial leukemia predisposition caused by PAX5 germline variants. Leukemia 2022, 36, 2338–2342. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zou, Y.; Ruan, M.; Chang, L.; Chen, X.; Wang, S.; Yang, W.; Zhang, L.; Guo, Y.; Chen, Y.; et al. Pediatric Acute Lymphoblastic Leukemia Patients Exhibit Distinctive Alterations in the Gut Microbiota. Front. Cell Infect. Microbiol. 2020, 10, 558799. [Google Scholar] [CrossRef] [PubMed]
- Buri, M.C.; Shoeb, M.R.; Bykov, A.; Repiscak, P.; Baik, H.; Dupanovic, A.; David, F.O.; Kovacic, B.; Hall-Glenn, F.; Dopa, S.; et al. Natural Killer Cell-Mediated Cytotoxicity Shapes the Clonal Evolution of B-cell Leukemia. Cancer Immunol. Res. 2025, 13, 430–446. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casado-García, A.; García-Aguilera, G.; Pozo, J.; Oak, N.; Barrena, S.; Ruiz-Corzo, B.; Lalchandani, J.; Chamorro-Vera, A.; Castillo-Robleda, A.; Soriano, B.; et al. PD-1 Expression Promotes Immune Evasion in B-ALL. Hematol. Rep. 2025, 17, 61. https://doi.org/10.3390/hematolrep17060061
Casado-García A, García-Aguilera G, Pozo J, Oak N, Barrena S, Ruiz-Corzo B, Lalchandani J, Chamorro-Vera A, Castillo-Robleda A, Soriano B, et al. PD-1 Expression Promotes Immune Evasion in B-ALL. Hematology Reports. 2025; 17(6):61. https://doi.org/10.3390/hematolrep17060061
Chicago/Turabian StyleCasado-García, Ana, Gonzalo García-Aguilera, Julio Pozo, Ninad Oak, Susana Barrena, Belén Ruiz-Corzo, Jaanam Lalchandani, Ana Chamorro-Vera, Ana Castillo-Robleda, Beatriz Soriano, and et al. 2025. "PD-1 Expression Promotes Immune Evasion in B-ALL" Hematology Reports 17, no. 6: 61. https://doi.org/10.3390/hematolrep17060061
APA StyleCasado-García, A., García-Aguilera, G., Pozo, J., Oak, N., Barrena, S., Ruiz-Corzo, B., Lalchandani, J., Chamorro-Vera, A., Castillo-Robleda, A., Soriano, B., Alemán-Arteaga, S., Sánchez, E. G., Martínez-Cano, J., López-Álvarez de Neyra, A., Somoza-Cotillas, P., Blanco, O., Riesco, S., Prieto-Matos, P., García Criado, F. J., ... Sánchez-García, I. (2025). PD-1 Expression Promotes Immune Evasion in B-ALL. Hematology Reports, 17(6), 61. https://doi.org/10.3390/hematolrep17060061







