AI Improves Agreement and Reduces Time for Quantifying Metabolic Tumour Burden in Hodgkin Lymphoma †
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Image Acquisitions
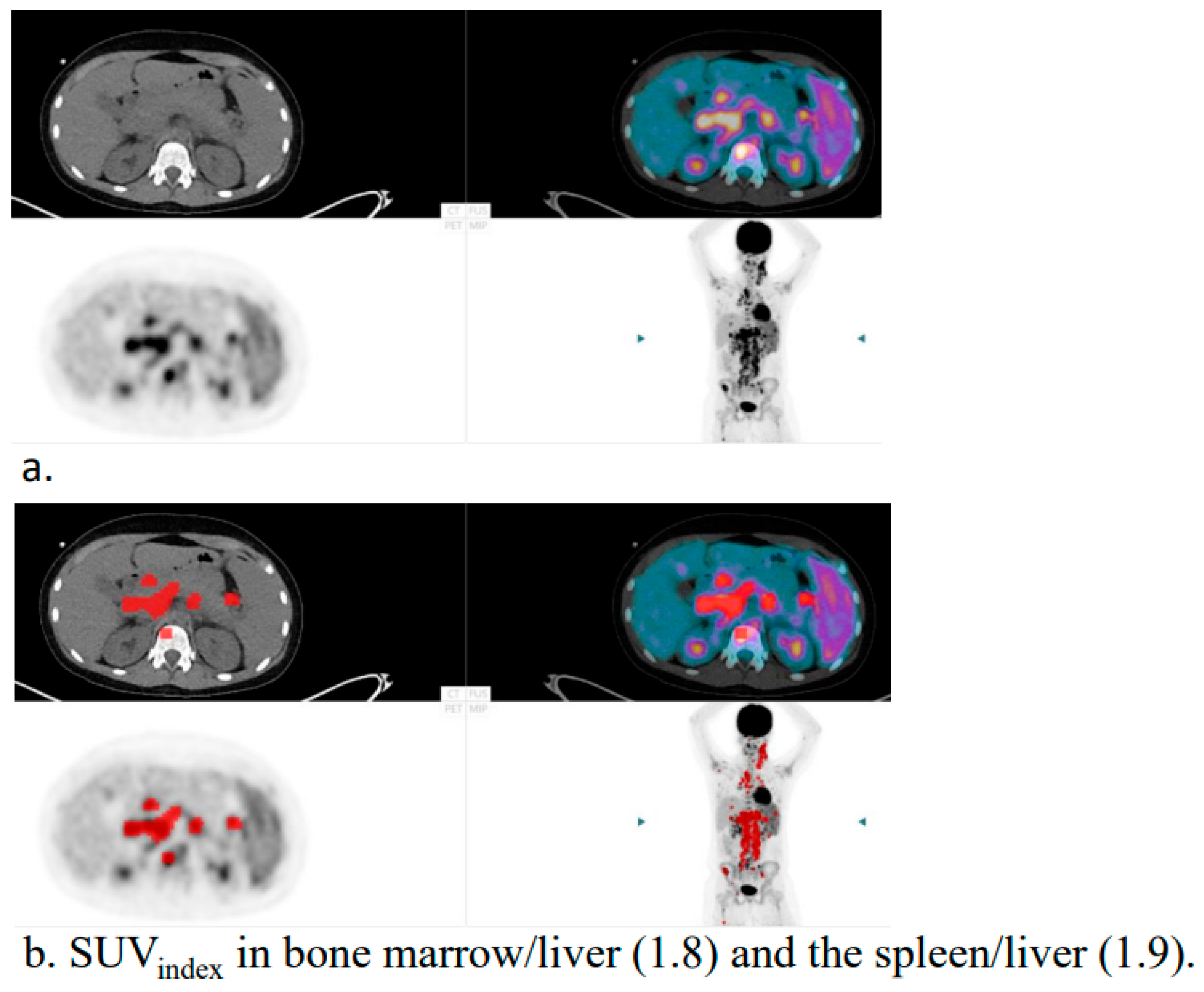
2.3. Image Interpretation
- Viable regions within lymph nodes showing increased FDG uptake;
- Focal FDG uptake in bone marrow or other extranodal sites;
- Focal FDG uptake in the spleen, regardless of splenic size;
- Diffuse splenic uptake exceeding liver uptake (spleen/liver ratio > 1.5 and bone marrow/liver ratio < 1.0), in the absence of reactive bone marrow changes.
2.4. AI Tool
2.5. Statistical Analysis
3. Results
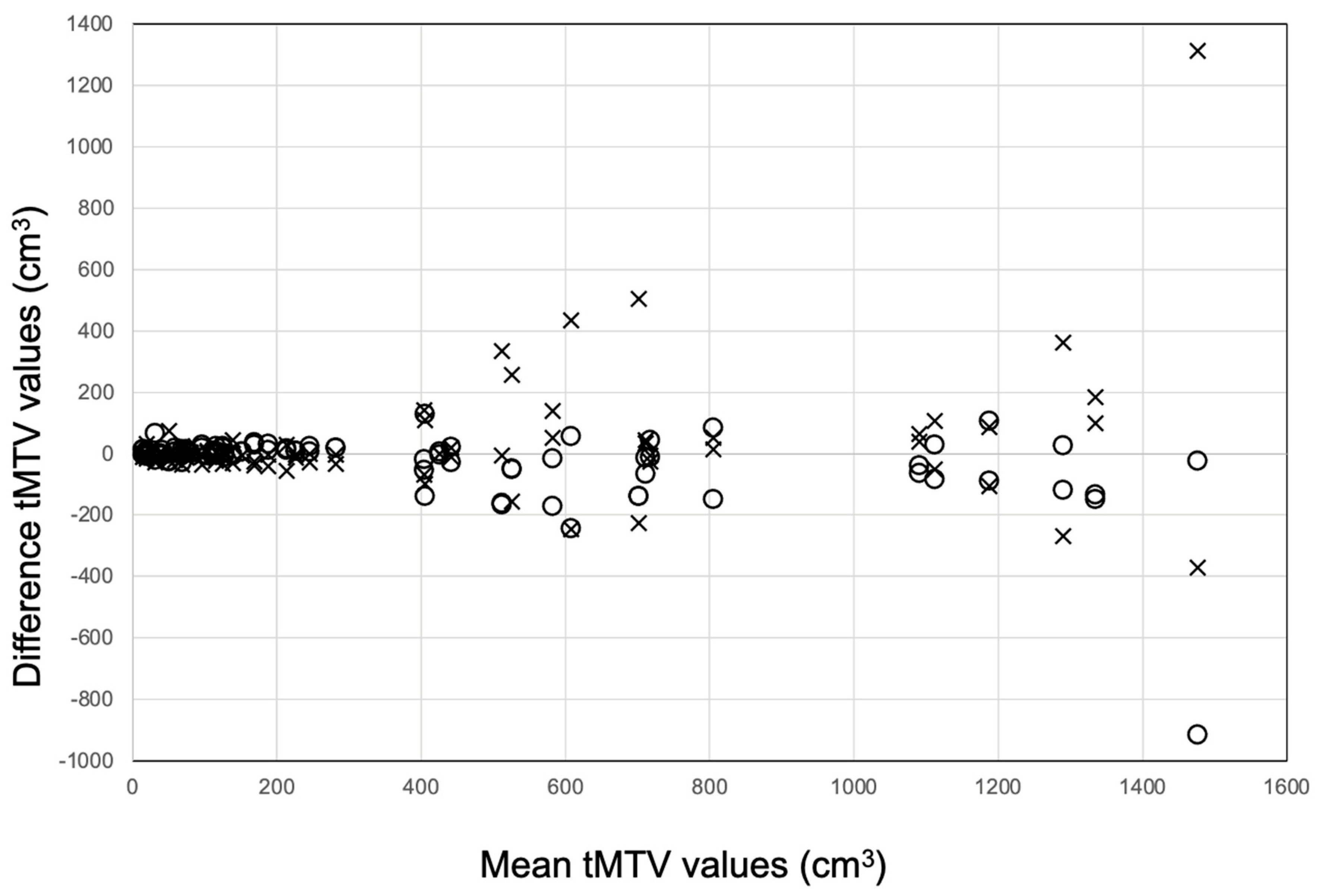
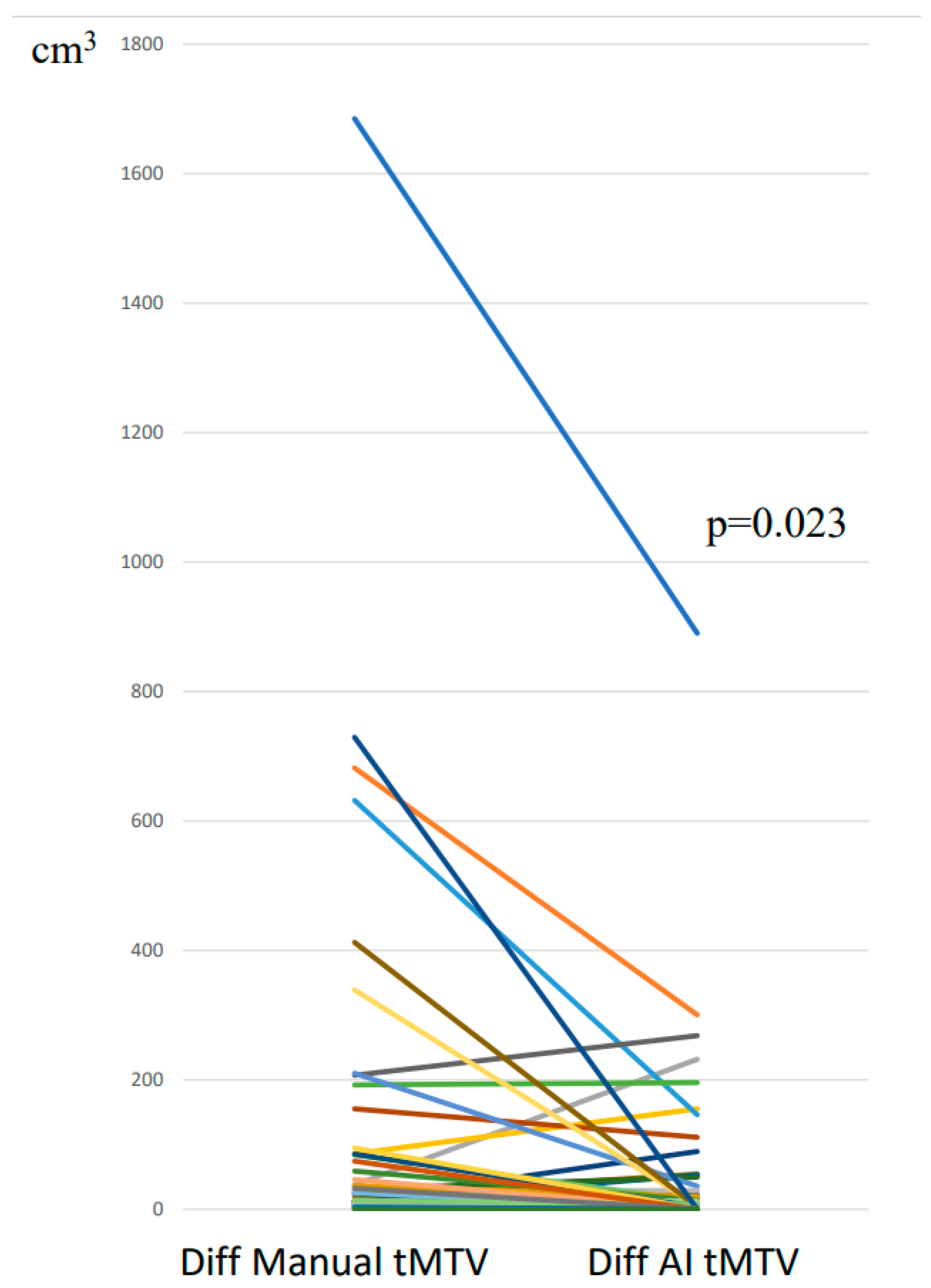
3.1. tMTV: Segmentations with and Without AI Tool
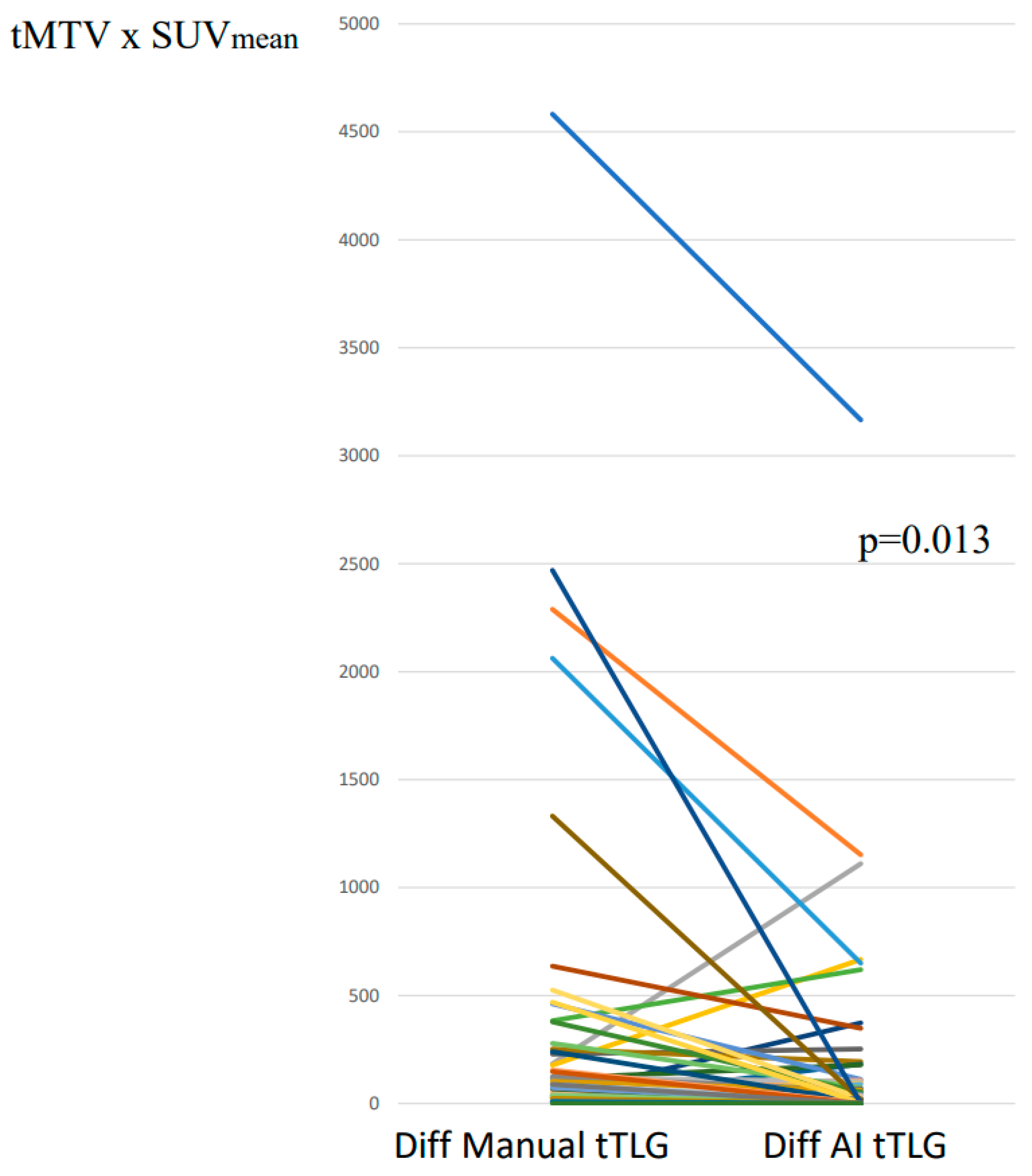
3.2. tTLG: Segmentations with and Without AI-Tool
3.3. Time Registration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrington, S.F.; Meignan, M. Time to Prepare for Risk Adaptation in Lymphoma by Standardizing Measurement of Metabolic Tumor Burden. J. Nucl. Med. 2019, 60, 1096–1102. [Google Scholar] [CrossRef]
- Weisman, A.J.; Kieler, M.W.; Perlman, S.; Hutchings, M.; Jeraj, R.; Kostakoglu, L.; Bradshaw, T.J. Comparison of 11 automated PET segmentation methods in lymphoma. Phys. Med. Biol. 2020, 65, 235019. [Google Scholar] [CrossRef]
- Barrington, S.F.; Zwezerijnen, B.G.J.C.; de Vet, H.C.W.; Heymans, M.W.; Mikhaeel, N.G.; Burggraaff, C.N.; Eertink, J.J.; Pike, L.C.; Hoekstra, O.S.; Zijlstra, J.M.; et al. Automated Segmentation of Baseline Metabolic Total Tumor Burden in Diffuse Large B-Cell Lymphoma: Which Method Is Most Successful? A Study on Behalf of the PETRA Consortium. J. Nucl. Med. 2021, 62, 332–337. [Google Scholar] [CrossRef]
- Pomykala, K.L.; Fendler, W.P.; Vermesh, O.; Umutlu, L.; Herrmann, K.; Seifert, R. Molecular Imaging of Lymphoma: Future Directions and Perspectives. Semin. Nucl. Med. 2023, 53, 449–456. [Google Scholar] [CrossRef]
- Sadik, M.; Barrington, S.F.; Trägårdh, E.; Saboury, B.; Nielsen, A.L.; Jakobsen, A.L.; Gongora, J.L.L.; Urdaneta, J.L.; Kumar, R.; Edenbrandt, L. Metabolic tumour volume in Hodgkin lymphoma-A comparison between manual and AI-based analysis. Clin. Physiol. Funct. Imaging 2024, 44, 220–227. [Google Scholar] [CrossRef]
- Sibille, L.; Seifert, R.; Avramovic, N.; Vehren, T.; Spottiswoode, B.; Zuehlsdorff, S.; Schäfers, M. 18F-FDG PET/CT Uptake Classification in Lymphoma and Lung Cancer by Using Deep Convolutional Neural Networks. Radiology 2020, 294, 445–452. [Google Scholar] [CrossRef]
- Sadik, M.; López-Urdaneta, J.; Ulén, J.; Enqvist, O.; Krupic, A.; Kumar, R.; Andersson, P.O.; Trägårdh, E. Artificial intelligence could alert for focal skeleton/bone marrow uptake in Hodgkin´s lymphoma patients staged with FDG-PET/CT. Sci. Rep. 2021, 17, 10382. [Google Scholar]
- Sadik, M.; Lind, E.; Polymeri, E.; Enqvist, O.; Ulén, J.; Trägårdh, E. Automated quantification of reference levels in liver and mediastinal blood pool for the Deauville therapy response classification using FDG-PET/CT in Hodgkin and non-Hodgkin lymphomas. Clin. Physiol. Funct. Imaging 2019, 39, 78–84. [Google Scholar] [CrossRef]
- Trägårdh, E.; Borrelli, P.; Kaboteh, R.; Gillberg, T.; Ulén, J.; Enqvist, O.; Edenbrandt, L. RECOMIA-a cloud-based platform for artificial intelligence research in nuclear medicine and radiology. EJNMMI Phys. 2020, 7, 51. [Google Scholar] [CrossRef]
- Boellaard, R.; Zwezerijnen, G.J.C.; Buvat, I.; Champion, L.; Hovhannisyan-Baghdasarian, N.; Orlhac, F.; Arens, A.I.J.; Lobeek, D.; Celik, F.; Mitea, C.; et al. Measuring Total Metabolic Tumor Volume from18F-FDG PET: A Reality Check. J. Nucl. Med. 2025, 66, 802–805. [Google Scholar] [CrossRef]
- Boellaard, R.; Buvat, I.; Nioche, C.; Ceriani, L.; Cottereau, A.S.; Guerra, L.; Hicks, R.J.; Kanoun, S.; Kobe, C.; Loft, A.; et al. International Benchmark for Total Metabolic Tumor Volume Measurement in Baseline 18F-FDG PET/CT of Lymphoma Patients: A Milestone Toward Clinical Implementation. J. Nucl. Med. 2024, 65, 1343–1348. [Google Scholar] [CrossRef]
- Prieto Prieto, J.C.; Casas, J.A.V.; Hatzimichael, E.; Fotopoulos, A.; Kiortsis, D.N.; Sioka, C. The contribution of metabolic parameters of FDG PET/CT prior and during therapy of adult patients with lymphomas. Ann. Nucl. Med. 2020, 34, 707–717. [Google Scholar] [CrossRef]
- Meignan, M.; Cottereau, A.S.; Specht, L.; Mikhaeel, N.G. Total tumor burden in lymphoma—An evolving strong prognostic parameter. Br. J. Radiol. 2021, 94, 20210448. [Google Scholar] [CrossRef]
- El-Galaly, T.C.; Villa, D.; Cheah, C.Y.; Gormsen, L.C. Pre-treatment total metabolic tumour volumes in lymphoma: Does quantity matter? Br. J. Haematol. 2022, 197, 139–155. [Google Scholar] [CrossRef]
- Meignan, M.; Cottereau, A.S.; Versari, A.; Chartier, L.; Dupuis, J.; Boussetta, S.; Grassi, I.; Casasnovas, R.O.; Haioun, C.; Tilly, H.; et al. Baseline metabolic tumor volume predicts outcome in high-tumor-burden follicular lymphoma: A pooled analysis of three multicenter studies. J. Clin. Oncol. 2016, 34, 3618–3626. [Google Scholar] [CrossRef]
- Cottereau, A.S.; Versari, A.; Loft, A.; Casasnovas, O.; Bellei, M.; Ricci, R.; Bardet, S.; Castagnoli, A.; Brice, P.; Raemaekers, J.; et al. Prognostic value of baseline metabolic tumor volume in early-stage Hodgkin lymphoma in the standard arm of the H10 trial. Blood 2018, 131, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Mikhaeel, N.G.; Heymans, M.W.; Eertink, J.J.; de Vet, H.C.W.; Boellaard, R.; Dührsen, U.; Ceriani, L.; Schmitz, C.; Wiegers, S.E.; Hüttmann, A.; et al. Proposed New Dynamic Prognostic Index for Diffuse Large B-Cell Lymphoma: International Metabolic Prognostic Index. J. Clin. Oncol. 2022, 40, 2352–2360. [Google Scholar] [CrossRef]
- Weisman, A.J.; Kim, J.; Lee, I.; McCarten, K.M.; Kessel, S.; Schwartz, C.L.; Kelly, K.M.; Jeraj, R.; Cho, S.Y.; Bradshaw, T.J. Automated quantification of baseline imaging PET metrics on FDG PET/CT images of pediatric Hodgkin lymphoma patients. EJNMMI Phys. 2020, 7, 76. [Google Scholar] [CrossRef]
- Anand, A.; Morris, M.J.; Kaboteh, R.; Reza, M.; Trägårdh, E.; Matsunaga, N.; Edenbrandt, L.; Bjartell, A.; Larson, S.M.; Minarik, D. A Preanalytic Validation Study of Automated Bone Scan Index: Effect on Accuracy and Reproducibility Due to the Procedural Variabilities in Bone Scan Image Acquisition. J. Nucl. Med. 2016, 57, 1865–1871. [Google Scholar] [CrossRef]
- Anand, A.; Morris, M.J.; Kaboteh, R.; Båth, L.; Sadik, M.; Gjertsson, P.; Lomsky, M.; Edenbrandt, L.; Minarik, D.; Bjartell, A. Analytic Validation of the Automated Bone Scan Index as an Imaging Biomarker to Standardize Quantitative Changes in Bone Scans of Patients with Metastatic Prostate Cancer. J. Nucl. Med. 2016, 57, 41–45. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Anand, A.; Edenbrandt, L.; Bondesson, E.; Bjartell, A.; Widmark, A.; Sternberg, C.N.; Pili, R.; Tuvesson, H.; Nordle, Ö.; et al. Phase 3 Assessment of the Automated Bone Scan Index as a Prognostic Imaging Biomarker of Overall Survival in Men With Metastatic Castration-Resistant Prostate Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018, 4, 944–951. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadik, M.; Barrington, S.F.; Ulén, J.; Enqvist, O.; Trägårdh, E.; Saboury, B.; Lerberg Nielsen, A.; Loft, A.; Loaiza Gongora, J.L.; Lopez Urdaneta, J.; et al. AI Improves Agreement and Reduces Time for Quantifying Metabolic Tumour Burden in Hodgkin Lymphoma. Hematol. Rep. 2025, 17, 60. https://doi.org/10.3390/hematolrep17060060
Sadik M, Barrington SF, Ulén J, Enqvist O, Trägårdh E, Saboury B, Lerberg Nielsen A, Loft A, Loaiza Gongora JL, Lopez Urdaneta J, et al. AI Improves Agreement and Reduces Time for Quantifying Metabolic Tumour Burden in Hodgkin Lymphoma. Hematology Reports. 2025; 17(6):60. https://doi.org/10.3390/hematolrep17060060
Chicago/Turabian StyleSadik, May, Sally F. Barrington, Johannes Ulén, Olof Enqvist, Elin Trägårdh, Babak Saboury, Anne Lerberg Nielsen, Annika Loft, Jose Luis Loaiza Gongora, Jesus Lopez Urdaneta, and et al. 2025. "AI Improves Agreement and Reduces Time for Quantifying Metabolic Tumour Burden in Hodgkin Lymphoma" Hematology Reports 17, no. 6: 60. https://doi.org/10.3390/hematolrep17060060
APA StyleSadik, M., Barrington, S. F., Ulén, J., Enqvist, O., Trägårdh, E., Saboury, B., Lerberg Nielsen, A., Loft, A., Loaiza Gongora, J. L., Lopez Urdaneta, J., Kumar, R., van Essen, M., & Edenbrandt, L. (2025). AI Improves Agreement and Reduces Time for Quantifying Metabolic Tumour Burden in Hodgkin Lymphoma. Hematology Reports, 17(6), 60. https://doi.org/10.3390/hematolrep17060060






